Abstract
Radiation exposure during the peri-pubertal period is a proven risk factor for breast cancer, whereas parity is an established protective factor. The present study investigated whether parity imposes differential protective effects against radiation-induced rat mammary carcinoma depending on the age at exposure. Pre- and post-pubertal female rats, irradiated or left unirradiated, were mated and allowed to nurse until weaning or left unmated. Appearance of mammary tumors was monitored, and serum concentrations of estradiol and progesterone were measured following weaning. Carcinomas were evaluated by immunohistochemistry for estrogen receptor, progesterone receptor, and the cell proliferation marker Ki-67. Parity reduced the risk of carcinoma in unirradiated and pre-pubertally irradiated rats but not post-pubertally irradiated rats. Although radiation exposure increased serum progesterone level, parity after pre-pubertal exposure significantly decreased the elevated progesterone to a normal level, reflecting a protective effect. Moreover, parity significantly decreased the proportion of hormone receptor–positive carcinomas after pre-pubertal exposure. Parity was also related to the observed positive association between progesterone receptor and Ki-67 indices in cancer tissue, implying progesterone receptor–dependent cell proliferation. Thus, parity protects against radiation-induced rat mammary carcinogenesis depending on the age at exposure; the mechanisms may involve changes in hormone levels and cancer tissue.
Introduction
Breast cancer is the most common cancer for women in both developed and developing countries1. Epidemiologists have identified several risk factors for breast cancer such as diet and reproductive history2,3, with exposure to ionizing radiation from both medical and accidental exposures a proven risk factor4,5. The occurrence of breast cancer as a second cancer is of great concern for women who received radiation therapy in childhood, with a longer timeframe in which to manifest a radiation-induced cancer than for those treated as adults6. Mammary glands are thought to be highly susceptible to radiation-induced cancer around the time of puberty because of the onset of rapid growth7. In addition to this age factor, population data suggest that lifestyle, diet, and reproductive history act as risk modifiers for both spontaneous and radiation-induced breast cancer4. Epidemiological studies have documented that pregnancy and lactation at an early age reduces a woman’s lifetime risk of breast cancer8, and studies of Japanese atomic-bomb survivors have also shown that pregnancy decreases the radiation-related risk of breast cancer9,10. However, the risk of Hodgkin’s lymphoma following radiotherapy is not reduced by parity11, suggesting that the effects of parity on radiation-induced cancer are complex and potentially site-specific. This complexity is further underscored by a study of childhood cancer survivors, which revealed that breast cancer risk was sharply reduced among women who received radiation therapy as a child with a high dose of exposure to the ovaries5.
Rodent models have been widely used to study the protective effects of parity with respect to mammary tumorigenesis12, including thorough investigations of parity and chemically induced mammary carcinoma13,14. Rat models have been used to study the risk and underlying mechanisms of both chemically and radiation-induced mammary cancer because these models mimic both the pathogenesis of human breast cancer and the expression of hormone receptors (HRs), such as the estrogen receptor (ER) and progesterone receptor (PR), in diseased tissue15. To our knowledge, however, only one study has assessed the effects of radiation in female rats prior to pregnancy16. Therefore, the combined effects of parity and radiation on the risk of mammary cancer remain unknown.
Several mechanisms underlying the protective effect of parity against breast cancer have been proposed8,17. Some epidemiological studies have reported that parity reduces the risk of HR-positive, but not HR-negative, breast cancer18–20. Several lines of evidence have also shown that both parity and exposure to radiation alter the levels of certain circulating hormones such as estradiol and growth hormone21–24. However, the observed changes in hormonal status have not been consistently reproduced in experimental settings, and thus it remains unclear how radiation exposure affects hormonal status during tumorigenesis.
We previously reported that pre-pubertal, but not post-pubertal, radiation exposure induces premature cessation of the regular estrous cycle25–27, consistent with the documented effects of radiation on ovarian function5 and the fact that tumor subtypes differ between mammary carcinomas induced by pre- or post-pubertal radiation exposure27. Also, certain experimental and computational studies have addressed the mechanisms underlying the difference between the effects of radiation exposure during pre- and post-pubertal stages28,29. However, it is unknown whether there is an interaction between the age at radiation exposure and parity with respect to the risk of mammary cancer. Understanding any combined effects of age at radiation exposure and parity may improve the accuracy of predicting and controlling the risk of second breast cancer after radiation therapy in childhood. Moreover, such an understanding would provide a firmer biological basis on which to model radiation-induced cancer risk in future epidemiology analyses.
Given the aforementioned lines of evidence, we hypothesized that the effects of parity on radiation-induced mammary cancer would differ with respect to pre- or post-pubertal irradiation because age at exposure (pre- vs. post-pubertal) has been shown to affect the HR status of radiation-induced tumors. We therefore examined whether the effect of parity on the risk of mammary cancer depends on the age at which rats were exposed to radiation. Our findings indicate that parity indeed has differential effects on mammary cancer risk posed by pre- or post-pubertal radiation exposure; parity reduced the development of HR-positive tumors in rats irradiated before puberty, but not after. Postulated mechanisms underlying the age effect include changes in hormonal and tumor status, involving progesterone level and consequent proliferation of cancer cells, in parous rats.
