Significance
Nanocavities in cellular compartments or molecular assemblies are commonplace in nature. Nanoconfinement has demonstrated significant effects on the folding of biomolecules, which is mediated by hydration waters. Therefore, understanding the property of water molecules in nanocavities is instrumental to elucidate the confinement effect. By exploiting the known numbers of waters lost during the folding of DNA G-quadruplex and i-motif structures, we quantified that water activities decreased within smaller DNA origami nanocages. This effect is more pronounced than that induced by cosolutes such as polyethylene glycol (PEG). In addition, loss of water molecules during the folding of G-quadruplex and i-motif governs the folding of biomolecules in nanoconfinement.
Keywords: water activity, G-quadruplex, i-motif, nanoconfinement, DNA origami nanocage
Abstract
Due to the small size of a nanoconfinement, the property of water contained inside is rather challenging to probe. Herein, we measured the amount of water molecules released during the folding of individual G-quadruplex and i-motif structures, from which water activities are estimated in the DNA nanocages prepared by 5 × 5 to 7 × 7 helix bundles (cross-sections, 9 × 9 to 15 × 15 nm). We found water activities decrease with reducing cage size. In the 9 × 9-nm cage, water activity was reduced beyond the reach of regular cosolutes such as polyethylene glycol (PEG). With this set of nanocages, we were able to retrieve the change in water molecules throughout the folding trajectory of G-quadruplex or i-motif. We found that water molecules absorbed from the unfolded to the transition states are much fewer than those lost from the transition to the folded states. The overall loss of water therefore drives the folding of G-quadruplex or i-motif in nanocages with reduced water activities.
Confined space plays a significant role in the folding/unfolding of proteins and nucleic acid structures such as G-quadruplexes and i-motifs. It is believed that a small volume of nanoconfinement facilitates the population of a folded state, which can be accommodated inside the confined space much better than the unfolded conformation (1–5). Thus, the entropic penalty for unfolded species to stay in a confined space is significantly increased with respect to the open space, tilting the equilibrium toward the folded state in nanoconfinement. Inside cells, many biomolecules fold and unfold in the nanocavities of cellular compartments or molecular assemblies that are filled with water molecules (1, 2). Since folding/unfolding transitions are mediated by solvation waters, the driving force for these transitions inside nanoconfinement can be understood by elucidating the property of water molecules inside confined space. It is well known that, during the folding of many biomolecules in open space, water molecules well aligned at the unfolded state are lost to the bulk as disordered species (6, 7). The entropy gained in this process drives the folding.
The behavior of water molecules in confined volume, however, is rather different from the open space. For example, water molecules inside carbon nanotubes are ice-like (8, 9). The melting temperature of these ice-like structures approaches to the boiling point of the bulk water. These observations suggest that water structures inside a carbon nanotube are highly ordered. This is surprising as the hydrophobic carbon nanotube is expected to have relatively weak interactions with polar water molecules. With a surface of increased hydrophilicity, the interaction between the surface and the water becomes stronger. It is conceivable that water molecules become more ordered inside hydrophilic nanoconfinement. Since ordered water molecules, such as ice, have decreased vapor pressure, measurement of water activity, which is the ratio of the vapor pressure of an aqueous solution to that of pure water (10), can depict the orderliness of the water in nanoconfinement.
Given that confined space contains only a limited number of water molecules, it is rather challenging to measure water activity in the nanoconfinement. In this report, we estimated the water activity inside DNA origami nanocages (cross-sections, 9 × 9 to 15 × 15 nm) by exploiting the fact that, during the folding of a human telomeric G-quadruplex, a fixed number of water molecules is lost to the environment (6). Therefore, the observed change in the free energy of G-quadruplex folding is correlated with the water activity inside nanoconfinement. We found that water activity decreases with reducing nanocage size, suggesting that water molecules become more ordered inside smaller nanocages. The estimation of water activity in nanocages by this method is further validated by the number of water molecules lost during the folding of DNA i-motif, which is close to the value obtained from conventional thermo-melting experiments (11). Finally, by exploiting different water activities inside different origami nanocages, we retrieved the change in water molecules for the folded, the unfolded, and the transition states during the entire folding/unfolding processes of biomolecules such as G-quadruplex and i-motif. We found that loss of the water molecules during the folding is not continuous. Water molecules released from transition states to the fully folded states are much more than those changed from the unfolded states to the transitions states.
