Significance
Glucosylation of host proteins is a common pathogenicity mechanism of bacterial protein toxins. Prominent examples are Clostridium difficile toxins A and B, which inactivate Rho proteins by glucosylation and cause diarrhea and pseudomembranous colitis in C. difficile infections. So far, host factors involved in uptake and/or intracellular stabilization of the toxins are not known. Here we show that TRiC/CCT chaperonins interact with glycosylating toxins and play an essential role in the intoxication process. Knockdown of chaperonins or inhibition of TRiC/CCT functions by compound HSF1A prevents the cytotoxic effects of glucosylating toxins. In contrast, HSP90, which is essential for ADP-ribosylating toxins, does not affect the uptake of glucosylating toxins. Our data provide insight into the cellular actions of glucosylating toxins.
Keywords: chaperonin, toxin uptake, glycosyltransferase toxins, Clostridium difficile toxins, TRiC/CCT
Abstract
Various bacterial protein toxins, including Clostridium difficile toxins A (TcdA) and B (TcdB), attack intracellular target proteins of host cells by glucosylation. After receptor binding and endocytosis, the toxins are translocated into the cytosol, where they modify target proteins (e.g., Rho proteins). Here we report that the activity of translocated glucosylating toxins depends on the chaperonin TRiC/CCT. The chaperonin subunits CCT4/5 directly interact with the toxins and enhance the refolding and restoration of the glucosyltransferase activities of toxins after heat treatment. Knockdown of CCT5 by siRNA and HSF1A, an inhibitor of TRiC/CCT, blocks the cytotoxic effects of TcdA and TcdB. In contrast, HSP90, which is involved in the translocation and uptake of ADP ribosylating toxins, is not involved in uptake of the glucosylating toxins. We show that the actions of numerous glycosylating toxins from various toxin types and different species depend on TRiC/CCT. Our data indicate that the TRiC/CCT chaperonin system is specifically involved in toxin uptake and essential for the action of various glucosylating protein toxins acting intracellularly on target proteins.
Glycosylating protein toxins are major virulence factors of various pathogenic bacteria, including Clostridia, Legionella, Yersinia, Photorhabdus species, and Escherichia coli (1, 2). Prototypical members of this toxin/effector family are toxins A (TcdA) and B (TcdB) from Clostridium difficile. This pathogen is the causative agent of antibiotic-associated diarrhea and pseudomembranous colitis (3–5) and has emerged as a major healthcare threat in clinical settings (6). The clostridial glucosylating toxins TcdA and TcdB play pivotal roles in C. difficile infections (7). Related glucosylating toxins from Clostridium sordellii and Clostridium novyi cause gas gangrene, necrotizing fasciitis, and toxic shock syndrome (8, 9).
TcdA and TcdB have been in the focus of intensive research for two decades. The two toxins are related multidomain proteins with an N-terminal glucosyltransferase domain (10), followed by an autoprotease domain (11) and a large middle part of the toxins, which is crucial for toxin translocation into the cytosol (3, 12–15). The C-terminal part of the toxins participates in binding to target cells, involving possibly three cell membrane receptors: Frizzled (16), poliovirus receptor-like 3 (PVRL3) (17), and chondroitin sulfate proteoglycan 4 (18). After endocytosis of the toxin-receptor complex, the toxins translocate from an acidic endosomal compartment into the cytosol, where the autoprotease domain is activated by inositol hexakisphosphate (11, 19) to release the glucosyltransferase domain. TcdA and TcdB glucosylate and inhibit small GTP-binding proteins of the Rho family, including Rho, Rac, and Cdc42 proteins, which are master regulators of the cytoskeleton and involved in numerous cellular processes (20, 21).
Up to date, the translocation of the glycosylating toxins into the cytosol is the least understood process of the toxins’ actions. Pore formation by TcdA and TcdB has been described (22, 23); however, how the large toxins cross the endosomal membrane and how the glucosyltransferase is reactivated in the cytosol remain unknown. Moreover, the question of reactivation and refolding of bacterial glycosyltransferases during and after translocation is enigmatic for many toxins/effectors known. In this study, we found that chaperonin TCP-1 ring complex (TRiC)/ chaperonin containing TCP-1 (CCT) plays a pivotal role in toxin translocation and/or refolding. TRiC/CCT is a molecular machine involved in proper folding of a large number of newly synthesized eukaryotic proteins (24, 25). Approximately 10% of cytosolic proteins appear to interact with TRiC/CCT (25, 26). The chaperonin is essential for the folding of many cytoskeleton proteins, including actin (27–29) and tubulin (30, 31). We report that not only TcdA and TcdB, but also numerous other glycosylating toxins, including toxins/effectors from Clostridia and Photorhabdus species, depend on the TRiC/CCT system. These findings provide insight into the actions and properties of glycosylating toxins that are translocated into the cytosol of host cells.
Results
TRiC/CCT Interacts with TcdB and Other Glycosyltransferase Toxins.
