Abstract
Background
Peripheral blood mononuclear cells (PBMNCs) and purified CD34+ cells (PCCs) are increasingly being used at treating no-option critical limb ischaemia (NO-CLI). We aimed to compare the efficacies and uncover the advantages associated with each treatment approach.
Methods
A randomised single-blinded non-inferiority trial (Number: NCT 02089828) was performed. NO-CLI patients were 1:1 randomised to the PBMNCs and PCCs groups, and compared in relation to safety and efficacy outcomes. The primary efficacy outcomes included major amputation and total amputation over 12 months. The major amputation-free survival (MAFS) and total amputation-free survival (TAFS) rates were calculated.
Findings
Fifty patients (25 per group, 47 with thromboangiitis obliterans and 3 with other angiitis) were enrolled, with a median follow-up period of 24.5 months (interquartile range: 17–34 months). One patient in the PCCs group was lost at 2 months and one major amputation occurred in the PBMNCs group at 3 months post-transplantation. The total amputation rates at 6 months post-transplantation were 28.0% in the PCCs group and 16.0% in the PBMNCs group (p = 0.343), and remained unchanged at 12 months. The groups did not differ regarding the MAFS and TAFS (Breslow-Wilcoxon test: p = 0.3014 and p = 0.3414). The PCCs group had a significantly higher probability of rest pain relief than the PBMNCs group (Breslow-Wilcoxon test: p = 0.0454).
Interpretation
PCCs was not inferior to PBMNCs at limb salvage in the treatment of angiitis-induced NO-CLI and appeared to induce earlier ischaemia relief. Each cell type had specific advantages. These outcomes require verification from longer-term trials involving larger numbers of patients.
Fund
Training program for outstanding academic leaders of Shanghai health and family planning system (Hundred Talent Program,Grant No. 2018BR40); China National Natural Science Funds (Grant No. 30801122); The excellent core member training programme at Zhongshan Hospital, Fudan University, China (Grant No. 2015ZSYXGG02); and Zhongshan Funds for the Institute of Vascular Surgery, Fudan University, China.
Clinical trial registration
This study is registered with ClinicalTrials.gov (NCT 02089828).
Keywords: Critical limb ischaemia, Cell therapy, Purified CD34+ cells, Peripheral blood mononuclear cells, Limb salvage
Research in context.
Evidence before this study
CD34 is one of the widely recognised surface markers for endothelial progenitor cells (EPCs), which might play a dominant role in angiogenic and vasculogenic efficacy in postnatal neovascularisation. Intramuscular or intra-arterial infusion of the EPCs targeted at proliferation of arteriole and capillary bed in the ischemic muscle area, thus accelerating the blood flow perfusion even though the input pressure is unchanged.This method might bring light to patients with critical limb ischemia (CLI) when bypass or endovascular procedure does not fit. Mobilised or non-mobilised peripheral blood mononuclear cells (PBMNCs), which contain EPCs, are increasingly used in the clinical trials of stem cell therapy for CLI. Most of them have shown positive results for limb salvage. However, the quality control is difficult and efficacies of individual cell types can hardly be explained because the cell types are mixed and the EPCs' concentrations are low in the transplants.Theoretically, purified CD34+ cells (PCCs) are enriched in endothelial progenitor cells and might induce greater levels of neovascularizationand less inflammation reaction followingtreatment than non-purified mononuclear cells. However, more than half of the CD34+ cells are lost during PCCs isolation, and the separatedCD34- cells might also contributeto angiogenesis synergistically via their paracrine activity. It was unknown weather removal of CD34- fraction could bring beneficial or adverse influence to efficacy of mononuclear cell transplantation.
We searched PubMed articles published until March 1st, 2018, without language restrictions, reporting on trials and treatments for cell therapy in CLI. We used terms (“critical limb ischemia” OR “critical limb ischaemia”) AND (“mononuclear cell” OR “CD34+” OR “CD34 positive”) AND (“cell therapy” OR “therapy” OR “treatment” OR “transplantation” OR “implantation”). No previous prospective randomised trials comparing PCCs and PBMNCs were reported. A pilot single-armed study (Kawamoto et al. Stem Cells, 2009) firstly indicated the feasibility and safety of G-CSF mobilisedCD34+ cells in patients with CLI (no major amputation or death in 12 weeks). This trial used a dose-escalation design (105/Kg, 5 × 105/Kg, 106/Kg) and showed no difference of therapeutic efficacy among these doses. It provided evidence for dose selection of CD34+ cells in the subsequent studies. However, the drawback of this study was its relatively small sample size (17 patients). The study team also reported a 4-year major amputation-free survival of 76.5% (13/17, no major amputation, 4 deaths unrelated to cell therapy) in its long-term result (Makoto Kinoshita et al. Atherosclerosis, 2012). A double-blinded, randomised, controlled phase I/IIastudy further provided evidence of favorable trends toward reduced rate of all amputations at 12 months in autologous CD34+ cell-treated (5/16) versus placebo-control (9/12) subjects with CLI(ACT34-CLI trial, Losordo et al. CircCardiovascInterv. 2012). However, the trial failed to demonstrate improved cell therapy-related major amputation-free survival, and it was not powered enough to detect differences among the cell treatment and control, due to its small sample size. In our center, we did a single-armed pilot study of transplantation of PCCs from G-CSF mobilised PBMNCs in the treatment of CLI (Dong et al. Journal of vascular surgery. 2013) and reported a six-month major amputation-free survival rate of 84% (21/25), which paved the way for the current trial.
