Abstract
Our recent study showed that bradykinin increases cell cycling progression and migration of human cardiac c‐Kit+ progenitor cells by activating pAkt and pERK1/2 signals. This study investigated whether bradykinin‐mediated Ca2+ signalling participates in regulating cellular functions in cultured human cardiac c‐Kit+ progenitor cells using laser scanning confocal microscopy and biochemical approaches. It was found that bradykinin increased cytosolic free Ca2+ () by triggering a transient Ca2+ release from ER IP3Rs followed by sustained Ca2+ influx through store‐operated Ca2+ entry (SOCE) channel. Blockade of B2 receptor with HOE140 or IP3Rs with araguspongin B or silencing IP3R3 with siRNA abolished both Ca2+ release and Ca2+ influx. It is interesting to note that the bradykinin‐induced cell cycle progression and migration were not observed in cells with siRNA‐silenced IP3R3 or the SOCE component TRPC1, Orai1 or STIM1. Also the bradykinin‐induced increase in pAkt and pERK1/2 as well as cyclin D1 was reduced in these cells. These results demonstrate for the first time that bradykinin‐mediated increase in free via ER‐IP3R3 Ca2+ release followed by Ca2+ influx through SOCE channel plays a crucial role in regulating cell growth and migration via activating pAkt, pERK1/2 and cyclin D1 in human cardiac c‐Kit+ progenitor cells.
Keywords: bradykinin; cell cycle progression; human cardiac c‐Kit+ progenitor cells; inositol 1,4,5‐triphosphate receptor; migration; store‐operated Ca2+ entry
1. INTRODUCTION
Cardiac progenitor cells, also known as cardiac stem cells, are self‐renewing, clonogenic and pluripotent and can differentiate into a minimum of three cell types including cardiomyocytes, vascular smooth muscle cells and vascular endothelial cells.1 Among cardiac progenitor cells, c‐Kit+ progenitor cells are a widely studied cell type for myocardial repair. They reconstitute the myocardium with new vessels and myocytes after being injected into the ischaemic heart.2, 3 Therefore, the cardiac c‐Kit+ progenitor cell is believed to be a cell source for transplantation to treat ischaemic cardiomyopathy. A clinical trial showed that the cardiac c‐Kit+ progenitor cells isolated from patients with ischaemic cardiomyopathy may significantly improve heart function and the quality of life when transplanted back into the patients via intracoronary injection;4, 5 however, current cell therapy with adult cardiac c‐Kit+ progenitor cells for ischaemic cardiomyopathy is limited by the poor survival and retention in the heart and the lack of sufficient de novo differentiation of transplanted stem cells into mature cardiac cell types,6, 7, 8 which is at least in part due to the poor understanding of cellular biology and physiology of cardiac c‐Kit+ progenitor cells.
An earlier study reported that spontaneous oscillations of cytosolic free Ca2+ () play an important role in regulating human cardiac c‐Kit+ progenitor cell growth.9 Our recent study demonstrated that the endogenous peptide bradykinin promotes cell cycling progression and migration via activating PI3K, phospholipase C (PLC), pAkt, pERK1/2 and cyclin D1 in human cardiac c‐Kit+ progenitor cells.10 However, it is unknown whether bradykinin‐induced cycle progression and migration are related to Ca2+ signalling in human cardiac c‐Kit+ progenitor cells. This study was therefore designed to investigate the detailed molecular mechanisms of whether/how Ca2+ signalling‐related channels/receptors are involved in bradykinin‐induced increase in cell growth and migration in human cardiac c‐Kit+ progenitor cells with multiple biochemical/molecular biological approaches. Our results demonstrated that bradykinin‐induced increase by activating Ca2+ release via inositol triphosphate receptor 3 (IP3R3) followed by Ca2+ influx through store‐operated Ca2+ entry (SOCE) channel, which plays a crucial role in regulating cell cycle progression and migration via activating pAkt, pERK1/2 and cyclin D1 in human cardiac c‐Kit+ progenitor cells.
2. MATERIALS AND METHODS
2.1. Experimental solutions and reagents
The experimental solutions and reagents used in this study were described in online Supporting Information (Supplementary Materials and Methods).
2.2. Cell culture
Human cardiac c‐Kit+ progenitor cells were isolated from human atrial specimens from patients undergoing coronary artery bypass surgery as described previously.11, 12, 13, 14 The tissue collection was approved by the Ethics Committee of the University of Hong Kong (UW‐10‐174) with patients’ consent. The study conforms with the declaration of Helsinki the Declaration of Helsinki (see Cardiovascular Research 1997;35:2‐4) for using human tissue. The cells were maintained in α‐MEM supplemented with 15% FBS, 2 mmol L−1 l‐glutamine, 5 ng/mL bFGF, 5 ng/mL EGF, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. The cells at 3‐6 passages used in this study were from 2 female patients (54 and 56 years old) and 2 male patients (48 and 61 years old).
2.3. Cytosolic Ca2+ measurement
Cytosolic free Ca2+ () was measured with a laser scanning confocal microscopy technique in human cardiac c‐Kit+ progenitor cells loaded with the free Ca2+ indicator Fluo‐4 AM (Thermo Fisher Scientific Inc.) following manufacturer's instruction.13 Briefly, cells were seeded on 35‐mm plates at a density of approximately 1 × 104 cells for 24 hours, and then loaded with 1 μmol L−1 Fluo‐4 AM in FBS‐free α‐MEM for 30 minutes at 37°C in the dark. Afterwards, the cells were washed three times with Tyrode's solution to remove the residual fluorescent dye. was monitored every 5 seconds using the laser scanning confocal microscope Leica SP5‐II at room temperature (23‐25°C).
2.4. Small interfering RNA
Gene silencing was conducted with small interfering RNA (siRNA) technique as described previously.11, 13 Briefly, human cardiac c‐Kit+ progenitor cells were seeded in six‐well plates or 96‐well plates at a confluence of 60%‐80% overnight. Then the cells were transfected with different siRNA molecules (Santa Cruz Biotech) at 10 or 40 nmol L−1 using Lipofectamine 2000 reagent (Thermo Fisher Scientific) for 48‐72 hours. The control siRNA, which had no known target in the human genome, was used as negative control.
2.5. Reverse transcription‐polymerase chain reaction
Reverse transcription‐polymerase chain reaction was employed to determine mRNA expression in cells with silenced IP3Rs, TRPC channels or SOCE channels for siRNA efficacy as described previously.10, 13 Briefly, total RNA was extracted from human cardiac c‐Kit+ progenitor cells transfected with corresponding siRNA for 48 hours using TRIzol reagent. The amount of total RNA was quantified by spectrophotometry, and reverse transcription reaction was performed using 2 μg of total RNA to transcribe into complementary DNA with Advantage® RT‐for‐PCR Kit (Takara biotech Co., Ltd, Dalian, China) following manufacturer's instruction. Primers for the corresponding targets are shown online in Supporting Information (Table S1).