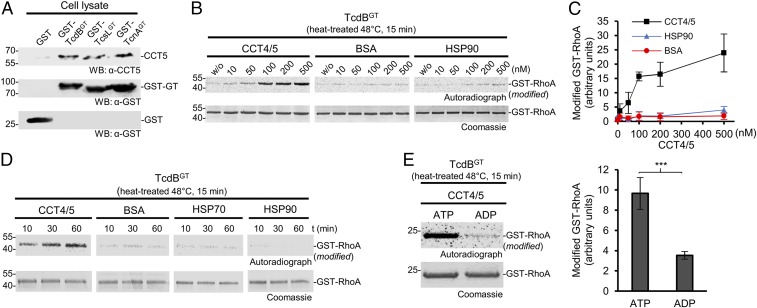
The initial aim of the study was to identify cytosolic interaction partners of glycosylating toxins in host cells. To this end, we used C. difficile toxin TcdB and Legionella pneumophila effector SetA, a glucosyltransferase that is translocated into eukaryotic cells by the Dot/Icm type-IV secretion system (32–34). Glucosyltransferase SetA modified ∼60- and ∼40-kDa proteins in Jurkat cell lysate in the presence of UDP-[14C]glucose. Further studies focused on the 60-kDa protein that was identified by 2D gel electrophoresis and mass spectrometry as the T-complex protein 1ε (CCT5) of the chaperonin TRiC/CCT complex (SI Appendix, Fig. S1). Subsequently, we observed that the glucosyltransferase domain of TcdB (TcdBGT) also directly interacted with CCT5 in precipitation experiments, although it did not glucosylate CCT subunits (Fig. 1A). To analyze a direct interaction of TcdB with CCT in more detail, we purified recombinant CCT4 and CCT5 and performed binding studies using surface plasmon resonance spectrometry. These studies revealed an affinity in the low micromolar range between TcdBGT and CCT4 or CCT5 (SI Appendix, Fig. S2 A–D). Moreover, a similar interaction with CCT5 was observed with the glucosyltransferase domains of C. sordellii lethal toxin (TcsL) and C. novyi alpha toxin (TcnA) (Fig. 1A).
Fig. 1.
CCT4/5 mediates recovery of TcdBGT activity after heat treatment. (A) Western blot analysis of CCT5 pulldown experiments from HeLa cells using GST-TcdBGT–, GST-TcsLGT–, and GST-TcnAGT–coupled beads. GST-coupled beads served as control. Bound CCT5 was detected by Western blot analysis using anti-CCT5 antibody. GST and GST-TcdBGT were detected by anti-GST antibody. (B) Heat-treated TcdBGT was incubated in the presence of CCT4/5 (100 nM), BSA (100 nM), or HSP90 (100 nM) in a buffer containing 0.5 mM ATP for 1 h at 30 °C. Subsequently, recovery of TcdBGT activity was determined by glucosylation of GST-RhoA using UDP-[14C]glucose. (C) Quantification of the recovery of TcdBGT by CCT4/5 shown in B. Error bars indicate ± SD (n = 3) (D) In vitro [14C]glucosylation of GST-RhoA by heat-treated TcdBGT after the indicated recovery times in the presence of CCT4/5, BSA, HSP70, or HSP90. (E, Left) Autoradiograph and Coomassie gel of the [14C]glucosylation of GST-RhoA by TcdBGT (200 nM) after heat treatment (48 °C, 15 min) of the toxin with ATP or ADP (0.5 mM each) in the presence of CCT4/5 (200 nM). (E, Right) Quantification of four experiments. Values are average ± SD. Student’s t test was applied for statistical comparisons. ***P < 0.001.
To study whether TRiC/CCT participates in the stabilization or refolding of glucosylating toxins, we used CCT4/5, because it has been reported that CCT4 and CCT5 can form biologically active homo-oligomers (35). We pretreated TcdB at different temperatures (37°, 42°, 48°, and 55 °C) for 15 min in the presence of ATP. Then the reaction mixture was cooled to 30 °C in the presence of CCT4/5, followed by toxin-catalyzed glucosylation of GST-tagged RhoA or Rac1 (Fig. 1 B–D and SI Appendix, Fig. S3 B and C). We used a 1:1 mixture of CCT4 and CCT5, because this combination was more effective than the single components (SI Appendix, Fig. S3A). CCT4/5 largely restored the glucosyltransferase activity of TcdB in a concentration- and time-dependent manner, while BSA and heat shock proteins HSP90 and HSP70 had no or only minimal effects (Fig. 1 B–D). A similar restoration of enzyme activity after heat treatment was observed with the glucosyltransferase domains of TcsL, TcnA, and Photorhabdus asymbiotica toxin PaTox, which is a tyrosine-modifying GlcNAc-transferase (SI Appendix, Fig. S3 D and E).
The function of TRiC/CCT depends on ATP binding and hydrolysis (31). We tested the nucleotide dependence of the restoration of TcdB activity by CCT4/5. While ATP largely enhanced the restoration after heat treatment in the presence of CCT4/5, ADP had no effect (Fig. 1E). The addition of nucleotides did not affect the glucosyltransferase activity under control conditions (SI Appendix, Fig. S3F).
We next investigated whether other enzyme activities of bacterial toxins are protected and restored by CCT4/5. To this end, we studied the binary ADP ribosylating toxin CDT from C. difficile and compared the effects of CCT4/5 with BSA on the restoration of the enzyme activity after heat treatment. In contrast to TcdB, the ADP ribosylating toxin CDT was not protected from heat inactivation by CCT4/5, indicating a specific effect of the chaperonin on glycosylating toxins (SI Appendix, Fig. S4).
Effects of the TRiC/CCT Inhibitor HSF1A.