Results
Differential effect of parity on rat mammary carcinogenesis induced by pre- or post-pubertal radiation exposure
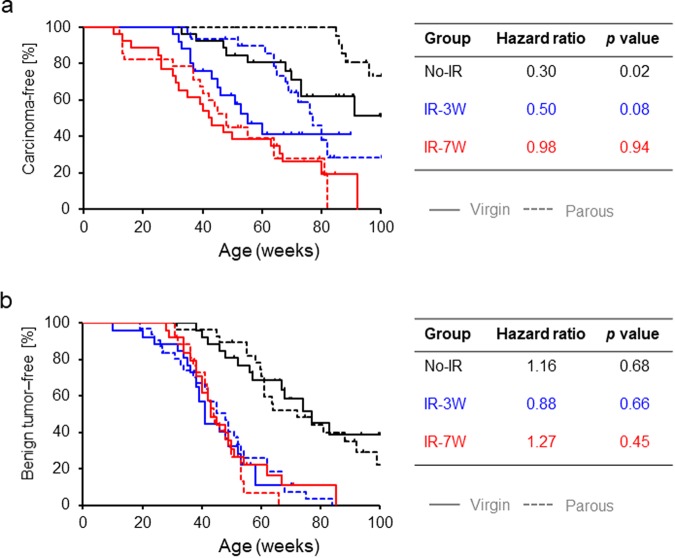
To investigate possible interactions between the age at ionizing radiation (IR) exposure and parity with respect to the development of rat mammary tumors, we established six experimental groups: pre-pubertal irradiation (IR-3W), post-pubertal irradiation (IR-7W), and nonirradiated (No-IR), with virgin and parous groups for each. The experimental procedure is schematically shown in Fig. 1. The percentage of rats that ultimately developed mammary carcinoma by age 100 weeks was not significantly different between the parous rats and the exposure-matched virgin groups (Table 1). However, the rate of carcinoma manifestation (the mean number of postmortem-confirmed carcinomas detected per week) was significantly decreased by parity in the IR-3W group. In addition, the mean time to first palpation of mammary carcinoma was significantly delayed by parity in both the IR-3W and No-IR groups. The incidence, rate, and latency of mammary carcinomas were unaffected by parity in the IR-7W group.
Figure 1.
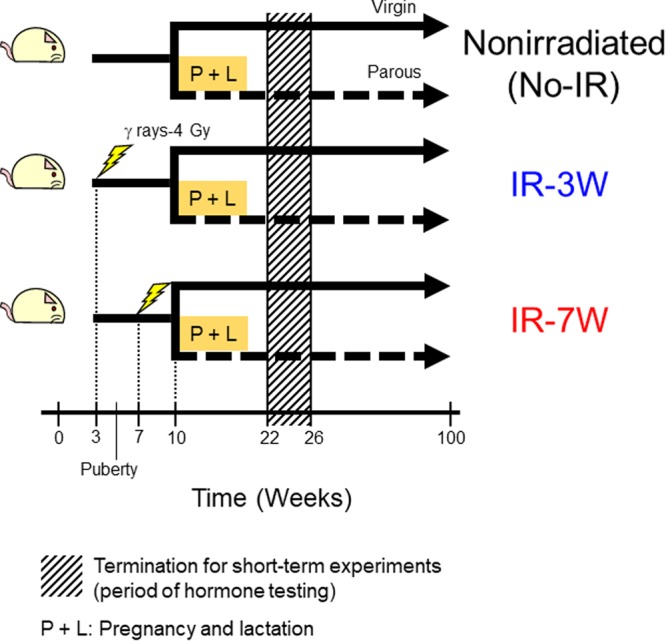
Schematic overview of the animal experiment. Pre- or post-pubertal female rats (3 or 7 weeks of age, respectively) were irradiated with 4 Gy of γ rays from 137Cs or left untreated. Half of the rats in each group were mated at 10 weeks of age. Mated rats that completed delivery, lactation (for 3 weeks), and weaning by the time they were 18 weeks old were used as parous rats. The experiment for evaluating mammary tumor risk was terminated at age 100 weeks. To assess hormonal status during the period of tumorigenesis separate experimental groups were established in the same manner. Parous rats in proestrus were autopsied at 5 weeks after weaning (age 22–26 weeks), and likewise for age-matched virgin rats. In the figure, the period denoted by hatched shading indicates the termination of the short-term experiment for the hormone tests. P + L, pregnancy and lactation; IR-3W, irradiation at age 3 weeks (pre-puberty); IR-7W, irradiation at age 7 weeks (post-puberty); No-IR, nonirradiated control; each group comprised virgin and parous subgroups.
Table 1.
Summary of mammary tumorigenesis in virgin and parous rats.
| Age at exposure (week) | Parity | Rats with tumor (%) | Tumors observed per week (10−2) | Age at first palpation (week) |
|---|---|---|---|---|
| Carcinoma | ||||
| —a | Virgin | 11/26 (42 ± 10) | 2.1 ± 0.5 | 60 ± 15 |
| Parous | 7/28 (25 ± 8) | 1.5 ± 0.2 | 90 ± 6** | |
| 3 | Virgin | 15/26 (58 ± 10) | 4.5 ± 0.9 | 49 ± 13 |
| Parous | 17/31 (55 ± 9) | 1.9 ± 0.3* | 66 ± 13*** | |
| 7 | Virgin | 21/26 (81 ± 8) | 3.5 ± 0.6 | 45 ± 19 |
| Parous | 21/28 (75 ± 8) | 3.2 ± 0.5 | 43 ± 22 | |
| Benign tumor (fibroadenoma/adenoma) | ||||
| — | Virgin | 16/26 (62 ± 10) | 2.9 ± 0.4 | 69 ± 18 |
| Parous | 20/28 (71 ± 9) | 3.0 ± 0.4 | 76 ± 17 | |
| 3 | Virgin | 22/26 (85 ± 7) | 5.2 ± 0.6 | 49 ± 14 |
| Parous | 29/31 (94 ± 4) | 5.4 ± 0.6 | 59 ± 17*** | |
| 7 | Virgin | 21/26 (81 ± 8) | 5.2 ± 0.5 | 53 ± 15 |
| Parous | 25/28 (89 ± 6) | 5.3 ± 0.6 | 58 ± 17 | |
aNonirradiated. *p < 0.05; **p < 0.005; ***p < 0.001 vs. matched virgin (i.e. same exposure treatment) by Mann-Whitney’s U test. Percentage and tumor number are mean ± SE; age at palpation is mean ± SD.
To further assess tumor incidence, time to first palpation of a mammary tumor in each rat (as confirmed retrospectively with a pathology report) was analyzed using the Kaplan-Meier method and Cox’s proportional hazards model. For the No-IR group, parous rats had a significantly decreased and delayed incidence of mammary carcinoma compared with virgin rats (black lines in Fig. 2a). This protective effect of parity offset the risk of pre-pubertal irradiation-induced mammary carcinoma (blue lines in Fig. 2a) but had no effect on the risk induced by post-pubertal irradiation (red lines in Fig. 2a). Despite the similar curves for the IR-7W parous and virgin rats, the relative effect of post-pubertal radiation appeared to be greater for parous rats given their lower rate of spontaneous incidence. In agreement with our previous studies25–27, the incidence of carcinoma was higher in IR-7W virgin rats than IR-3W virgin rats (hazard ratio = 1.72, p = 0.12). Although the risk of benign tumors was also increased by radiation, parity did not affect the rates of incidence of those tumors (Fig. 2b). Table 1 presents detailed information on mammary tumorigenesis.