2.6. Cell proliferation assay
Cell proliferation was detected with 3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyltetrazolium bromide (MTT) and 5‐bromo‐2‐deoxy uridine (BrdU) in human cardiac c‐Kit+ progenitor cells transfected with siRNAs targeting IP3Rs, TRPCs and SOCEs for 60 hours as described previously11, 12, 13, 14 and online in Supporting Information (Materials and Methods).
2.7. Flow cytometry analysis
The cell cycle distribution involved in the proliferation process was detected by flow cytometry in human cardiac c‐Kit+ progenitor cells as described previously.11, 12, 13, 14 Briefly, cells were dissociated with 0.25% trypsin, washed three times with phosphate‐buffered saline (PBS) and fixed with cold 70% ethanol at 4°C over night. The ethanol was removed by centrifuge, and the cell pellets were washed with PBS for three times. Then, the propidium iodide/PBS staining buffer (propidium iodide 20 μg/mL, RNase A 10 μg/mL and 0.1% Triton‐X 100) was used to stain the cells at 37° for 30 minutes. Data were acquired with a Beckman Coulter FC500, and the percentages of G0/G1‐phase, S‐phase and G2/M‐phase cells were calculated with MODFIT LT software (BD Biosciences, San Jose, CA, USA).
2.8. Cell mobility assay
The effects of bradykinin on human cardiac c‐Kit+ cells transfected with corresponding siRNA were determined with wound‐healing and transwell assay as described previously11, 12, 13, 14 and online in Supporting Information (Materials and Methods).
2.9. Western blot analysis
Western blot was conducted to determine the protein expression in human cardiac c‐Kit+ progenitor cells as described previously.10 The silencing efficiency of IP3Rs, TRPC channels, STIM1 and Orai1 was determined in cells transfected with the corresponding siRNAs for 72 hours, and cyclin D1, Akt and ERK1/2 as well as their phosphorylated levels were determined in these cells. Briefly, the cells were lysed with RIPA buffer and mixed with sample buffer, heated to 95°C for at least 5 minutes and cooled on ice. Samples were electrophoresed on SDS‐PAGE gels and then transferred onto PVDF membranes (Bio‐Rad, Hercules, CA, USA). The membranes were blocked with 5% non‐fat milk or 5% bovine serum albumin in Tween‐20 Tris‐buffer saline buffer (TTBS). Then, the membranes were incubated with corresponding primary antibodies at dilutions 1:1000 to 1:2000 in blocking buffer at 4°C overnight with agitation. After washed with TTBS, the membranes were then incubated with HRP‐conjugated secondary antibody at dilutions 1:5000‐1:10 000 at room temperature for 1 hour with agitation. The membranes were then washed 3 times with TTBS. Electrogenerated chemiluminescence was applied to develop X‐ray film with the membranes. The relative band intensities of Western blots were measured by quantitative scanning densitometry and the image analysis using Image J (https://imagej.nih.gov/ij/).
2.10. Statistical analysis
All data were presented as mean ± SEM. Unpaired Student's t test was used as appropriate to evaluate the statistical significance of differences between two group means. One‐way ANOVA followed by Tukey's test was applied for multiple group comparison. A value of P < .05 was considered to indicate statistical significance.
3. RESULTS
3.1. Effects of bradykinin on in human cardiac c‐Kit+ progenitor cells
In our previous study, bradykinin at 10 nmol L−1 remarkably stimulates cell growth and migration.10 We therefore utilize the 10 nmol L−1 bradykinin in this study to investigate whether the stimulation of cell growth and migration is linked to Ca2+ signalling induced by bradykinin.
Figure 1A illustrates that bradykinin induces a significant increase in in human cardiac c‐Kit+ progenitor cells. Changes in induced by bradykinin show a quick transient increase followed by a sustained low level increase in (Figure 1A), and the effect was fully prevented by the bradykinin type 2 receptor (B2R) antagonist HOE140 (30 nmol L−1), but not the B1R antagonist R715 (300 nmol L−1) (Figure 1B). Omitting extracellular Ca2+ (Ca2+‐free medium plus 1 mmol L−1 EGTA) abolished the sustained component without affecting the transient increase by bradykinin (Figure 1C). These results suggest that the sustained increase is dependent on Ca2+ influx and the transient increase results from Ca2+ release from intracellular calcium stores, similar to previous observations in cultured human foreskin fibroblasts15, 16, 17 but different from observations in afferent sensory neurons18 and vascular endothelial cells.19
Figure 1.
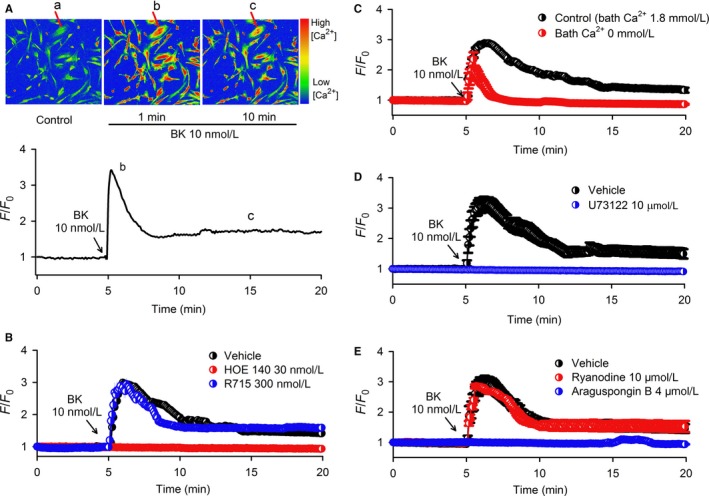
Increase in by bradykinin (BK) in human cardiac c‐kit+ progenitor cells. A, Pseudocolour images (upper panel) showing changes in fluorescence intensity (ie ) induced by 10 nmol L−1 bradykinin at different time points indicated in the lower panel in human cardiac c‐Kit+ progenitor cells. The F/F0 represents level, where the F is the changes in cell fluorescence intensity of Fluo‐4 AM, and the F0 is the initial level of fluorescence intensity. B, increase induced by bradykinin was prevented in cells pre‐treated with 30 nmol L−1 HOE140 (B2R inhibitor) (n = 80 cells of 5 experiments), but not the B1R inhibitor R715 (300 nmol L−1) (n = 80 cells of 5 experiments). C, Only a transient increase induced by bradykinin was observed in cells exposure to Ca2+‐free Tyrode's solution with 1 mmol L−1 EGTA (n = 96 cells of 6 experiments). D, Bradykinin‐induced Ca2+ increase was absent in cells pre‐treated with the PLC blocker U73122 (10 μmol L−1) (n = 80 cells of 5 experiments). E, increase induced by bradykinin was abolished by the IP3R blocker araguspongin B (4 μmol L−1) (n = 80 cells of 5 experiments), but not by the RyR inhibitor ryanodine (10 μmol L−1) (n = 96 cells of 6 experiments). Traces are shown as mean ± SEM in corresponding experiments
To analyse the molecular mechanisms underlying Ca2+ signalling by bradykinin in human cardiac c‐Kit+ progenitor cells, we utilized pharmacological tools followed by siRNA approach to identify the molecules related to intracellular Ca2+ release from calcium stores and/or Ca2+ influx.
