Significance
The interendothelial slit (IES) is the narrowest circulatory pathway in the human spleen where aged and diseased red blood cells (RBCs) are filtered. We use a two-component RBC model to probe the dynamics of healthy and diseased RBCs traversing IES. Our simulations reveal that the spleen not only senses and clears RBCs with abnormal shapes and deformability but also alters the geometries of RBCs that contain protein defects arising from hereditary blood disorders. The framework presented here is sufficiently general to be extended to elucidate the pathophysiological roles of the spleen in other blood diseases.
Keywords: spleen, hereditary spherocytosis, hereditary elliptocytosis, vesiculation, cell fragmentation
Abstract
In red blood cell (RBC) diseases, the spleen contributes to anemia by clearing the damaged RBCs, but its unique ability to mechanically challenge RBCs also poses the risk of inducing other pathogenic effects. We have analyzed RBCs in hereditary spherocytosis (HS) and hereditary elliptocytosis (HE), two typical examples of blood disorders that result in membrane protein defects in RBCs. We use a two-component protein-scale RBC model to simulate the traversal of the interendothelial slit (IES) in the human spleen, a stringent biomechanical challenge on healthy and diseased RBCs that cannot be directly observed in vivo. In HS, our results confirm that the RBC loses surface due to weakened cohesion between the lipid bilayer and the cytoskeleton and reveal that surface loss may result from vesiculation of the RBC as it crosses IES. In HE, traversing IES induces sustained elongation of the RBC with impaired elasticity and fragmentation in severe disease. Our simulations thus suggest that in inherited RBC disorders, the spleen not only filters out pathological RBCs but also directly contributes to RBC alterations. These results provide a mechanistic rationale for different clinical outcomes documented following splenectomy in HS patients with spectrin-deficient and ankyrin-deficient RBCs and offer insights into the pathogenic role of human spleen in RBC diseases.
The spleen is the largest secondary immune organ in the human body, and it consists of two functionally distinct compartments, the white pulp and the red pulp (1, 2). The white pulp is responsible for initiating immune reactions to blood-borne antigens, whereas the red pulp serves as a primary blood filter to sequester and remove pathogenic microorganisms as well as senescent or diseased red blood cells (RBCs) from circulation (3–6). RBCs traversing the red pulp move from the cords into venous sinuses, where they have to squeeze through the narrow apertures, known as interendothelial slits (IESs), which are located between the elongated endothelial cells of the sinus wall. Two distinct mechanisms lead to filtering of RBCs in the red pulp: (i) physicochemical filtration, which involves adherence of surface-altered RBCs to reticular connective tissue and macrophages, followed by removal of adhered RBCs through phagocytosis, and (ii) mechanical filtration, whereby the IES of the sinus wall functions as a physical barrier to prohibit RBCs with abnormal size, shape, and deformability from returning to general circulation. These two sequential processes constantly control the quality of circulating RBCs and prevent microcirculatory complications elsewhere in the body.
Healthy RBCs have remarkable deformability and stability, which enable them to undergo repeated deformation in microcirculation during their life span of ∼120 d. In blood disorders of hereditary spherocytosis (HS) and hereditary elliptocytosis (HE), however, defects in RBC membrane proteins weaken the cohesion between the lipid bilayer and the cytoskeleton (in HS) or the integrity of the cytoskeleton (in HE), thereby compromising the deformability and stability of RBCs (7). In HS, defects in cytoskeletal proteins or membrane proteins that anchor the cytoskeleton to the lipid bilayer destabilize the RBC membrane, leading to membrane surface loss through release of vesicles (8, 9). Loss of membrane surface causes a decrease in the ratio of surface area-to-volume (S/V) of RBCs and promotes the formation of spherical RBCs. Due to their reduced deformability, spherical RBCs are removed prematurely by the spleen, resulting in mild to severe forms of anemia, dependent on the extent of surface area loss (10). In HE, defects in the cytoskeletal proteins impair the elasticity of RBCs by disrupting the integrity of the membrane cytoskeleton (9, 11). As a result, RBCs in HE undergo irreversible elongation after large deformation and progressively transform into elliptical shape (12). In severe forms of HE, RBCs become mechanically unstable, resulting in cell fragmentation and lysis (9, 11). The ensuing ill-shaped RBCs and cell fragments are cleared from circulation by the spleen, causing hemolytic anemia.
The function of spleen in sensing and clearing RBCs with alterations in their size, shape, and deformability has been elucidated through in vivo (13, 14), ex vivo (3–5), and in vitro (15–17) experiments and computational modeling (18–21). However, the role of IES in filtering RBCs with significant membrane protein defects, as seen in HS and HE, has not been investigated in sufficient detail. To address these questions, we use a two-component coarse-grained molecular dynamics (CGMD) RBC model (22) to simulate the traversal dynamics of healthy, HS, and HE RBCs through IES. In particular, we introduce specific transmembrane protein defects into the RBC model and correlate the molecular basis of the diseases to different clinical expressions of RBCs in HS and HE.
Results
IES Model and Two-Component RBC Model.