Figure 2.
Kaplan-Meier plot for first palpation of a mammary tumor. (a) Carcinoma. (b) Benign tumors (fibroadenoma or adenoma). p values calculated with the log-rank test (virgin vs. parous) are indicated. Hazard ratios for the parous groups are shown, evaluated with the Cox’s model with the respective virgin groups as reference. Solid and dashed lines indicate virgin and parous groups, respectively.
Hormonal status during mammary tumorigenesis
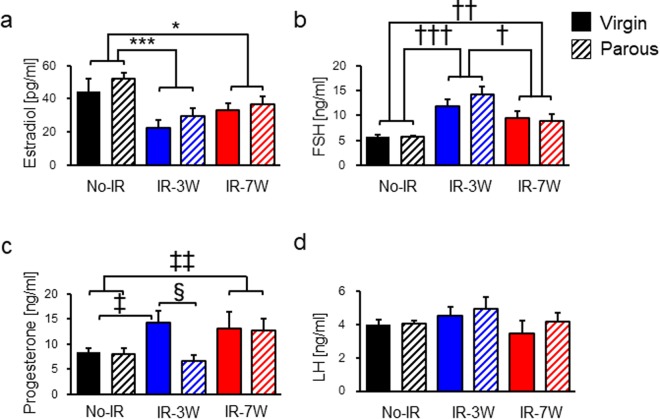
As in previous studies26,27, earlier cessation of the estrous cycle was observed here in virgin and parous rats irradiated before but not after puberty (Supplementary Table 1), implying that hormonal alteration associated with radiation-induced ovarian damage depends on the age at exposure. To investigate changes in hormonal status during radiation-induced mammary tumorigenesis, we measured the levels of serum estradiol, progesterone, follicle stimulating hormone (FSH, a pituitary hormone that stimulates ovarian follicle growth), luteinizing hormone (LH, a pituitary hormone that triggers ovulation and development of the corpus luteum), growth hormone, and prolactin (related to mammary tumorigenesis21,30) as well as thyroxine and corticosterone (associated with mammary cancer31,32); measurements were made between 22 and 26 weeks of age, i.e., after pregnancy and lactation but before the cessation of regular estrous cycling. Mean estradiol level was significantly decreased by radiation exposure regardless of parity or age at exposure (Fig. 3a), whereas mean FSH level showed the opposite trend (Fig. 3b) consistent with a radiation-induced ovarian dysfunction as seen in aging rats33. Pre-pubertal irradiation led to a significant increase in mean progesterone level, which was offset by parity; post-pubertal irradiation also increased progesterone level, which remained unchanged by parity (Fig. 3c). This observation is consistent with the previously observed increase in progesterone level in irradiated rats, which was associated with a decreased number of ovarian follicles and increased size of luteinized tissues34. In addition, pre-pubertally irradiated virgin rats bearing mammary carcinomas showed a significant increase in progesterone level compared with the tumor-free rats, whereas such an increase was not observed in parous rats (Supplementary Fig. 1). Although temporary elevation of LH in the circulation is required for formation of the corpus luteum and consequent secretion of progesterone, mean LH level was not significantly affected by either radiation exposure or parity (Fig. 3d). Similarly, the levels of other hormones analyzed did not differ significantly among the groups (Supplementary Fig. 2).
Figure 3.
Hormonal status during mammary tumorigenesis. (a–d) Estradiol, follicle stimulating hormone (FSH), progesterone, and luteinizing hormone (LH), respectively. The number of rats was as follows: No-IR virgin, 8; No-IR parous, 14; IR-3W virgin, 9; IR-3W parous, 8; IR-7W virgin, 5; IR-7W parous, 9. *Student’s t-test after 2 × 3 ANOVA; †Welch’s t-test after 2 × 3 ANOVA; ‡Welch’s t-test after 2 × 2 ANOVA; §Student’s t-test after 2 × 2 ANOVA. Single, double, and triple symbols indicate p < 0.05, <0.005, and <0.001, respectively. Error bars indicate ± SE.
HR status in mammary carcinomas and the effect of parity
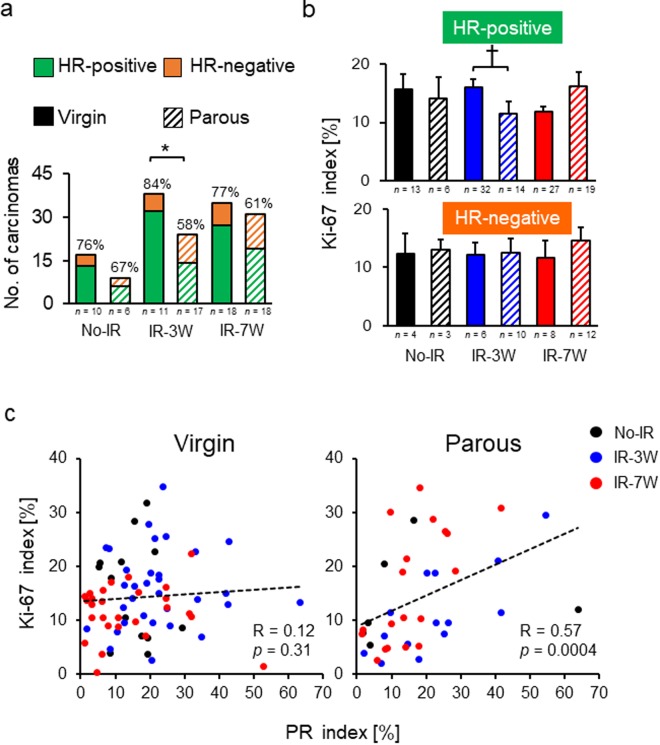
Epidemiological studies have demonstrated that parity reduces the risk of developing HR-positive mammary tumors18–20. To determine how parity modifies the HR status in radiation-induced rat mammary carcinomas, we performed immunohistochemistry for ER and PR as well as the cell proliferation marker Ki-67 to understand their association. Overall, the rat mammary carcinomas were mostly HR positive (virgins, 80%; parous, 61%), yet the percentage of HR-positive mammary carcinomas was significantly decreased by parity in the IR-3W group (84% vs. 58%) (Fig. 4a), a trend not significant in the IR-7W group (77% vs. 61%). For the HR-positive carcinomas remaining in the parous IR-3W group, the Ki-67 was significantly decreased compared with similar tumors in the IR-3W virgin rats. The Ki-67 index was marginally increased (p = 0.06) by parity in the HR-positive tumors of the IR-7W group, whereas the Ki-67 index of HR-negative tumors was not changed by parity in any group (Fig. 4b). Thus, parity decreased cell proliferation in HR-positive carcinomas but only in pre-pubertally irradiated rats. Indeed, the delay in age at first palpation by parity seen in the IR-3W and No-IR groups was specific to the HR-positive tumors (Supplementary Table 2). Unexpectedly, irrespective of the age at irradiation, the Ki-67 index was significantly correlated with the PR index in HR-positive mammary carcinomas from parous rats (Fig. 4c; all parous groups combined, R = 0.57, p = 0.0004; No-IR only, p = 0.18; IR-3W only, p = 0.0095; IR-7W only, p = 0.0029), but not in virgin rats (Fig. 4c). It thus appears that cell proliferation in carcinomas is independent of the PR index in virgin rats, whereas it is related to the PR index in parous rats. The changes in the hormonal status alone (Fig. 3) are unable to fully explain this observation; thus, the results suggest that another mechanism such as hormone responsiveness of the mammary gland is involved in the progesterone-dependent proliferation in HR-positive carcinomas. A similar correlation was not observed between the Ki-67 and ER indices in the HR-positive carcinomas from either virgin or parous rats (Supplementary Fig. 3). Moreover, while the mammary carcinoma cells were strongly stained with PR, normal mammary glands were only weakly stained and no drastic difference was observed between the No-IR and IR groups (irrespective of the tumor-bearing status; Supplementary Fig. 4).