3.2. Effects of pharmacological inhibitors on Ca2+ signalling induced by bradykinin
Figure 1D,E shows that increase by bradykinin was abolished in cells pre‐treated with the PLC inhibitor U73122 (10 μmol L−1) or the inositol 1,4,5‐trisphosphate (IP3) receptor (IP3R) inhibitor araguspongin B20 (4 μmol L−1), but not ryanodine receptor (RyR) inhibitor ryanodine (10 μmol L−1). In addition, we found that cardiac RyR2 protein was expressed in human atria, but not in human cardiac c‐Kit+ progenitor cells (Supporting Information Figure S1). These results support the notion that in human cardiac c‐Kit+ progenitor cells, as in human skin fibroblasts,16 B2R activation stimulates PLC and generates IP3, which then activates ER IP3Rs to release Ca2+ followed by Ca2+ influx.
To determine the possible ion channel involvement in Ca2+ influx by bradykinin, pharmacological blockers for different channels that mediate Ca2+ entry were tested in human cardiac c‐Kit+ progenitor cells. An earlier study reported that bradykinin‐induced Ca2+ influx was mediated by L‐type Ca2+ channels in cultured human skin fibroblasts.15 However, this is not the case in human cardiac c‐Kit+ progenitor cells, as bradykinin‐induced increase was not affected by pre‐treatment with the L‐type Ca2+ channel blocker nifedipine (10 μmol L−1) (Figure 2A). This is supported by Western blot analysis which showed that cardiac Cav1.2 protein was expressed in human atria, but not in human cardiac c‐Kit+ progenitor cells (Supporting Information Figure S1). In addition, we tested whether the reverse‐mode of Na+/Ca2+ exchanger mediates the agonist‐induced Ca2+ entry by pre‐treating the cells with 10 μmol L−1 KB‐R7943 (an inhibitor of the reverse Na+/Ca2+ exchanger). The bradykinin‐induced increase was also not affected in these cells (Figure 2A), suggesting that the reverse‐mode of Na+/Ca2+ exchanger is not involved in the increase.
Figure 2.
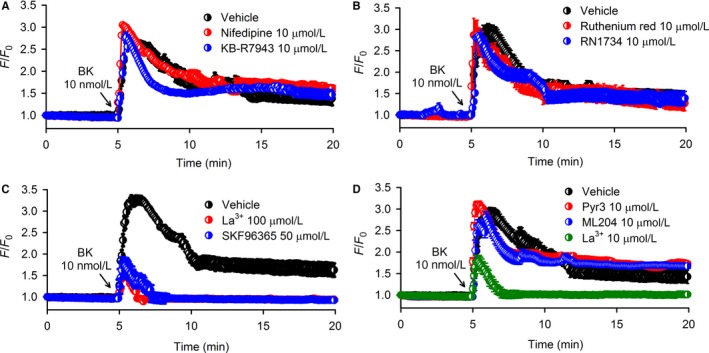
Effects of membrane channel blockers on increase by bradykinin (BK) in human cardiac c‐Kit+ progenitor cells. A, increase was not affected by the L‐type Ca2+ channel inhibitor nifedipine (10 μmol L−1) (n = 96 cells of 6 experiments) or NCX inhibitor KB‐R7943 (10 μmol L−1) (n = 96 cells of 6 experiments). B, increase was not affected by the TRPV2 channel blocker ruthenium red (10 μmol L−1) (n = 96 cells of 6 experiments) or the TRPV4 blocker RN1734 (10 μmol L−1) (n = 96 cells of 6 experiments). C, General TRP channel blocker La3+ (100 μmol L−1) (n = 96 cells of 6 experiments) or the non‐selective TRPC channel blocker SKF96365 (50 μmol L−1) (n = 80 cells of 5 experiments) inhibited the sustained Ca2+ influx, but not Ca2+ transient release induced by bradykinin. D, Selective SOCE channel blocker (La3+, 10 μmol L−1), but not selective TRPC3 blocker (Pyr3, 10 μmol L−1) (n = 96 cells of 6 experiments) or selective TRPC4 channel blocker (ML204, 10 μmol L−1) (n = 96 cells of 6 experiments), inhibited the sustained Ca2+ influx, but not Ca2+ transient release induced by bradykinin. Summarized traces are shown as mean ± SEM in corresponding experiments
Our previous study demonstrated that functional TRPV2 and TRPV4 channels were predominantly expressed in human cardiac c‐Kit+ progenitor cells.11 However, TRPV2 and TRPV4 channels are not involved in bradykinin‐induced increase because the increase was not affected by the TRPV2 blocker ruthenium red (10 μmol L−1) or the TRPV4 blocker RN1734 (10 μmol L−1) (Figure 2B). In cells pre‐treated with the broad spectrum TRP channel blocker La3+ (100 μmol L−1) or the non‐selective TRPC channel blocker SKF96365 (50 μmol L−1), bradykinin‐induced a transient increase (Figure 2C), suggesting that the Ca2+ influx is mediated by TRPC channels.
We then tested the selective TRPC3 blocker Pyr321 and the selective TRPC4 blocker ML204;22 however, neither Pyr3 (10 μmol L−1) nor ML204 (10 μmol L−1) affected the Ca2+ activity induced by bradykinin (Figure 2D). This implies that TRPC3 and TRPC4 are not involved in mediating bradykinin‐induced Ca2+ influx. La3+ is a well‐known TRP channel blocker; however, a recent study reported that a low concentration of La3+ at 10 μmol L−1 can selectively block SOCE channel.23 We therefore used 10 μmol L−1 La3+ to test whether SOCE channel is involved in mediating the bradykinin‐induced Ca2+ influx. The results (Figure 2D) show that 10 μmol L−1 La3+ fully inhibited bradykinin‐induced Ca2+ influx without affecting ER IP3R Ca2+ release, indicating that bradykinin‐induced Ca2+ influx is mediated by SOCE channel in human cardiac c‐Kit+ progenitor cells.
3.3. Effects of siRNAs targeting IP3Rs and TRPC channels on Ca2+ signalling by bradykinin
To further determine the molecular identities of proteins involved in bradykinin‐mediated Ca2+ signalling in human cardiac c‐Kit+ progenitor cells, was determined in cells transfected with siRNA targeting B2R, IP3R1, IP3R2, IP3R3, TRPC1, TRPC3 or TRPC4, and also the SOCE channel components STIM1 (Stromal interaction molecule 1) and Orai1 (calcium release‐activated calcium channel protein 1). Genes and proteins of B2R, IP3R1, IP3R2, IP3R3, TRPC1, TRPC3, TRPC4, Orai1 and STIM1 were remarkably reduced in cells transfected with 10 and 40 nmol L−1 of the corresponding siRNAs (n = 5, P < .01 vs control siRNA) (Supporting Information Figures S2‐S4).
Bradykinin could not induce increase in cells with silenced B2R (Figure 3A). Interestingly, bradykinin‐induced increase was significant in cells with silenced IP3R1 or IP3R2, but not in cells with silenced IP3R3 (Figure 3B). This indicates that the bradykinin‐induced increase in human cardiac c‐Kit+ progenitor cells is initiated by activating ER IP3R3 to release Ca2+ from intracellular calcium stores followed by Ca2+ influx.
