Abstract
The rice blast fungus Magnaporthe oryzae is the most serious pathogen of cultivated rice and a significant threat to global food security. To accelerate targeted mutation and specific genome editing in this species, we have developed a rapid plasmid-free CRISPR-Cas9-based genome editing method. We show that stable expression of Cas9 is highly toxic to M. oryzae. However efficient gene editing can be achieved by transient introduction of purified Cas9 pre-complexed to RNA guides to form ribonucleoproteins (RNPs). When used in combination with oligonucleotide or PCR-generated donor DNAs, generation of strains with specific base pair edits, in-locus gene replacements, or multiple gene edits, is very rapid and straightforward. We demonstrate a co-editing strategy for the creation of single nucleotide changes at specific loci. Additionally, we report a novel counterselection strategy which allows creation of precisely edited fungal strains that contain no foreign DNA and are completely isogenic to the wild type. Together, these developments represent a scalable improvement in the precision and speed of genetic manipulation in M. oryzae and are likely to be broadly applicable to other fungal species.
Introduction
In recent years, the use of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated RNA-guided Cas9 endonuclease, has facilitated genome editing technologies and become the leading tool used to generate specific changes to DNA sequences in a wide range of species1. To generate double stranded breaks (DSBs) in the genome of a target organism, the CRISPR-Cas9 system requires Cas9 endonuclease, which cleaves target DNA at a genomic target sequence2–4, and a single RNA molecule, which in the CRISPR-Cas9 system, uses a linker sequence to join the nuclease-binding tracrRNA and the target specific crRNA molecules found in naturally occurring complexes in the source organism, Streptococcus pyogenes5. The sgRNA associates with the nuclease and directs it to a genomic target sequence5. The DSB created by the nuclease can then be repaired by non-homologous DNA-end joining (NHEJ) or using homologous recombination (HR), by introduction of donor DNA homologous to the sequence around the break, which allows very specific edits to the DNA sequence, or very precise insertions or deletions6. The only target sequence requirement necessary for CRISPR-Cas9 genome editing is the presence of the protospacer adjacent motif (PAM), a triplet NGG located immediately 3′ of the genomic target sequence7,8. Because HR-based repair can be used to introduce modifications at some distance to the DSB, for example up to 30 bp in human stem cells9, the majority of fungal genomes are accessible to manipulations using CRISPR-Cas9 editing.
CRISPR-Cas9 genome editing offers huge potential to accelerate the pace of research in key fungal research areas, such as biotechnology, medical mycology and plant pathology, by dramatically reducing the time required to undertake common objectives, such as targeted gene deletion, overexpression, single nucleotide changes, or tagging the products of genes of interest with fluorescent proteins10. The technique also permits targeting of gene families, making multiple mutations11, and generating mutants in dikaryotic, or polyploid fungi12. Moreover, the potential exists to carry out ‘selectable marker-free’ manipulations for precise genetic changes, a prerequisite for any commercial application. CRISPR-Cas9 generated edible mushrooms have already, for instance, bypassed the gene manipulation regulations to which crop species engineered by methods preceding CRISPR were subject13.
In the rice blast fungus Magnaporthe oryzae, a CRISPR-Cas9 genome editing system based on expression of Cas9 and CRISPR components in vivo has been reported to target single genes14. However, the generation of mutants using this procedure has not been widely adopted and the protocol requires labour-intensive cloning strategies, so that the deletion of multiple genes would not be practical. We therefore set out to look for an alternative method which might extend the range of applications for CRISPR-based gene editing technologies in this economically important pathogen of rice. Here, we report a ribonucleoprotein-CRISPR-Cas9 (RNP-CRISPR-Cas9) system for genome editing in M. oryzae, using purified nuclear-localised Cas9 (Cas9-NLS) and in vitro synthesised sgRNA, an approach pioneered in Caenorhabditis elegans15. This procedure generates highly efficient rates of mutation in M. oryzae at a genomic target sequence, when a donor DNA carrying a selectable marker sequence and capable of repairing the DSB is co-transformed with the RNP into fungal protoplasts. We have also established what we term a gene co-editing strategy, which allows single nucleotide edits to be made without any other changes in, or around, a given target locus. We have employed a novel selection strategy that exploits negative cross resistance to two fungicides to enable CRISPR mediated counterselection. This counter-selection method allows mutants to be created that are isogenic to an original wild-type strain. We believe that using RNP-CRISPR-Cas9 will permit precise and rapid gene manipulation in M. oryzae and other fungi, and thereby accelerate the pace of research in this economically important plant pathogen.
Results
Evidence of toxicity of Cas9 in Magnaporthe oryzae
We made multiple attempts to generate a strain stably expressing a Cas9 gene, together with small guide RNAs (sgRNA) targeting the melanin biosynthetic polyketide synthase-encoding genes ALB1 (MGG_07219) or RSY1 (MGG_05059), mutations which give rise to easily identifiable white (albino) or orange-red (rosy) colour phenotypes, respectively, to fungal colonies16. Although Arazoe and co-workers reported successful generation of rsy1 mutants in M. oryzae using CRISPR-Cas9, it is likely that their constructs (and hence Cas9) were only transiently present in the cell (although this was not tested in their work)14. Despite many attempts, and using three different versions of Cas9 under control of two different promoters (see methods for details), we were never able to generate mutants showing altered pigmentation, among the few transformants which resulted from transformation with either vector. Importantly, we were not able to reproduce the generation of mutants reported previously, even when the same vectors were used14. We did, however, observe that the transformation of all constructs containing Cas9-encoding sequences, either with or without a sgRNA present, always gave rise to far fewer transformants than empty vector controls (Fig. 1 and Table S1.). Based on our observations and on the previous report14, it would seem that stable expression of Cas9 is very toxic to M. oryzae, but that genome editing using Cas9 might be possible by transient expression of the nuclease.
Figure 1.
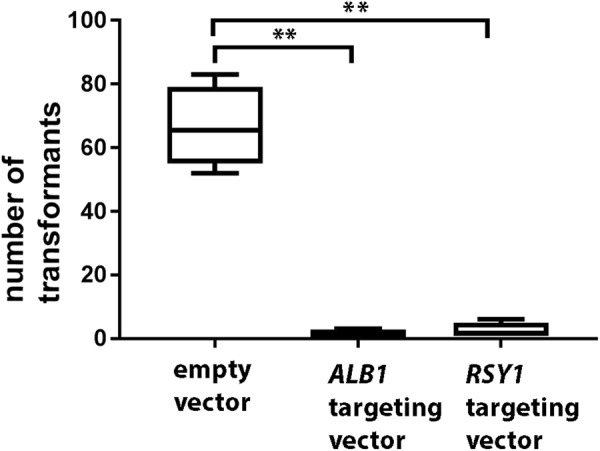
Toxicity of stably expressed Cas9. Binary vectors containing the gene encoding Cas9-NLS under the control of the TrpC promoter and terminator were introduced into Guy 11 using Agrobacterium-mediated transformation. Transformant numbers were assessed after 7 days on selective medium (transformants were subsequently sub-cultured for assessment of pigmentation after growth on CM).