Figure 4.
Number and Ki-67 index of hormone receptor–positive and –negative carcinomas, and correlation between progesterone receptor (PR) and Ki-67 expression levels in hormone receptor–positive mammary carcinomas derived from virgin and parous rats. (a) The number of carcinomas with positive and negative expression of a hormone receptor (estrogen receptor, progesterone receptor). Percentages indicate the proportion of hormone receptor–positive carcinomas in each group; n, number of rats. (b) The Ki-67 index for hormone receptor–positive and –negative carcinomas; n, number of carcinomas. *p < 0.05 by Fisher’s exact test; †p < 0.05 by Mann-Whitney’s U test. Error bars indicate ± SE. (c) Correlation between PR and Ki-67 expression in hormone receptor–positive carcinomas from virgin and parous rats. The Spearman’s correlation coefficient (R) and p values are shown in the panels. Circles indicate individual carcinomas.
A high frequency of overexpression of human epidermal growth-factor receptor 2 (HER2) has been observed in breast cancer tissue samples obtained from atomic-bomb survivors compared with women who were not exposed to radiation35. Although HER2 was overexpressed in approximately 14% of the rat mammary carcinomas in our study, this aspect was not significantly affected by parity or age at exposure (Supplementary Fig. 5).
Discussion
Our present study reveals a combined effect of the age at exposure and parity on the risk of mammary cancer. The risk induced by pre-pubertal radiation exposure was reduced by parity, whereas the risk induced by post-pubertal irradiation was not affected by parity. IR exposure altered the hormonal environment by reducing serum estradiol and increasing progesterone, which may be associated with ovarian dysfunction, whereas parity normalized the serum progesterone level if it was increased by pre-pubertal IR exposure but not post-pubertal exposure. Parity also seemed to be associated with progesterone-dependent cell proliferation in radiation-induced HR-positive carcinomas. These results suggest that parity differentially affects the onset of radiation-induced rat mammary carcinomas based on the age at exposure via multiple mechanisms. These results suggest that several mechanisms contribute to the differential effects of parity on the onset of radiation-induced rat mammary carcinomas as modulated by age at exposure.
Our results agree with those of previous studies in that parity decreased the risk of spontaneous mammary tumors, as observed in epidemiology studies and models of chemically induced tumorigenesis8,14. The present study is the first to demonstrate the preventive effect of parity with respect to the spontaneous development of mammary carcinoma in rats, and this was a consequence of the high frequency of spontaneous mammary tumor development in the present model, which is in agreement with our previous studies25,26. In addition, a previous study showed that parity had a negligible effect on the development of mammary carcinomas induced by post-pubertal IR exposure, consistent with the present study, when the rats were irradiated with their ovarian area shielded16. The results of the previous and present studies thus imply that the mechanisms underlying the absence of any protective effect of parity after post-pubertal IR exposure is not related to exposure of the ovary. Alternative mechanisms may include alteration in some specific molecular pathways in cancer. For example, pregnancy affords little protective effect for female carriers of BRCA1/BRCA2 mutations36,37. Moreover, a study of p53-null mice demonstrated the lack of a parity effect38. As these specific genomic aberrations have not been observed in radiation-induced rat mammary carcinoma39,40, further determination of altered pathways in mammary cancer will be necessary to fully understand the molecular basis of breast tumorigenesis.
Our results demonstrate that the risk of HR-positive carcinoma is reduced by parity, consistent with epidemiological studies18–20. In contrast, the present study did not reproduce the previously reported dependence of the subtype of mammary carcinomas on age at IR exposure27; differences between the former study and our study include: (i) a lower radiation dose (2 Gy in the previous study), resulting in a smaller number of carcinomas analyzed; (ii) feeding with a high-fat diet during the experiment; and (iii) a shorter experimental period (until 50 weeks of age vs. 100 weeks in our study). These differences could have contributed to the distinct results concerning carcinoma phenotype. In addition, our observation of the similar frequency of tumors with HER2 overexpression in IR-exposed and non-exposed rats is not consistent with the study of atomic bomb survivors, in which the frequency was higher in exposed women than non-exposed women35, but it is consistent with another, more statistically powerful study41, in which the frequency was similar.