Figure 3.
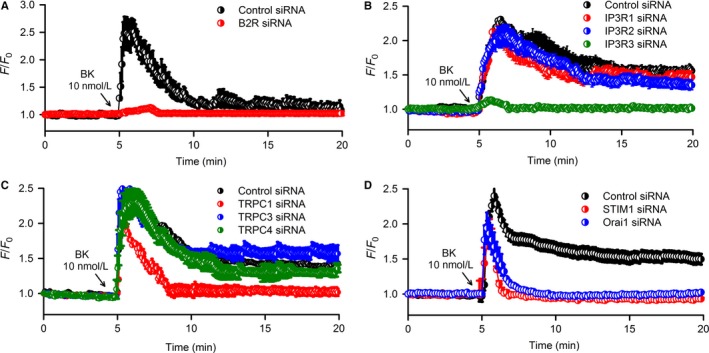
Effects of silencing related genes with corresponding siRNAs on increase induced by bradykinin (BK). A, Bradykinin did not induce increase in cells with silenced B2R (n = 80 cells of 5 experiments). B, Silencing IP3R3, but not IP3R1 or IP3R2, prevented the increase induced by bradykinin (n = 80 cells of 5 experiments). C, Silencing TRPC1, but TRPC3 or TRPC4, prevented the Ca2+ influx without affecting transient Ca2+ release induced by bradykinin (n = 96 cells of 6 experiments). D, Silencing Orai1 or STIM1 prevented the Ca2+ influx without affecting transient Ca2+ release induced by bradykinin (n = 96 cells of 6 experiments). Summarized traces are shown as mean ± SEM in corresponding experiments
Bradykinin‐induced Ca2+ influx through TRPC channel was further tested in cells with silenced TRPC1, TRPC3 or TRPC4 (Figure 3C), in which the Ca2+ influx was abolished only in cells transfected with TRPC1 siRNA, but not TRPC3 siRNA or TRPC4 siRNA.
Our recent study demonstrated that TRPC1, Orai1 and STIM1 are components of SOCE channel complex in human cardiac c‐Kit+ progenitor cells.13 If bradykinin‐induced Ca2+ influx is mediated solely by TRPC1 channel, the effect would not be affected in cells with silenced Orai1 or STIM1. However, in the cells with silenced Orai1 or STIM1, as in the cells with silenced TRPC1, bradykinin only induced a transient ER IP3R3 Ca2+ release without a subsequent Ca2+ influx. These results indicate that bradykinin‐induced Ca2+ influx is mediated by SOCE channel and depends on the integrity of channel complex.
3.4. Effects of silencing molecules of IP3Rs or SOCE on cell cycling progression and mobility
We have recently demonstrated that SOCE channel participates in regulating cell growth and migration,13 and bradykinin promotes cell cycling progression and mobility in cultured human cardiac c‐Kit+ progenitor cells.10 To investigate whether the stimulation of cell cycling progression and mobility by bradykinin is related to Ca2+ signalling. The effects of bradykinin on cell proliferation and migration were determined in cells with silenced IP3Rs and components molecules of SOCE channel.
Figure 4 illustrates the effects of bradykinin on cell proliferation determined by MTT (Figure 4A) and BrdU (Figure 4B,C) assays in cells transfected with corresponding siRNAs. Bradykinin 10 nmol L−1 significantly stimulated cell growth in cells with silenced IP3R1, IP3R2, TRPC3 or TRPC4 (n = 5 for each group, P < .01 vs vehicle) but had no such effect in cells with silenced IP3R3, TRPC1, Orai1 or STIM1 (n = 5 for each group, P = NS vs vehicle; P < .01 vs control siRNA with bradykinin 10 nmol L−1). These results indicate that the enhancement of cell growth by bradykinin in human cardiac c‐Kit+ progenitor cells is related to the Ca2+ signalling.
Figure 4.
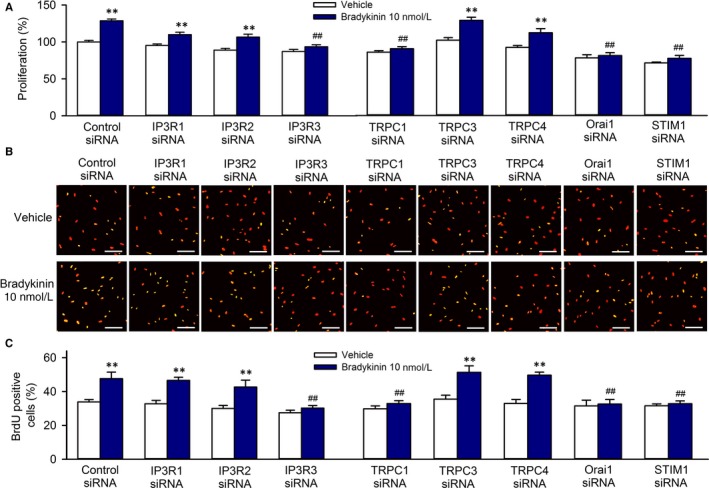
Effects of silencing IP3Rs or components of SOCE channel on cell growth and migration induced by bradykinin (BK). A, Cell proliferation determined with MTT assay in cells transfected with 40 nmol L−1 siRNAs targeting IP3R1, IP3R2, IP3R3, TRPC1, TRPC3, TRPC4, Orai1 or STIM1 in the absence or presence of 10 nmol L−1 bradykinin. B, Images of BrdU incorporation in cells transfected with 40 nmol L−1 siRNAs targeting IP3R1, IP3R2, IP3R3, TRPC1, TRPC3, TRPC4, Orai1 or STIM1 in the absence or presence of 10 nmol L−1 bradykinin. The proliferative cells show yellow colour from the merging of BrdU green staining with Propidium Iodide (PI) red nuclei staining. C, Percentage values of BrdU incorporation in cells transfected with 40 nmol L−1 siRNAs targeting IP3R1, IP3R2, IP3R3, TRPC1, TRPC3, TRPC4, Orai1 or STIM1 in the absence or presence of 10 nmol L−1 bradykinin. N = 5 experiments, **P < .01 vs control siRNA, ## P < .01 vs control siRNA with 10 nmol L−1 bradykinin
The cell cycling progression was then determined by flow cytometry analysis in cells with silenced IP3Rs, TRPCs, STIM1 or Orai1 in the absence or presence of bradykinin (Figure 5). Bradykinin significantly promoted cell cycling progression by decreasing G0/G1 population and increasing S‐phase population in cells transfected with control, IP3R1, IP3R2, TRPC3 or TRPC4 siRNA (40 nmol L−1), but not in cells transfected with IP3R3, TRPC1, STIM1 or Orai1 siRNA. These results indicate that bradykinin‐induced promotion of cell cycling progression is related to increase mediated by IP3R3 and SOCE channel in human cardiac c‐Kit+ progenitor cells.
Figure 5.