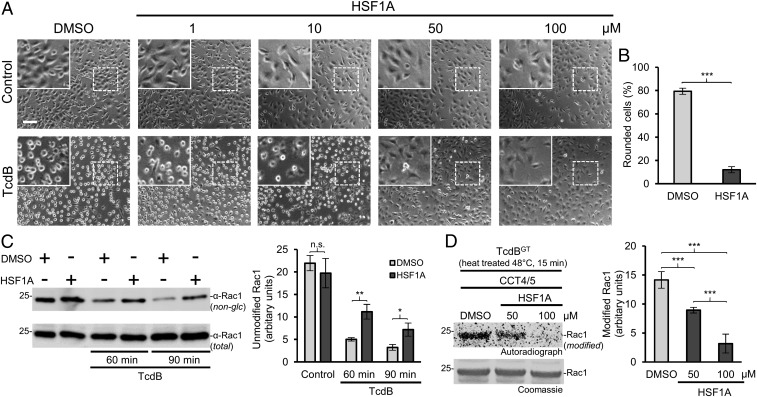
We next studied whether CCT4/5 plays a role in the intoxication of intact cells by TcdB. We used HSF1A, which was recently identified as a specific inhibitor of TRiC/CCT (36). TcdB (5 pM) induced almost complete rounding up of HeLa cells after treatment for 90 min (Fig. 2 A and B). HSF1A blocked the intoxication process in a concentration-dependent manner. While initial effects were observed at 10 µM, HSF1A at 50 µM completely blocked TcdB-induced cytotoxicity. We also tested the in vivo glucosylation of Rac1 protein, which is targeted by TcdB (Fig. 2C). Therefore, we used an anti-Rac1 antibody that recognizes only nonmodified Rac1 (37). Treatment of HeLa cells with TcdB for 90 min resulted in nearly complete glucosylation of Rac1, resulting in no detection by the anti-Rac1 antibody. In contrast, the addition of HSF1A increased staining by the anti-Rac1 antibody, indicating inhibition of Rac1 glucosylation in intact cells. A similar protection from TcdB was observed in mouse embryonic fibroblasts (MEFs) (SI Appendix, Fig. S5 A–C).
Fig. 2.
HSF1A inhibits TcdB-mediated intoxication of HeLa cells. (A) HeLa cells were pretreated with the indicated concentrations of HSF1A or DMSO as control for 1 h before intoxication with TcdB (5 pM). Pictures were taken after 90 min. (Scale bar: 100 µm.) (Insets) Magnifications of dotted areas. (B) Quantification of HeLa cell intoxication with TcdB (5 pM) after 90 min with pretreatment of DMSO or HSF1A (100 µM). The percentage of rounded cells per picture (>500 cells) is given as mean ± SD (n = 4). Student’s t test was applied for statistical comparison. ***P < 0.001. (C, Left) HeLa cells, pretreated for 1 h with DMSO (control) or HSF1A (100 µM), were incubated with or without TcdB (5 pM) for 60 or 90 min. Cell lysates were analyzed by Western blot with the specific anti-Rac1 antibody (MAB102), which does not recognize glucosylated Rac1. Anti-Rac1 (23A8) antibody served as input control. (C, Right) Quantification of three experiments. Values are average ± SD. Student’s t test was applied for statistical comparison. n.s., not significant (P > 0.05); *P < 0.05; **P < 0.01. (D, Left) CCT4/5-mediated recovery assay of heat-treated (48 °C) TcdBGT with the addition of DMSO or HSF1A (50 or 100 µM). Shown is the [14C]glucosylation of Rac1. (D, Right) Quantification of four experiments. Values are average ± SD. Student’s t test was applied for statistical comparisons. ***P < 0.001.
To exclude the possibility that HSF1A has a direct effect on the glucosyltransferase activity of TcdB, we performed an in vitro glucosylation reaction with Rac1 as substrate (SI Appendix, Fig. S6 A and B). This experiment showed that HSF1A did not affect the glucosylation of Rho proteins per se. In addition, we used HSF1A (50–100 µM) in the heat treatment experiment together with TcdB and CCT4/5 (Fig. 2D). The inhibitor clearly blocked the chaperone activity of CCT4/5 to restore the glucosyltransferase activity of heat-treated TcdB, indicating an interaction with these subunits of TRiC/CCT. Similarly, as was seen for TcdB, HSF1A inhibited the intoxication of HeLa cells by TcdA and TcsL and prevented glucosylation of Rac in intact cells (SI Appendix, Fig. S7 A–D).
Clostridial glucosylating toxins cause pore formation, which might be involved in toxin translocation into the cytosol of target cells (23). Therefore, the clostridial toxins release rubidium ions from preloaded cells, when the pH of the cell culture medium is reduced to pH <5 to mimic the low pH of endosomes. We studied the effects of HSF1A on TcdB-induced 86Rb+ release in Chinese hamster ovary (CHO) cells. The chaperonin inhibitor had no effect on rubidium release (SI Appendix, Fig. S8A), suggesting that CCTs play no major role in toxin-induced pore formation. We also tested the effects of HSF1A on the autoproteolytic cleavage of TcdB, which occurs in the cytosol in the presence of inositol hexakisphosphate as an activating cellular cofactor. Again, HSF1A had no effect, indicating that TRiC/CCT did not interfere in vitro with the autocleavage of TcdB (SI Appendix, Fig. S8B).