Added value of this study
To our knowledge, this is the first clinical trial specifically designed to evaluate therapeutic efficacies of PCCs versus PBMNCs transplantation in the treatment of no-option CLI. Furthermore, this is also the first randomised trial to evaluate a special cell type versus mixed cell types in cell therapy for limb ischemia. This study provides evidences for more precise application of cell therapy in terms of specific cell types.
Implication of all the available data
Our clinical results revealed that PCCs is not inferior to PBMNCs transplantation treating no option-CLIat limb salvage and improving quality of life,and might induce earlier ischaemia relief. Besides, the parallel animal experiments revealed that The PCCs, rather than CD34- cells, induced similar blood perfusion indexes and microvascular densities to the PBMNCs in the CLI model. Together with the previous pilot clinical studies, our findings lend support to efficacies of highly purified CD34+ cells transplantation to CLI and its compensation for the removal of CD34- cells.
Alt-text: Unlabelled Box
1. Introduction
Critical limb ischaemia (CLI) is associated with high major amputation and mortality rates and requires timely treatment to re-establish blood flow to the affected area. While bypass surgery and endovascular interventions are the mainstream treatments, 20–30% of CLI patients, or no-option CLI (NO-CLI) patients, are ineligible for these procedures [1,2], and the major amputation and mortality rates for these patients can reach 40% within 1 year and 25% at 6 months, respectively [3,4]. Treatment approaches involving cell therapy have produced promising limb salvage results in NO-CLI patients. Bone marrow mononuclear cells (BMMNCs), peripheral blood mononuclear cells (PBMNCs), and purified CD34+ cells (PCCs) have been used in transplantations. Compared with BMMNCs, PBMNCs [[5], [6], [7]] and PCCs [[8], [9], [10]] are increasingly being used and are advantageous because bone marrow aspiration and general anaesthesia are avoided. Dedicated clinical controlled studies that have evaluated PCCs versus PBMNCs in the treatment of NO-CLI patients are sparse; hence, how these approaches compare is unclear. Theoretically, higher purities of CD34+ cells might induce greater levels of angiogenesis and less inflammation following PCCtreatment [11,12]. However,more than half of CD34+ cells are lost during PCCs isolation, and the separatedCD34− cells can contribute to angiogenesis synergistically via their paracrine effects [13,14].Whether the angiogenesis induced by PCCs is subsequently impaired requires clarification. To date, whether PCCs or PBMNCs are more efficient or equally effective at treating NO-CLI patients remains unclear. Hence, we performed a prospective randomised single-blinded controlled trial to compare the efficacies of PCCs and PBMNCs in treating NO-CLI patients, disclose their respective potential advantages, and guide the selection of patients to ensure that these patients receive particular treatments.
2. Methods
2.1. Design and participants
This randomised single-blinded parallel-group controlled trial was undertaken from April 2014 to December 2017 and was approved by the Ethics Committee of Zhongshan Hospital, which is affiliated with Fudan University. All of the participants provided signed informed consent and were enrolled by the investigators. The study's inclusion criteria were as follows: the provision of signed informed consent before admission; patients aged 18 to 80 years; the presence of stenotic or occlusive lesions in the limb arteries, as confirmed by computed tomography angiography, magnetic resonance angiography, or digital subtraction angiography; CLI with a Rutherford classification of 4–5 that was anatomically unsuitable for surgery or an endovascular intervention; no improvement for at least 3 months following surgery or an endovascular intervention; rest pain that was not alleviated after at least 1 month of conservative treatments, including regular drug therapy, smoking cessation, dietary control and exercise therapy; and an area of tissue loss that had not diminished in size after at least 1 month of these treatments. The study's exclusion criteria were as follows: the occurrence of serious health events <3 months before admission, including but not limited to myocardial infarction, cerebral apoplexy, pulmonary embolism, severe hepatic dysfunction and renal dysfunction; a diagnosis or suspicion of cancer <5 years before admission; proliferative retinopathy; a life expectancy of <1 year; or contraindications for the administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF).
