Chemists are tinkering with a variety of different designs and means of propulsion, though practical uses for these mini-motors have yet to be realized.
Some of the smallest, most useful machines known to science are the biological molecules that keep living things living. The protein myosin drives the contraction and relaxation of muscle. Kinesin drags cellular cargo around the cell. Motor enzymes unwind, rewind, and maintain DNA, and bacteria use a molecular motor to rotate their whip-like flagella up to 100,000 times per minute, propelling them forward. These machines turn chemical energy into motion. They’re very efficient at their jobs.
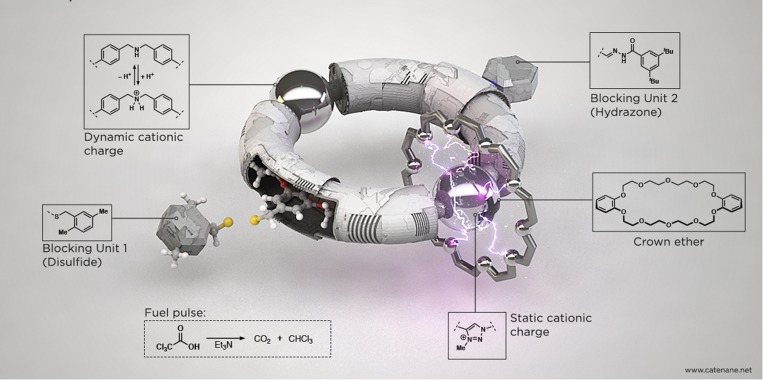
Seeking various ways to build molecular motors that can do meaningful work, researcher David Leigh came up with designs such as the one depicted here, a version of a molecular structure called a catenane that’s powered by chemical reactions. Illustration courtesy of Jo Richers (artist) and David Leigh (University of Manchester, Manchester, United Kingdom).
Now researchers are building synthetic cousins of these molecular machines, taking inspiration not only from their design but also their function. “Nature has worked with over 3 billion years of evolution to use them for every conceivable thing,” says chemist David Leigh at the University of Manchester in the United Kingdom, who has developed a variety of nanoscale machines. “So perhaps nature should tell us how and why to use them.”
The idea of using molecules to build minuscule machines that perform useful tasks dates back at least to a lecture given in 1959 by physicist Richard Feynman titled “There’s Plenty of Room at the Bottom.”* More recently, demonstrations of artificial molecular machines offer good reasons to think that such devices are feasible. Researchers have forged motors, shuttles, elevators, walkers, and pumps out of molecules, and powered them with electrical energy, chemical reactions, or light. Tiny motor by tiny motor, these demonstrations are inching toward future applications that could range from molecular electronics to artificial muscles.
Today, though, the work is so fundamental that no one really knows what to do with the machines or how they’ll be useful. “A Stone Age man makes a wheel and sticks it on an axle, but what does it do?” says Leigh. “He’s still a long way from the motor car—he can’t even envision a motorcar.”
Parallel efforts to develop larger microscopic machines—still smaller than a millimeter—have more clearly defined goals, such as designing medical microrobots that might swim around the human body and remove foreign objects or perform minor surgery (1). But building machines that operate at the molecular scale brings a raft of different problems and opportunities that will take decades to explore. “The biggest challenge for molecular machines is to avoid the trap of delivering something useful, now,” says chemist Wesley Browne at the University of Groningen in The Netherlands. “It has to stay fundamental.”
Driving Without a Map
In 2011, Nobel Laureate Ben Feringa and his group at University of Groningen made headlines when they unveiled a miniature “car” just a few nanometers long that could drive along a copper surface, powered by electrons delivered from the tip of a scanning tunneling microscope (2). And in February 2018, his team announced that they had created threads that could flex like muscles when exposed to light (3). These hydrogel strands were 95% water and contained motor molecules that had self-assembled with the help of calcium ions. Boasting enough strength to lift a weight of 0.4 milligrams, the threads demonstrated how an assembly of light-driven molecular motors can perform macroscopic work.
As with many scientific frontiers, there’s no clear roadmap for these devices. But there are at least two overarching concepts that guide their design. Feringa’s nanocar, for example, illustrates an approach in which researchers shrink everyday mechanical mechanisms down to the nanoscale: The Lilliputian vehicle rolls along a copper surface like a car rolls along a road.