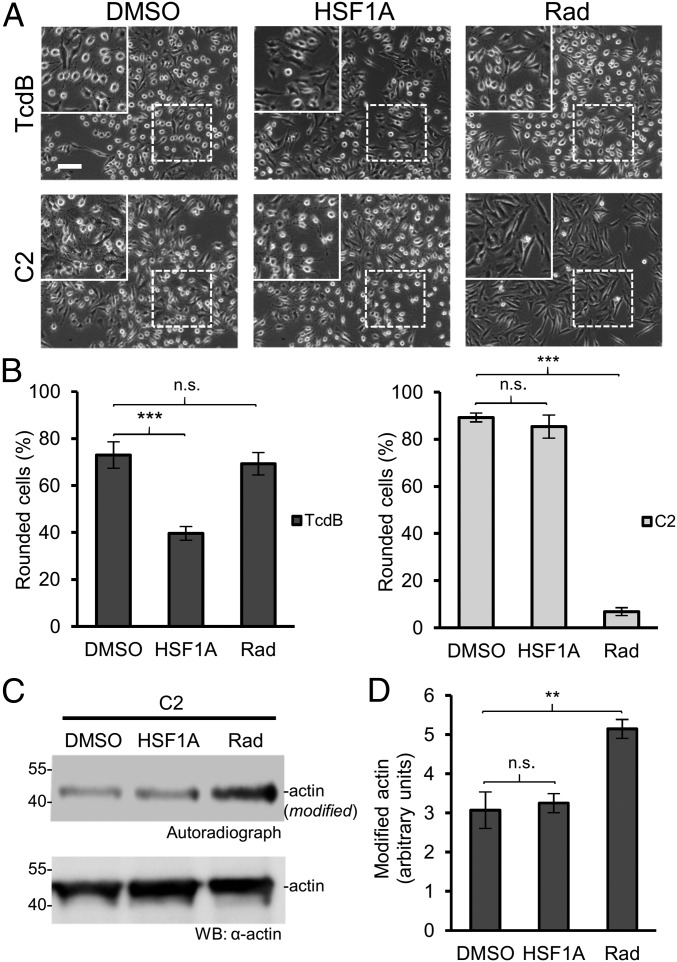
In intact cells, we studied whether HSF1A could interfere with the cytotoxic effects of ADP ribosylating toxins. For these experiments, Clostridium botulinum C2 toxin was used, because it enters HeLa cells readily. Like other ADP ribosylating toxins, the uptake of C2 toxin into host cells depends on the HSP90 system (38). Therefore, we compared the effect of HSF1A on the cytotoxic action of C2 toxin and of TcdB with that of radicicol, which is a specific inhibitor of HSP90 (Fig. 3) (39). While HSF1A blocked the cytotoxic effects of TcdB, radicicol had no effect. In contrast, HSF1A was not able to protect cells from the effects of C2 toxin, whereas radicicol reduced the cytotoxicity of C2 toxin. In line with these findings, radicicol, but not HSF1A, prevented the ADP ribosylation of actin during the intoxication of intact HeLa cells with C2 toxin. This caused an enhanced signal after an additional C2 toxin-induced [32P]ADP ribosylation in the cell lysate (Fig. 3 C and D). Because uptake of binary ADP ribosylating toxins (e.g., C2 toxin, CDT) depends on a preformed pore-forming toxin component, we asked whether other ADP ribosylating toxins, which are taken up differently, are affected by CCT. To this end, we employed diphtheria toxin (40). Similarly, as found for the binary ADP ribosylating toxins, CCT4/5 did not affect the cytotoxic effects of diphtheria toxin, whereas radicicol was inhibitory (SI Appendix, Fig. S9).
Fig. 3.
HSF1A does not inhibit C. botulinum C2-mediated intoxication of HeLa cells. (A) HeLa cells were pretreated with 50 µM HSF1A or with 50 µM of the HSP90 inhibitor radicicol (Rad) for 1 h at 37 °C before intoxication. DMSO served as control. The cells were intoxicated with TcdB (5 pM) or C2 toxin (50 ng/mL C2I plus 100 ng/mL C2IIa). Pictures were taken after 100 min. (Scale bar: 100 µm.) (Insets) Magnifications of dotted areas. (B) The percentage of rounded cells per picture (>200 cells) is given as mean ± SD (n = 8). Student’s t test was applied for statistical comparison. ***P < 0.001; n.s., not significant (P > 0.05). (C) Autoradiograph and Western blot analysis of lysates of C2 toxin-intoxicated cells, treated with additional C2I (40 nM) and radiolabeled [32P]NAD (1 mM) after 100 min of intoxication. (D) Quantification of the autoradiograph shown in C. Values are average ± SD (n = 3). Student’s t test was applied for statistical comparison. **P < 0.01; n.s., not significant (P > 0.05).
Knockdown of CCT5 by siRNA.
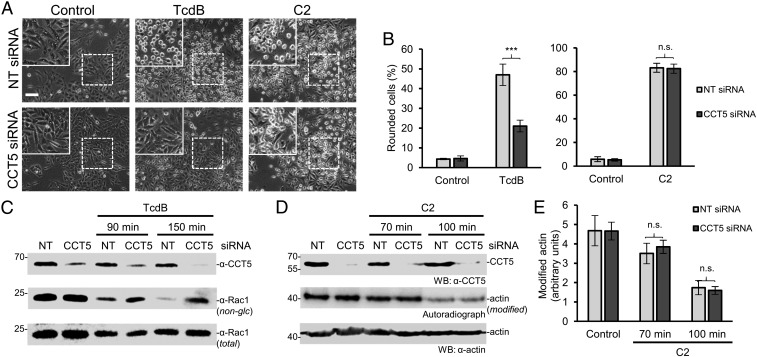
To confirm the crucial role of TRiC/CCT in the action of TcdB, we used knockdown of CCT5 by siRNA (Fig. 4). We used a transient transfection protocol with Lipofectamine, which resulted in reduced expression of CCT5 by 60–90%, while nontargeting siRNA had no effect (Fig. 4 C and D). As was seen with HSF1A, knockdown of CCT5 inhibited the cytotoxic effects of TcdB. Moreover, the knockdown resulted in strongly reduced glucosylation of Rac1 protein. In contrast, the cytotoxic effects of C2 toxin were not affected by the knockdown of CCT5 (Fig. 4 A and B). Accordingly, the second in vitro ADP ribosylation of actin with [32P]NAD, carried out in the cytosol of C2 toxin-pretreated cells, was not affected by the siRNA, although CCT5 expression was strongly reduced (Fig. 4 D and E).
Fig. 4.