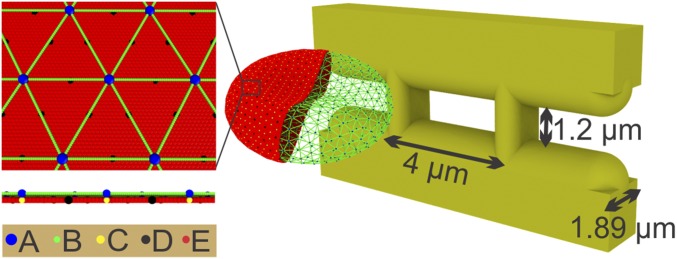
To capture the geometry of IES in the human spleen, we construct a model that comprises four solid elements, as illustrated in Fig. 1. The two vertical bars represent annular fibers with a width of 1 m, whereas the two horizontal bars represent endothelial cells. The thickness of the slit wall is 1.89 m, and the width and height of the slit are 4 m and 1.2 m, respectively, based on the experimentally documented slit geometry (19).
Fig. 1.
Simulating RBC passage through IES. The membrane of the RBC is explicitly represented by CG particles. A: actin junctions; B: spectrin particles; C: glycophorin particles; D: band-3 particles; E: lipid particles. The width and height of the simulated slit are 4 m and 1.2 m, respectively. The width of the vertical bars is 1 m, and the thickness of the slit wall is 1.89 m.
We use the computer code OpenRBC (22) to model healthy and diseased RBCs. As shown in Fig. 1, the lipid bilayer and the cytoskeleton as well as transmembrane proteins are explicitly represented in the RBC model. This allows simulations of RBC vesiculation and of cell morphological changes induced by protein defects in blood disorders. Further details of the RBC model can be found in SI Appendix.
Pressure Gradients and S/V Ratio Determine the Passage of RBCs through IES.
Over the life span of about 120 d, the cytoskeleton of the RBC stiffens and its membrane components undergo degradation, resulting in loss of surface area through release of vesicles (23–25). Senescent RBCs with reduced surface area become less deformable and thus are amenable to sequestration and removal in the spleen. In this section, we simulate healthy RBCs with reduced surface area traversing the splenic IES. Pressure gradients of 3, 5, 8, 10, 15, and 20 Pam−1 are applied to drive the RBC through IES. These pressure gradients fall within the range found in in vitro experiments (17)—that is, 1 to 30 Pam−1. Such values were sufficient to reproduce the physiological dynamics of RBCs transiting through IES in rat spleen (14). First, we model an RBC with a surface area of 140 m2 and volume of 90 m3. Our simulation results show that when driven by a pressure gradient of 3 Pam−1, the RBC is retained by IES (Fig. 2 A and B). When the pressure gradients are equal to or larger than 5 Pam−1, RBCs are able to pass through IES. This critical pressure gradient is on the same order of magnitude as the pressure gradient of 1 Pam−1, which was sufficient to drive RBCs of all sizes found in blood through the slits in microsphere experiments and ex vivo perfusion of human spleen (5, 15). The origins of the difference between the critical pressure gradient inferred from this work and the values reported from previous studies are discussed in detail in SI Appendix.
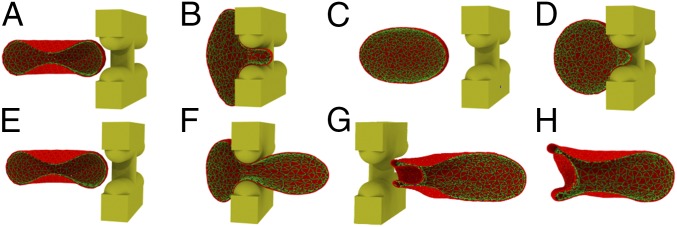
Fig. 2.
(A and B) An RBC with surface area of 140 m2 and volume of 90 m3 is retained by IES under a pressure gradient of 3 Pam−1 because of an insufficient driving force. (C and D) An RBC with surface area of 110 m2 and volume of 90 m3 is retained by IES under a pressure gradient of 8 Pam−1 due to reduced surface area. (E–H) Four sequential snapshots of the RBC with surface area of 140 m2 and volume of 90 m3 passage through IES under a pressure gradient of 20 Pam−1 (see Movie S1). Only one half of the RBC is displayed for clarity.
Fig. 2 E–H illustrate a sequence of shape changes of the RBC (surface area of 140 m2 and volume of 90 m3) during its deformation through IES. When the RBC moves into the slit (Fig. 2F), the portion inside the slit is being squeezed, whereas the rest of the RBC membrane is expanded to accommodate the excluded volume, thus forming a dumbbell shape. As the RBC moves toward the downstream side of IES, the downstream bulge expands, while the upstream bulge shrinks. Subsequently, the cell membrane in the slit infolds toward the cell body and creates a concave region, as shown in Fig. 2G. After crossing the IES, the deformed RBC gradually spreads out the inward-folded membrane, turning to a bullet shape (Fig. 2H). The dynamics of the RBC model passage through IES are consistent with the in vivo microscopic observations of the transilluminated rat spleen (14) and the in vitro microfluidic study of human RBCs passage through IES-like slits (17). When driven by increased pressure gradients, the RBC dynamics are similar except that the higher pressure gradients lead to faster RBC traversal.