A second approach more directly takes its cues from nature. “The fact that molecular machines are used in natural systems means we already have a proof of concept,” says chemist Nathalie Katsonis at the University of Twente in Enschede, The Netherlands. So instead of trying to mimic macroscale machines, some chemists design tiny machines that can do jobs similar to those done by known biological motors, such as myosin. A nanocar is spectacular, Leigh says, but “that’s not how biology does transport.”
Borrowing ideas from nature is not without its problems. Biological systems are so complex that researchers haven’t determined all of the dynamics of their individual parts, says Leigh. And compared with the macroscopic world, “everything works differently” at the molecular level, adds Katsonis.
That’s partly because molecular machines are by nature floppy, like the soft matter that makes up the human body, whereas macroscopic machines are typically made from rigid materials such as metal. But it’s also a consequence of scale. Although the laws of physics don’t change in the nanoworld, their relative influences do. Concepts such as inertia and momentum—critical to the design of machines like cars and planes—become irrelevant. So does gravity, because molecules have such a small mass. Movement at the nanoscale is dominated instead by viscosity and Brownian motion, the random bumbling of individual molecules caused by thermal fluctuations.
Katsonis calls this molecular environment a “Brownian storm.” In a 2007 article on the physics of nanoscale machines, physicist R. Dean Astumian at the University of Maine in Orono, ME, likened the challenges to swimming in molasses and walking in a hurricane (4).
So how to conquer the nanoscale tempest? Either overcome Brownian motion or harness it. Both strategies require a way to throw the motor out of equilibrium with its surroundings, and that involves adding energy. That may provide enough oomph for the molecule to rotate or move or simply allow it to be nudged by Brownian motion in a particular direction.
How researchers add energy to the system depends in part on whether they’re mimicking nature or miniaturizing a macro-machine. Researchers such as Leigh may use chemical reactions to destabilize the system, mirroring the action of adenosine triphosphate in cellular processes. The idea is to orient the machine in such a way that its response to the Brownian storm moves the machine in the desired direction. Conversely, those who favor a miniaturization approach might use light or electricity to drive a “power stroke,” a reference to internal combustion engines, which need an energy boost (from tiny explosions) to generate motion.
Although these two strategies yield motors that work at the same scale, and might even do similar jobs, they present separate sets of challenges and possibilities. For now, there is no consensus on which is the best option. “It’s very much an open playing field,” says chemist J. Fraser Stoddart at Northwestern University in Evanston, IL, “and I would hesitate to say that one approach is better than the other approach.”
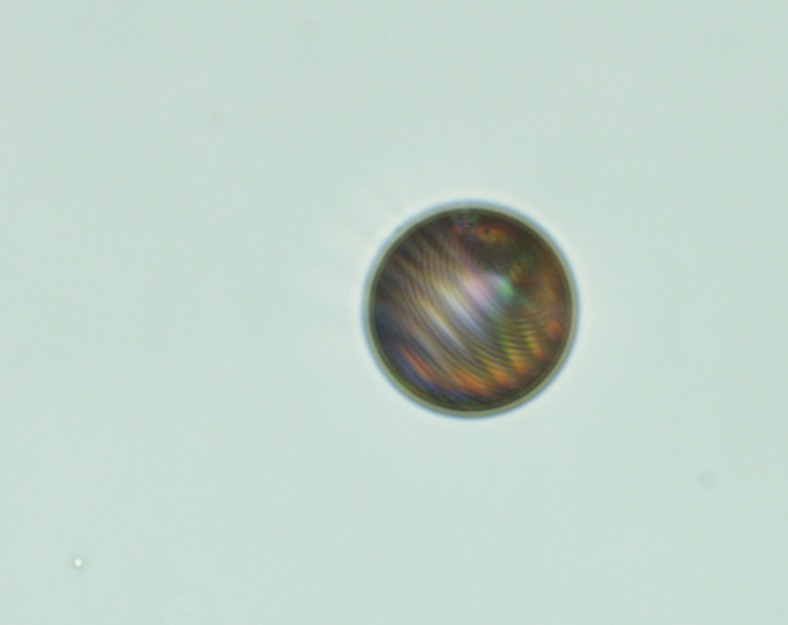
At the University of Twente, Nathalie Katsonis and her group have shown how light-driven motors embedded in liquid crystals can change those crystals. The embedded motor caused the pattern shown here to emerge in the droplet. Image courtesy of Nathalie Katsonis and Federico Lancia (University of Twente, Enschede, The Netherlands).