Knockdown of CCT5 via siRNA inhibits intoxication by TcdB but not by C2 toxin. (A) siRNA (NT, nontargeting siRNA; CCT5 siRNA)-treated HeLa cells were intoxicated with TcdB (1 pM) or C2 toxin (50 ng/mL C2I plus 100 ng/mL C2IIa). Pictures were taken after 150 min for TcdB and after 100 min for C2-intoxicated cells. (Scale bar: 100 µm.) (Insets) Magnifications of dotted areas. (B) Percentage of rounded cells per picture (>400 cells) is given as mean ± SD (n = 8). Student’s t test was applied for statistical comparisons. ***P < 0.001; n.s., not significant (P > 0.05). (C) Cell lysates of TcdB-treated HeLa cells were analyzed after the indicated incubation times (90 and 150 min) by Western blot with the specific anti-Rac1 antibody (MAB102), which does not recognize glucosylated Rac1. Anti-Rac1 (23A8) antibody served as input control, and anti-CCT5 antibody was used to confirm CCT5 knockdown. (D) Modification of C2 toxin-intoxicated cells was analyzed via ADP ribosylation of actin in cell lysates with additional C2I (40 nM) and radiolabeled [32P]NAD (1 mM) after intoxication of intact cells for the indicated times. Shown is an autoradiograph of SDS/PAGE and a Western blot of the cell lysates with anti-CCT5 antibody to confirm CCT5 knockdown. (E) Quantification of the autoradiograph of the [32P]ADP ribosylation of actin shown in D (n = 3). Student’s t test was applied for statistical comparison. n.s., not significant (P > 0.05).
Discussion
Toxin uptake into the cytosol of target cells is one of the least understood processes of glycosylating toxins. According to current models, these toxins form pores and must be unfolded for translocation into the cytosol of host cells, where they refold to restore their activity (23, 41, 42). Our findings indicate that the TRiC/CCT chaperonin system plays a major role in this process and likely is essentially involved in the refolding processes, occurring subsequently or concomitantly with toxin translocation. Several findings support our conclusion. First, CCT4/5 subunits of the TRiC/CCT chaperonin complex interact directly with TcdB and with various other glycosylating toxins. This interaction occurs with low micromolar affinity, similar to that reported for the interactions of TRiC/CCT with actin and tubulin (43). Second, CCT4 and CCT5 efficiently restore the activities of glycosyltransferase toxins after heat treatment. This effect is ATP-dependent, a finding in favor of specific interaction and function (44). Notably, CCT4/5 also restores the enzyme activity of PaTox, a toxin from P. asymbiotica that is distantly related to clostridial glucosylating toxins and modifies Rho proteins by attachment of N-acetylglucosamine at tyrosine residues (45). Third, using the TRiC/CCT-inhibitor HSF1A (36), we show that the chaperonin system plays a pivotal role in the uptake and intoxication process of TcdB in intact cells. HSF1A largely prevents the TcdB-induced rounding up of cells. Similarly, HSF1A blocks the cytotoxic effects of TcdA and TcsL. Finally, knockdown of CCT5 by a specific siRNA, which reduces the protein levels by 60–90%, significantly inhibits the cytotoxic effects of TcdB.
We suggest that the inhibition of the cytotoxicity of glycosylating toxins caused by CCT5 knockdown or the TRiC/CCT inhibitor HSF1A is the consequence of blockade of the reestablishment of the toxins’ activity after the translocation process. In line with this is the finding that the TRiC/CCT inhibitor prevents the toxin-induced glucosylation of Rac1 in intact cells. HSF1A does not affect the toxin-catalyzed glucosylation of Rho proteins in vitro, indicating that the effects in intact cells are not due to direct inhibition of TcdB or TcdA.
The cytotoxicity of various ADP ribosylating toxins like CDT, C2 toxin, and diphtheria toxin is not affected by HSF1A or CCT5 knockdown (e.g., C2 toxin). Thus, TRiC/CCT likely is not involved in the uptake of ADP ribosylating toxins. Accordingly, CCT4/5 does not enhance the recovery of the ADP ribosyltransferase activity of CDT after heat treatment of the toxin. We and others have previously shown that HSP90 is involved in the uptake process of CDT and of other ADP ribosylating toxins, including C2 toxin, Clostridium perfringens iota toxin, and diphtheria toxin (38, 46, 47). Along with HSP90, peptidyl-prolyl-cis-trans-isomerases (PPIases) of the cyclophilin and FKBP families participate in these effects (47, 48). Notably, these chaperones do not facilitate the uptake of glucosyltransferase toxins (38), which we studied in the present investigation. Thus, the uptake of the different types of toxins is facilitated by different chaperone systems that strictly differentiate between ADP ribosyltransferases and glucosyltransferases. While ADP ribosylating toxins depend on HSP90 and PPIases of the cyclophilin and FKBP families, glycosylating toxins require TRiC/CCT. In line with this hypothesis is our finding that HSP90 is not able to restore the glucosyltransferase activity of TcdB after heat treatment.
The precise functions of TRiC/CCT in the uptake of glucosylating toxins are not clear. Several viral proteins require TRiC/CCT for proper function (49–51). It has been suggested that the glucosylating toxins are unfolded during the translocation process across the membrane of the acid endosomal compartments (3, 23, 41). Thus, the toxins may occur in the cytosol as a linear chain, similar to how they are synthesized at ribosomes. Here the chaperones may come into play to facilitate toxin folding. Whether the support of refolding of toxin domains and subdomains in the cytosol can affect the dynamics and directionality of toxin translocation in a manner characterized as entropic pulling remains to be studied (52). The clostridial toxins TcdA and TcdB are multidomain proteins. It has been shown that TRiC/CCT is able to fold multidomain proteins by partial encapsulation (53). This also may be the case with the large multidomain toxins. However, CCT4/5 also interacted with the apparently folded glucosyltransferase domains in vitro and were precipitated in pull-down experiments. Although the structural determinants of this interaction remain elusive, because all studied glucosylating toxins belong to the same GT-A type family of glucosyltransferases (2), which share a similar folding, and all ADP ribosylating toxins exhibit a similar 3D structure, which largely differs from that of GT-A type glycosyltransferases, we speculate that structural differences, including folding intermediates, and folding dynamics are involved in chaperone recognition (54).