Next we show results for cases where the pressure gradient is 5 Pam−1 and the surface area of RBCs is reduced from 140 m2 to 100 m2 in decrements of 10 m2. For the surface areas of 130 m2 and 120 m2, RBCs are still able to pass through IES. However, in the case of the surface area of 110 m2, representing about 21% surface area loss, the simulated RBC is retained by IES (Fig. 2 C and D). This result is consistent with prior ex vivo experimental observations that RBCs with more than 18% surface area loss were mostly entrapped in the spleen (5). This finding is also validated by an analytical model (19, 26) that defines the relationship between the critical surface area and volume for healthy RBCs, beyond which traversal through IES is predicated to be compromised. Given the geometry of the slit, the minimum surface area (), below which the RBCs with fixed volume () are retained by the slit, is given by (19, 26)
| [1] |
where . All other symbols are defined in SI Appendix. This model predicts a critical surface area of 113.1 m2 for the RBC volume of 90 m3, which validates the critical area obtained from our simulations. Then, we increase the pressure gradients to 8, 10, 15, and 20 Pam−1, respectively, and we find that RBCs with surface areas of 110 m2 and 100 m2 still cannot pass through IES when driven by these increased pressure gradients.
Traversing IES Causes Intrasplenic Vesiculation of RBCs in HS.
In HS, defects occur in the RBC membrane proteins, such as ankyrin, protein 4.2, band-3, and spectrin (8, 10). These protein deficiencies alter the RBC membrane in two distinct ways (8). In RBCs with deficiencies in the band-3 or protein 4.2, the vertical connections between the spectrin filaments and the lipid bilayer are diminished, whereas the spectrin content is normal (Fig. 3B). On the other hand, spectrin-deficient or ankyrin-deficient RBCs are characterized by depleted spectrin content and reduced numbers of actin junction complexes, while the overall structure of the membrane cytoskeleton is preserved (Fig. 3C). These alterations in the RBC membrane weaken the vertical linkages between the cytoskeleton and the lipid bilayer, causing surface area loss through release of vesicles (8, 10). HS RBCs with reduced surface area transform progressively from a biconcave shape to a near-spherical shape. The concomitant decrease in cell deformability leads to RBC retention and premature removal by the spleen (5, 27). Although the genetic basis and clinical consequences of HS are known, the mechanics of membrane loss has hitherto not been explored in detail. In this section, we simulate band-3–deficient and spectrin-deficient RBCs traveling through IES. Varying degrees of protein deficiency are examined for these two types of HS RBCs. The deformability of the HS RBCs following reduction in surface area is evaluated by stretching the RBCs, similar to the manner in which RBC deformation was induced in optical tweezers experiments (28, 29). A deformability index (DI) is computed based on the deformation of the stretched RBC. Details of the calculations of DI are given in SI Appendix.
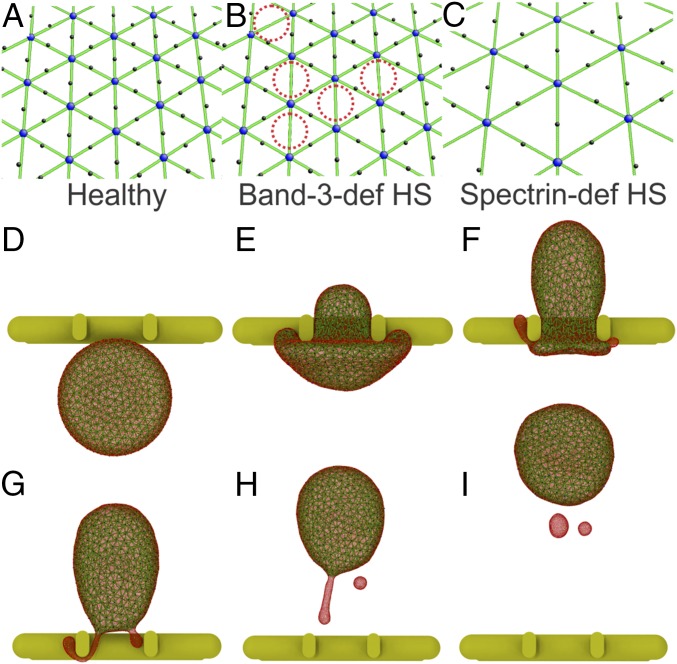
Fig. 3.
(A) Cytoskeleton in a healthy RBC model. (B) In the band-3–deficient HS RBC model, the band-3 sites are randomly removed (highlighted by red dotted circles) to represent the effect of band-3 deficiency. (C) In the spectrin-deficient HS RBC model, the density of spectrin network is reduced to represent the effect of spectrin deficiency. (D–I) Six sequential snapshots (top views) of an HS RBC with a vertical connectivity of 60% passage through IES under a pressure gradient of 10 Pam−1(see Movie S2). Reduced vertical connectivity leads to the detachment of the lipid bilayer from the cytoskeleton and subsequent RBC vesiculation. The lipid CG particles (red particles) are plotted at a smaller size to visualize the cytoskeleton below (green particles).