2.2. Randomisation and masking
The investigators recorded the participants' demographic data, Rutherford classifications, CLI aetiologies, comorbidities, other disease histories, and medical and surgical histories before randomisation. Then, the eligible patients were randomly allocated 1:1 to the PCCs or PBMNCs groups using a computer-generated randomisation schedule. All of the patients were masked before and after the interventions. During treatment, the surgeons who performed the cell transplantations knew which cellular treatment each patient received but did not participate in the patients' follow-up assessments or data collection or analysis. Another group of surgeons who were blinded to the patient allocation to the groups made independent decisions about major or minor amputations after the cell transplantations. The staff who managed the patients' follow-up assessments, collected the data, and undertook the statistical analyses were also masked. The masking was removed if a serious adverse event occurred that was related to the trial, a patient died or was lost to follow-up, or if an emergency required unmasking. When the 12-month follow-up period ended, the database was locked, and all of the investigators were unmasked.
2.3. Procedures
After admission, all of the participants received subcutaneous injections of rhG-CSF (Neupogen®; Amgen, Thousand Oaks, CA, USA) (5–10 μg/kg per day for 4 days) to mobilise the bone marrow cells. Additionally, enoxaparin (4000 IU/day) was administered daily to prevent hypercoagulable states. On the fifth day, a suspension of PBMNCs (200 mL) was collected via leukapheresis (COM.TEC; Fresenius Hemocare GmbH, Bad Homburg, Germany). For the patients in the PBMNCs group, the cells that were separated by leukapheresis were washed 3 times and resuspended in an ethylenediaminetetra-acetic acid-phosphate buffered saline solution(200 mL) that contained 0.5% human albumin. For the patients in the PCCs group, CD34+ cells were purified using a magnetic cell sorting system (MiltenyiBiotec GmbH, BergischGladbach, Germany) immediately after leukapheresis. The final cell products were assessed by leukocyte counting and flow cytometry using CD34 antibody. The pharmacists ensured that the implanted CD34+ cell doses ranged from 105 to 106 per kg body weight. The surgeons implanted the cells into the calves/arms and feet/hands of the ischaemic limbs via equidistant intramuscular injections (0.5 mL/site) while the patients were under general anaesthesia. Severely infected wounds were debrided.
2.4. Outcomes
The safety outcomes included all of the adverse events and all-cause mortality from the time at which the cells were mobilised until 2 weeks after treatment; pathological retinal angiogenesis; and the leukocyte counts during hospitalisation and at 1, 2, 3, 6, and 12 months during the follow-up period. The primary efficacy outcomes included major amputation (above the ankle), minor amputation (below the ankle), and total amputation. The major amputation-free survival (MAFS) and total amputation-free survival (TAFS) rates were calculated. The secondary efficacy outcomes were defined as described below. Complete wound healing was defined as skin integrity without superficial tissue loss in the form of a skin ulcer or gangrene compared with baseline and was confirmed by a specialist clinician who inspected and photographed the patients' limbs before treatment and during follow-up. The Wong-Baker Faces Pain Rating Scale (WBFPS), on which a score of 0 represents no pain and a score of 10 represents the greatest pain, was used to measure the intensity of a patient's rest pain. The patients were taught to use the WBFPS, and they recorded their pain scores in the supine position in the absence of the use of analgesic agents at baseline and every week after cell transplantation. The data were transferred to the investigators at each follow-up visit. The Rutherford classification was used to define the severity of the limb ischaemia, and the non-CLI ratios were calculated in both groups at each time point. The restoration of the blood supply to an ischaemic extremity was assessed according to the pain-free walking time (PFWT) at 2.5 km/h and at a 10% incline on a treadmill, the ankle-brachial index (ABI), the toe-brachial index (TBI), and the transcutaneous oxygen pressure (TcPO2) before treatment and during follow-up. Quality of life (QoL) was assessed using the 36-item Short Form Health Survey (version 2)at baseline and at 12 months after cell transplantation [15,16].
2.5. In vivo neovascularisation potential
A parallel animal experiment was performed with the transplanted cells to validate the clinical results, particularly from a histological perspective. We used a classical non-obese diabetic/severe combined immunodeficiency mouse CLI model to assess the in vivo neovascularisation potentials of the cell products. Forty-eight male mice, aged 8–12 weeks, were randomly assigned to 4 groups(n = 12/group), and PCCs (5 × 105/kg, which is the typical dose); PBMNCs (5 × 107MNCs/kg) that included 5 × 105 CD34+ cells/kg; CD34− cells (4.95 × 107/kg) derived from the negative fraction of the magnetic cell sorting process; or endothelial cell base medium-2 as the blank control were injected into the gastrocnemius muscles of the animals' ischaemic limbs 20 min after the induction of unilateral hind limb ischaemia. The hind limb blood perfusion values were measured bilaterally using laser speckle contrast imaging (PeriCam PSI System; PerimedAB, Järfälla, Sweden) before modelling, at the time of the injections, and on days 1, 4, 8, 16, and 24 after the injections. The blood perfusion indexes (BPIs) were calculated as the ratios of the blood perfusion values in the ischaemic limbs to those in the contralateral limbs. The microvascular densities (MVDs) of the ischaemic gastrocnemius muscles were assessed using immunostaining for mouse-specific CD31 on day 8 (n = 4/group) after transplantation. Five fields at a magnification of 200× were randomly selected from the animals' tissue sections, and the MVD was calculated as the mean number of isolated positive regions per muscle fibre.