The interval between IR exposure and pregnancy is a possible factor that influences the protective effect of parity. Epidemiological studies have shown decreased protective effects of parity with increasing age at delivery42 that are thought to be attributable to an increase in the number of mutated cells with age43,44 and in the proliferation of cells during pregnancy45. Because mating was started at a young age of 10 weeks in the present study, pre- and post-pubertal rats had a 7- and 3-week interval, respectively, between IR exposure and mating. Thus, the difference in this interval may have altered the parity-induced protective effect against rat mammary carcinoma. In this regard, a previous study proposed that the frequency of chromosomal translocations in irradiated rat mammary glands does not significantly change with the increase in the interval between IR exposure and analysis46. If this scenario applies to our present study, the frequency of genetic aberrations at the time of mating may not differ regardless of the difference in the interval between IR exposure (pre- and post-pubertal) and mating. Further animal and epidemiological studies will be necessary to fully understand the effect of the interval between radiation exposure and pregnancy.
Parity seems to influence mammary carcinogenesis via both systemic and local mechanisms. Rat mammary carcinomas exhibit a hormone dependency that is similar to that of human breast cancer. It has been reported that long-term tamoxifen treatment reduces the incidence of radiation-induced mammary cancer in rats47, suggesting that the hormonal environment greatly influences radiation-related mammary cancer risk. In the present study, the pre-pubertal exposure resulted in premature cessation of regular estrous cycling, like that observed for young female patients who received radiation therapy and experienced premature menopause48. Because the sensitivity of oocytes to radiation in immature rats is greater than that in adult rats49, our results suggest that this differential sensitivity causes differential parity-induced changes in hormonal status. Progesterone stimulates cell proliferation in normal mammary glands50 and also in mammary carcinomas51. Considering such a general progesterone function for both the normal mammary gland and mammary carcinoma, the increase in serum progesterone as a consequence of radiation exposure may have contributed to the observed increase in the risk of mammary carcinoma. However, it remains unclear why parity after pre-pubertal irradiation normalizes the increased progesterone level. A possible mechanism is related to regression of the corpus luteum after parturition52. The high level of progesterone from the corpus luteum during pregnancy is normalized in the postpartum phase, which is related to prolactin-induced corpus luteum regression53. The mechanism of the postpartum regression of corpus luteum may be unique in that it is independent of the Caspase-3-mediated apoptosis, whereas the involution in regular menstrual cycles is related with Caspase-3 expression54. Such mechanisms may be related to the normalized progesterone levels in the present experiment. Nevertheless, these explanations are hypothetical and further studies are needed. Several studies have reported that parity alters the metabolic function of the mammary gland, liver, and uterus55–57, and this may be caused by parity-induced hormonal changes. Further studies of parity-induced systematic and local changes may help clarify the differential effects of age at exposure. In addition to the systemic changes in progesterone level, the present study postulates that parity may be associated with stronger PR dependency of cancer-cell proliferation, as evidenced by the correlation between PR and Ki-67 in the induced carcinomas. The morphology of the post-involution mammary gland in females is distinguishable from that of a virgin58. Moreover, several studies have reported that the mammary gland undergoes parity-associated epigenetic modifications, e.g., DNA methylation and histone modifications59,60. Because our finding that cell proliferation is altered in mammary carcinoma cannot be readily explained by theories invoking parity-induced changes in hormonal status, further epidemiological and animal studies are needed.
The present study also provides new insights into the observed differences in cancer incidence between virgin rats irradiated at different ages. The risk of mammary carcinomas was lower for IR-3W virgins than IR-7W virgins, with earlier cessation of the normal estrous cycle observed only in IR-3W virgins, reproducing our previous result27. That study, however, did not address the effects of hormone levels prior to carcinogenesis. In the present study, estrogen and progesterone levels were comparable among IR-3W and IR-7W virgins at 22–26 weeks of age, which was before the onset of carcinogenesis. Thus, any fluctuations in the levels of these hormones seemingly cannot account for the observed differential effect of age at irradiation, implying that any hormonal changes associated with the cessation of estrous cycles may have had an impact in this regard.
In conclusion, the complex combined effects of parity and radiation exposure on mammary carcinogenesis may include both systemic and local mechanisms. The effect of parity on radiation-induced mammary carcinoma was found to depend on the age at exposure and correlated with serum progesterone level and the responsiveness of the cancer tissue to progesterone.
Methods
Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences (approval No. 11–1027 and 16–1030), and were performed in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. Detailed procedures for the animal experiments are described in our previous reports25,26. Briefly, male and female Sprague-Dawley (Jcl:SD) rats were obtained from Clea Japan. Pre- or post-pubertal (3 or 7 weeks of age, respectively) rats were subjected to single, whole-body irradiation (4 Gy from 137Cs; 0.5 Gy/min) or left untreated. Rats were palpated weekly for detection of mammary tumors. At age 10 weeks, half of the rats were paired with males; two females were housed together with one male for 2 weeks. The parous rats were allowed to carry a litter to term and to nurse 6 pups for 3 weeks. Mated female rats that failed to wean pups by the time the mothers were 18 weeks old were excluded from the experiment. No significant difference was observed in the weaning rate between the groups (Supplementary Table 1). All rats were fed a CE-2 diet (Clea Japan) throughout the experiment. The estrous cycle for each rat in a subset (n = 6 to 9 per group) was monitored for five consecutive days every second week based on the cytology of vaginal smears. Age at cessation of the regular estrous cycle was determined as the age when the cycle was irregular for two consecutive observations. Observation was terminated when rats showed any sign of general deterioration, died, or reached 100 weeks of age. Collected mammary tumors were fixed in 10% neutral buffered formalin, and approximately 4-μm-thick paraffin-embedded sections were stained with hematoxylin and eosin for histological evaluation15.