We first simulate the band-3–deficient RBCs by reducing the connectivity between the band-3 proteins and spectrin filaments (vertical connectivity) in the RBC model. The vertical connectivity is reduced from 100% to 0% in decrements of 20%, representing elevated degrees of band-3 deficiency. The surface area and volume of the simulated RBCs are 140 m2 and 90 m3, respectively. Fig. 3 D–I show a sequence of shape changes of an HS RBC with a vertical connectivity of 60% as it passes through an IES (top views). Due to weakened cohesion between the lipid bilayer and the cytoskeleton, the lipid bilayer detaches from the cytoskeleton when the RBC traverses IES (Fig. 3F). One detachment separates from the RBC and forms a vesicle, whereas the other develops into a tubular vesicle and passes through IES behind the RBC (Fig. 3 G and H). Eventually, this tubular vesicle detaches from the RBC and reshapes into a separate spherical vesicle (Fig. 3I). Detailed discussion of this vesiculation process can be found in SI Appendix. These simulations confirm that reduced vertical connectivity compromises the cohesion between the cytoskeleton and the lipid bilayer and that surface area loss of RBCs in HS occurs when they traverse the IES, thereby clearly establishing the connection between the spleen and shape alterations in diseased RBCs.
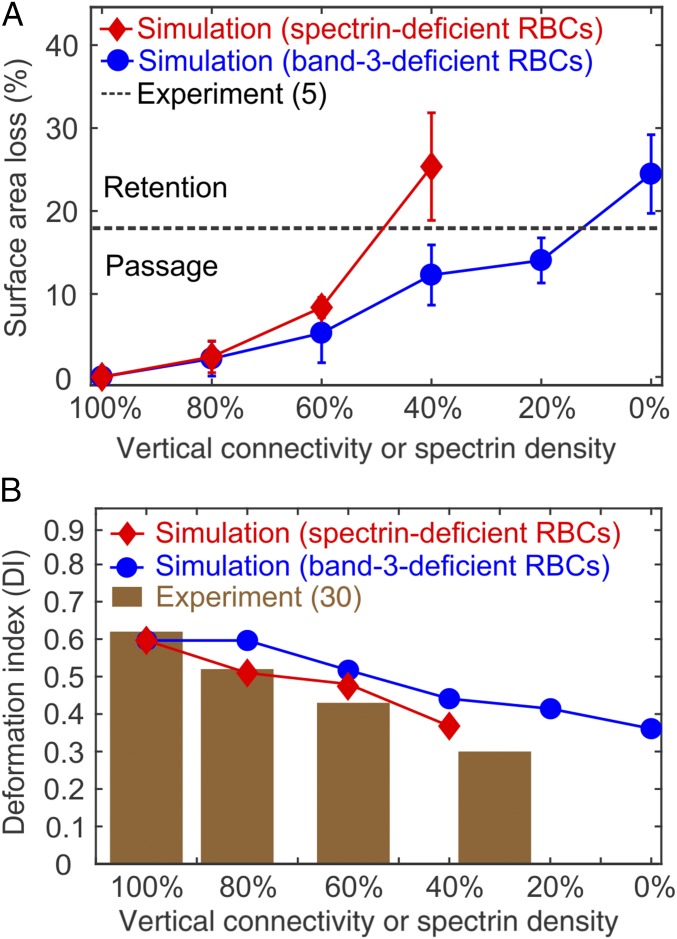
We also examine RBCs with various vertical connectivities. At each vertical connectivity, pressure gradients of 5, 8, 10, 15, and 20 Pam−1 are applied. The blue curve in Fig. 4A shows that RBCs shed more surface area as the vertical connectivity is reduced. In other words, the increased degree of band-3 deficiency exacerbates membrane loss of the HS RBCs. Notably, the fraction of surface area loss from the HS RBCs with a vertical connectivity of 0% is larger than the IES retention threshold (the black dashed line in Fig. 4A) inferred from ex vivo experiments (5), implying that these HS RBCs do not successfully pass through IES. The corresponding DI of the band-3–deficient RBCs (the blue curve in Fig. 4B) decreases as the degree of band-3 deficiency increases. These results illustrate the correlation between the degree of protein deficiency and clinical expressions of RBCs in HS.
Fig. 4.
(A) Fractional surface area loss of HS RBCs after passage through IES. For the band-3–deficient RBCs, the surface area loss is increased with the decreased vertical connectivity. For the spectrin-deficient RBCs, the surface area loss is increased with the decreased spectrin density. The error bars are computed based on pressure gradient values of 5, 8, 10, 15, and 20 Pam−1. The black dashed line highlights the critical fraction of surface area loss that determines the retention of RBCs reported by ex vivo experiment (5). (B) DI of the band-3–deficient and the spectrin-deficient RBCs at different levels of HS-related protein deficiency. The brown bar graph shows DI of spectrin-deficient RBCs measured by osmotic gradient ektacytometry at a fixed osmolality of 300 mOsmol/kg (30).