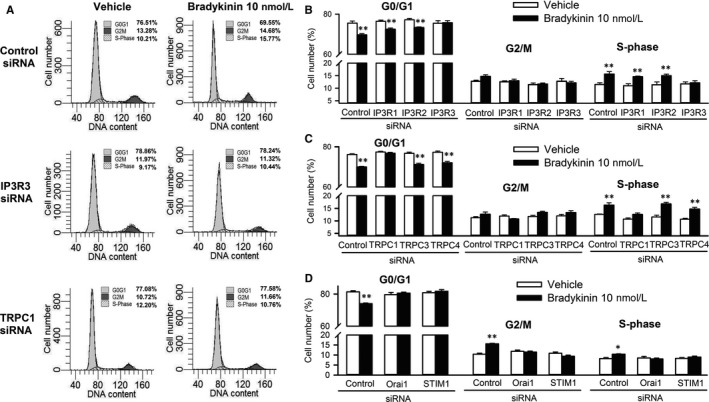
Effects of silencing IP3Rs or components of SOCE channel on cell cycling progression induced by bradykinin (BK). A, Representative flow cytometry graphs in cells transfected with control, IP3R3 or TRPC1 siRNA (40 nmol L−1) in the absence or presence of 10 nmol L−1 bradykinin. B, Percentage values of cell population at different cycling phases in cells transfected with control, IP3R1, IP3R2 or IP3R3 siRNA (40 nmol L−1) in the absence or presence of 10 nmol L−1 bradykinin. C, Percentage values of cell population at different cycle phases in cells transfected with control, TRPC1, TRPC3 or TRPC4 siRNA (40 nmol L−1) in the absence or presence of 10 nmol L−1 bradykinin. D, Percentage values of cell population at different cycle phases in cells transfected with control, Orai1 or STIM1 siRNA (40 nmol L−1) in the absence or presence of 10 nmol L−1 bradykinin treatment. N = 5, experiments *P < .05, **P < .01 vs control siRNA
The effects of bradykinin on cell migration were determined with wound‐healing assay (Figure 6A,B) and transwell assay (Figure 6C,D) in cells transfected with 40 nmol L−1 control, IP3R1, IP3R2, IP3R3, TRPC1, TRPC3, TRPC4, Orai1 or STIM1 siRNA molecules. The cells migrated to the acellular area or the lower membrane surface of transwell were increased by bradykinin in cells transfected with control, IP3R1, IP3R2, TRPC3 or TRPC4 siRNA, but not in cells transfected with IP3R3, TRPC1, Orai1 or STIM1 siRNA. These results indicate that cell migration induced by bradykinin is related to increase via both IP3R3 and SOCE channel in human cardiac c‐Kit+ progenitor cells.
Figure 6.
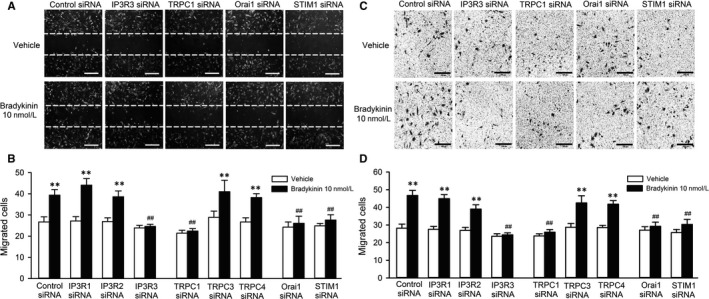
Effects of silencing IP3Rs or components of SOCE channel on migration induced by bradykinin (BK). A, Representative images of wound‐healing assay in cells transfected with control, IP3R3, TRPC1, Orai1 or STIM1 siRNA in the absence or presence of 10 nmol L−1 bradykinin treatment for 8 h. B, Mean values of cell number migrated into the acellular area in cells transfected corresponding siRNAs in the absence and presence of 10 nmol L−1 bradykinin. C, Images of migrated cells on the lower surface membrane in transwell assay in cells transfected with control, IP3R3, TRPC1, Orai1 or STIM1 siRNA in the absence or presence of 10 nmol L−1 bradykinin treatment for 8 h. D, Mean number of migrated cells on the lower surface membrane in cells transfected corresponding siRNAs in the absence or presence of 10 nmol L−1 bradykinin. N = 6 experiments, **P < .01 vs control siRNA, ## P < .01 vs control siRNA with 10 nmol L−1 bradykinin
3.5. Ca2+ signalling by bradykinin and intracellular signal molecules is involved in regulating cell cycling progression and migration
Our recent study has showed that activation of pAkt, pERK1/2 and cyclin D1 is involved in regulation of proliferation and/or migration by bradykinin in human cardiac c‐Kit+ progenitor cells.10 To determine whether pAkt, pERK1/2 and cyclin D1 expression is mediated by increase, these signal molecules were determined in cells with silenced IP3Rs, TRPCs and SOCE components. Figure 7 illustrates the Western blot analysis of pAkt, pERK1/2 and cyclin D1 in cells transfected with IP3Rs, TRPCs or SOCE siRNAs. The increased expression of pAkt, pERK1/2 and cyclin D1 by bradykinin was seen in cells transfected with control, IP3R1, IP3R2, TRPC3 or TRPC4 siRNA, but not in cells transfected with IP3R3, TRPC1, Orai1 or STIM1 siRNA. These results support the notion that stimulation of cell cycling progression and migration by bradykinin is related to activating pAkt, pERK1/2 and cyclin D1 by increasing increase in human cardiac c‐Kit+ progenitor cells.
Figure 7.