Results and Discussion
Nanoconfinement Facilitates the Formation of i-Motif.
G-quadruplex and i-motif are non-B DNA structures found in the genome of many organisms including humans (12). While G-quadruplex consists of a stack of G-tetrads each with four interconnected guanine residues, i-motif is made of intercalated hemiprotonated cytosine–cytosine pairs. Both structures have tetraplex conformation with four DNA strands. These tetraplexes formed in promoter regions have shown regulatory roles for transcriptions (13, 14). The formation of tetraplexes in promoter regions faces competing process of reannealing in duplex DNA. It has been demonstrated that the roles of water in the folding/unfolding of the tetraplex DNA are different from the duplex DNA (6, 11, 15). When processed by enzymes such as polymerase or telomerase inside cells, a certain section of single-stranded or duplex DNA can be constrained in the nanometer cavities of the enzymes (16, 17). However, the knowledge of the nanoconfinement effect on the formation of tetraplex or duplex DNA is limited (18).
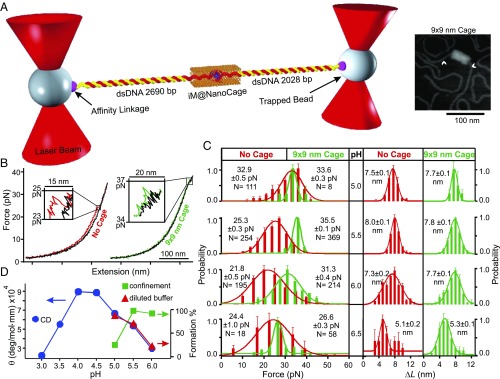
Previously, the transition kinetics of G-quadruplex has been studied inside a nanoconfinement (18). Here, we investigated the effect of confined space on the folding/unfolding of single i-motif molecules in DNA origami nanocages (Fig. 1A). To probe the nanoconfinement effect, the nanocage was designed such that the contour length of the i-motif–forming sequence was comparable with the cross-section of the nanocage. Two ends of an origami nanocage were left open to thread through an i-motif–forming DNA, which was subsequently attached to the two optically trapped beads. The i-motif hosting sequence was secured inside the nanocage with the help of the guide and capture strands (SI Appendix, Fig. S3). The final construct was illuminated with 365-nm UV to break the guide strands via the photolabile group. This is to avoid unwanted strain on the nanocage during the application of force.
Fig. 1.
The pH titration of telomeric i-motif in the 9 × 9-nm DNA origami nanocage. (A) Experimental setup for the mechanical unfolding of a single i-motif (iM) inside the nanocage using optical tweezers. Atomic force microscopy (AFM) image of the 9 × 9-nm DNA construct is shown to the Right. The arrowheads indicate the dsDNA handles attached to the nanocage. (B) Typical force vs. extension curves. The colored and black traces depict stretching and relaxing curves, respectively. (C) Histograms for the force and change in contour length of i-motif at different pH values. The red and green colors represent the i-motif unfolding outside and inside the nanocage, respectively. N represents the number of data points collected in each experiment. (D) Comparative pH plot of molar ellipticity obtained by CD (Left) and the percentage i-motif formation obtained by mechanical unfolding (Right). Error bars represent SDs.