Next, we reduce the spectrin density in the cytoskeleton by decreasing the number of actin junction complexes in the RBC model (Fig. 3C) to mimic spectrin-deficient RBCs. To capture the wide spectrum of clinical severity observed in HS, the spectrin density is decreased from 100% (healthy) to 40% in decrements of 20%. When the spectrin-deficient RBCs pass through IES, the lipid bilayer in the spectrin-depleted area can bud off and develop into vesicles (8). More discussion regarding the vesiculation process is given in SI Appendix. Fig. 4A (red curve) illustrates that the fractional surface area loss of spectrin-deficient RBCs increases with decreased spectrin density, in agreement with clinical evidence (8). Fig. 4B shows that reduced spectrin density leads to decreased DI of the spectrin-deficient RBCs (red curve). In particular, the values of DI are consistent with the values experimentally measured by ektacytometry at a fixed osmolality of 300 mOsmol/kg (30, 31), indicating that the trends predicted by the present results are validated by clinical observations.
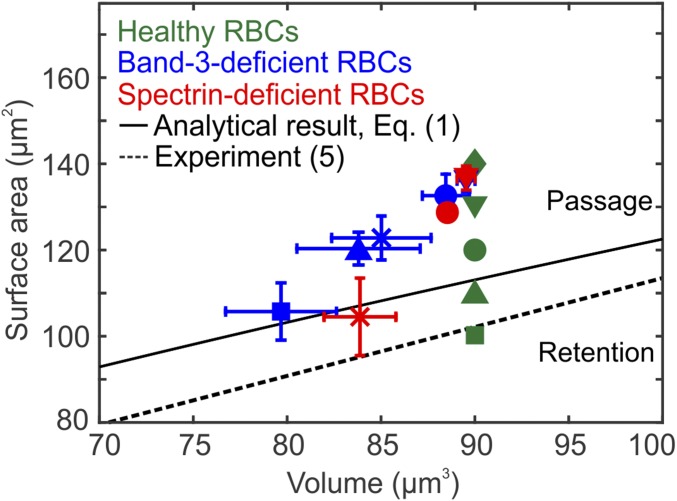
The surface area and volume of HS RBCs following the formation of vesicles are plotted in Fig. 5. The blue and red symbols in Fig. 5 show that as the HS-related protein defects increase, the S/V ratios of HS RBCs gradually decrease toward the retention threshold predicted by Eq. 1 (black solid line) as well as the retention threshold reported by ex vivo experiments (black dashed line) (5). This plot implies that HS RBCs with low degrees of protein deficiency could pass IES despite reduced surface area. Therefore, these RBCs undergo a gradual decrease in their life spans, leading to mild anemia. Conversely, RBCs with high degrees of protein deficiency undergo rapid drops of S/V ratio after crossing IES. As a result, the expected life spans of these RBCs are shortened markedly, leading to severe anemia. These findings provide a mechanistic rationale for the connections among molecular defects, RBC alterations, and the broad spectrum of clinical severity found in HS.
Fig. 5.
Prediction of splenic IES retention for healthy RBCs (no protein deficiency) and HS RBCs. Healthy RBCs (green color symbols) with surface area of 140 (), 130(), 120 (), 110 (), and 100 m2 () and a fixed volume of 90 m3 are examined, respectively. The surface area and volume of HS RBCs after their passage through IES are plotted. Blue color symbols denote the band-3–deficient RBCs with vertical connectivities of 80% (), 60% (), 40% (), 20% (), and 0% (). Red color symbols denote the spectrin-deficient RBCs with spectrin density of 80% (), 60% (), and 40% (). The error bars are computed based on pressure gradient values of 5, 8, 10, 15, and 20 Pam−1. The black solid and dashed lines highlight the RBC retention threshold predicted by an analytical model given by Eq. 1 (19, 26) and the threshold reported by ex vivo experiment (5), respectively. RBCs with surface area and volume above these thresholds are able to cross IES; otherwise, RBCs are retained by IES.
Fig. 4A shows that as the level of HS-related protein deficiency increases, the spectrin-deficient RBCs with dispersed cytoskeleton shed more membrane surface than the band-3–deficient RBCs whose cytoskeleton is dense. Fig. 5 also shows that as the spectrin density decreases, the spectrin-deficient RBCs (red symbols) undergo a more rapid drop of S/V ratio than the band-3–deficient RBCs (blue symbols) for the same decrease in volume. These results suggest that the spectrin-deficient RBCs are more amenable to vesiculation than the band-3–deficient RBCs when traversing IES. This finding provides a compelling mechanistic rationale for the clinically documented observation that splenectomy prolongs the survival of spectrin/ankyrin-deficient RBCs but not band-3–deficient RBCs (32). The distinct clinical manifestations between these two types of HS RBCs are likely attributed to the fact that the band-3–deficient RBCs in the spleen shed surface area to the same extent as they do through other mechanisms, such as by antibody-triggered vesiculation or trogocytosis (32), while the spectrin-deficient RBCs lose membrane surface predominantly in the spleen.
Traversing IES Causes Shape Transition and Fragmentation of RBCs in HE.