CRISPR-Cas9 genome editing using purified Cas9 and sgRNAs
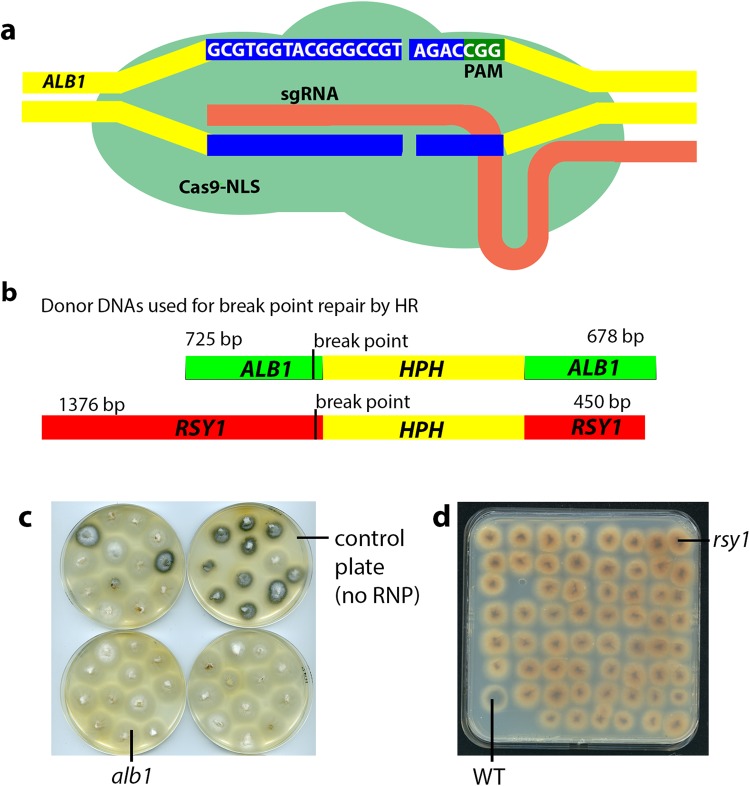
In view of the likely toxicity of Cas9 nuclease to M. oryzae cells, we set out to assess whether CRISPR-Cas9 genome editing might instead be accomplished by transient introduction of purified Cas9 protein along with in vitro synthesised sgRNAs, into protoplasts of M. oryzae. To this end, we purchased nuclear-localised Cas9 (Cas9-NLS) from commercial suppliers (see Methods for details) and complexed this to sgRNAs capable of directing Cas9 to the ALB1 locus (Fig. 2a). We independently tested a second RNP that targets the RSY1 locus. We introduced these Cas9-NLS-sgRNA ribonucleoprotein complexes (RNPs) independently into a wild type M. oryzae strain Guy11, together with donor DNAs which would introduce an insertion containing a selectable marker (HPH – the hygromycin phosphotransferase gene cassette) near the 5′ end of the coding regions of these genes, by repair of the DSB by homologous recombination with donor DNAs containing homologous regions on either side of the selectable marker gene (Fig. 2b). In both cases the selectable marker gene was expected to integrate close to the DSB, normally 3–4 bp from the PAM site. We were able to demonstrate very efficient targeting of both genes, as shown in Fig. 2c,d, and Table S2. Remarkably, mutation of RSY1 was near to 100% efficient in multiple experiments (see for example Fig. 2d) and the efficiency of targeting ALB1 was also greater than 50% in every test, with typically 70–80% albino transformants generated (Table S2). As expected, the rates of mutation using donor-only controls were more typical of rates reported for gene deletion using conventional gene deletion strategies (see Table S2). These observations provide evidence that RNP-CRISPR-Cas9 generated DSBs strongly induce HR repair and can be exploited for efficient gene manipulation in M. oryzae.
Figure 2.
CRISPR-Cas9 genome editing using purified Cas9 and sgRNAs. (a) Illustration of the genomic target sequence for the ALB1-targeting RNP-CRISPR-Cas9 complex used showing a typical double stranded break in relation to the PAM site. (b) An illustration of the donor DNA sequences used to repair DSBs created by RNP-CRISPR-Cas9 complexes and showing the position of break point relative to the selection marker hygromycin -phosphotransferase (HPH) used. (c) Transformants picked from ALB1-targeting RNP-CRISPR-Cas9 + donor DNA transformation plates (before pigmentation was normally apparent) and growing on CM + hygromycin showing albino mutants and also (top right) transformants picked from a donor only control plate. (d) Transformants picked RSY1-targeting RNP-CRISPR-Cas9 + donor DNA transformation plates (before pigmentation was apparent) and growing on CM + hygromycin showing the rsy phenotype (rosy or normally orange-red) pigmentation (one wild-type pigmented transformant is also indicated (WT) in the bottom left hand side of the plate).
Highly efficient mutation of genes using CRISPR-Cas9 induced recombination of micro-homologous donor DNAs
The efficient mutation of ALB1 by CRISPR-Cas9 induced homologous recombination of a donor DNA, prompted us to define the minimum length of homologous DNA that would facilitate efficient genome editing. Recent reports in some fungi suggest that micro-homologous regions are sufficient to allow repair by short donor DNAs of CRISPR-induced DSBs with high efficiency17–19. To test whether this was possible in M. oryzae, we amplified the BAR gene which confers resistance to the herbicide glufosinate ammonium20, with 30 bp and 40 bp flanking regions on either side of the selectable marker. In this way we were able to demonstrate that a 30 bp region of homology was sufficient to induce repair by HR of the CRISPR-Cas9 generated DSB and result in mutation of ALB1, with efficiencies approaching those achieved with the much longer donor DNAs (see Table S3). The increased rates of mutation, compared to those observed with donor-only controls, provided further evidence that RNP-CRISPR-dependent gene replacement is efficient, and also demonstrated that RNP-CRISPR-Cas9 gene inactivation can be generated without the need for laborious cloning strategies.