Measuring hormone concentrations
Rats were grouped as in the aforementioned animal experiment to measure serum estradiol, progesterone, FSH, LH, growth hormone, prolactin, thyroxine, and corticosterone. Five weeks after weaning (age 22–26 weeks), vaginal smears were checked at midmorning. Rats were autopsied between noon and 4 pm on the day of proestrus under isoflurane anesthesia (4% in air). Each serum sample was obtained from blood collected by cardiac puncture and then stored at −80 °C until used. The serum level of estradiol was measured by liquid chromatography-tandem mass spectrometry analysis carried out by the Oriental Yeast Co. Ltd. (Japan) using a liquid chromatography system consisting of a Nexera chromatograph (Shimadzu, Japan) coupled to a SCIEX API 5000 triple-quadrupole tandem mass spectrometer. The electrospray ionization (TurboIonSpray) source was operated in the positive-ion mode to generate estradiol ions. Each serum sample (200 μl) was mixed with 50 μl isotopically labeled estradiol (13C4, 100 pg/50 μl) as internal controls. The estradiol retention time was 5.11 min. The transitions of estradiol and [13C4]estradiol (m/z) were 544.2/339.0 and 548.2/343.2, respectively. The limit of quantitation was 0.5 pg/μl. Throughout the experiments, the coefficient of variation and recoveries of internal standards were in the range of 0.7 to 8.4% and 82.3 to 103.9%, respectively. The method was validated according to FDA guidance on bioanalytical method validation61. Serum progesterone, FSH, LH, growth hormone, prolactin, thyroxine, and corticosterone were analyzed by competitive immunoassays using europium-labeled antibodies prepared in a commercial laboratory (Protein Purify Ltd., Japan). The steroid hormones were extracted with ether (for progesterone) or methanol (for thyroxine and corticosterone), followed by antibody incubations. Serum was diluted at optimal levels, which were between 2- and 60-fold, and then analyzed. The results for FSH, LH, growth hormone, and prolactin are expressed in terms of the NIH rat FSH RP-2, rat LH RP-3, rat growth hormone RP-2, and rat prolactin RP-2 standard preparations, respectively. Measurements were carried out in triplicate, with the intra-assay coefficient of variation (%) and estimated limit of detection (ng/ml) being 3.7%/0.01 (progesterone), 5.9%/0.69 (FSH), 6.0%/0.16 (LH), 7.5%/0.25 (growth hormone), 3.7%/0.11 (prolactin), 3.1%/0.03 (thyroxine), and 6.4%/0.02 (corticosterone). For the measurement of estradiol, progesterone, FSH, and LH, some samples had a very low estradiol level (<10 pg/μl), as expected for proestrus62; these samples were considered as outliers as a result of misreading of the vaginal cytology and were thus excluded from further evaluation. The number of excluded samples was as follows: No-IR virgin, 1 sample of 9 total; IR-3W parous, 2 of 10 samples; IR-7W virgin, 2 of 7 samples; and IR-7W parous, 1 of 10 samples. The estradiol and progesterone levels in the No-IR groups were comparable with those reported in a previous study63.
Classification of mammary carcinomas based on HR expression
Formalin-fixed paraffin-embedded tissues were sectioned at approximately 4 μm. Primary antibodies for ER (clone 6F11; Leica, USA; dilution, 1:200), PR (clone PR10A9; GeneTex, USA; dilution, 1:400), Ki-67 (clone SP6; Spring Bioscience, USA; dilution, 1:200), and HER2 (clone e2-4001 + 3B5; Thermo Scientific, USA; dilution, 1:100) were used for immunohistochemistry. After the tissue sections were dewaxed, antigen retrieval was performed by autoclaving the sections at 120 °C for 15 min in 0.1 M tris(hydroxymethyl)aminomethane (pH 8.0), deionized water, and 10 mM sodium citrate buffer (pH 6.0) for ER, PR, and Ki-67, respectively. The tissue sections were treated with 0.3% hydrogen peroxide in methanol at room temperature for 15 min, incubated with 10% normal goat serum (Cedarlane Laboratories, Canada) in a blocking solution (Dako Protein Block Serum-Free; Agilent Technologies, USA) at room temperature for 60 min, and then incubated with a primary antibody at 4 °C overnight. Subsequently, sections were reacted with a peroxidase-conjugated secondary antibody (Histofine SimpleStain MAX-PO (M or R) kit; Nichirei Biosciences, Japan) at room temperature for 60 min. Peroxidase activity was visualized with a 3,3′-diaminobenzidine peroxidase staining kit (SK-4100; Vector Laboratories, USA). Staining for HER2 was performed with an enhancer kit (Super Sensitive™ Polymer-HRP IHC Detection System/DAB, BioGenex, USA) following the autoclaving of the sections in sodium citrate buffer (pH 6.0). Sections were counterstained with hematoxylin after immunohistochemical staining. Stained sections were scanned with a NanoZoomer Digital Pathology system (Hamamatsu Photonics, Japan). Two independent researchers (among M.T., T.I., H.M., and M.N.) randomly chose at least 10 areas (magnification, 40×) from each carcinoma, and then the HR index in the tumor epithelium was measured using Tissue Studio image analysis software (Definiens, Germany). HR-positive tumors were defined as those with positive staining of over 1% of cells for both ER and PR64. Tumors were classified as HER2 overexpression (3+, positive) if there was strong membrane staining in 30% or more of tumor cells65.
Statistical analysis
All statistical tests were done using R software (http://www.r-project.org)66 with the graphical user interface EZR67. Differences in the number per unit time and the age at first palpation of mammary tumors, the number of pups, and the age at the cessation of a regular estrous cycle were analyzed with the Kruskal-Wallis test, followed by pairwise comparisons with the Mann-Whitney’s U test. The log-rank test and Cox proportional hazard analysis were performed to analyze the time to first palpation of mammary tumors for each rat. Differences in serum levels of estradiol, progesterone, FSH, LH, growth hormone, prolactin, thyroxine, and corticosterone were evaluated with the multi-way analysis of variance (ANOVA), followed by pairwise comparisons with the Student’s t-test or Welch’s t-test. The distribution of both tumor subtypes and expression of Ki-67 were compared between virgin and parous groups by the Fisher’s exact test and Mann-Whitney’s U test, respectively. Correlations were evaluated by the Spearman’s rank correlation coefficient. A p value of less than 0.05 was considered to reflect a statistically significant difference.