The mechanical unfolding experiments of the i-motif–hosting constructs at pH 5.5 inside a 9 × 9-nm nanocage gave higher rupture force (35.5 pN) with faster refolding events with respect to those without nanocages (Fig. 1B). As the change in contour length for the unfolding (ΔL = 7.8 nm) matches with that of the i-motif structures (see SI Appendix for calculation), the unfolded structures were likely i-motifs. The increased rupture force and faster refolding are likely due to the confined space effect as observed by G-quadruplex inside nanocages (18). Since formation of i-motif is pH dependent (19), we varied pH (5.0, 5.5, 6.0, and 6.5) to verify that these features were indeed due to the unfolding of i-motif. All of the molecules inside the nanocages in this pH range showed ΔL values (∼7.7 nm, Fig. 1C) consistent with i-motif unfolding. They also demonstrated higher rupture forces and faster refolding events compared with those without nanocage at the same pH (Fig. 1B). At pH 6.5, the ΔL was reduced to ∼5 nm, suggesting the formation of a partially folded structure such as triplex (20). Since these species are not fully folded i-motif, pH 6.5 was not considered for the calculation of percentage formation of i-motif.
As shown in Fig. 1D, the percentage i-motif formation decreases with increasing pH. This confirms that rupture events observed above are indeed from the unfolding of the i-motif. Compared with the i-motif outside nanocage, the percentage formation of i-motif inside the nanocage is higher. At pH 5.0, formation of i-motif inside the nanocage was suddenly decreased (mechanical unfolding of i-motif could not be carried out at pH < 5.0 where DNA tether was no longer stable). In comparison, such a drastic decrease was observed at pH 3.5 for circular dichroism (CD) experiments in which ∼285-nm signal was plotted to reflect the i-motif formation (21) (SI Appendix, Fig. S8).
The intriguing shift in the pH profiles of i-motif populations suggests that effects of hydronium ions are different within and without nanocages. The formation of an i-motif structure requires hemiprotonation of cytosine (C)–cytosine pairs (see CH+:C in Eq. 1):
| [1] |
Such a requirement suggests that acidic conditions are necessary for i-motif formation [pH ∼ pKa (4.3) of cytosine (22)]. However, further reduction in pH leads to the protonation of both cytosine residues (CH+), disassembling the CH+:C pairs, which unfold the i-motif (Eq. 2):
| [2] |
Compared with the i-motif population in open space, the pH profile of the i-motif in nanocages shifted to the higher pH (Fig. 1D), suggesting the effective pH inside the nanocages is more acidic than the open space. The increased acidity can result from reduced water activity inside the nanoconfinement. It has been known that, for a linear polyelectrolyte such as DNA, the counterion condensation (23) results in a well-shielded electric field around the DNA backbone. However, for a charged plane such as a DNA origami surface, the electric field originated from phosphate groups in DNA is maintained (24), leading to well-organized water molecules attracted to the origami surface by ion–dipole interactions. Similar to ice, these organized water molecules have reduced vapor pressure, decreasing the overall water activity inside nanocages. The reduced water activities then shift the equilibrium toward the product sides in both Eqs. 1 and 2, making an apparently more acidic environment that shifts the formation profile of i-motif to the higher pH range inside nanocages (Fig. 1D).
Measurement of Water Activity Inside Nanoconfinement.
Next, we measured water activities in the DNA origami nanocages to test whether water activities are reduced in nanocages. Due to the small size of DNA origami nanocages, conventional water activity measurement by vapor pressure determination is not feasible. Given that the number of water molecules absorbed upon unfolding of G-quadruplex (Δn) is known (6), we used this property to estimate the water activity inside DNA origami nanocages.