Direct demonstration of marker free mutation of the ALB1 melanin biosynthesis gene using donor free CRISPR-Cas9 RNPs
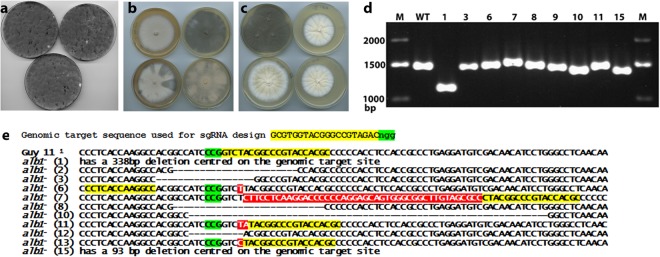
Our observation of efficient gene replacement when RNPs were introduced into M. oryzae with donor DNA fragments, suggested that the Cas9-NLS enzyme creates DSBs and, in so doing, induces repair by the homologous recombination pathway. However, because these experiments used donor DNAs to repair the breaks using a selectable marker gene, they were genotypically indistinguishable from alb1 or rsy1 strains that would be generated from an experiment using the donor DNA only to disrupt each gene– as in ‘traditional’ gene disruption approaches. We therefore set out to examine CRISPR events more directly by introduction of the RNP complex targeting ALB1 only, without donor DNAs to direct repair by homologous recombination. In the absence of donor DNA, the resultant DSB can be repaired by the non-homologous DNA end-joining (NHEJ) pathway which, because it is frequently inaccurate, should result in alb1 (albino) mutants. Although such events were found to be rare under the conditions tested, we were able to observe albino mutants among a large background (showing confluent growth) of normally pigmented regenerants (Fig. 3a). Control transformations without RNPs yielded no such white patches. Unfortunately, three attempts to dilute the transformants to a concentration where 10–20,000 individuals could be isolated, failed to yield any albino colonies, suggesting that much less than 0.01% of regenerated protoplasts harbour alb1 mutations. These observations suggest that the efficiency of the delivery system used would make marker-less gene targeting impracticable in the absence of an easily identifiable phenotype, because this would necessitate analysis of more than 20,000 individuals. Nevertheless, the albino mutants generated allowed us to directly demonstrate that RNPs are functional in vivo and to understand the nature of mutations generated through NHEJ. To this end, we purified albino mutants by several rounds of subculture followed by single spore isolation (Fig. 3b,c) and in a few cases, the insertion or deletion was large enough to be apparent by gel electrophoresis of the amplicons (Figs 3d and S1). In one case, no amplification was possible, indicating that a larger deletion had removed the sites where one or both primers, anneal. Sequencing of DNA around the genomic target site of the RNP in these albino mutants revealed true CRISPR mutations, showing a range of insertions or deletions close to the PAM site, as shown in Fig. 3e. These results demonstrated that the RNP complex functions by creating a DSB at the expected site and that marker-less single mutations are feasible, but not at a frequency which would be useful in the absence of an easily identifiable phenotype.
Figure 3.
Marker-less RNP-CRISPR-Cas9 gene targeting of ALB1 without donor DNA. (a) Transformation plates from ALB1-targeting RNP-CRISPR-Cas9 without donor DNA showing rare albino patches (b) Primary colonies obtained by picking from the white patches shown in a. (c) Purified regenerants following a combination of subculture (hyphal tip isolation) and single spore isolation in most cases gave pure albino colonies. (d) Gel electrophoresis of the PCR products generated using the genomic DNA of the purified albino regenerants and primers PKS-ck-F and PKS-ck-R which flank the ALB1-targeting RNP-CRISPR-Cas9 genomic target sequence showing visible variation in product size. The gel image shown here was cropped, but no other bands were present. The full gel is given in Fig. S1. (e) Sequences of the amplicons shown in (d), showing a range of mutations and indels.
Specific single nucleotide gene edits can be very efficiently accomplished using short oligonucleotide donor DNA
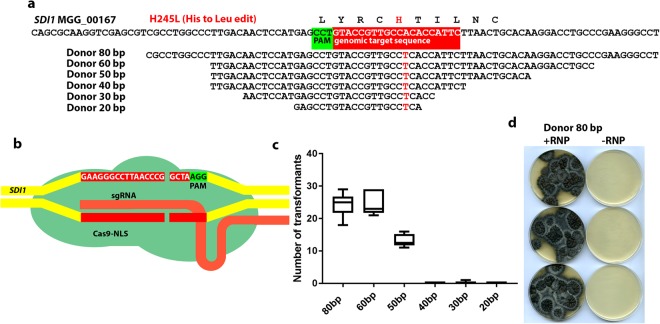
One of the attractive features of CRISPR-induced genome editing is the ability to make highly specific changes to the coding sequence of a gene that could, for example, give rise to a single amino acid change in a protein product. To test whether single nucleotide edits were feasible using RNP-CRISPR-Cas9 in M. oryzae, we attempted to edit the gene SDI1, which encodes a subunit of the succinate dehydrogenase enzyme. We designed a SDI1-targeting RNP, to introduce a mutation that leads to an amino acid change in the enzyme known to confer resistance to the fungicide carboxin (see Fig. 4a)21. At the same time we attempted to test how short homologous regions on the donor DNA can be, while still efficiently editing the target gene. By introduction of a SDI1-targeting RNP (Fig. 4b) and oligonucleotide donor DNAs of varied lengths (Fig. 4a) into Guy 11 protoplasts, we were able to demonstrate that a 50–80 bp double stranded (ds) oligonucleotide donor DNA, containing the desired single base edit, was sufficient to efficiently edit SDI1, as shown in Fig. 4c,d. We therefore employed 80 bp donor DNAs in all of our subsequent experiments.
Figure 4.
Oligonucleotide-mediated RNP-CRISPR-Cas9 genome editing of SDI1 to confer carboxin resistance. (a) Illustration of the substitution required to give a carboxin resistant form of the succinate dehydrogenase subunit product of MGG_00167 and the oligonucleotide donor DNAs capable of introducing the required one nucleotide change necessary tested. Also indicated is the genomic target sequence of the SDI1-targeting RNP-CRISPR-Cas9 complex employed. (b) Diagram showing the RNP used and the predicted DSB at the SDI1-targeting RNP-CRISPR-Cas9 genomic target sequence. (c) Graph showing the number of transformants obtained using the different donor DNAs indicated in a. in combination with the RNP illustrated in b. (d) Transformants from the 80 bp long donor DNA shown in panel a. transformed together with the RNP complex illustrated in panel b. and also showing control plates where only the donor DNA without RNP was transformed (although no transformants are visible on the control plates, using the RNP+ the 30 bp donor one carboxin resistant transformant was obtained which may indicate that very rarely the short oligos can recombine in the absence of the RNP complex; no other carboxin resistant transformants were obtained in the other controls).