Natural Limits
In 2015, Stoddart’s team built the first artificial molecular pump, which uses chemical reactions to squeeze molecules together (5). It is based on a rotaxane, a widely used design for molecular machines in which interlocked components aren’t held together with covalent bonds and are free to move relative to each other. They look a little like bracelets trapped on the bar of a dumbbell. Other designs used for molecular machines include catenanes, which look like interlocked rings, and rotors, which can rotate around a central axis.
Stoddart was inspired by carrier proteins that ferry ions or small molecules across a cell membrane, working against an energy gradient to push them from a low-concentration zone to a high-concentration zone. His artificial pump uses chemical energy to pull charged, ring-shaped molecules out of solution and drive them into a collection area. There, two rings can sit just a few nanometers apart—a feat that would be impossible under ordinary circumstances, because the charged rings would repel each other.
Leigh has been developing rotaxane-based molecular machines that act rather like the ribosome, a macromolecule in cells that can read a strand of RNA and use that information to connect amino acids in the right order to build a particular protein. In April, his team showed off an assembly machine containing a ring-shaped molecule that follows an inert track of polystyrene, collecting small molecules in its path and using chemical reactions to build a larger molecule (6). And in work published in 2016, Leigh’s group introduced a molecular machine that runs around a circular track. The machine goes in the desired direction not because it’s pushed but because strategically placed and timed chemical reactions prevent it from moving in the wrong direction (7). “The reaction blocks the movement of the components in directions that you don’t want,” says Leigh.
Nature’s molecular machines offer chemists a valuable guide for their own devices, says Leigh, but they also point the way to potential applications for their synthetic counterparts. “What does biology use them for?” he asks. “I think that will be where progress comes from.”
There are drawbacks to the biological approach, though. When artificial molecular motors break down, they need to be fixed or replaced, and cells have their own built-in repair systems that can keep everything running smoothly. “But with a synthetic system you don’t have those repair tools,” says Browne. “It’s like doing the Indy 500 or the Le Mans without a repair crew.”
It’s not practical to incorporate artificial repair systems that work like complex cellular machinery. So Browne sees more promise in building synthetic machines that act like miniaturized macroscopic motors, propelled by electricity or light, rather than trying to mimic cellular mechanisms. “We have to do it in a way that doesn't do exactly what nature has done,” he says.
Aside from his electron-powered nanocar, Feringa has also created a series of rotary molecular motors that are driven by light. In a pioneering example from 1999 (8), the rotor contained two blade-like hydrocarbon groups joined by a double bond, which acted as an axle. When irradiated with a burst of ultraviolet light, the blades changed their relative positions and rotated around the axle. Feringa's creation does have a parallel in nature, because bonds between molecules in mammalian eyes undergo a similar shift when they absorb photons, making vision possible. At the same time, the synthetic motor pumps light and uses a power stroke, in a way that is unlike anything found in nature.
Light makes an appealing energy source because it’s effectively unlimited, it produces no waste, its wavelength and intensity are easily controlled, and it doesn't require any physical contact with the molecular machine. Multiple groups have built increasingly sophisticated light-driven machines, and Browne says that they are generally easier to design, control, and test than chemically driven alternatives.
“Biology has had some time to think about this, and it has settled on catalysis-driven motors.”
—R. Dean Astumian
Artificial-light–driven motors also tend to be more efficient, says Astumian. “So the kneejerk reaction is to say it’s so much easier to build a light-driven motor,” he says. But the issue is far from settled: “Biology has had some time to think about this, and it has settled on catalysis-driven motors.”
Ultimately, says Browne, both approaches are valuable because they will reveal new knowledge about matter and movement on the nanoscale. “They’ll allow us to start answering questions we didn't know we should ask.”
Useless but Exciting
In April 2017, in the spirit of Feringa’s landmark 2011 nanocar, chemists Christian Joachim at the Centre for Materials Elaboration and Structural Studies and Gwénaël Rapenne at the University of Toulouse–Paul Sabatier organized the first international nanocar race. It attracted six entrants to Toulouse, France, each with a different design. Rapenne's team, the Toulouse Nanomobile Club, named their speedster the “Green Buggy.”
These nanocars didn't incorporate motors into their design like Feringa’s 2011 version. Instead, they sped along a gold track, powered by reactions to—or interactions with—electrons delivered by a scanning tunneling microscope. They were not allowed to be towed or pushed by the microscope tip.
First place awards were given to the Nanocar Dipolar Racer, which travelled 1 micron in 29 hours and was designed by an American–Austrian team, and the Swiss Nano Dragster, which travelled 133 nanometers in 6.5 hours. The race had little practical value, but like much molecular machine research, it served to show what was possible—and, with its series of livestreamed events, the stunt drew attention to the field.