Unfolding of a G-quadruplex (GQ) in a solution without cosolutes such as polyethylene glycol (PEG) can be described by the following expression:
| [3] |
where M+ represents monovalent cations such as Na+ or K+ and Δm depicts the change in the number of cations during the unfolding. The thermodynamic unfolding equilibrium constant (Kunfold) is expressed as follows:
| [4] |
Here, and are activities of water and metal cations, respectively; and Kobs is the observed unfolding equilibrium constant calculated by the concentration ratio of the unfolded vs. folded species, Kobs = [(GQ)unfolded]/[(GQ)folded]. After taking natural logarithms of Eq. 4 and rearranging, we have the following:
| [5] |
Given that Kunfold is a constant and assuming the change in metal cations plays an insignificant role, with respect to water, on the Kobs of the G-quadruplex transitions (6), a partial derivative of ln(Kobs) vs. in Eq. 5 yields the following:
| [6] |
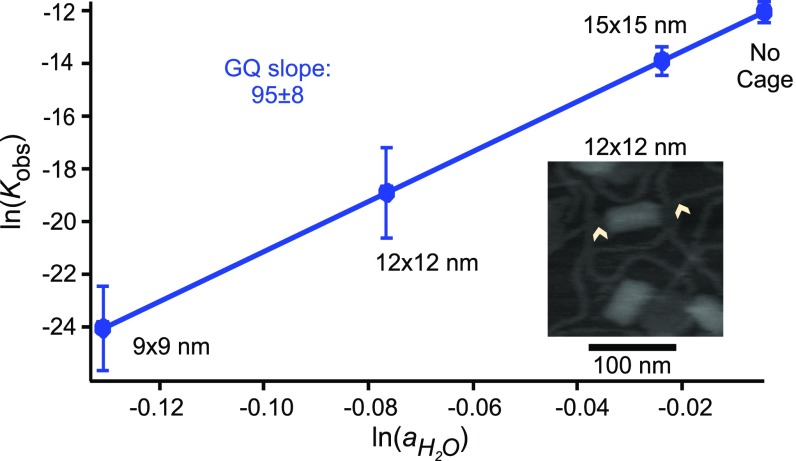
Therefore, by plotting ln(Kobs) vs. , the change in the water molecules (Δn) can be obtained using Eq. 6. With this method, thermo-melting experiments (6) have determined that ∼95 water molecules are released during the formation of telomeric DNA G-quadruplex in the buffers with different water activities, which were introduced by addition of 10–40% PEG 200 to aqueous solutions (6). In our experiments, we obtained different Kobs from mechanical unfolding of the same telomeric G-quadruplex outside or inside nanocages with different cross-sections (see SI Appendix, Fig. S6 for the 12 × 12-nm nanocage; see ref. 18 for other nanocages). From the known water activity outside the nanocage ( = 0.99) (6) and the slope of the ln(Kobs) vs. (Δn = 95), we were able to estimate the values in nanocage with cross-sections ranging from 9 × 9 to 15 × 15 nm (Fig. 2).
Fig. 2.
Log–log plot of observed unfolding equilibrium constant (Kobs) of telomeric G-quadruplex vs. water activity () outside and inside DNA origami nanocages with different cross-sections. Error bars represent SDs.
To validate this water activity measurement, we used this set of water activities in nanocages to retrieve the number of water released during the folding of telomeric DNA i-motif, which was determined as ∼69 by the UV melting experiments at pH 5.5 (11). To this end, we performed mechanical unfolding of the i-motifs at pH 5.5 outside and inside nanocages with different cross-sections (6 × 6, 9 × 9, 12 × 12, and 15 × 15 nm) using the strategy outlined in Fig. 1A (18).
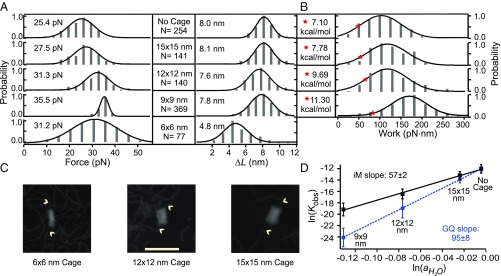
Similar to Fig. 1B, mechanical unfolding of telomeric i-motif showed higher rupture forces followed by faster refolding events (SI Appendix, Fig. S4) compared with those outside the cage (Fig. 1B), suggesting these features are due to the transition of i-motif structures inside the nanocages. Except for the smallest nanocage (6 × 6 nm, SI Appendix, Fig. S4), the ΔL of the unfolding events inside the nanocages (9 × 9-, 12 × 12-, and 15 × 15-nm cross-sections) was found to be ∼8 nm, a value expected for the telomeric i-motif (see SI Appendix for calculation). We argue that insufficient space in the 6 × 6-nm nanocage prohibits the formation of the i-motif, resulting in partially folded structures (20). Therefore, the 6 × 6-nm nanocage was not considered in the calculation of released water molecules during the i-motif folding. Overall, the rupture force of i-motif increases with decreasing nanocage sizes, with the lowest rupture force observed for the i-motif outside the nanocage (Fig. 3A). These results indicate that mechanical stability of i-motif increases within smaller nanocages, a trend consistent with the telomeric G-quadruplex (18).