It is accepted that at least the glucosyltransferase and the protease domain are translocated into the cytosol, where the protease domain is activated by inositol hexakisphosphate, releasing the glucosyltransferase domain (11, 19, 55). The chaperonin inhibitor HSF1A has no effect on the protease activity of TcdB in vitro; however, we cannot exclude the possibility that TRiC/CCT5 plays a role in the refolding of the autoprotease domain of the clostridial glucosylating toxins. It was recently reported that TRiC/CCT is involved in the action of anthrax toxin (56). These studies focused on the lethal toxin (LFN) of the tripartite anthrax toxin, which is a zinc-dependent metalloprotease. The binding and translocation component of anthrax toxin is the protective antigen PA, which exhibits significant sequence similarity with the binding and translocation components of the binary toxins CDT and C2 toxin. Our finding that the uptake of ADP ribosylating toxins CDT and C2 toxin is not affected by HSF1A or knockdown of CCT5 is in line with the view that the refolding process, but not pore formation, is affected by the chaperonin.
In summary, here we have identified TRiC/CCT as an essential helper system for the uptake action of TcdA and TcdB. Moreover, we present evidence that the uptake and refolding of other glycosylating protein toxins depend on this chaperone system. Whether a cytosolic system and if so, which one, is involved in the uptake of these types of toxins has remained elusive. Our findings provide insight into the cellular uptake and action of an ever-growing group of important bacterial toxins and effectors.
Materials and Methods
Additional information on materials, cloning and recombinant protein expression, 2D gel electrophoresis, mass spectrometry, surface plasmon resonance spectrometry, the 86Rb+ release assay, and the TcdB autoproteolytic cleavage assay are provided in the SI Appendix.
Antibodies.
The following antibodies were used in the experiments: anti-TCP1ε (CCT5, dilution 1:10,000; Abcam), anti-GST (1:2,000; GE Healthcare), anti-Rac1non-Glc (clone 102, dilution 1:5,000; BD Biosciences), anti-Rac1total (clone 23A8, dilution 1:2,500; Merck Millipore), HRP-linked anti-mouse (Merck Millipore), HRP-conjugated anti-rabbit (New England BioLabs), and anti-actin (clone 2G2, dilution 1:3,000; Hypermol).
Cell Culture.
HeLa cells (human cervix epithelioid carcinoma cells), MEF cells, and Jurkat cells (human T lymphocytes) were cultured in DMEM, and CHO-K1 cells were cultured in Ham’s F-12 medium. Complete medium was supplemented with 10% FCS, 1% nonessential amino acids, 4 mM penicillin, 4 mM streptomycin, and 1% sodium pyruvate (Biochrom). Cells were cultivated in a humidified atmosphere of 5% CO2 at 37 °C. For intoxication, cells were starved in DMEM or Ham’s F-12 medium without supplements. DMSO, HSF1A, or radicicol was added to the medium 1 h before intoxication. For SDS/PAGE and Western blot analysis, cells were lysed with 1× RIPA buffer (20 mM Tris⋅HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin, and cOmplete protease inhibitor). For autoradiography, cells were lysed in 50 mM Tris⋅HCl pH 7.4, 100 mM NaCl, 2 mM MgCl2, and cOmplete protease inhibitor.
Intoxication of Cells after CCT5 Knockdown with siRNA.
HeLa cells were transfected with Silencer Select predesigned siRNA (Life Technologies) targeting CCT5 (sense: 5′-CAAAUGGGCUUGAUAAGAUtt-3′; antisense: 5′-AUCUUAUCAAGCCCAUUUgt-3′) or Silencer Select Negative control #1 siRNA using Lipofectamine 2000 transfection reagent (Life Technologies) according to the manufacturer’s instructions. After a 24-h incubation at 37 °C, cells were treated with 1 pM TcdB for 90 and 150 min or with C. botulinum C2 toxin (50 ng/mL C2I plus 100 ng/mL C2IIa) for 70 and 100 min before analysis via microscopy, Western blot, or autoradiography.
Microscopy and Image Acquisition.
Microscopic pictures were acquired with a Zeiss Axiovert 25 CFL microscope with an Axiocam HRC high-resolution camera driven by AxioVision release 4.9 software. Cells were counted using the ImageJ Fiji cell counter plugin (51).
Recovery Reaction.
Recombinant bacterial glycosyltransferases and ADP ribosyltransferase CDT-A (100 nM, if not specified otherwise) were heat-treated for 15 min at different temperatures (37, 42, 48, and 55 °C). Then CCT protein, BSA, or HSP70/90 was added together with 0.5 mM ATP (or other nucleotides) to induce CCT-mediated restoration of enzyme activity. Unless noted otherwise, recovery incubation conditions were 1 h at 30 °C. After recovery, glycosylation or an ADP ribosylation reaction was performed.
Glycosylation Reaction.
Nontreated TcdBGT or PaToxG or recovery reaction mixtures were incubated with 10 µM UDP-[14C]glucose or UDP-[14C]N-acetylglucosamine in a buffer containing 50 mM Hepes pH 7.4, 2 mM MgCl2, 1 mM MnCl2, and 1 M KCl for 30 min at 30 °C in the presence of recombinant Rac1, GST-RhoA, or cell lysates. The total volume was 20 µL. Proteins were analyzed via SDS/PAGE and phosphorimaging.
ADP-Ribosylation Reaction.
The CDT-A containing recovery reactions were incubated together with 1 mM [32P]NAD (3.5 µCi) and 1.2 µM rabbit α-actin (Cytoskeleton) for 10 min at 30 °C in ADP ribosylation buffer containing 20 mM Tris⋅HCl pH 7.4, 1 mM EDTA, and 5 mM MgCl2. The total reaction volume was 20 µL. Proteins were analyzed via SDS/PAGE and phosphorimaging. Cell lysates of C2 toxin-intoxicated cells were treated with additional recombinant C2I (40 nM) and radiolabeled [32P]NAD (1 mM, 3.5 µCi) in ADP ribosylation buffer in a total reaction volume of 25 µL for 20 min at 30 °C, followed by SDS/PAGE and phosphorimaging.