Electronic supplementary material
Acknowledgements
The authors thank Dr. Eric J. Grant for critical reading of the manuscript and making helpful suggestions. The authors also thank Dr. Airo Tsubura and Dr. Jose Russo for their kindly informing us about the protocols for Ki-67 and HER2 staining, respectively. We are very grateful to all laboratory members for their assistance and the Laboratory Animal and Genome Sciences Section of National Institute of Radiological Sciences for animal management. The animal tissues used in this study are part of the J-SHARE Project (Japan-StoreHouse of Animal Radiobiology Experiments) under construction by the National Institute of Radiological Sciences. This work was supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science Fellows (No. 24-7555) to M.T., a Grant-in-Aid for Young Scientists (No. 16K19876) to M.T., and the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University.
Author Contributions
M.T., S.K., M.F., and Y.S. conceived and designed the experiments. M.T., K.D., T.I., Y.N., K.S., A.H., H.M., and M.N. performed the primary experiments, including animal experiments. M.T., K.D., T.I., T.K., H.M., and M.N. evaluated pathology. M.T. analyzed the results, wrote the draft manuscript, and prepared the figures and tables. K.D., T.I., B.J.B., and Y.S. reviewed the draft manuscript. All authors reviewed and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masaru Takabatake, Email: takabatake.masaru@qst.go.jp.
Yoshiya Shimada, Email: shimada.yoshiya@qst.go.jp.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32406-1.
References
- 1.Abdulrahman, G. O. & Rahman, G. A. Epidemiology of breast cancer in Europe and Africa. J. Cancer Epidemiol. 2012 (2012). [DOI] [PMC free article] [PubMed]
- 2.Farvid MS, et al. Dietary fiber intake in young adults and breast cancer risk. Pediatrics. 2016;137:e20151226. doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol. Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 4.Preston DL, et al. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat. Res. 2002;158:220–235. doi: 10.1667/0033-7587(2002)158[0220:REOBCR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Inskip PD, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J. Clin. Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inskip PD, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int. J. Radiat. Oncol. Biol. Phys. 2016;94:800–807. doi: 10.1016/j.ijrobp.2015.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2004;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier-Abt F, Bentires-Alj M. How pregnancy at early age protects against breast cancer. Trends Mol. Med. 2014;20:143–153. doi: 10.1016/j.molmed.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Land CE, et al. A case-control interview study of breast cancer among Japanese A-bomb survivors. II. Interactions with radiation dose. Cancer Causes Control. 1994;5:167–176. doi: 10.1007/BF01830263. [DOI] [PubMed] [Google Scholar]
- 10.Goodman MT, Cologne JB, Moriwaki H, Vaeth M, Mabuchi K. Risk factors for primary breast cancer in Japan: 8-year follow-up of atomic bomb survivors. Prev. Med. 1997;26:144–153. doi: 10.1006/pmed.1996.9979. [DOI] [PubMed] [Google Scholar]
- 11.Cooke R, et al. Breast cancer risk following Hodgkin lymphoma radiotherapy in relation to menstrual and reproductive factors. Br. J. Cancer. 2013;108:2399–2406. doi: 10.1038/bjc.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ. Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina D, Smith GH. Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J. Natl. Cancer Inst. 1999;91:967–969. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Yoshizawa K, Nandi S, Tsubura A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosourea-induced mammary carcinomas in female Lewis rats. Carcinogenesis. 1999;20:623–628. doi: 10.1093/carcin/20.4.623. [DOI] [PubMed] [Google Scholar]
- 15.Russo J. Significance of rat mammary tumors for human risk assessment. Toxicol. Pathol. 2015;43:145–170. doi: 10.1177/0192623314532036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shellabarger C, Aponte G, Cronkite E, Bond V. Studies on radiation-induced mammary gland neoplasia in the rat: VI. the effect of changes in thyroid function, ovarian function, and pregnancy. Radiat. Res. 1962;17:492–507. doi: 10.2307/3571175. [DOI] [PubMed] [Google Scholar]
- 17.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr. Relat. Cancer. 2007;14:907–933. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 18.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat. 2014;144:1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambertini M, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 2016;49:65–76. doi: 10.1016/j.ctrv.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Thordarson G, et al. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16:2847–2853. doi: 10.1093/carcin/16.11.2847. [DOI] [PubMed] [Google Scholar]
- 22.Dorgan JF, et al. Relationships of age and reproductive characteristics with plasma estrogens and androgens in premenopausal women. Cancer Epidemiol. Biomarkers Prev. 1995;4:381–386. [PubMed] [Google Scholar]
- 23.Arslan AA, et al. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:2123–2130. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- 24.Grant EJ, et al. Associations of ionizing radiation and breast cancer-related serum hormone and growth factor levels in cancer-free female A-bomb survivors. Radiat. Res. 2011;176:678–687. doi: 10.1667/RR2631.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imaoka T, et al. Age modifies the effect of 2-MeV fast neutrons on rat mammary carcinogenesis. Radiat. Res. 2017;188:419–425. doi: 10.1667/RR14829.1. [DOI] [PubMed] [Google Scholar]
- 26.Imaoka T, et al. Influence of age on the relative biological effectiveness of carbon ion radiation for induction of rat mammary carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:1134–1140. doi: 10.1016/j.ijrobp.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Imaoka T, et al. Pre‐and postpubertal irradiation induces mammary cancers with distinct expression of hormone receptors, ErbB ligands, and developmental genes in rats. Mol. Carcinog. 2011;50:539–552. doi: 10.1002/mc.20746. [DOI] [PubMed] [Google Scholar]
- 28.Takabatake M, et al. DNA Methylation patterns in rat mammary carcinomas induced by pre-and post-pubertal irradiation. PLoS One. 2016;11:e0164194. doi: 10.1371/journal.pone.0164194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, et al. Irradiation of juvenile, but not adult, mammary gland increases stem cell self‐renewal and estrogen receptor negative tumors. Stem Cells. 2014;32:649–661. doi: 10.1002/stem.1533. [DOI] [PubMed] [Google Scholar]
- 30.Anderson GM, Grattan DR, van den Ancker W, Bridges RS. Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology. 2006;147:4688–4694. doi: 10.1210/en.2006-0600. [DOI] [PubMed] [Google Scholar]