2.6. Statistical analyses
We calculated the study's sample size using a non-inferiority test of 2 proportions with a randomisation ratio of 1:1. Regarding a reference group parameter, MAFS at 6 months after the transplantation of PBMNCs into the CLI patients was estimated to be approximately 93%, which was based on the findings from previous clinical trials [6,17,18].Three publications that describe studies investigating PCC transplantation suggested that the MAFS rate at 6 months post-transplantation ranged from 69% to 94% [[8], [9], [10]].Based on these data and our experience, a non-inferiority margin of −19%(20% of the MAFS in the reference group) was formulated. Assuming a 1-tailed type I error of 5% and a power of no <80%,a sample size of 23 patients/group was needed to detect the non-inferiority of MAFS at 6 months post-transplantation in CLI patients who had been treated with PCCs or PBMNCs. Given a withdrawal rate of <10%, sample sizes of 25 patients/group were finally determined.
The quantitative data are presented as the means and standard deviations (SDs) or as the medians with the interquartile ranges (IQRs), depending on the distribution of the data. The categorical data are presented as numbers and percentages. We used an intention-to-treat approach, considering all of the patients randomly assigned to the study, to evaluate all of the primary outcomes and some of the safety and secondary outcomes, including all-cause mortality, complete wound healing, rest-pain alleviation, and Rutherford class. Any patient who was lost to follow-up was considered the worst-case scenario. Pearson's chi-squared test with or without Yete's continuity correction or Fisher's exact test were used to compare the groups in relation to these results. A linear mixed model was used to analyse the effects of the cell type on the longitudinal changes of the continuous variables and to determine the presence of any interactions between the individual groups and the time point. The Wilcoxon signed-rank test was used to compare the QoL scores at baseline and at 1-year post-transplantation. The MAFS,TAFS, and rest-pain relief probabilities were depicted by plotting Kaplan-Meier curves and compared using the Breslow-Wilcoxon test. Regarding the parallel experiment, the differences among >2 groups in relation to the continuous variables at the longitudinal time points were analysed using two-way analysis of variance, and the comparisons between the groups were assessed using Dunnett's t-tests with adjustments for significance. All of the tests were 2-sided, and a p value < 0.05 was considered statistically significant. The statistical analyses were performed using PASW software, version 19 (IBM Corporation, Armonk, NY, USA).
3. Results
From April 2014 to December 2016, 61 NO-CLI patients were screened, and 50 patients were eligible for and enrolled in the trial, including 47 patients with thromboangiitis obliterans (TAO), 1 patient with systemic lupus erythaematosus and 2 patients with hypereosinophilic syndrome. Twenty-five patients were allocated to each study group. All of the patients presented with NO-CLI in 1 limb, except for 2 patients who had>2 ischaemic limbs, the most ischaemic of which, namely, those with ABIs <0.4 or TBIs <0.3, were included in the study. The included patients were characterised by low risks for cardiovascular and cerebrovascular disease risk factors, except for high frequencies of smoking history (84% in the PCCs group and 92% in the PBMNCs group).Notably, none of the baseline characteristics differed significantly between the groups (Table 1). Forty-eight patients completed the 12-month follow-up period, and the median follow-up period spanned 24.5 months(IQR: 17–34 months) (Fig. 1).One patient in the PCCs group was lost to follow-up at 2 months after transplantation, and 1 patient in the PBMNCs group underwent an above-the-knee amputation at 3 months post-transplantation. A total of 17/21 (81.0%) patients in the PCCs group and 20/23 (87.0%) patients in the PBMNCs group had quit smoking during the 12-month follow-up period.
Table 1.
Patients' baseline characteristics.