Fig. 3.
Cage titration of telomeric i-motif at pH 5.5. (A) Rupture force and change-in-contour-length histograms during the unfolding of i-motif outside and inside different nanocages. N represents the number of data points collected in each experiment. (B) Unfolding work histograms for i-motif outside and inside different nanocages. The red stars depict ΔGunfold values. (C) AFM images of the DNA constructs that contain origami nanocages with cross-sections of 6 × 6, 12 × 12, and 15 × 15 nm. (Scale bar: 100 nm.) Arrowheads indicate dsDNA handles. (D) Log–log plot of the observed unfolding equilibrium constant (Kobs) vs. water activity () outside and inside DNA origami nanocages with different cross-sections. Error bars represent SDs.
Next, we used Jarzynski equality (25) to calculate the observed change in the free energy of unfolding, ΔGunfold, of the i-motifs in nanocages (Fig. 3B; see SI Appendix for details). Using the expression, Kobs = exp(−ΔGunfold/RT), where R is gas constant and T is absolute temperature, we retrieved the observed equilibrium constants (Kobs) for the unfolding of i-motif in nanocages. By plotting ln(Kobs) vs. , we found excellent linearity (R2 = 0.99) between ln(Kobs) and (Fig. 3D), confirming insignificant effects of monovalent cations on the variation of Kobs (Eq. 5) as observed in literature (6). From the slope of the plot in Fig. 3D, we concluded that ∼57 ± 2 water molecules were released upon the folding of the i-motif (Eq. 6). This number is close to the UV melting result (68.7 ± 4.2 molecules) (11), validating our water activity measurement inside DNA origami nanocages.
Such a measurement supports our argument that it is the decreased water activity inside nanocavity that leads to the shift in the pH profile of the i-motif populations (Fig. 1D). As activity of water decreases, the power of hydronium ions to protonate cytosines becomes stronger (Eqs. 1 and 2), which facilitates hemiprotonation and full protonation of cytosine residues, two boundaries that define the pH profile of stable i-motif structures. It is significant that water activity decreases with smaller cage size. As shown in SI Appendix, Fig. S5, the low water activity inside the 9 × 9-nm nanocage provides the highest driving force to form fully folded i-motif in the nanocage. In fact, inside the 9 × 9-nm nanocage, the water activity is reduced beyond that projected for pure PEG 200 (equivalent to 102% PEG; SI Appendix, Fig. S9). We anticipated such capability is instrumental to investigate properties of biomolecules at extreme conditions, such as those found in the prebiotic era (26).
Change in Water Molecules During the Folding/Unfolding of DNA Tetraplexes.
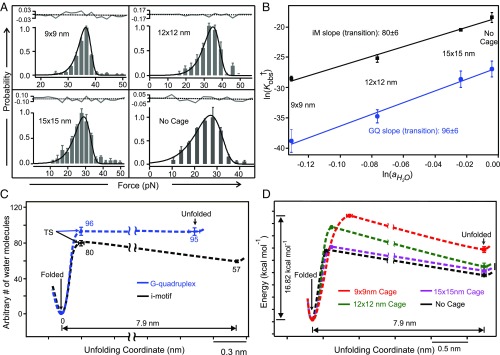
With the water activities determined inside different DNA nanocages, next, we proceeded to obtain the profile of change in water molecules during the folding/unfolding trajectory of the telomeric G-quadruplex and i-motif. To this purpose, we first calculated the loss of water molecules from the unfolded state to the transition state. Fitting the unfolding force histograms using the equation proposed by Dudko (27) (see SI Appendix for details) allowed us to retrieve the energy barrier of the unfolding (ΔG†unfold) (Fig. 4A and Table 1). With the expression, Kobs† = exp(−ΔG†unfold/RT), we estimated the observed equilibrium constants at the transition state (Kobs†) in different DNA nanocages. These values were then plotted against water activities in different nanocages (Fig. 4B and Eq. 6). Slope of the linear fitting of ln(Kobs†) vs. then allowed us to retrieve the change in the water molecules when folded tetraplex reaches to the transition state (Fig. 4B). With this information, we reconstructed the change of water molecules in the three main states (the folded, the transition, and the unfolded states) during the folding/unfolding of DNA G-quadruplex or i-motif (Fig. 4C).