In HE, defects occur in cytoskeletal proteins of RBCs, such as -spectrin, -spectrin, and protein 4.1R (11). The - and -spectrin deficiencies, which account for nearly 95% of HE cases, disrupt the self-association of spectrin dimers, whereas the protein 4.1R deficiency, responsible for 5% of HE cases, alters the cohesion of the spectrin–actin–protein 4.1R junctional complexes (11, 33). The characteristics of RBCs in HE are increased cell fragility and shape transition from the biconcave to the elliptical shape (9, 11). In severe forms of HE, cell fragments have been detected in patients’ blood smear (9). The prevailing hypothesis is that RBCs in HE become damaged during their transit through narrow pathways in microcirculation (33, 34), although little clinical evidence is available to support this hypothesis. Here, we examine RBCs displaying different degrees of HE-related protein deficiency as they travel through IES and explore how the traversal through IES contributes to the pathological alterations of RBCs in HE. We break the spectrin filaments in the RBC model (Fig. 6 A, Inset), mimicking the disrupted spectrin tetramers. As the severity of HE varies according to the degree of the impaired cytoskeleton (33), we reduce the percentage of the intact spectrin filaments (horizontal connectivity) from 100% to 20% in decrements of 10%. At each horizontal connectivity, pressure gradients of 5, 8, 10, 15, and 20 Pam−1 are examined.
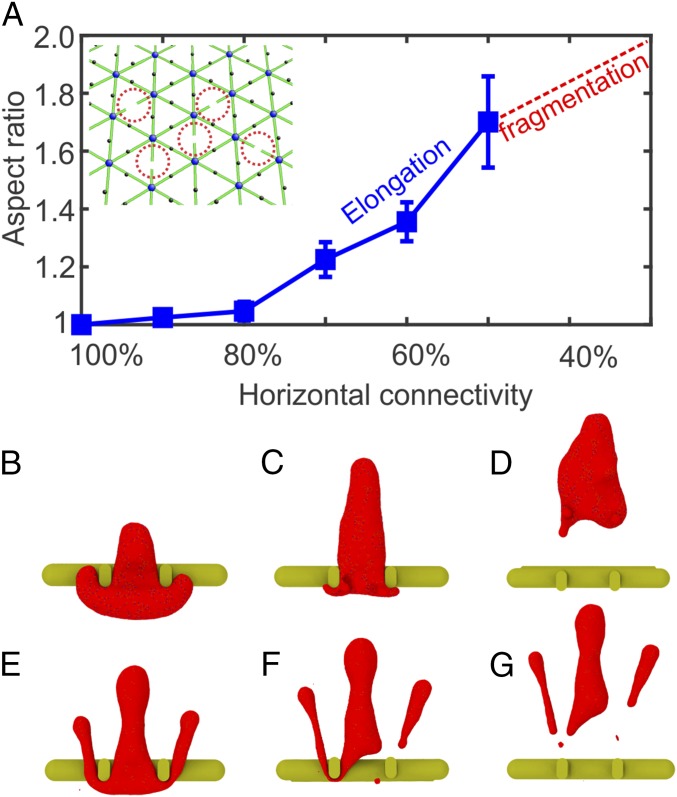
Fig. 6.
(A) Aspect ratios of HE RBCs after passage through IES. When the horizontal connectivity is reduced to 40% or less, HE RBCs break into fragments due to reduced cytoskeleton integrity. The error bars are computed based on pressure gradient values of 5, 8, 10, 15, and 20 Pam−1. Inset shows that in the spectrin-deficient HE RBC model, spectrin filaments are randomly disassociated (highlighted by red dotted circles), mimicking the disrupted spectrin tetramers. (B–D) Three sequential snapshots (top views) of an HE RBC with a horizontal connectivity of 50% crossing IES (see Movie S3). (E–G) Three sequential snapshots (top views) of an HE RBC with a horizontal connectivity of 20% breaking into fragments after crossing IES (see Movie S4).
A notable feature of HE RBCs is the elongation of RBCs after passage through IES (Fig. 6 B–D). Although vesiculation of HE RBCs is also observed, it is less pronounced compared with that of HS RBCs. Here, we quantify the shape transition of HE RBCs by computing the aspect ratio of cells after their egress from IES. The aspect ratio is defined as , where is the length of the RBC along its moving direction and is the length of the RBC perpendicular to its moving direction. As shown in Fig. 6A, RBCs with decreased horizontal connectivity undergo further elongation during their passage through IES. These elongated HE RBCs cannot fully recover their original biconcave shape due to impaired cell elasticity, leading to the progressive shape transition to elliptical shape. When the horizontal connectivity is 40% or less (30% and 20% in our simulations), the model predicts that the HE RBCs break into cell fragments. Fig. 6 E–G illustrate three sequential snapshots of an RBC with a horizontal connectivity of 20% passage through IES. It is noted that the portions of RBCs protruding into the luminal sides of IES are elongated and subsequently break apart from the RBC, forming two cell fragments. These simulations provide a mechanistic rationale for RBC fragmentation in HE and for the presence of cell fragments in the blood smear of HE patients (9, 11).