| PCCs group (n = 25) | PBMNCs group (n = 25) | P value | |
|---|---|---|---|
| Age, years | 40.80 (10.70) | 42.12 (11.15) | 0.671 |
| Male, n (%) | 25 (100) | 25 (100) | – |
| BMI, kg/m2 | 23.47 (3.59) | 23.41 (3.18) | 0.945 |
| Underlying disease | |||
| TAO | 23 (92) | 24 (96) | 1.0 |
| Collagen disease | 2 (8) | 1 (4) | 1.0 |
| Smoking history, n (%) | 21 (84) | 23(92) | 0.663 |
| Hypertension, n (%) | 2(8) | 1(4) | 1.0 |
| Diabetes mellitus, n (%) | 2(8) | 1 (4) | 1.0 |
| Hyperlipidaemia, n (%) | 2(8) | 2(8) | 1.0 |
| Disease history | |||
| Coronary artery disease, n (%) | 1 (4) | 1 (4) | 1.0 |
| Cerebrovascular disease, n (%) | 0 (0) | 0 (0) | 1.0 |
| Pulmonary embolism, n (%) | 0 (0) | 0 (0) | 1.0 |
| Treated limbs | |||
| Upper/lower extremity, n | 1/24 | 0/25 | – |
| Right/left, n | 20/5 | 12/13 | – |
| Ulcer only, n(%) | 8 (32) | 10 (40) | 0.556 |
| Gangrene, n(%) | 16 (64) | 14 (56) | 0.564 |
| Rutherford class | |||
| 4, n (%) | 1 (4) | 1 (4) | 1.0 |
| 5, n (%) | 24 (96) | 24 (96) | 1.0 |
| ABI (median, IQR) | 0.46(0.31–0.54) | 0.54 (0.40–0.70) | 0.089 |
| TBI (median, IQR) | 0.21(0.16–0.36)a | 0.22 (0.20–0.46)b | 0.332 |
| TcPO2, mmHg | 18.40 (12.92) | 20.88 (12.31) | 0.491 |
| Medication history | |||
| Aspirin, n (%) | 17 (68) | 21 (84) | 0.185 |
| Clopidogrel, n (%) | 0 (0) | 1 (4) | 1.0 |
| Cilostazol, n (%) | 20 (80) | 19 (76) | 0.733 |
| Prostaglandins, n (%) | 19 (76) | 21 (84) | 0.480 |
| Warfarin, n (%) | 1 (4) | 0 (0) | 1.0 |
| Ca2+ channel blocker | 0 (0) | 1 (4) | 1.0 |
| ACEI/ARB, n (%) | 1 (4) | 1 (4) | 1.0 |
| Surgical history | |||
| Bypass, n (%) | 0 (0) | 2 (8) | 0.490 |
| Endarterectomy, n (%) | 0 (0) | 1(4) | 1.0 |
| Stent grafting, n (%) | 3 (12) | 1 (4) | 0.602 |
| Balloon angioplasty, n (%) | 5 (20) | 2 (8) | 0.415 |
| Sympathectomy, n (%) | 0 (0) | 2 (8) | 0.490 |
| Thrombolysis, n (%) | 7 (28) | 4 (16) | 0.306 |
| Thrombectomy, n (%) | 1 (4) | 1 (4) | 1.0 |
| Major amputation, n (%) | 0 (0) | 1 (4) | 1.0 |
The data presented are the numbers (%) and the means (standard deviations), except for the ABI and TBI that are presented as the medians and the interquartile ranges.
PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells; BMI, body mass index; TAO, thromboangiitis obliterans; ABI, ankle-brachial index; TBI, toe-brachial index; IQR, interquartile range;TcPO2, transcutaneous oxygen pressure; Ca, calcium; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
n = 9.
n = 13.
Fig. 1.
Trial design. PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells.
3.1. Quality of the cell products
Each cell transplant comprised a CD34+ cell dose of no <105/kg, except for 2 subjects in the PCCs group who received 3.54 × 104 cells/kg and 4.56 × 104 cells/kg and 1 subject in the PBMNCs group who received 5.53 × 104 cells/kg; the median dose administered was 6.29 × 105 cells/kg (IQR: 3.36–12.08 × 105cells/kg). The median final transplant volumes were 39 mL (IQR: 38–40 mL) in the PCCs group and 80 mL (IQR: 60–110 mL) in the PBMNCs group (p < 0.001). The total cell counts and white blood cell (WBC) concentrations were significantly higher in the PBMNCs group than in the PCCs group (p < 0.001). In the PBMNCs group, the median (IQR) number of transplanted MNCs was 2.63 × 108 (1.43–3.22 × 108) per kg. The median CD34+ cell concentrations in the PCCs group (8 × 108/L [IQR: 3.96–9.92 × 108/L]) and the PBMNCs group (8.61 × 108/L [IQR:4.35–21.6 × 108/L])did not differ (p = 0.662). The median purity of the CD34+ cells was significantly higher in the PCCs group (69.00%[IQR: 47.00–84.00%]) than in the PBMNCs group (0.31%[IQR: 0.18–0.51%]) (p < 0.001) (Table 2).The median CD34+ cell acquisition rate after purification was 26.61% (IQR: 17.16–48.24%) in the PCCs group.
Table 2.
Characteristics of the cell products.
| PCCs (n = 25) | PBMNCs (n = 25) | P value | |
|---|---|---|---|
| Final volume, mL | 39 (38–40) | 80 (60–110) | <0.001 |
| Total WBC count,×106 | 54.8 (34.7–89.9) | 25,800 (15,200–44,100) | <0.001 |
| WBC concentration,×109/L | 1.48 (0.88–2.25) | 264 (153–640) | <0.001 |
| CD34+ cell concentration,×108/L | 8.00 (3.96–9.92) | 8.61 (4.35–21.6) | 0.662 |
| CD34+ cells/WBCs,% | 69.00(47.00–84.00) | 0.31 (0.18–0.51) | <0.001 |
The data presented are the medians (interquartile ranges).
PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells; WBC, white blood cell; CD, cluster of differentiation.
3.2. Safety
No serious adverse events, including death, cardiovascular events, cerebrovascular events, hepatic dysfunction or renal dysfunction, occurred during the treatment period. Twelve adverse events were associated with the mobilisation of the cells, which included slight fevers, transient headaches, back pains, and pruritus. Pain at the injection site occurred significantly more frequently in the PBMNCs group (14/25, 56%) than in the PCCs group (2/25, 8%) (p < 0.001). Pathological retinal angiogenesis was not observed during follow-up. Sustained elevations in the WBC levels for >3 days after transplantation were observed in 1 patient in the PCCs group and in 2 patients in the PBMNCs group, and these rises were ascribed to focal infections of the affected limbs.
3.3. Amputations
Simultaneous debridement with or without a minor amputation was performed on 2 patients in each group during the transplantation because they had severe infections. These amputation events were not included in the subsequent analysis. At 3 months post-transplantation, 1 patient (4%) in the PBMNCs group underwent a major amputation. No additional major amputations were performed on any of the patients in the 2 groups at 6 months or 12 months post-transplantation. The total amputation rates at 3 months post-transplantation were 24.0% in the PCCs group and 8.0% in the PBMNCs group (p = 0.274). At 6 months post-transplantation, the total amputation rates were 28.0% in the PCCs group and 16.0% in the PBMNCs group (p = 0.343) (Table 3). The groups did not differ with respect to the probabilities of MAFS and TAFS (Breslow-Wilcoxon test: p = 0.3014 and p = 0.3414, respectively) (Fig. 2).Among the 47 patients with TAO, the major amputation rate was 4%, and the total amputation rate was 19.1% at the 12-month follow-up assessment.
Table 3.
Comparisons of the groups using the intention-to-treat principle based on worst-case scenarios.
| PCCs group (n = 25) | PBMNCs group (n = 25) | P value | |
|---|---|---|---|
| Major amputation, n (%) | |||
| At 3 months | 1/25 (4) | 1/25 (4) | 1.000 |
| At 6 months | 1/25 (4) | 1/25 (4) | 1.000 |
| At 12 months | 1/25 (4) | 1/25 (4) | 1.000 |
| Minor amputation, n (%) | |||
| At 3 months | 5/25 (20) | 1/25 (4) | 0.192 |
| At 6 months | 6/25 (24) | 3/25 (12) | 0.462 |
| At 12 months | 6/25 (24) | 3/25 (12) | 0.462 |
| Total amputation, n (%) | |||
| At 3 months | 6/25 (24.0) | 2/25 (8.0) | 0.274 |
| At 6 months | 7/25 (28.0) | 4/25 (16.0) | 0.343 |
| At 12 months | 7/25 (28.0) | 4/25 (16.0) | 0.343 |
| All-cause mortality, n (%) | |||
| At 3 months | 1/25 (4) | 0/25 (0) | 1.000 |
| At 6 months | 1/25 (4) | 0/25 (0) | 1.000 |
| At 12 months | 1/25 (4) | 0/25 (0) | 1.000 |
| Patients with an unhealed wound, n (%) | |||
| At 3 months | 19/25 (76.0) | 18/25 (72.0) | 0.747 |
| At 6 months | 11/25 (44.0) | 8/25 (28.0) | 0.382 |
| At 12 months | 8/25 (32.0) | 3/25 (12.0) | 0.088 |
| Complete wound healing, n (%) | |||
| At 3 months | 5/24 (20.8) | 7/24 (29.2) | 0.505 |
| At 6 months | 13/24 (54.2) | 17/24 (70.8) | 0.233 |
| At 12 months | 16/24 (66.7) | 21/24 (87.5) | 0.086 |
| Rest pain alleviation, n (%) | |||
| At 1 week | 6/25 (24.0) | 4/25 (16.0) | 0.480 |
| At 2 weeks | 17/25 (68.0) | 8/25 (32.0) | 0.011 |
| At 3 weeks | 21/25 (84.0) | 15/25 (60.0) | 0.059 |
| At 1 month | 22/25 (88.0) | 21/25 (84.0) | 1.000 |
| At 3 months | 23/25 (92.0) | 24/25 (96.0) | 1.000 |
| At 6 months | 25/25 (100.0) | 24/25 (96.0) | 1.000 |
| At 12 months | 25/25 (100.0) | 24/25 (96.0) | 1.000 |
| Rutherford class at 3 months | |||
| 0–3 | 5/25 (20.0) | 6/25 (24.0) | 1.000 |
| 4 | 1/25 (4.0) | 1/25 (4.0) | 1.000 |
| 5 | 17/25 (68.0) | 17/25 (68.0) | 1.000 |
| 6 | 1/25 (4.0) | 1/25 (4.0) | 1.000 |
| Rutherford class at 6 months | |||
| 0–3 | 11/25 (44.0) | 16/25 (64.0) | 0.156 |
| 4 | 3/25 (12.0) | 1/25 (4.0) | 0.602 |
| 5 | 10/25 (40.0) | 7/25 (28.0) | 0.370 |
| 6 | 1/25 (4.0) | 1/25 (4.0) | 1.000 |
| Rutherford class at 12 months | |||
| 0–3 | 17/25 (68.0) | 21/25 (84.0) | 0.185 |
| 4 | 0/25 (0) | 1/25 (4.0) | 1.000 |
| 5 | 7/25 (28.0) | 2/25 (8.0) | 0.141 |
| 6 | 1/25 (4.0) | 1/25 (4.0) | 1.000 |
The data presented are the numbers (%). PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells.