- 31.Cristofanilli M, et al. Thyroid hormone and breast carcinoma. Cancer. 2005;103:1122–1128. doi: 10.1002/cncr.20881. [DOI] [PubMed] [Google Scholar]
- 32.De la Roca-Chiapas JM, et al. Impact of stress and levels of corticosterone on the development of breast cancer in rats. Psychol. Res. Behav. Manag. 2016;9:1–6. doi: 10.2147/PRBM.S94177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Steger R, Bruni J, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103:1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- 34.Jarrell J, et al. An analysis of the effects of increasing doses of ionizing radiation to the exteriorized rat ovary on follicular development, atresia, and serum gonadotropin levels. Am. J. Obstet. Gynecol. 1986;154:306–309. doi: 10.1016/0002-9378(86)90661-7. [DOI] [PubMed] [Google Scholar]
- 35.Oikawa M, et al. Significance of genomic instability in breast cancer in atomic bomb survivors: analysis of microarray-comparative genomic hybridization. Radiat. Oncol. 2011;6:168. doi: 10.1186/1748-717X-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milne RL, et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2010;119:221–232. doi: 10.1007/s10549-009-0394-1. [DOI] [PubMed] [Google Scholar]
- 37.Cullinane CA, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int. J. Cancer. 2005;117:988–991. doi: 10.1002/ijc.21273. [DOI] [PubMed] [Google Scholar]
- 38.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 39.Iizuka D, et al. DNA copy number aberrations and disruption of the p16Ink4a/Rb pathway in radiation-induced and spontaneous rat mammary carcinomas. Radiat. Res. 2010;174:206–215. doi: 10.1667/RR2006.1. [DOI] [PubMed] [Google Scholar]
- 40.Imaoka T, et al. High relative biologic effectiveness of carbon ion radiation on induction of rat mammary carcinoma and its lack of H-ras and Tp53 mutations. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:194–203. doi: 10.1016/j.ijrobp.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Castiglioni F, et al. Radiation effects on development of HER2-positive breast carcinomas. Clin. Cancer Res. 2007;13:46–51. doi: 10.1158/1078-0432.CCR-06-1490. [DOI] [PubMed] [Google Scholar]
- 42.Lambe M, et al. Transient increase in the risk of breast cancer after giving birth. New Engl. J. Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 43.Grist S, McCarron M, Kutlaca A, Turner D, Morley A. In vivo human somatic mutation: frequency and spectrum with age. Mutat. Res. 1992;266:189–196. doi: 10.1016/0027-5107(92)90186-6. [DOI] [PubMed] [Google Scholar]
- 44.Sun B, Shima N, Heddle JA. Somatic mutation in the mammary gland: influence of time and estrus. Mutat. Res. 1999;427:11–19. doi: 10.1016/S0027-5107(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 45.Temko D, Cheng Y-K, Polyak K, Michor F. Mathematical modeling links pregnancy-associated changes and breast cancer risk. Cancer Res. 2017;77:2800–2809. doi: 10.1158/0008-5472.CAN-16-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakano M, et al. Fetal irradiation of rats induces persistent translocations in mammary epithelial cells similar to the level after adult irradiation, but not in hematolymphoid cells. Radiat. Res. 2014;181:172–176. doi: 10.1667/RR13446.1. [DOI] [PubMed] [Google Scholar]
- 47.Peterson NC, et al. Tamoxifen resistance and Her2/neu expression in an aged, irradiated rat breast carcinoma model. Carcinogenesis. 2005;26:1542–1552. doi: 10.1093/carcin/bgi103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Leeuwen FE, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J. Natl. Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 49.Mandl AM. A quantitative study of the sensitivity of oocytes to X-irradiation. Proc. R. Soc. Lond. B. Biol. Sci. 1959;150:53–71. doi: 10.1098/rspb.1959.0007. [DOI] [PubMed] [Google Scholar]
- 50.Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens progestogens normal breast cell proliferation and breast cancer risk. Epidemiol. Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 51.Manni A, Wright C, Badger B, Demers L, Bartholomew M. Polyamines and autocrine control of N-nitrosomethylurea-induced rat mammary tumor growth in vitro by progesterone. Cancer Res. 1988;48:3058–3061. [PubMed] [Google Scholar]
- 52.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 53.Sugino N, Okuda K. Species-related differences in the mechanism of apoptosis during structural luteolysis. J. Reprod Dev. 2007;53:977–986. doi: 10.1262/jrd.19047. [DOI] [PubMed] [Google Scholar]
- 54.Takiguchi S, et al. Differential regulation of apoptosis in the corpus luteum of pregnancy and newly formed corpus luteum after parturition in rats. Biol. Reprod. 2004;70:313–318. doi: 10.1095/biolreprod.103.018853. [DOI] [PubMed] [Google Scholar]
- 55.Goddard ET, et al. The rodent liver undergoes weaning-induced involution and supports breast cancer metastasis. Cancer Discov. 2017;7:177–187. doi: 10.1158/2159-8290.CD-16-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dearth RK, et al. Parity-induced decrease in systemic growth hormone alters mammary gland signaling: a potential role in pregnancy protection from breast cancer. Cancer Prev. Res. (Phila). 2010;3:312–321. doi: 10.1158/1940-6207.CAPR-09-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keiler AM, et al. Comparison of estrogenic responses in bone and uterus depending on the parity status in Lewis rats. J. Steroid Biochem. Mol. Biol. 2013;133:101–109. doi: 10.1016/j.jsbmb.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 58.Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7:131–142. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhury S, et al. Molecular profiling of human mammary gland links breast cancer risk to a p27 + cell population with progenitor characteristics. Cell stem cell. 2013;13:117–130. doi: 10.1016/j.stem.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russo J, et al. Pregnancy‐induced chromatin remodeling in the breast of postmenopausal women. Int. J. Cancer. 2012;131:1059–1070. doi: 10.1002/ijc.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Food and Drug Administration (FDA). FDA Guidance for Industry: Bioanalytical Method Validationhttps://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (2001).
- 62.Butcher R, Collins W, Fugo N. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson ME, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156:2492–2502. doi: 10.1210/en.2014-1890. [DOI] [PubMed] [Google Scholar]
- 64.Coates AS, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker RA, et al. HER2 testing in the UK: further update to recommendations. J. Clin. Pathol. 2008;61:818–824. doi: 10.1136/jcp.2007.054866. [DOI] [PubMed] [Google Scholar]
- 66.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2014).
- 67.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.