Fig. 4.
Change in the water molecules during the folding/unfolding of DNA tetraplexes. (A) Fittings using Dudko equation (27) (solid curves) on the unfolding force histograms of the i-motif structures outside and inside nanocages with different cross-sections. The gray solid and black dotted curves above each histogram represent the residual analysis and the corresponding linear fitting, respectively. (B) Log–log plots of the observed unfolding equilibrium constant at the transition state (Kobs†) for the G-quadruplex (blue) and i-motif (black) vs. water activity () outside and inside DNA origami nanocages with different cross-sections. (C) Profile of the change in the water molecules during the folding/unfolding of G-quadruplex (blue) and i-motif (black) structures. TS depicts transition state. See Table 1 for the information on the distance between the folded and transition states. (D) Free-energy trajectories for i-motif without and within nanocage cages of different sizes. Error bars represent SDs.
Table 1.
Thermodynamic (ΔGunfold) and kinetic (ΔGunfold† and kunfold) parameters for the telomeric i-motif within and without nanocages
| Cage size | ΔGunfold, kcal/mol | kunfold, s−1 | x†, nm | ΔG†unfold, kcal/mol |
| No cage | 7.10 ± 0.48 | 5.76 ± 0.38 × 10−3 | 0.21 ± 0.05 | 10.86 ± 0.70 |
| 15 × 15 nm | 7.78 ± 0.80 | 1.31 ± 0.01 × 10−3 | 0.24 ± 0.01 | 12.07 ± 0.04 |
| 12 × 12 nm | 9.69 ± 1.16 | 5.21 ± 2.60 × 10−4 | 0.25 ± 0.01 | 14.85 ± 0.44 |
| 9 × 9 nm | 11.30 ± 0.99 | 3.09 ± 3.43 × 10−8 | 0.56 ± 0.08 | 16.82 ± 0.22 |
ΔGunfold, change in free energy of unfolding; ΔGunfold†, activation energy of unfolding; kunfold, unfolding rate constant; x†, distance between the folded and the transition states. Error bars represent SDs.
From the overall profile of water molecules (Fig. 4C), the less stable structure of i-motif with respect to G-quadruplex in nanocages can be ascribed to the decreased entropic driving force for the i-motif folding (number of water molecules released from unfolded to folded states, Δn = 57 ± 2) with respect to the G-quadruplex (Δn = 95 ± 8). Detailed inspection on Fig. 4C revealed that the change in hydration water molecules is not monotonic during the i-motif folding. While 23 water molecules were absorbed during the long transitions (∼8 nm) from the unfolded to the transition states for the telomeric i-motif, far more (∼80) water molecules were lost in the subsequent short transitions (<0.5 nm) to reach the folded state. Significantly, the general profiles of hydration water molecules follow the free-energy trajectories of folding/unfolding very well (Fig. 4D and Table 1). The striking similarity confirms it is the change in water molecules that drives the folding/unfolding of the DNA i-motif. For G-quadruplex, however, the number of water molecules does not change significantly between the transition and the unfolded states, suggesting water may not be the driving force for this part of the reaction trajectory. Given that metal ions such as K+ are critical for the G-quadruplex formation, it is possible that absorption of K+ ions may serve as the rate-determining step from the unfolded to the transition states of G-quadruplex.