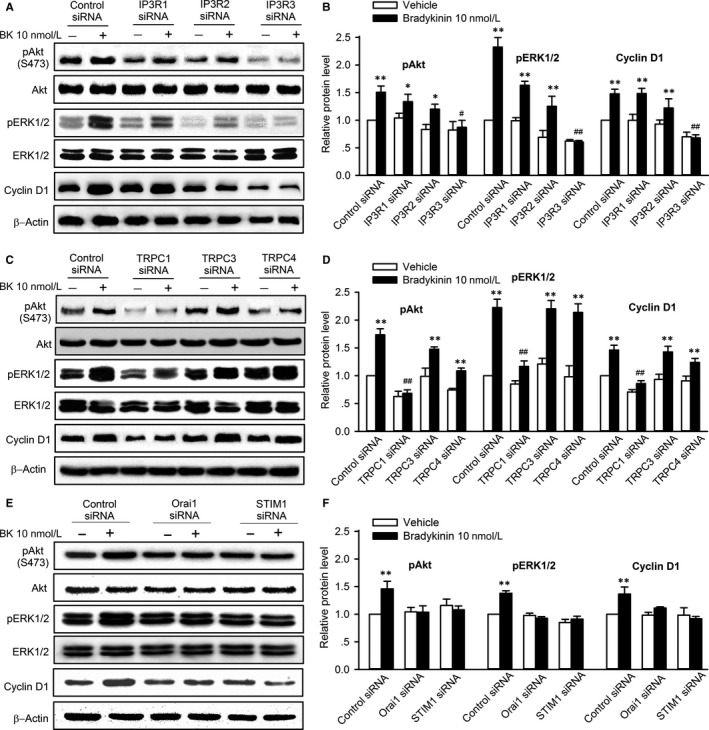
Effects of silencing IP3Rs or components of SOCE channel on increase of pAkt, pERK1/2 and cyclin D1 by bradykinin (BK). A, Western blots show that bradykinin (10 nmol L−1) increased pAkt, pERK1/2 or cyclin D1 expression in cells transfected with control, IP3R1 and IP3R2 siRNA, but not with IP3R3 siRNA. B, Relative protein levels of pAkt, pERK1/2 and cyclin D1 in cells transfected with corresponding IP3R siRNAs. C, Western blots show that bradykinin (10 nmol L−1) increased pAkt, pERK1/2 and cyclin D1 level in cells transfected with control, TRPC3 or TRPC4 siRNA, but not with TRPC1 siRNA. D, Relative protein levels of pAkt, pERK1/2 and cyclin D1 in cells transfected with control, TRPC1, TRPC3 or TRPC4 siRNA. E, Western blots show that bradykinin (10 nmol L−1) increased pAkt, pERK1/2 and cyclin D1 level in cells transfected with control siRNA, but not with Orai1 or STIM1 siRNA. F, Relative protein levels of pAkt, pERK1/2 and cyclin D1 in cells transfected with control, Orai1 or STIM1 siRNA. N = 5, **P < .01 vs control siRNA treated with vehicle, ## P < .01 vs control siRNA with 10 nmol L−1 bradykinin
4. DISCUSSION
Although human cardiac c‐Kit+ progenitor cells is a promising cell source for repairing ischaemic cardiomyopathy,4, 5 one of limitations for this regenerative therapy is the poor survival rate of transplanted cells in the host myocardium.6, 7, 8 Previous studies have explored different strategies to enhance the survival rate of the donor cells using growth factor genes24 or preconditioning donor stem cells with different interventions including nitric oxide donor,25 HO‐1 inducer CoPP26 and exposure to hypoxia.27, 28 Our recent report10 and the present study demonstrated that the endogenous peptide bradykinin can initiate proliferation and migration of human cardiac c‐Kit+ progenitor cells.
The present study has demonstrated that stimulation of cell cycling progression and migration by bradykinin is related to increase via Ca2+ release‐induced Ca2+ influx mechanism: (1) bradykinin binds to B2R to stimulate PLC and generate IP3, (2) IP3 activates ER IP3R3 to release Ca2+ and (3) Ca2+ release induces a reduced ER Ca2+ level and activates SOCE channel to mediate Ca2+ influx. The bradykinin‐induced Ca2+ signalling plays a crucial role in regulating cell cycling progression and mobility via activating pAkt, pERK1/2 and cyclin D1 in human cardiac c‐Kit+ progenitor cells (Supporting Information Figure S5); however, we cannot rule out that other signalling pathways, such as NAADP and TPC1‐2, could be involved in bradykinin‐induced intracellular Ca2+ release.
It is well recognized that cytosolic free Ca2+ is a secondary messenger which plays crucial roles in regulating many cellular activities, for example, muscle contraction, gland secretion, cell growth, differentiation, migration, survival and apoptosis.29, 30, 31 In endothelial progenitor cells, Ca2+ oscillations play a key role in stimulating colony cells proliferation and tubulogenesis.32 In a number of cardiac progenitor and stem cells, Ca2+ signalling mediates cardiovascular regeneration.9, 33 Bradykinin is an endogenous peptide that not only participates in the mitogenesis process,34 myocardial35, 36, 37 and neuronal38, 39 protection, but also mediates respiratory allergic reactions, septic shock, acquired angioedema etc.40 These physiological effects or pathophysiological responses may result from different Ca2+ signalling pathways in different types of cells/organs. In bovine coronary artery, bradykinin‐induced vasodilation is related to endothelial increase mediated by cyclic ADP ribose‐RyRs Ca2+ signalling pathway, but not PLC‐IP3Rs Ca2+ signalling pathway, because the PLC inhibitor U73122 and the IP3R inhibitor 2‐aminoethoxydiphenyl borate do not affect the increase induced by bradykinin.41 However, an earlier study demonstrated that bradykinin induces an increase in inositol 1,4,5‐triphosphate (IP3) in neonatal rat cardiomyocytes42 and bradykinin‐mediated short nocifensive responses is related to activating PLC, followed by Orai1 in afferent sensory neurons.18
In rat C6 glioma cells, bradykinin triggers Ca2+ influx, resulting in calcium store Ca2+ release, which is associated with nitric oxide generation for regulating permeability of blood‐tumour barrier,43 while in primary cultured rat brain microvascular endothelial cells, bradykinin‐triggered Ca2+ influx‐induced Ca2+ release is involved in regulation of the junction protein claudin‐5.19 A recent study shows that Ca2+ signalling induced by bradykinin results from initial Ca2+ release from ER IP3Rs followed by Ca2+ entry through Ca2+ release‐activated channels in normal pancreatic stellate cells.44 This is similar to the earlier report in human foreskin fibroblasts,16 and the present observation in human cardiac c‐Kit+ progenitor cells.
Nonetheless, most of the previous reports on various types of cells were obtained using pharmacological tools, and the molecular identities of Ca2+ signal pathway involved in bradykinin‐mediated are not fully understood. Although in this study, we found some molecules involved in bradykinin‐induced Ca2+ signalling and modulation of cell cycling progression and migration using both pharmacological tools and biochemical/molecular biological approaches, we cannot completely exclude the involvement of other potential molecules that would mediate Ca2+ signalling by bradykinin in c‐Kit+ progenitor cells.
Our previous study has shown that B2Rs, but not B1Rs, are expressed in human cardiac c‐Kit+ progenitor cells.10 Mediation of Ca2+ signalling by B2Rs is demonstrated in cells pre‐treated with HOE140 or transfected with B2R siRNA, in which bradykinin no longer induces increase. Involvement of PLC and IP3Rs in Ca2+ signalling is confirmed by the PLC inhibitor U73122 and the IP3R blocker raguspongin B. Ryanodine did not affect the Ca2+ signals, supporting the previous report that RyRs are not expressed in human cardiac c‐Kit+ progenitor cells.9
Inositol 1,4,5‐trisphosphate receptors (IP3Rs) play a key role in intracellular calcium signalling.45 It is not only the primary cytosolic target responsible for the initiation of intracellular calcium (Ca2+) signalling, but can also control apoptosis, intracellular pH, the initiation and regulation of neuronal Ca2+ signalling, exocytosis and gene expression by coupling with modulatory protein.46 There are three types of IP3R subunits (IP3R1, IP3R2 and IP3R3) expressed in mammalian cells, which co‐assemble to form homo‐ or heterotetrameric IP3R channels47 and mediate Ca2+ release from ER/SR in different types of cells. Although the three types of IP3Rs are expressed in human cardiac c‐Kit+ progenitor cells, we found that only IP3R3 mediates the bradykinin‐induced Ca2+ release and the subsequent Ca2+ influx through SOCE channel.