Development of a gene co-editing strategy in M. oryzae
We observed that mutants generated by NHEJ using RNPs without a selectable marker did not arise at a frequency that would make gene targeting practicable. We therefore set out to develop a different method to enrich for gene-edited transformants. We decided to adopt a co-targeting approach in which two independent RNPs are transformed together into M. oryzae. We reasoned that a proportion of cells would take up both complexes and be edited at both loci. If one of the genes had an easily scorable phenotype that could be used as a selectable marker, we should be able to select transformants more easily and then determine whether the second locus had also been edited. As a proof of principle, we introduced RNPs targeting the succinate dehydrogenase subunit-encoding gene SDI1 and ALB1, simultaneously, together with the 80 bp oligonucleotide donor dsDNA, which we had already established was able to convert the SDI1 gene to an allele bestowing carboxin resistance. We employed two different ALB1-targeting donor DNAs, both of which introduce a premature stop codon in the gene, close to its 5′ end (Fig. S2). One of the donor DNAs introduces the edit within the genomic target sequence. The other donor is predicted to generate an edit 40 bp from the PAM allowing us to assess whether we can create edits at some distance from the DSB. We found that when Guy 11 was used as a recipient, these donor DNAs integrated in approximately 50% of the albino transformants, which represented 1–2% of all the carboxin resistant transformants, as shown in Table S4. Furthermore, the donor DNA that introduces an edit outside the genomic target sequence was as efficient at editing as the other donor. Albino mutants that lacked the integrated donor DNAs exhibited indels, typically 3–4 bp 5′ from the PAM. These are indicative of mutations generated by NHEJ of the CRISPR-generated DSB. Surprisingly, in one instance an albino mutant arose by integration of the SDI1-targeting donor DNA at the ALB1 locus. By contrast, when we employed a Δku70 mutant22 that lacks the NHEJ pathway, all albino mutants generated showed precise integration of the ALB1 targeting donor DNA. Moreover, the efficiency of co-editing both loci increased, although the number of overall transformants was reduced (Table S4). To determine whether this approach, which we henceforth refer to as co-editing, was applicable to other genes, we set out to co-edit both the ILV2 and TUB2 genes, which encode acetolactate synthase and β-tubulin, respectively. These genes can be edited at a single nucleotide to give rise to alleles encoding sulfonylurea and benomyl resistant mutants, respectively (see Fig. S3; refs23,24). RNPs were first created to introduce these edits and then tested individually (Fig. S3). We then conducted a co-editing experiment by transforming the ILV2 and TUB2 targeting RNPs, together with the two corresponding oligonucleotide donors. We selected for sulfonylurea resistance and then calculated the proportion of sulfonylurea resistant transformants that were also benomyl resistant. Consistently, we observed ~1% efficiency of co-editing (Table S4). We were able to confirm the edits that had occurred in these transformants by direct sequencing of amplicons containing the target sequence. Mutations generated by NHEJ repair would in most conceivable instances, not be selected for by these experiments. Together, these experiments demonstrated that we are able to generate marker-less mutations in M. oryzae at target loci by employing a straightforward co-editing strategy.
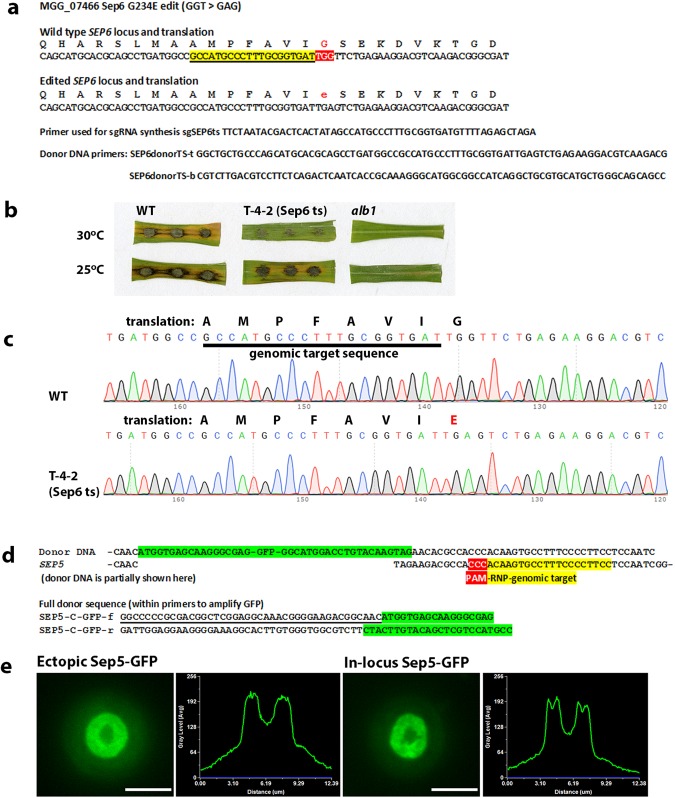
Co-editing allows generation of in-locus GFP-tagged gene fusions and conditional mutant alleles in M. oryzae without a selectable marker
To demonstrate that co-editing could be employed to generate novel genotypes in any gene of interest, we decided to tag the SEP5 septin-encoding gene25 with GFP, using CRISPR co-editing at the native locus. We also tested whether we could exploit co-editing to introduce a two nucleotide edit into the SEP6 septin-encoding gene to create a temperature-sensitive allele. We generated a G234E substitution into SEP6, which corresponds to a mutation (G247E) that in the Sep6 orthologue Cdc12 in Saccharomyces cerevisiae, gives rise to a temperature sensitive (ts) form of the septin (Fig. 5a)26. Using CRISPR-mediated co-editing we were able to generate both a sep6G234D allele (Fig. 5b,c) and a SEP5-GFP strain, as shown in Fig. 5d,e. To confirm that the correct genotype had been created at the corresponding loci, we sequenced amplicons of both genomic regions (for the sep6G234D strain’s edit see Fig. 5c). The SEP5-GFP strain was identified by examination of 200 transformants, while the sep6G234D mutation was identified among 79 transformants. These experiments confirmed that co-editing can be employed to rapidly and precisely manipulate genes in M. oryzae. We confirmed that the sep6G234D mutation leads to a temperature sensitive loss of virulence and is unable to cause rice blast symptoms at the non-permissive temperature of 30 °C, as shown in Fig. 5b. Replacement of SEP5 with a SEP5-GFP gene fusion at the native locus meanwhile leads to visualisation of a GFP-tagged septin ring at the M. oryzae appressorium pore (Fig. 5e), identical to that previously reported for an ectopically integrated gene fusion25.
Figure 5.
RNP-CRISPR-Cas9 gene co-editing to introduce a N-terminal GFP tag to Sep5 and a temperature sensitive mutation in Sep6. (a) An illustration of the genomic target sequence and donor DNAs employed for generation of a temperature sensitive (ts)-encoding allele of SEP6 by co-editing at the native locus. (b) Leaf sections showing the infection of rice cultivar Co-39 with conidia of a strain (T-4-2) with the ts allele of SEP6. (c) Confirmation of the introduction of the desired mutation in T-4-2 by sequencing of a 472 bp amplicon generated using the primers SEP6ts?-f CACACCCTGAAGCCCCTTGATATC and SEP6ts?-R CTCCTCGGTTGTGTGGATGAG (relevant section only shown; other regions of the amplicon corresponded to the WT sequence exactly). (d) An illustration of the genomic target sequence for the RNP and PCR-generated donor DNA used for generation of a strain where the GFP-encoding gene is inserted after the START codon of the Sep5-encoding gene potentially giving rise to a SEP5-GFP expressing strain with an in locus replacement of the native SEP5 gene. (e) Micrographs showing the appressorial Sep5-Gfp containing septin ring in a strain where SEP5 was replaced by the Sep5-Gfp-encoding gene in locus by RNP-CRISPR-Cas9 co-editing and the corresponding septin ring in the ectopically integrated SEP5-GFP-expressing strain constructed by Dagdas and co-workers26. The bar is 5 μm. The linescan graphs show the Sep5-GFP fluorescence in a transverse section of the individual appressoria shown in the micrographs.