Several research groups continue to strive to push new molecular machine capabilities, if not applications. Katsonis, at her lab in Twente, has shifted her focus away from finding new kinds of motors and fine-tuning power sources. Instead, she wants to exploit work that’s already been done to integrate molecular motors into other materials.
She recently co-led the design of liquid crystals containing embedded molecular motors and switches that can be driven by light, similar to those developed in Feringa’s lab. Liquid crystals are materials that flow like liquids, but their molecules are arranged in symmetric crystalline structures. Katsonis’ group reported in February that when they treated the embedded motors with ultraviolet light, the molecules rotated, dragging helical structures within the liquid crystal from one location to another (9).
Katsonis’ work is an example of emerging research that aims to unite molecular machines with soft matter, a broad class of materials that readily change shape at room temperature when compressed, stretched, or heated. This includes liquid crystals but also the tissues that make up the human body. Katsonis’ research may lead to strategies that use molecular motors to do macroscopic work in a way that could be useful in biological or medical systems.
Looking to biomedicine, researchers have investigated using DNA as a molecular building block or incorporating molecular motors into biomolecules such as peptides and antibiotics. In August 2017, James Tour’s group at Rice University in Houston reported using a Feringa-inspired molecular motor to drill holes in cancer cells (10), suggesting that nanodevices might eventually be deployed in the body to improve drug delivery. (Tour also helped design the winning Nanocar Dipole Racer in the nanocar race.) Meanwhile, large assemblies of artificial molecular motors, incorporated into materials such as polymers, might produce muscle-like actions on a macroscopic scale—just like Feringa’s weightlifting threads—which could improve prosthetics and find uses in robotics.
But many molecular engineers agree that it’s simply too soon to focus on serious applications. The science of molecular machines, Browne says, isn’t ready for market, and he worries that the pressure to produce commercial devices could divert valuable research efforts and funding away from the basic science. The danger, he says, is that this would slow progress in answering fundamental questions about the rules of motion on a molecular scale.
Indeed, the field’s wide horizon is part of what makes it so appealing. “We don’t know specifically what we will be able to do with it yet, but we see a lot of possibilities,” Katsonis says. “It’s an open field, so for the curious mind, it’s always exciting.”
Footnotes
*Feynman R, There’s Plenty of Room at the Bottom, Annual Meeting of the American Physical Society, December 29, 1959, Caltech, Pasadena, CA.
References
- 1.Ornes S. Inner workings: Medical microrobots have potential in surgery, therapy, imaging, and diagnostics. Proc Natl Acad Sci USA. 2017;114:12356–12358. doi: 10.1073/pnas.1716034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudernac T, et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature. 2011;479:208–211. doi: 10.1038/nature10587. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, et al. Artificial muscle-like function from hierarchical supramolecular assembly of photoresponsive molecular motors. Nat Chem. 2018;10:132–138. doi: 10.1038/nchem.2887. [DOI] [PubMed] [Google Scholar]
- 4.Astumian RD. Design principles for Brownian molecular machines: How to swim in molasses and walk in a hurricane. Phys Chem Chem Phys. 2007;9:5067–5083. doi: 10.1039/b708995c. [DOI] [PubMed] [Google Scholar]
- 5.Cheng C, et al. An artificial molecular pump. Nat Nanotechnol. 2015;10:547–553. doi: 10.1038/nnano.2015.96. [DOI] [PubMed] [Google Scholar]
- 6.De Bo G, et al. An artificial molecular machine that builds an asymmetric catalyst. Nat Nanotechnol. 2018;13:381–385. doi: 10.1038/s41565-018-0105-3. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MR, et al. An autonomous chemically fuelled small-molecule motor. Nature. 2016;534:235–240. doi: 10.1038/nature18013. [DOI] [PubMed] [Google Scholar]
- 8.Koumura N, Zijlstra RWJ, van Delden RA, Harada N, Feringa BL. Light-driven monodirectional molecular rotor. Nature. 1999;401:152–155. doi: 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- 9.Orlova T, et al. Revolving supramolecular chiral structures powered by light in nanomotor-doped liquid crystals. Nat Nanotechnol. 2018;13:304–308. doi: 10.1038/s41565-017-0059-x. [DOI] [PubMed] [Google Scholar]
- 10.García-López V, et al. Molecular machines open cell membranes. Nature. 2017;548:567–572. doi: 10.1038/nature23657. [DOI] [PubMed] [Google Scholar]