Discussion
Circulating healthy human RBCs have a life span of ∼120 d, during which they experience gradual degradation of membrane components and progressive loss of surface area through release of vesicles (23, 24, 35). Loss of surface area leads to alterations of the shape, size, and deformability of RBCs. Removal of aged RBCs from circulation mainly occurs in the spleen, where the less deformable RBCs are retained by IES and subsequently phagocytosed by macrophages (27). Our present simulation results show that the S/V ratio of RBCs is an essential determinant of their passage through IES, confirming the function of IES in filtering senescent RBCs (1, 3). In particular, our simulations illustrate that the critical surface area that allows RBCs with a fixed volume of 90 m3 to successfully cross IES is between 110 m2 and 120 m2. This finding is consistent with the value reported from ex vivo experiments (5) and with the prediction of an analytical model (19, 26). These results demonstrate the capability of our model in quantitatively assessing RBC filtration function in the spleen.
Altered RBCs in HS are characterized by spherical shape and impaired deformability, which results from the reduced surface area (8–10). Our simulations illustrate that HS RBCs lose surface area through shedding vesicles when traversing IES because of the weakened cohesion between cytoskeleton and lipid bilayer. Loss of surface area from HS RBCs becomes more pronounced as the degree of inherited protein deficiency increases. Our simulation results also demonstrate that the reduction in surface area compromises the deformability of RBCs, potentially leading to their splenic retention by IES. As shown in Fig. 5, the S/V ratios of HS RBCs after losing surface area decrease toward the retention thresholds (black solid and dashed lines). HS RBCs with low levels of protein deficiency undergo a small drop of S/V ratio after passage through IES, indicating a gradual decrease in their life spans, whereas HS RBCs with higher protein deficiency undergo a larger drop of S/V ratio, implying a marked decrease in their life spans. These findings correlate well with the severity of anemia and earlier occurrence of chronic complications induced by hemolysis in patients with severe forms of HS. In addition, our simulation results suggest that the spectrin-deficient RBCs are more amenable to vesiculation than the band-3–deficient RBCs when traversing IES. This result provides a possible explanation of the distinct clinical outcomes of splenectomy in HS patients with different pathogenesis (32).
The present simulations do not account for the effect of a putatively impaired splenic RBC filtration function in HS patients (15, 36). Therefore, the prediction of RBC retention in Figs. 4A and 5 may overestimate the function of IES in retaining the altered RBCs in HS. In addition, the reduced surface area in HS RBCs can provoke a transient dehydration, leading to a decrease in cell volume and thus an attendant increase in the S/V ratio (5, 37). As a result, a greater than expected proportion of HS RBCs may escape splenic retention and return to circulation. Although our present study strongly suggests that the surface area loss from HS RBCs occurs when they cross IES in the spleen, this pathogenic process may begin even earlier when reticulocytes (young RBCs) exit from the bone marrow, a process not devoid of mechanical constraints (37). These two mechanisms of surface area loss are not mutually exclusive, as reticulocytes exit the bone marrow only once but cross IES in the spleen many times during their life span.
The major molecular basis of HE is the - or -spectrin deficiency, which disrupts the self-association of spectrin dimer into tetramers or oligomers, leading to a weak and fragile membrane cytoskeleton (9, 11). Elongated cell shape and decreased membrane stability are the clinical features of RBCs in HE (12). Our simulations demonstrate that due to impaired cytoskeleton, HE RBCs are elongated after traversing IES, contributing to their shape transition to ellipsoids. When the severity of HE-related protein deficiency is high, RBCs break into fragments during their passage through IES. These findings clarify the relationship among the genetics, degree of protein deficiency in cytoskeleton, and clinical expressions of RBCs in HE.
Taken together, our simulations provide unique insights into the dual pathophysiological function of the spleen. The spleen not only assesses the mechanical fitness of the healthy and diseased RBCs in circulation but also contributes directly to the alterations of diseased RBCs. Our results also elucidate the connections among the molecular basis of inherited RBC disorders, the different clinical manifestations of RBCs, the resulting symptoms, and the clinical outcomes of splenectomy. The computational framework presented in the current work thus offers possibilities to study the complex role of the spleen in the pathogenesis of RBC disorders.
Supplementary Material
Acknowledgments
H.L. and X.L. thank Dr. Zhangli Peng for setting up dissipative particle dynamics simulations for model comparison. The work was supported by National Institutes of Health Grants U01HL114476 and U01HL116323. This research used resources of the Argonne Leadership Computing Facility under Contract DE-AC02-06CH11357 and resources of the Oak Ridge Leadership Computing Facility under Contract DE-AC05-00OR22725. S.S. acknowledges support from the Distinguished University Professorship at Nanyang Technological University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806501115/-/DCSupplemental.