A novel selection strategy allowing construction of isogenic gene edited mutants in M. oryzae
It is likely that expression of antibiotic resistance genes has unknown consequences in fungi. Ultimately, it would therefore be desirable to generate a mutant that contains a specific edit, but that is in all other respects, isogenic to the progenitor strain. We reasoned that the reported negative cross resistance of certain benomyl resistant mutations to the compound diethofencarb27,28, might provide a novel counterselection strategy that would allow us to generate isogenic CRISPR mutants in M. oryzae. We were able to confirm in plate growth tests that the wild type M. oryzae strain, Guy11, can exhibit normal growth in the presence of 10 μg mL−1 diethofencarb, whereas a benomyl-resistant strain harbouring a TUB2 allele with a E198A mutation cannot grow under the same conditions (Fig. 6a). We therefore used a TUB2-targeting RNP and an 80 bp oligo donor that restores the TUB2 sequence to wild type (Fig. 6b), and introduced this into a benomyl resistant transformant, previously created using RNP-CRISPR-Cas9. This led to the generation of diethofencarb-resistant transformants at a very high frequency (Fig. 6c). The diethofencarb-resistant transformants were as sensitive to benomyl as Guy 11. No diethofencarb-resistant transformants were generated in the absence of the TUB2-targeting RNP. Furthermore, we observed that the counterselection is very tight, because no wild type TUB2 strains can grow on 10 µg mL−1 benomyl, and no benomyl-resistant TUB2E198A strains are able to grow at all on 10 µg mL−1 diethofencarb as shown in Fig. 6a. We conclude that CRISPR-Cas9-RNP mediated generation of TUB2E198A benomyl resistant strains of M. oryzae, followed by a second round of CRISPR-Cas9-RNP to restore a wild type TUB2 sequence, bestowing diethofencarb resistance, provides a means of generating isogenic, markerless, genome-edited mutants. The counter selection strategy also represents an excellent way by which to build multiple mutations in a single strain of the fungus, as it is straightforward to cycle between the two states, and thereby introduce further specific edits or other manipulations each time by co-editing.
Figure 6.
Counterselection exploiting the diethofencarb sensitivity of strains expressing E198A beta-tubulin. (a) Plates illustrating the negative cross resistance of benomyl resistant transformants to diethofencarb. benR-1, benR-2 and benR-3 are three different benomyl resistant strains generated using RNP-CRISPR while the WT (wild type) is the strain Guy 11 which is benomyl sensitive/diethofencarb resistant control. (b) Illustration of the genomic target sequence for the RNP used and the oligonucleotide donors used to make the sequence edit for reversion to a WT TUB2 sequence as well as the donor DNA used for the creation of the benomyl resistant strains shown in a. (c) Plates showing the transformation of the benomyl resistant strain number 2 with the TUB2-targeting RNP-CRISPR-Cas9 and a donor DNA (shown in b) conferring the reversion to the wild type TUB2 sequence and diethofencarb resistance.
Assessment of off target effects in albino mutants generated by RNP-CRISPR without donor DNA
One potential constraint on the use of CRISPR-Cas9 genome editing is the possibility of off-target mutations at sites showing significant similarity to the genomic target sequence29. Although no mismatch is tolerated in the seed sequence proximal to the PAM, in some species up the 5 bp mismatches are tolerated at the PAM-distal end of the genomic target sequence, resulting in potential for off-target cleavage30. For this reason, most programmes used for automated protospacer selection search for a protospacer with minimal potential for off target cleavage. The degree of off target cleavage, however, varies considerably between organisms and is, of course, also a function of genome size. However, careful sgRNA design and limited longevity of the Cas9-SgRNA complex in a cell, seem to be major means to maintain editing specificity30. An advantage of using RNP-CRISPR genome editing is that there is evidence that the transient presence of the CRISPR machinery in the cell may also limit off target effects31.
In order to assess whether off target mutations had occurred in CRISPR generated mutants, we randomly selected two alb1 mutants generated by CRISPR-Cas9-RNP and, as a control, two strains that had been through the transformation procedure and regenerated but not exposed to RNP complexes. The genomes of these M. oryzae strains were sequenced (Table S5). The presence of SNPs and small INDELs was then determined in alb1 mutant strains, compared to the control strains. Potential off-site mutations were detected based on the presence of an insertion or deletion (INDEL) within 3 bp of the PAM site or in regions showing sequence identity at the mutant position, of at least 10 bases to the guide RNA. We found that the alb1_3 mutant had 44 SNPs and 30 INDELS, while the alb1_6 mutant had 40 SNPs + 33 INDELS across the whole genome, when compared to the control strains, which represent the sequence of the progenitor Guy11 isolate. Our analysis showed that there are 115 sites in the M. oryzae genome with at least 10 bp identity to the gRNA, but we found that none of these corresponded to mutations detected in either of the alb1 mutants. We conclude that no off-target CRISPR generated mutations occurred in the alb1 mutants of M. oryzae.
Discussion
In this report, we have demonstrated the efficient generation of CRISPR induced gene edits in the rice blast fungus using purified CRISPR machinery components. We were motivated to develop this method because stable expression of Cas9 appears to be very toxic to M. oryzae in the same way as reported in some other species, including fission yeast32–34. The transient nature of CRISPR-Cas9-ribonucleoprotein expression provides an excellent means of circumventing the problems associated with Cas9 toxicity and rapidly generating gene edited mutants in M. oryzae. This contrasts with the previously published method requiring expression of the Cas9-encoding gene14, which we were unable to reproduce in spite of extensive efforts. The key advantages of RNP-mediated CRISPR-Cas9 editing are its efficiency, accuracy, and especially its speed. Use of oligonucleotide-based or PCR-amplified donor DNAs obviates the need for labour-intensive DNA cloning and thereby dramatically reduces the time and cost required to make precise gene manipulations in this species. Additionally, we demonstrated that RNP-CRISPR editing is highly specific, because we saw no evidence of off-target mutations in the genomes of two CRISPR generated mutants. RNP-CRISPR therefore is an extremely useful and adaptable addition to the Magnaporthe gene manipulation toolbox as a simple amendment to existing transformation protocols. Furthermore, as the price of the necessary components of the RNP complex falls over time, we predict that RNP-CRISPR-Cas9 editing will become a standard manipulation in a very short time in M. oryzae.