SOCE channel is a complex composed of STIM1, Orai1 and also TRPC1. STIM1 functions as an ER Ca2+ sensor, and Orai1 is a pore subunit located on the plasma membrane.48 Although there exist arguments on whether TRPC1 is a component of SOCE channel,49 our recent results from human cardiac c‐Kit+ progenitor cells13 support the notion that TRPC1 works together with Orai1 to form discrete STIM1‐gated SOCE channel, mediate distinct Ca2+ signals and regulate specific cellular functions.50, 51, 52
The present study shows that bradykinin‐induced Ca2+ influx in c‐Kit+ progenitor cells results from PLC‐IP3R3 ER‐Ca2+ release and activates the STIM1‐Orai1‐TRPC1 complex of SOCE channel to mediate extracellular Ca2+ entry. The involvement of TRPC1 in SOCE channel is demonstrated by the fact that inhibition of TRPC1 with the silencing TRPC1 abolishes the bradykinin‐induced Ca2+ influx. Similar results were observed in cells with silenced STIM1 or Orai1, in which bradykinin only induces a Ca2+ transient release without subsequent Ca2+ influx. This indicates that bradykinin‐induced Ca2+ influx relies on the SOCE channel as a STIM1‐Orai1‐TRPC1 complex in human cardiac c‐Kit+ progenitor cells.
In Ca2+ signalling, very low cytoplasmic Ca2+ concentration can increase in a specific manner to trigger downstream cellular events, and every cell type in the human body utilizes some form of Ca2+ signalling to function or survive.29 Ferreira‐Martins and colleagues reported that spontaneous Ca2+ oscillations promote human cardiac c‐Kit+ progenitor cell proliferation.9 We have previously found that Ca2+ signalling‐related channels (SOCE, TRPV2 and TRPV4) are involved in cell cycling progression and migration in human cardiac c‐Kit+ progenitor cells.11, 13 It has been demonstrated that division of c‐Kit+ cardiac stem cells in the mouse is promoted by spontaneous Ca2+ spikes, which dictate the pattern of stem cell replication and the generation of myocyte progeny at all phases of prenatal life and up to 1 day after birth.53
The endogenous peptide bradykinin has an extensive range of biological activities in different organs, including regulation of mitogenesis in different types of cells.54 Our previous study showed that bradykinin stimulated cell cycling progression and migration via activating pAkt and pERK1/2 as well as expression of cyclin D1 in human cardiac c‐Kit+ progenitor cells.10 The present study demonstrates the novel molecular mechanisms of bradykinin that involves Ca2+ signalling mediated by PLC‐IP3R3 ER‐Ca2+ release followed by Ca2+ influx through SOCE channel (STIM1‐Orai1‐TRPC1 complex); the cell growth, migration as well as pAkt, pERK1/2 and cyclin D1 are no longer increased by bradykinin in cells with silenced IP3R3, STIM1, Orai1 or TRPC1.
In the present study, we did not explore why IP3R3s, but not IP3R1s and IP3R2s, are activated by bradykinin. However, this limitation would not affect the conclusion that bradykinin is coupled with B2R to activate PLC and generate IP3, which then stimulates ER IP3R3 to release Ca2+ followed by Ca2+ influx through SOCE channel in human cardiac c‐Kit+ progenitor cells.
Collectively, cell proliferation and migration of cardiac c‐Kit+ progenitor cells are an essential cellular function for the heart development53 and cardiac repair, in which cardiac stem/progenitor cells are required to migrate to the infarcted myocardial zone and proliferate.55, 56 The present study demonstrates that bradykinin‐induced Ca2+ signalling via stimulating ER IP3R3 to release Ca2+ followed by Ca2+ influx through SOCE channel initiates Akt and ERK1/2 phosphorylation and cyclin D1 expression and promotes cell proliferation and migration, suggesting that bradykinin may be useful for preconditioning cardiac progenitor cells to promote myocardial repair.
CONFLICT OF INTEREST
The authors confirm that there are no conflict of interests.
Supporting information
ACKNOWLEDGEMENT
This study was supported in part by a General Research Fund (771712M) from Research Grant Council of Hong Kong, Hong Kong, China, a Joint Fund (U1605226) for Science and Technology Cooperation across the Taiwan Straits from the National Natural Science Foundation and Fujian Province, China and a Key Cardiovascular Laboratory Fund (3502Z20150050) from Department of Xiamen Science and Technology, Xiamen, Fujian, China. The authors thank Ms Hai‐Ying Sun for the excellent technical support.
Li G, Che H, Wu W‐Y, et al. Bradykinin‐mediated Ca2+ signalling regulates cell growth and mobility in human cardiac c‐Kit+ progenitor cells. J Cell Mol Med. 2018;22:4688–4699. 10.1111/jcmm.13706
Contributor Information
Yan Wang, Email: wy@medmail.com.cn.
Gui‐Rong Li, Email: grli8@outlook.com.
REFERENCES
- 1. Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941‐961. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763‐776. [DOI] [PubMed] [Google Scholar]
- 3. Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068‐14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong KU, Bolli R. Cardiac stem cell therapy for cardiac repair. Curr Treat Opt Cardiovasc Med. 2014;16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Hong KU, Li QH, Guo Y, et al. A highly sensitive and accurate method to quantify absolute numbers of c‐kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30‐day‐old infarction. Circulation. 2010;121:293‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Q, Guo Y, Ou Q, et al. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira‐Martins J, Rondon‐Clavo C, Tugal D, et al. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res. 2009;105:764‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li G, Wang Y, Li GR. Bradykinin regulates cell growth and migration in cultured human cardiac c‐Kit+ progenitor cells. Oncotarget. 2017;8:10822‐10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Che H, Xiao GS, Sun HY, Wang Y, Li GR. Functional TRPV2 and TRPV4 channels in human cardiac c‐kit(+) progenitor cells. J Cell Mol Med. 2016;20:1118‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang YY, Li G, Che H, et al. Effects of BKCa and Kir2.1 channels on cell cycling progression and migration in human cardiac c‐kit+ progenitor cells. PLoS ONE. 2015;10:e0138581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Che H, Li G, Sun HY, Xiao GS, Wang Y, Li GR. Roles of store‐operated Ca2+ channels in regulating cell cycling and migration of human cardiac c‐kit+ progenitor cells. Am J Physiol Heart Circ Physiol. 2015;309:H1772‐H1781. [DOI] [PubMed] [Google Scholar]
- 14. Zhang YY, Li G, Che H, et al. Characterization of functional ion channels in human cardiac c‐kit+ progenitor cells. Basic Res Cardiol. 2014;109:407. [DOI] [PubMed] [Google Scholar]
- 15. Baumgarten LB, Toscas K, Villereal ML. Dihydropyridine‐sensitive L‐type Ca2+ channels in human foreskin fibroblast cells. Characterization of activation with the growth factor Lys‐bradykinin. J Biol Chem. 1992;267:10524‐10530. [PubMed] [Google Scholar]
- 16. Byron KL, Babnigg G, Villereal ML. Bradykinin‐induced Ca2+ entry, release, and refilling of intracellular Ca2+ stores. Relationships revealed by image analysis of individual human fibroblasts. J Biol Chem. 1992;267:108‐118. [PubMed] [Google Scholar]