Fig. 2.
Kaplan-Meier curves showing the probabilities of (A) major amputation-free survival and (B) total amputation-free survival in both groups. The P values were calculated using the Breslow-Wilcoxon test.PCCs, purified CD34+ cells, PBMNCs, peripheral blood mononuclear cells.
3.4. Wound healing and rest-pain alleviation
At baseline, 24 patients in each group had foot ulcers or gangrene, which healed at 3 months post-transplantation in 5 patients (20.8%) in the PCCs group and in 7 patients (29.2%) in the PBMNCs group. At 6 months post-transplantation, the foot ulcers and gangrene had healed in 13 patients (54.2%) in the PCCs group and in 17 patients (70.8%) in the PBMNCs group. By the 12-month follow-up assessments, the ulcers had healed in 16 patients (66.7%) in the PCCs group and in 21 patients (87.5%) in the PBMNCs group (Fig. 3). One patient in the PBMNCs group who only had rest pain at baseline developed a foot ulcer at 1 month post-transplantation, which had healed by the 12-month follow-up assessment. The complete wound healing rates did not differ significantly between the groups at 3, 6, and12 months (p = 0.505, p = 0.233, and p = 0.086, respectively) (Table 3). At 1 week post-transplantation, the estimated margin mean (EMM) WBFPS scores declined significantly from baseline in the PCCs group (ΔWBFPS: -0.800;standard error [SE]:±0.359; p = 0.036) and in the PBMNCs group [ΔWBFPS: -0.480;SE: ± 0.227; p = 0.043], and the declines remained significant until the 12-month follow-up assessment (p < 0.001)(Fig. 4A). The general linear mixed model revealed an interaction of borderline significance between the mean (SD) WBFPS score at 2 weeks post-transplantation and the group (PCCs-PBMNCs ΔWBFPS: -1.200 [0.634]; p = 0.061).Pain relief occurred in 17 patients(68%) in the PCCs group and in 8 patients(32%) in the PBMNCs group (p = 0.011) at 2 weeks. The Kaplan-Meier curves showed that the patients in the PCCs group achieved more rapid rest-pain relief than those in the PBMNCs group (median times for pain relief: 2 weeks and 3 weeks, respectively; Breslow-Wilcoxon test: p = 0.0454) (Fig. 4B).
Fig. 3.
Longitudinal pictures of the treated limb of patient 3 in the PCCs group. The patient had gangrene on the second toe and an ulcer on the dorsum of his left foot before cell therapy (A). He underwent a foot debridement during the cell transplantation (B). The wound area reduced quickly in one month (C). The foot healed in the following two months (D, E) and sustained non-ischemic for 3 years (F).
Fig. 4.
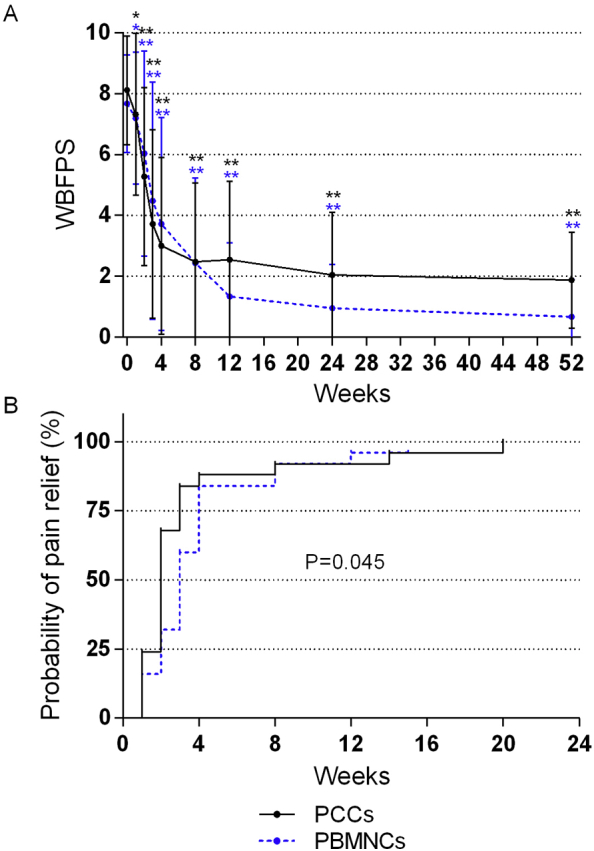
Longitudinal changes in the (A) Wong-Baker FacesPain Rating Scale(WBFPS) and (B)probability of rest pain relief. (A) The longitudinal changes in the WBFPS in both groups are depicted as linear graphs that show the mean values and thestandard deviation bars. *represents p < 0.05, ** represents p < 0.01 (intra-group comparison with baseline, based on a general linear mixed model).(B) Theprobability of rest pain relief is depicted as Kaplan-Meier curves, and the p value was calculated using the Breslow-Wilcoxon test. PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells; WBFPS, Wong-Baker Faces Pain Rating Scale.