We observed that although mutations resulting from RNP-CRISPR and NHEJ-dependent repair were possible without a selectable marker, these occurred at a frequency too low to be practically exploited. One interpretation of this result is that NHEJ may be highly accurate in M. oryzae, but a more likely explanation is that a very small proportion of fungal protoplasts actually take up the RNP. It is therefore possible that RNP complexes might be more efficiently delivered by other means, such as electroporation or biolistic delivery. We were, however, able to overcome this potential limitation by developing a gene co-targeting strategy that we termed co-editing. This co-editing approach significantly enriches for specific edits in a marker-less fashion without introduction of a further selectable marker sequence at the locus of interest. Co-editing was found to reproducibly occur in 0.5–2% of transformants that were also edited to bestow carboxin, benomyl or sulfonylurea resistance, respectively. This required a second RNP and donor DNA targeting a gene of interest, in addition to the RNP donor pair generating the mutation to confer antibiotic resistance. Using oligonucleotide donor DNAs also had the additional advantage that it generated very large numbers of antibiotic-resistant transformants, from which it was straightforward to select co-edited mutants.
When the gene co-editing method is combined with the benomyl/diethofencarb-based counterselection strategy, we have provided a mechanism to generate gene edited mutants in M. oryzae that are truly isogenic to a progenitor wild type. This is very advantageous, not only for basic research (where studying a single mutation in isolation from any other genome perturbations is the best possible method), but also in fungal biotechnological applications where the lack of a resistance gene marker is important from a regulatory perspective. Although the efficiency of gene co-editing that we report here is rather low, it is likely that optimisation by adjustment of the ratio of the RNPs and/or donor DNAs may be possible in future. Furthermore, in the current report we show that 1 or 2 base changes are possible with an 80 bp donor DNA, but preliminary results in our laboratory indicate that similarly sized donor DNAs can efficiently and precisely delete small sections of genes of 40–50 bp, that would facilitate simple PCR-based screens for gene-inactivated mutants. Additionally, the generation of small deletions may be a more attractive method for high-throughput gene functional analysis because they may be more stable than changes to a single nucleotide. The time saved by not having to construct vectors for gene manipulation makes co-editing an attractive option, even if it necessitates sequencing 100 or more transformants to identify the specific mutant required.
Our study does, however, raise some important questions too, that we will address in future. It is apparent, for instance, that some RNPs work more efficiently than others as has been reported in other species35,36. In making the most efficient use of co-editing, it is important that we better understand which protospacers are likely to generate the best results. Additionally, it has been suggested that protoplast-mediated transformation is in itself, mildly mutagenic37, and in the long term it would be worth investing time to explore other means to deliver RNP complexes to M. oryzae. Electroporation, for example, might represent a less disruptive means of delivering the RNP-CRIPSR-Cas9 complex. If RNP complexes can be delivered at the same time as DNA donors via electroporation, it would, for instance, be possible to directly compare SNPs and indel frequencies in the genomes of fungal strains manipulated by these different methods to test whether electroporation or other delivery systems really are less mutagenic than protoplast generation.
In summary, the current study demonstrates that RNP-CRISPR-Cas9 genome editing is a simple, precise, reproducible, and rapid means by which gene manipulation can be carried out in M. oryzae. It is our hope that researchers investigating Magnaporthe species and related fungi will adopt the procedures described here and that this will empower researchers and accelerate discovery towards understanding and ultimately combatting the devastating rice blast fungus M. oryzae and similarly important pathogenic fungal species.
Materials and Methods
Strains and Culture conditions and infection assays
The wild type strain Guy 11 and the NHEJ deficient mutant Δku70 were grown in a controlled temperature room at 25 °C with a 12 h light/dark cycle. For tests of temperature sensitivity an incubator at 30 °C with a 12 h light/dark cycle was used to conduct cut leaf infection assays. Infection assays used two cm long leaf sections cut from 3 week old leaves of the dwarf indica rice cultivar CO-39 and were assessed after 5 days incubation at either 25 °C or 30 °C with a 12 h light/dark cycle. For glufosinate and sulfonylurea selection, we used Basta defined complex medium (BDCM)38. For standard growth, we used CM39. For selection using hygromycin, benomyl, carboxin or diethofencarb we used OCM which is CM osmotically stabilised with 0.8 M Sucrose. Selective agents were used at a final concentration of 200 µg mL−1 for hygromycin or 150 µg mL−1 for sulfonylurea (chlorimuronethyl) 40 µg mL−1 glufosinate or 10 µg mL−1 benomyl 50 µg mL−1 carboxin or 10 µg mL−1 diethofencarb.
SgRNA synthesis and RNP formation
SgRNA for complexing with Cas9-NLS was synthesised using the EnGen sgRNA synthesis kit available from New England Biolabs (NEB #E3322), according to the instructions provided, and purified before complexing to Cas9 using the RNA Clean & Concentrator-25 kit (Zymo Research). Cas9-NLS was purchased from New England Biolabs (NEB Catalog #: M0646M). Cas9-NLS was complexed with the sgRNA by a 10 minute incubation at room temperature. The sgRNA selection was carried out using the E-CRISP program at http://www.e-crisp.org/E-CRISP/ 40.
Fungal transformation and introduction of RNPs and donor DNAs
PEG-mediated fungal transformation of Glucanex-generated protoplasts was achieved, as previously described39. The RNP complexes and donor DNAs were introduced together before the step in the standard transformation procedure, where 50% PEG is added and the mixture then incubated for 25 min. During optimisation for reproducibility of the method, sgRNAs were always freshly synthesised and purified on the day of transformation and kept on ice following formation of the RNP complex. All experiments except co-targeting experiments using the SDI1-targeting RNP, used 2 μg of donor DNA and 6 μg of Cas9 mixed at a 1:1 molar ratio with the respective sgRNA (pre-complexed together for 10 min at RT) and protoplasts, in a volume of 150 μl at a concentration of 1.5 × 108 protoplasts/ml. For the co-targeting experiments using the SDI1-targeting RNP, we used 1 μg of Cas9 precomplexed with 0.2 μg of sgRNA. For Agrobacterium-mediated transformation of Cas9-containing vectors, conidia of Guy11 were transformed as previously described41.
Oligonucleotide design and source
Oligonucleotides used in this study were designed with the aid of the Primer3 program (http://primer3.ut.ee/) or as templates for sgRNA synthesis were designed with the aid of NEB’s online design tool (http://nebiocalculator.neb.com/#!/sgrna). All the oligonucleotides used in this study were purchased desalted from Eurofins Genomics and are listed in Table S6. For generation of PCR products to recombine in vivo in S. cerevisiae 40–45 bp regions of homology to the recipient vector were added to the 5′ end of the primer sequence.