Effector Pulldown Assay.
GST-TcdBGT and GST were expressed in E. coli TG1 and purified by affinity chromatography with glutathione-Sepharose beads. HeLa cells were lysed in ice-cold buffer A (50 mM Tris⋅HCl pH 7.4, 100 mM NaCl, 2 mM MgCl2, and cOmplete protease inhibitor) and cellular debris was removed by centrifugation (3 min at 17,000 × g). The lysate was incubated for 30 min at 4 °C together with GST-TcdBGT or GST immobilized on glutathione-Sepharose beads. The suspensions were washed three times on Pierce Micro Spin Columns (Thermo Fisher Scientific) with ice-cold buffer B (50 mM Tris⋅HCl pH 7.4, 100 mM NaCl, 2 mM MgCl2, 1% Nonidet P-40, and 10% glycerol). The proteins were eluted from the beads with buffer C (50 mM Tris⋅HCl pH 8.0, 100 mM NaCl, and 10 mM reduced glutathione) and finally subjected to SDS/PAGE and transferred onto a PVDF membrane (Carl Roth).
Supplementary Material
Acknowledgments
We thank Peter Gebhardt for the excellent technical support. This study was supported by the Deutsche Forschungsgemeinschaft (AK6/16-4, to K.A. and SFB 746; Project A17, to K.A. and T.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807658115/-/DCSupplemental.
References
- 1.Lu Q, Li S, Shao F. Sweet talk: Protein glycosylation in bacterial interaction with the host. Trends Microbiol. 2015;23:630–641. doi: 10.1016/j.tim.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Jank T, Belyi Y, Aktories K. Bacterial glycosyltransferase toxins. Cell Microbiol. 2015;17:1752–1765. doi: 10.1111/cmi.12533. [DOI] [PubMed] [Google Scholar]
- 3.Aktories K, Schwan C, Jank T. Clostridium difficile toxin biology. Annu Rev Microbiol. 2017;71:281–307. doi: 10.1146/annurev-micro-090816-093458. [DOI] [PubMed] [Google Scholar]
- 4.Voth DE, Ballard JD. Clostridium difficile toxins: Mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orrell KE, Zhang Z, Sugiman-Marangos SN, Melnyk RA. Clostridium difficile toxins A and B: Receptors, pores, and translocation into cells. Crit Rev Biochem Mol Biol. 2017;52:461–473. doi: 10.1080/10409238.2017.1325831. [DOI] [PubMed] [Google Scholar]
- 6.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: Pathogenesis and host defence. Nat Rev Microbiol. 2016;14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter GP, et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. MBio. 2015;6:e00551. doi: 10.1128/mBio.00551-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens DL, Aldape MJ, Bryant AE. Life-threatening clostridial infections. Anaerobe. 2012;18:254–259. doi: 10.1016/j.anaerobe.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Genth H, et al. Haemorrhagic toxin and lethal toxin from Clostridium sordellii strain vpi9048: Molecular characterization and comparative analysis of substrate specificity of the large clostridial glucosylating toxins. Cell Microbiol. 2014;16:1706–1721. doi: 10.1111/cmi.12321. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann F, Busch C, Aktories K. Chimeric clostridial cytotoxins: Identification of the N-terminal region involved in protein substrate recognition. Infect Immun. 1998;66:1076–1081. doi: 10.1128/iai.66.3.1076-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egerer M, Giesemann T, Jank T, Satchell KJ, Aktories K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J Biol Chem. 2007;282:25314–25321. doi: 10.1074/jbc.M703062200. [DOI] [PubMed] [Google Scholar]
- 12.Genisyuerek S, et al. Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Mol Microbiol. 2011;79:1643–1654. doi: 10.1111/j.1365-2958.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- 13.Jank T, Aktories K. Structure and mode of action of clostridial glucosylating toxins: The ABCD model. Trends Microbiol. 2008;16:222–229. doi: 10.1016/j.tim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Chumbler NM, et al. Crystal structure of Clostridium difficile toxin A. Nat Microbiol. 2016;1:15002. doi: 10.1038/nmicrobiol.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012;2:28. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao L, et al. Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature. 2016;538:350–355. doi: 10.1038/nature19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaFrance ME, et al. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc Natl Acad Sci USA. 2015;112:7073–7078. doi: 10.1073/pnas.1500791112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan P, et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 2015;25:157–168. doi: 10.1038/cr.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reineke J, et al. Autocatalytic cleavage of Clostridium difficile toxin B. Nature. 2007;446:415–419. doi: 10.1038/nature05622. [DOI] [PubMed] [Google Scholar]
- 20.Just I, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 21.Just I, et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 22.Giesemann T, et al. Cholesterol-dependent pore formation of Clostridium difficile toxin A. J Biol Chem. 2006;281:10808–10815. doi: 10.1074/jbc.M512720200. [DOI] [PubMed] [Google Scholar]
- 23.Barth H, et al. Low pH-induced formation of ion channels by Clostridium difficile toxin B in target cells. J Biol Chem. 2001;276:10670–10676. doi: 10.1074/jbc.M009445200. [DOI] [PubMed] [Google Scholar]