References
- 1.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 2.Petroianu A. The Spleen. Bentham Science Publishers; Hilversum, The Netherlands: 2011. [Google Scholar]
- 3.Buffet PA, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood. 2006;107:3745–3752. doi: 10.1182/blood-2005-10-4094. [DOI] [PubMed] [Google Scholar]
- 4.Safeukui I, et al. Retention of plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112:2520–2528. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]
- 5.Safeukui I, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120:424–430. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndour PA, et al. Role of the Spleen in Human Malaria. Springer; New York: 2015. [Google Scholar]
- 7.Narla J, Mohandas N. Red cell membrane disorders. Int J Lab Hematol. 2017;39:47–52. doi: 10.1111/ijlh.12657. [DOI] [PubMed] [Google Scholar]
- 8.Eber S, Lux SE. Hereditary spherocytosis—Defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin Hematol. 2004;41:118–141. doi: 10.1053/j.seminhematol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa L, Galimand J, Fenneteau O, Mohandas N. Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev. 2013;27:167–178. doi: 10.1016/j.blre.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411–1426. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher PG. Hereditary elliptocytosis: Spectrin and protein 4.1R. Semin Hematol. 2004;41:142–164. doi: 10.1053/j.seminhematol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Diez-Silva M, Dao M, Han J, Lim CT, Suresh S. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull. 2010;35:382–388. doi: 10.1557/mrs2010.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groom A, Schmidt E, MacDonald I. Microcirculatory pathways and blood flow in spleen: New insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc. 1991;5:159–173. [PubMed] [Google Scholar]
- 14.MacDonald I, Ragan D, Schmidt E, Groom A. Kinetics of red blood cell passage through interendothelial slits into venous sinuses in rat spleen, analyzed by in vivo microscopy. Microvasc Res. 1987;33:118–134. doi: 10.1016/0026-2862(87)90011-2. [DOI] [PubMed] [Google Scholar]
- 15.Deplaine G, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood. 2011;117:e88–e95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picot J, et al. A biomimetic microfluidic chip to study the circulation and mechanical retention of red blood cells in the spleen. Am J Hematol. 2015;90:339–345. doi: 10.1002/ajh.23941. [DOI] [PubMed] [Google Scholar]
- 17.Gambhire P, et al. High aspect ratio sub-micrometer channels using wet etching: Application to the dynamics of red blood cell transiting through biomimetic splenic slits. Small. 2017;13:1700967. doi: 10.1002/smll.201700967. [DOI] [PubMed] [Google Scholar]
- 18.Freund JB. The flow of red blood cells through a narrow spleen-like slit. Phys Fluids. 2013;25:110807. [Google Scholar]
- 19.Pivkin IV, et al. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci USA. 2016;113:7804–7809. doi: 10.1073/pnas.1606751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salehyar S, Zhu Q. Deformation and internal stress in a red blood cell as it is driven through a slit by an incoming flow. Soft Matter. 2016;12:3156–3164. doi: 10.1039/c5sm02933c. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q, Salehyar S, Cabrales P, Asaro RJ. Prospects for human erythrocyte skeleton-bilayer dissociation during splenic flow. Biophysical J. 2017;113:900–912. doi: 10.1016/j.bpj.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YH, et al. OpenRBC: A fast simulator of red blood cells at protein resolution. Biophys J. 2017;112:2030–2037. doi: 10.1016/j.bpj.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutera S, et al. Age-related changes in deformability of human erythrocytes. Blood. 1985;65:275–282. [PubMed] [Google Scholar]
- 24.Waugh RE, et al. Rheologic properties of senescent erythrocytes: Loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 25.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Pivkin IV, et al. Correction for Pivkin, et al., Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci USA. 2017;114:E4521–E4521. doi: 10.1073/pnas.1706577114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, Van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol. 2014;5:79. doi: 10.3389/fphys.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dao M, Lim CT, Suresh S. Mechanics of the human red blood cell deformed by optical tweezers. J Mech Phys Sol. 2003;51:2259–2280. [Google Scholar]
- 29.Suresh S, et al. Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2005;23:3–15. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Suresh S. Mechanical response of human red blood cells in health and disease: Some structure-property-function relationships. J Mater Res. 2006;21:1871–1877. [Google Scholar]
- 31.Walensky L. Disorders of the Red Blood Cell Membrane. Lippincott Williams & Wilkins; Philadelphia: 2003. pp. 1709–1858. [Google Scholar]
- 32.Reliene R, et al. Splenectomy prolongs in vivo survival of erythrocytes differently in spectrin/ankyrin-and band 3–deficient hereditary spherocytosis. Blood. 2002;100:2208–2215. [PubMed] [Google Scholar]
- 33.An X, Mohandas N. Disorders of red cell membrane. Br J Haematol. 2008;141:367–375. doi: 10.1111/j.1365-2141.2008.07091.x. [DOI] [PubMed] [Google Scholar]
- 34.Mohandas N, Gallagher PG. Red cell membrane: Past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, Bitensky MW. A detailed study of time-dependent changes in human red blood cells: From reticulocyte maturation to erythrocyte senescence. Br J Haematol. 2006;135:395–404. doi: 10.1111/j.1365-2141.2006.06279.x. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan GR, Holtkamp CA. Pocked erythrocyte counts in patients with hereditary spherocytosis before and after splenectomy. Am J Hematol. 1987;25:253–257. doi: 10.1002/ajh.2830250304. [DOI] [PubMed] [Google Scholar]
- 37.Da Costa L, et al. Temporal differences in membrane loss lead to distinct reticulocyte features in hereditary spherocytosis and in immune hemolytic anemia. Blood. 2001;98:2894–2899. doi: 10.1182/blood.v98.10.2894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.