Cloning of ALB1 and RSY1 targeting donor DNAs
To generate cloned donor DNA for repair of RNP-CRISPR generated DSBs, in the case of ALB1 we amplified a 1.4 kb ALB1 fragment using the primers ALB1-for-EcoRI and ALB1-rev-SpeI. The resultant PCR product was digested with EcoRI and XhoI gel purified and ligated to EcoRI + SalI digested pCAMBIA0380 thus destroying the SalI site in this vector’s multi-cloning site. The resultant vector pCAMB-ALB1 was linearized using a SalI site in the middle of the ALB1 fragment and ligated to the HPH cassette, conferring hygromycin resistance excised from plasmid pCB163638 using SalI to create pCAMB-ALB-HPT. A donor DNA was generated by PCR amplification of the HPH interrupted ALB1 fragment from pCAMB-ALB-HPT using the primers ALB1-for and ALB1-rev. In the case of RSY1, RSY-donor-f and RSY-donor-r were used to amplify a 1.8 kb RSY1 fragment which was cloned into the vector pGEMT-easy (Promega) to give pGEM-RSY. pGEM-RSY was digested with XhoI and ligated to a SalI HPH fragment to give pGEM-RSY-HPT which could then be used as a template for amplification of the required donor DNA (HPT interrupted RSY1) using RSY-donor-f and RSY-donor-r.
Cloning of Agrobacterium tumefaciens compatible Cas9 expressing vectors
To generate an Agrobacterium compatible vector to introduce Cas9 and an ALB1 targeting sgRNA transcribing sequence into M. oryzae, we used the nuclear localised, Cas9 codon-optimised for Neurospora crassa in vector p415-PtrpC-Cas9-TtrpC-CYC1t42 as a template for high fidelity PCR amplification of Cas9-NLS under control of the Aspergillus nidulans TrpC promoter and terminator sequences, using the primers Cas9-recom-f and Cas9-recom-r. We then used yeast recombination in Saccharomyces cerevisiae strain DS94 (MATα, ura3-52, trp1-1, leu2–3, his3-111, and lys2-80143) to recombine this fragment into a 12,869 bp XhoI-BamHI fragment of the “soft-landing” vector pS315 pMMag_Cbx-mCherry, which will integrate at the SDI1 locus as a single copy to confer carboxin resistance (pS315-pMMag-Cbx-mCherry was a kind gift from Prof. Gero Steinberg, University of Exeter). The resultant vector p315-Cas9-csr-1 was then further modified to introduce sgRNA transcribing sequences targeting ALB1 or RSY1. This was achieved by generating a PCR-amplified fragment containing the desired guide RNA encoding sequence created by using the plasmid p426-SNR52p-gRNA.csr-1.Y-SUP4t as a template, with primers PKS1gRNA-f and PKS1gRNA-r for ALB1 and in the case of SDH1 the PKS1gRNA-r primer was replaced with the primer SDH1-sg-r. This PCR results in the replacement of the N. crassa csr-1 targeting guide with the M. oryzae ALB1 or RSY1 targeting guide under the control of the SNR52 promoter. The resultant amplicons were then used as a template to generate recombination competent fragments using the primers gRNAto315Cas-R and sgRNA-rec-R. The products of this amplification were recombined separately in yeast into XbaI digested p315-Cas9-csr-1. Correct assembly of the vectors in yeast was confirmed following extraction from yeast strains, transformation of, and purification from E. coli followed by analysis by restriction digests and partial sequencing. The vectors p415-PtrpC-Cas9-TtrpC-CYC1t and p426-SNR52p-gRNA.csr-1.Y-SUP4t were gifts from Christian Hong (Addgene plasmid numbers 68059 and 68060 respectively). Additionally, to test the Cas9-NLS promoter gene derived from p415-PtrpC-Cas9-TtrpC-CYC1t, a TEF1 promoter-Cas9 with a glucoamylase-encoding gene terminator sequence14 was used. Note that the codon optimised Cas9 allele used by these authors differs from the native Cas9 amino acid sequence at two amino acids, but is reported as functional despite these changes. We also tested our own TrpCp-Cas9-TrpC terminator construct (M. oryzae codon optimised Cas9-NLS) generated by gene synthesis. These constructs were generated by a yeast recombination cloning strategy using the vector pMMag_Cbx-mCherry as detailed above and replace the mCherry ORF with the appropriate Cas9 ORF or replace the promoter and gene in the case of the TEF1 promoter-Cas9 construct. The sgRNA expressing constructs tested used the S. cerevisiae SNR52 promoter42 or the U6-1 or U6-2 promoters of M. oryzae14.
Bioinformatic analysis for detection of potential off target mutations
Genomic DNA from two regenerant colonies (not exposed to RNP but which have been through the transformation procedure) and two hygromycin resistant alb mutants was extracted using the Qiagen Plant DNAeasy mini kit and was sequenced on Illumina HiSeq2500 using standard reagents and protocols producing 125 base paired-end reads by the University of Exeter Sequencing Service. Reads were filtered using the fastq-mcf program from the ea-utils package, ea-utils44: “Command-line tools for processing biological sequencing data”; https://github.com/ExpressionAnalysis/ea-utils). Genomes were assembled ‘de novo’ using SPAdes 3.11.045. For SNP and INDEL calling reads were aligned against the M. oryzae reference genome46 using BWA47. SNPs and INDEL were identified with a bespoke pipeline using bcftools and vcfutils from the SAMtools package as well as SnpSift48,49. SNPs called from the two alb1 mutants were compared to two regenerated strains that had been subjected to the transformation protocol but not exposed to RNPs, based on analysis made by Schuster and co-workers38.
Electronic supplementary material
Acknowledgements
This work was funded by a European Research Council Advanced Investigator Award to NJT under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement No. 294702 GENBLAST and by a BBSRC grant (BB/BB/N009959/1) to N.J.T.
Author Contributions
N.J.T. and A.F. designed the experiments, oversaw the study, and wrote the manuscript. A.J.F., M.M.U., X.Y., and S.W. carried out experimental work. D.S. conducted the genomic analysis of off-target effects.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files) or are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32702-w.