- 24.Lopez T, Dalton K, Frydman J. The mechanism and function of group II chaperonins. J Mol Biol. 2015;427:2919–2930. doi: 10.1016/j.jmb.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 26.Yam AY, et al. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stemp MJ, Guha S, Hartl FU, Barral JM. Efficient production of native actin upon translation in a bacterial lysate supplemented with the eukaryotic chaperonin TRiC. Biol Chem. 2005;386:753–757. doi: 10.1515/BC.2005.088. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Sullivan DS, Huffaker TC. Two yeast genes with similarity to TCP-1 are required for microtubule and actin function in vivo. Proc Natl Acad Sci USA. 1994;91:9111–9115. doi: 10.1073/pnas.91.19.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leroux MR, Hartl FU. Protein folding: Versatility of the cytosolic chaperonin TRiC/CCT. Curr Biol. 2000;10:R260–R264. doi: 10.1016/s0960-9822(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe MB, et al. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 31.Frydman J, et al. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992;11:4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidtman M, Chen EJ, Moy MY, Isberg RR. Large-scale identification of Legionella pneumophila Dot/Icm substrates that modulate host cell vesicle trafficking pathways. Cell Microbiol. 2009;11:230–248. doi: 10.1111/j.1462-5822.2008.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belyi Y, Jank T, Aktories K. Effector glycosyltransferases in legionella. Front Microbiol. 2011;2:76. doi: 10.3389/fmicb.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jank T, et al. Domain organization of Legionella effector SetA. Cell Microbiol. 2012;14:852–868. doi: 10.1111/j.1462-5822.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- 35.Sergeeva OA, et al. Human CCT4 and CCT5 chaperonin subunits expressed in Escherichia coli form biologically active homo-oligomers. J Biol Chem. 2013;288:17734–17744. doi: 10.1074/jbc.M112.443929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neef DW, et al. A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Reports. 2014;9:955–966. doi: 10.1016/j.celrep.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genth H, et al. Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett. 2006;580:3565–3569. doi: 10.1016/j.febslet.2006.04.100. [DOI] [PubMed] [Google Scholar]
- 38.Haug G, et al. The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J Biol Chem. 2003;278:32266–32274. doi: 10.1074/jbc.M303980200. [DOI] [PubMed] [Google Scholar]
- 39.Roe SM, et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 40.Murphy JR. Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process. Toxins (Basel) 2011;3:294–308. doi: 10.3390/toxins3030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, et al. Translocation domain mutations affecting cellular toxicity identify the Clostridium difficile toxin B pore. Proc Natl Acad Sci USA. 2014;111:3721–3726. doi: 10.1073/pnas.1400680111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geny B, Popoff MR. Bacterial protein toxins and lipids: Pore formation or toxin entry into cells. Biol Cell. 2006;98:667–678. doi: 10.1042/BC20050082. [DOI] [PubMed] [Google Scholar]
- 43.Melki R, Batelier G, Soulié S, Williams RC., Jr Cytoplasmic chaperonin containing TCP-1: Structural and functional characterization. Biochemistry. 1997;36:5817–5826. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
- 44.Meyer AS, et al. Closing the folding chamber of the eukaryotic chaperonin requires the transition state of ATP hydrolysis. Cell. 2003;113:369–381. doi: 10.1016/s0092-8674(03)00307-6. [DOI] [PubMed] [Google Scholar]
- 45.Jank T, et al. A bacterial toxin catalyzing tyrosine glycosylation of Rho and deamidation of Gq and Gi proteins. Nat Struct Mol Biol. 2013;20:1273–1280. doi: 10.1038/nsmb.2688. [DOI] [PubMed] [Google Scholar]
- 46.Haug G, Aktories K, Barth H. The host cell chaperone Hsp90 is necessary for cytotoxic action of the binary iota-like toxins. Infect Immun. 2004;72:3066–3068. doi: 10.1128/IAI.72.5.3066-3068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser E, Pust S, Kroll C, Barth H. Cyclophilin A facilitates translocation of the Clostridium botulinum C2 toxin across membranes of acidified endosomes into the cytosol of mammalian cells. Cell Microbiol. 2009;11:780–795. doi: 10.1111/j.1462-5822.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser E, et al. FK506-binding protein 51 interacts with Clostridium botulinum C2 toxin and FK506 inhibits membrane translocation of the toxin in mammalian cells. Cell Microbiol. 2012;14:1193–1205. doi: 10.1111/j.1462-5822.2012.01788.x. [DOI] [PubMed] [Google Scholar]
- 49.Inoue Y, et al. Chaperonin TRiC/CCT participates in replication of hepatitis C virus genome via interaction with the viral NS5B protein. Virology. 2011;410:38–47. doi: 10.1016/j.virol.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Lingappa JR, et al. A eukaryotic cytosolic chaperonin is associated with a high molecular weight intermediate in the assembly of hepatitis B virus capsid, a multimeric particle. J Cell Biol. 1994;125:99–111. doi: 10.1083/jcb.125.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, et al. Cellular chaperonin CCTγ contributes to rabies virus replication during infection. J Virol. 2013;87:7608–7621. doi: 10.1128/JVI.03186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goloubinoff P, De Los Rios P. The mechanism of Hsp70 chaperones: (Entropic) pulling the models together. Trends Biochem Sci. 2007;32:372–380. doi: 10.1016/j.tibs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Rüßmann F, et al. Folding of large multidomain proteins by partial encapsulation in the chaperonin TRiC/CCT. Proc Natl Acad Sci USA. 2012;109:21208–21215. doi: 10.1073/pnas.1218836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boczek EE, et al. Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 2015;112:E3189–E3198. doi: 10.1073/pnas.1424342112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, et al. Critical roles of Clostridium difficile toxin B enzymatic activities in pathogenesis. Infect Immun. 2015;83:502–513. doi: 10.1128/IAI.02316-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slater LH, Hett EC, Clatworthy AE, Mark KG, Hung DT. CCT chaperonin complex is required for efficient delivery of anthrax toxin into the cytosol of host cells. Proc Natl Acad Sci USA. 2013;110:9932–9937. doi: 10.1073/pnas.1302257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.