Conclusions
In summary, by probing the change in water molecules during the folding/unfolding of human telomeric G-quadruplex or i-motif in nanoconfinement, we measured the water activity inside DNA origami nanocages. We found water activity decreases with the nanocage size. Using these experimentally determined water activities, we retrieved the change in water molecules during the three main states (folded, transition, and unfolded states) along the folding/unfolding trajectories of G-quadruplex or i-motif structure. The net release of water molecules during the folding tilts the equilibrium to the folded structure as water activity decreases in smaller nanocages. Given that water molecules are absorbed during the hybridization of the dsDNA (15), we predict duplex DNA will be less stable in nanoconfinement, which may further facilitate the formation of the competing species, DNA tetraplexes, in nanometer enzyme cavities.
We anticipate the method described here has broad applications to probe water activities in confined space not amenable to conventional approaches, as well as to provide chemical and biochemical systems with water activities far beyond the reach of cosolutes added in aqueous solutions. On the other hand, elucidation of the mechanism for the folding of the G-quadruplex and i-motif offers insights to understand and modulate the formation of these structures with well-demonstrated biological functions.
Materials and Methods
Mechanical unfolding experiments were performed in the optical tweezer using microfluidics platform. The unfolding and refolding forces were measured with the change in contour length (ΔL) in respective events using the worm-like chain model (28, 29). The change in free energy of unfolding (ΔGunfold) and kinetic parameters were calculated using Jarzynski equality calculation (25) and Dudko equation (27), respectively. See SI Appendix for details.
Supplementary Material
Acknowledgments
This project was supported by the Japan Society for the Promotion of Science (JSPS) and the National Science Foundation (NSF) under the JSPS–NSF International Collaborations in Chemistry [CHE-1415883 (to H.S. and H.M.)]. H.M. also acknowledges support from the NSF (CHE-1609514). M.E. acknowledges supports from JSPS KAKENHI (Grant-in-Aid for Scientific Research) (Grants 24104002, 15H03837, and 16K14033).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.G.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805939115/-/DCSupplemental.
References
- 1.Tang Y-C, et al. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell. 2006;125:903–914. doi: 10.1016/j.cell.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Brinker A, et al. Dual function of protein confinement in chaperonin-assisted protein folding. Cell. 2001;107:223–233. doi: 10.1016/s0092-8674(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H-X. Protein folding and binding in confined spaces and in crowded solutions. J Mol Recognit. 2004;17:368–375. doi: 10.1002/jmr.711. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H-X, Dill KA. Stabilization of proteins in confined spaces. Biochemistry. 2001;40:11289–11293. doi: 10.1021/bi0155504. [DOI] [PubMed] [Google Scholar]
- 5.Lucent D, Vishal V, Pande VS. Protein folding under confinement: A role for solvent. Proc Natl Acad Sci USA. 2007;104:10430–10434. doi: 10.1073/pnas.0608256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyoshi D, Karimata H, Sugimoto N. Hydration regulates thermodynamics of G-quadruplex formation under molecular crowding conditions. J Am Chem Soc. 2006;128:7957–7963. doi: 10.1021/ja061267m. [DOI] [PubMed] [Google Scholar]
- 7.Privalov PL. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25:281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 8.Koga K, Gao GT, Tanaka H, Zeng XC. Formation of ordered ice nanotubes inside carbon nanotubes. Nature. 2001;412:802–805. doi: 10.1038/35090532. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal KV, Shimizu S, Drahushuk LW, Kilcoyne D, Strano MS. Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes. Nat Nanotechnol. 2017;12:267–273. doi: 10.1038/nnano.2016.254. [DOI] [PubMed] [Google Scholar]