References
- 1.Wright AV, Nuñez JK, Doudna JA. Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell. 2016;164:29–44. doi: 10.1016/j.cell.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jinek M, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.12580962014. [DOI] [PubMed] [Google Scholar]
- 6.DiCarlo JE, et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojica FJ, Díez-Villaseñor C, García-Martínez J, Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, et al. Optimization of scarless human stem cell genome editing. Nucleic Acids Res. 2013;41:9049–9061. doi: 10.1093/nar/gkt555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi T-Q, et al. CRISPR/Cas9-based genome editing of the filamentous fungi: the state of the art. Appl. Microbiol. Biotechnol. 2017;101:7435–7443. doi: 10.1007/s00253-017-8497-9. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Lee X. Multiple Applications of a Transient CRISPR-Cas9 Coupled with Electroporation (TRACE) System in the Cryptococcus neoformans Species Complex. Genetics. 2018;208:1357–1372. doi: 10.1534/genetics.117.300656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G-C, et al. Construction of a Quadruple auxotrophic mutant of an industrial polyploid Saccharomyces cerevisiae strain by using RNA-guided Cas9 nuclease. Appl. Environ. Microbiol. 2014;80:7694–7701. doi: 10.1128/AEM.02310-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waltz E. Gene-edited CRISPR mushroom escapes US regulation. Nature. 2016;532:293. doi: 10.1038/nature.2016.19754. [DOI] [PubMed] [Google Scholar]
- 14.Arazoe T, et al. Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol Bioeng. 2015;112:2543–2549. doi: 10.1002/bit.25662. [DOI] [PubMed] [Google Scholar]
- 15.Cho SW, Lee J, Carroll D, Kim JS, Lee J. Heritable gene knockout in Caenorhabditis elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics. 2013;195:1177–1180. doi: 10.1534/genetics.113.155853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chumley FG, Valent B. Genetic analysis of melanin-deficient, nonpathogenic mutants of Magnaporthe grisea. MPMI. 1990;3:135–143. doi: 10.1094/MPMI-3-135. [DOI] [Google Scholar]
- 17.Nødvig Christina S., Hoof Jakob B., Kogle Martin E., Jarczynska Zofia D., Lehmbeck Jan, Klitgaard Dorte K., Mortensen Uffe H. Efficient oligo nucleotide mediated CRISPR-Cas9 gene editing in Aspergilli. Fungal Genetics and Biology. 2018;115:78–89. doi: 10.1016/j.fgb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Pohl C, Kiel JAKW, Driessen AJM, Bovenberg RAL, Nygård. Y. CRISPR/Cas9 Based Genome Editing of Penicillium chrysogenum. ACS Synth. Biol. 2016;5:754–764. doi: 10.1021/acssynbio.6b00082. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Meng X, Wei X, Lu L. Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus. Fungal Genet. Biol. 2016;86:47–57. doi: 10.1016/j.fgb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Leung H, Loomis P, Pall ML. Transformation of Magnaporthe grisea to phosphinothricin resistance using the bar gene from Streptomyces hygroscopicus. Fungal Genet. Newsl. 1995;42:41–43. doi: 10.4148/1941-4765.1341. [DOI] [Google Scholar]
- 21.Guo M, Zhu X, Li H, Tan L, Pan Y. Development of a novel strategy for fungal transformation based on a mutant locus conferring carboxin-resistance in Magnaporthe oryzae. AMB Express. 2016;6:57. doi: 10.1186/s13568-016-0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kershaw MJ, Talbot NJ. Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc Natl Acad Sci USA. 2009;106:15967–15972. doi: 10.1073/pnas.0901477106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Naqvi NI. Sulfonylurea resistance reconstitution as a novel strategy for ILV2-specific integration in Magnaporthe oryzae. Fungal Genet. Biol. 2014;68:71–76. doi: 10.1016/j.fgb.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Kachroo P, Potnis A, Chattoo B. Transformation of the rice blast fungus Magnaporthe grisea to benomyl resistance. World J Microbiol Biotechnol. 1997;13:185–187. doi: 10.1023/A:1018589714357. [DOI] [Google Scholar]
- 25.Dagdas YF, et al. Septin-mediated plant cell invasion by the rice blast fungus. Magnaporthe oryzae. Science. 2012;336:1590–1595. doi: 10.1126/science.1222934. [DOI] [PubMed] [Google Scholar]
- 26.Weems AD, Johnson CR, Argueso JL, McMurray MA. Higher-Order Septin Assembly Is Driven by GTP-Promoted Conformational Changes: Evidence From Unbiased Mutational Analysis in Saccharomyces cerevisiae. Genetics. 2014;196:711–727. doi: 10.1534/genetics.114.161182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimura M, Oeda K, Inoue H, Kato T. A single amino-acid substitution in the beta-tubulin gene of Neurospora confers both carbendazim resistance and diethofencarb sensitivity. Curr Genet. 1992;21:399–404. doi: 10.1007/BF00351701. [DOI] [PubMed] [Google Scholar]
- 28.Koenraadt H, Jones AL. Resistance to benomyl conferred by mutations in codon-198 or codon-200 of the β-tubulin gene of Neurospora crassa and sensitivity to diethofencarb conferred by codon-198. Phytopathology. 1993;83:850–854. doi: 10.1094/Phyto-83-850. [DOI] [Google Scholar]
- 29.Peng R, Lin G, Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283:1218–1231. doi: 10.1111/febs.13586. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X-H, Tee LY, Wang X-G, Huang Q-S, Yang S-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther. Nucleic Acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Kim D, Cho SW, Kim J, Kim J-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Research. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs JZ, Ciccaglione KM, Tournier V, Zaratiegui M. Implementation of the CRISPR-Cas9 system in fission yeast. Nature Communications. 2014;5:5344. doi: 10.1038/ncomms6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan OW, et al. Selection of chromosomal DNA libraries using a multiplex CRISPR system. eLife. 2014;3:e03703. doi: 10.7554/eLife.03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Generoso WC, Gottardi M, Oreb M, Boles E. Simplified CRISPR-Cas genome editing for Saccharomyces cerevisiae. Microbiol Methods. 2016;127:203–205. doi: 10.1016/j.mimet.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR/Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson RA, Gurevich V, Filler S, Samach A, Levy AA. Comparative assessments of CRISPR-Cas nucleases’ cleavage efficiency in planta. Plant Mol Biol. 2015;87:143. doi: 10.1007/s11103-014-0266-x. [DOI] [PubMed] [Google Scholar]
- 37.Schuster M, Schweizer G, Reissmann S, Kahmann R. Genome editing in Ustilago maydis using the CRISPR-Cas system. Fungal Genet Biol. 2016;89:3–9. doi: 10.1016/j.fgb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Sweigard JA, Chumley F, Carroll AM, Farrall L, Valent B. A series of vectors for fungal transformation. Fungal Genet. Newsl. 1997;44:52–53. doi: 10.4148/1941-4765.128. [DOI] [Google Scholar]
- 39.Talbot NJ, Ebbole DJ, Hamer JE. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant cell. 1993;5:1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification. Nat. Methods. 2014;11:122–123. doi: 10.1038/nmeth.2812. [DOI] [PubMed] [Google Scholar]
- 41.Odenbach D, et al. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol. 2007;64:293–307. doi: 10.1111/j.1365-2958.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsu-Ura T, Baek M, Kwon J, Hong C. Efficient gene editing in Neurospora crassa with CRISPR technology. Fungal Biol Biotechnol. 2015;2:4. doi: 10.1186/s40694-015-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 44.Aronesty E. Comparison of Sequencing Utility Programs. TOBioiJ. 2013;7:1–8. doi: 10.2174/1875036201307010001. [DOI] [Google Scholar]
- 45.Bankevich A, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dean RA, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cingolani P, et al. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Frontiers in Genetics. 2012;3:35. doi: 10.3389/fgene.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, et al. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files) or are available from the corresponding author on reasonable request.