- 17. Pinheiro AR, Paramos‐de‐Carvalho D, Certal M, et al. Bradykinin‐induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun Signal. 2013;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szteyn K, Gomez R, Berg KA, Jeske NA. Divergence in endothelin‐1‐ and bradykinin‐activated store‐operated calcium entry in afferent sensory neurons. ASN Neuro. 2015;7:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou L, Yang B, Wang Y, Zhang HL, Chen RW, Wang YB. Bradykinin regulates the expression of claudin‐5 in brain microvascular endothelial cells via calcium‐induced calcium release. J Neurosci Res. 2014;92:597‐606. [DOI] [PubMed] [Google Scholar]
- 20. Gafni J, Munsch JA, Lam TH, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5‐trisphosphate receptor. Neuron. 1997;19:723‐733. [DOI] [PubMed] [Google Scholar]
- 21. Kiyonaka S, Kato K, Nishida M, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA. 2009;106:5400‐5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller MR, Shi J, Wu M, et al. Novel Chemical Inhibitor of TRPC4 Channels. Bethesda (MD): Probe Reports from the NIH Molecular Libraries Program; 2010. [Google Scholar]
- 23. Dragoni S, Laforenza U, Bonetti E, et al. Enhanced expression of Stim, Orai, and TRPC transcripts and proteins in endothelial progenitor cells isolated from patients with primary myelofibrosis. PLoS ONE. 2014;9:e91099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shintani S, Kusano K, Ii M, et al. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl. 1):S123‐S128. [DOI] [PubMed] [Google Scholar]
- 25. Teng L, Bennett E, Cai C. Preconditioning c‐kit‐positive human cardiac stem cells with a nitric oxide donor enhances cell survival through activation of survival signaling pathways. J Biol Chem. 2016;291:9733‐9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai C, Guo Y, Teng L, et al. Preconditioning human cardiac stem cells with an HO‐1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells. 2015;33:3596‐3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010;3:89‐102. [DOI] [PubMed] [Google Scholar]
- 28. Li L, Jaiswal PK, Makhoul G, et al. Hypoxia modulates cell migration and proliferation in placenta‐derived mesenchymal stem cells. J Thorac Cardiovasc Surg. 2017;154:543‐52 e3. [DOI] [PubMed] [Google Scholar]
- 29. Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clapham DE. Calcium signaling. Cell. 2007;131:1047‐1058. [DOI] [PubMed] [Google Scholar]
- 31. Fliniaux I, Germain E, Farfariello V, Prevarskaya N. TRPs and Ca2+ in cell death and survival. Cell Calcium. 2018;69:4‐18. [DOI] [PubMed] [Google Scholar]
- 32. Dragoni S, Laforenza U, Bonetti E, et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells. 2011;29:1898‐1907. [DOI] [PubMed] [Google Scholar]
- 33. Moccia F, Ruffinatti FA, Zuccolo E. Intracellular Ca(2)(+) signals to reconstruct a broken heart: still a theoretical approach? Curr Drug Targets. 2015;16:793‐815. [DOI] [PubMed] [Google Scholar]
- 34. Yang CM, Chien CS, Ma YH, Hsiao LD, Lin CH, Wu C. Bradykinin B2 receptor‐mediated proliferation via activation of the Ras/Raf/MEK/MAPK pathway in rat vascular smooth muscle cells. J Biomed Sci. 2003;10:208‐218. [DOI] [PubMed] [Google Scholar]
- 35. Leesar MA, Stoddard MF, Manchikalapudi S, Bolli R. Bradykinin‐induced preconditioning in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999;34:639‐650. [DOI] [PubMed] [Google Scholar]
- 36. Oldenburg O, Qin Q, Krieg T, et al. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286:H468‐H476. [DOI] [PubMed] [Google Scholar]
- 37. Potier L, Waeckel L, Vincent MP, et al. Selective kinin receptor agonists as cardioprotective agents in myocardial ischemia and diabetes. J Pharmacol Exp Ther. 2013;346:23‐30. [DOI] [PubMed] [Google Scholar]
- 38. Torres‐Rivera W, Perez D, Park KY, et al. Kinin‐B2 receptor exerted neuroprotection after diisopropylfluorophosphate‐induced neuronal damage. Neuroscience. 2013;247:273‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mechirova E, Danielisova V, Domorakova I, et al. Bradykinin preconditioning affects the number of degenerated neurons and the level of antioxidant enzymes in spinal cord ischemia in rabbits. Acta Histochem. 2014;116:252‐257. [DOI] [PubMed] [Google Scholar]
- 40. Golias C, Charalabopoulos A, Stagikas D, Charalabopoulos K, Batistatou A. The kinin system–bradykinin: biological effects and clinical implications. Multiple role of the kinin system–bradykinin. Hippokratia. 2007;11:124‐128. [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang G, Teggatz EG, Zhang AY, et al. Cyclic ADP ribose‐mediated Ca2+ signaling in mediating endothelial nitric oxide production in bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2006;290:H1172‐H1181. [DOI] [PubMed] [Google Scholar]
- 42. Kudoh A, Kudoh E, Katagai H, Takazawa T. Insulin potentiates bradykinin‐induced inositol 1,4,5‐triphosphate in neonatal rat cardiomyocytes. J Cardiovasc Pharmacol. 2002;39:621‐627. [DOI] [PubMed] [Google Scholar]
- 43. Wang YB, Liu YH. Initial bradykinin triggers calcium‐induced calcium release in C6 glioma cells and its significance. Neurosci Bull. 2009;25:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Petersen OH. Ca(2+) signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca(2+) channel blockade. J Physiol. 2016;594:281‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5‐trisphosphate receptor isoforms. Biophys J. 2005;88:1046‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choe CU, Ehrlich BE. The inositol 1,4,5‐trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006;2006(363):re15. [DOI] [PubMed] [Google Scholar]
- 47. Monkawa T, Miyawaki A, Sugiyama T, et al. Heterotetrameric complex formation of inositol 1,4,5‐trisphosphate receptor subunits. J Biol Chem. 1995;270:14700‐14704. [DOI] [PubMed] [Google Scholar]
- 48. Srikanth S, Gwack Y. Orai1, STIM1, and their associating partners. J Physiol. 2012;590:4169‐4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prakriya M, Lewis RS. Store‐operated calcium channels. Physiol Rev. 2015;95:1383‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store‐operated Ca2+ entry. Curr Top Membr. 2013;71:149‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ambudkar IS, de Souza LB, Ong HL. TRPC1, Orai1, and STIM1 in SOCE: friends in tight spaces. Cell Calcium. 2017;63:33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC‐mediated Ca2+ entry suggests that a TRPC: Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA. 2009;106:3202‐3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferreira‐Martins J, Ogorek B, Cappetta D, et al. Cardiomyogenesis in the developing heart is regulated by c‐kit‐positive cardiac stem cells. Circ Res. 2012;110:701‐715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Liebmann C. Bradykinin signalling to MAP kinase: cell‐specific connections versus principle mitogenic pathways. Biol Chem. 2001;382:49‐55. [DOI] [PubMed] [Google Scholar]
- 55. Latham N, Ye B, Jackson R, et al. Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation. 2013;128:S105‐S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155‐1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