- 10.Atkins PW, de Paula J. Physical Chemistry. 9th Ed W. H. Freeman and Company; New York: 2009. [Google Scholar]
- 11.Zhao C, Ren J, Qu X. Single-walled carbon nanotubes binding to human telomeric i-motif DNA under molecular-crowding conditions: More water molecules released. Chemistry. 2008;14:5435–5439. doi: 10.1002/chem.200800280. [DOI] [PubMed] [Google Scholar]
- 12.Huppert JL, Balasubramanian S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui-Jain A, Grand CL, Bearss DJ, Hurley LH. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc Natl Acad Sci USA. 2002;99:11593–11598. doi: 10.1073/pnas.182256799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang H-J, Kendrick S, Hecht SM, Hurley LH. The transcriptional complex between the BCL2 i-motif and hnRNP LL is a molecular switch for control of gene expression that can be modulated by small molecules. J Am Chem Soc. 2014;136:4172–4185. doi: 10.1021/ja4109352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano S, Karimata H, Ohmichi T, Kawakami J, Sugimoto N. The effect of molecular crowding with nucleotide length and cosolute structure on DNA duplex stability. J Am Chem Soc. 2004;126:14330–14331. doi: 10.1021/ja0463029. [DOI] [PubMed] [Google Scholar]
- 16.Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson LI, et al. Structural basis of template-boundary definition in Tetrahymena telomerase. Nat Struct Mol Biol. 2015;22:883–888. doi: 10.1038/nsmb.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha P, et al. Confined space facilitates G-quadruplex formation. Nat Nanotechnol. 2017;12:582–588. doi: 10.1038/nnano.2017.29. [DOI] [PubMed] [Google Scholar]
- 19.Gehring K, Leroy JL, Guéron M. A tetrameric DNA structure with protonated cytosine.cytosine base pairs. Nature. 1993;363:561–565. doi: 10.1038/363561a0. [DOI] [PubMed] [Google Scholar]
- 20.Dhakal S, et al. Coexistence of an ILPR i-motif and a partially folded structure with comparable mechanical stability revealed at the single-molecule level. J Am Chem Soc. 2010;132:8991–8997. doi: 10.1021/ja100944j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzini G, Yathindra N, Xodo LE. Evidence for intramolecularly folded i-DNA structures in biologically relevant CCC-repeat sequences. Nucleic Acids Res. 1994;22:4634–4640. doi: 10.1093/nar/22.22.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen JJ, Rytting JH, Izatt RM. Thermodynamics of proton dissociation in dilute aqueous solution. 8. pK, change in heat content, and change in entropy values for proton ionization from several pyrimidines and their nucleosides at 25 degrees. J Phys Chem. 1967;71:2700–2705. doi: 10.1021/j100867a047. [DOI] [PubMed] [Google Scholar]
- 23.Manning GS. Limiting laws and counterion condensation in polyelectrolyte solutions I. Colligative properties. J Chem Phys. 1969;51:924–933. [Google Scholar]
- 24.Zimm BH, Le Bret M. Counter-ion condensation and system dimensionality. J Biomol Struct Dyn. 1983;1:461–471. doi: 10.1080/07391102.1983.10507455. [DOI] [PubMed] [Google Scholar]
- 25.Jarzynski C. Nonequilibrium equality for free energy differences. Phys Rev Lett. 1997;78:2690–2693. [Google Scholar]
- 26.He C, Gállego I, Laughlin B, Grover MA, Hud NV. A viscous solvent enables information transfer from gene-length nucleic acids in a model prebiotic replication cycle. Nat Chem. 2017;9:318–324. doi: 10.1038/nchem.2628. [DOI] [PubMed] [Google Scholar]
- 27.Dudko OK, Hummer G, Szabo A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc Natl Acad Sci USA. 2008;105:15755–15760. doi: 10.1073/pnas.0806085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann CG, Smith SB, Bloomfield VA, Bustamante C. Ionic effects on the elasticity of single DNA molecules. Proc Natl Acad Sci USA. 1997;94:6185–6190. doi: 10.1073/pnas.94.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Mao H. Non-B DNA structures show diverse conformations and complex transition kinetics comparable to RNA or proteins–A perspective from mechanical unfolding and refolding experiments. Chem Rec. 2013;13:102–116. doi: 10.1002/tcr.201200021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.