3.5. Remission of critical limb ischaemia
A non-CLI status was defined as Rutherford classes of 0–3. In the PCCs group, the non-CLI rate increased from 0% to 20% at 3 months, to 44.0% at 6 months, and to 68.0% at 12 months post-transplantation. In the PBMNCs group, the non-CLI rate increased from 0% to 24.0% at 3 months, to 64.0% at 6 months, and to 84.0% at 12 months post-transplantation. No significant differences were observed between the groups in relation to the non-CLI rate at any time point (Table 3).
3.6. Supply restoration
At 1 month post-transplantation, the EMM (±SE) ABI increase from baseline was significant in the PBMNCs group (ΔABI: 0.068 [±0.028]; p = 0.021) and was of borderline significance in the PCCs group (ΔABI: 0.082 [± 0.042]; p = 0.054).The increases in the ABI values remained significant until 12 months post-transplantation in both groups (Fig. 5A). The EMM (±SE) TBI increased significantly at 2 months post-transplantation in the PCCs group (ΔTBI: 0.114[±0.045]; p = 0.039) and in the PBMNCs group (ΔTBI: 0.102 [± 0.035]; p = 0.028)compared with the baseline values. These significant improvements persisted until 12 months post-transplantation in both groups (Fig. 5B). The EMM (± SE) TcPO2 values increased significantly at 2 months post-transplantation in the PCCs group (ΔTcPO2: 12.428[±4.736]mmHg; p = 0.011) and the PBMNCs group (ΔTcPO2: 10.885[±4.667]mmHg; p = 0.023) compared with the baseline values. The increases in the EMM (± SE) TcPO2 values remained significant at 6 months post-transplantation (12.928[±4.699]mmHg; p = 0.007), but they were nonsignificant at 12 months post-transplantation(7.234[±4.116]mmHg; p = 0.082) in the PBMNCs group. The increases in the EMM (±SE) TcPO2 values were significant at 3 months post-transplantation (11.076[±4.435] mmHg; p = 0.015) in the PCCs group, but they were nonsignificant at 6 months post-transplantation(0.161[±4.854] mmHg; p = 0.974) and 12 months post-transplantation(4.213[±4.521]mmHg; p = 0.355) (Fig. 5C). Significant interactions were not observed, except for the interaction between the change in the EMM (± SE) TBIs at one time point [(month 3 - baseline) × (PCCs-PBMNCs) = 0.111[±0.052]; p = 0.034)].
Fig. 5.
Longitudinal changes in blood perfusion restoration and functional improvement. The assessments of blood perfusion restoration included the (A) ankle-brachial index,(B) toe-brachial index, and (C) transcutaneous oxygen pressure, and the functional improvement was assessed using (D) the pain-free walking time. The values are presented in linear graphs that show the means and standard deviations. *represents p < 0.05, **represents p < 0.01 (intra-group comparison with baseline, based on a general linear mixed model),and Φ represents p < 0.05 for the interaction between a time point and the group, based on a general linear mixed model.ABI, ankle-brachial index; TBI, toe-brachial index; TcPO2, transcutaneous oxygen pressure; PFWT, pain-free walking time,PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells.
3.7. Functional improvement
Five patients were ineligible for the treadmill test because they had plantar wounds, serious foot deformities, undergone a previous contralateral limb amputation, or had upper limb involvement. At baseline, 10 patients in the PCCs group and 6 patients in the PBMNCs group failed to take the test because they could not tolerate the treadmill's speed, and their PFWTs were recorded as zero. Of these patients, 6 in the PCCs group and 3in the PBMNCs group were able to take the test within 6 months of transplantation, and 7 in the PCCs group and 4 in the PBMNCs group were able to take the test within 12 months of transplantation. The EMM (± SE) PFWT values improved significantly at 1 month post-transplantation in the PCCs group (ΔPFWT: 160[±75] sec; p = 0.039) and in the PBMNCs group (ΔPFWT: 177[±85] sec; p = 0.045). These improvements remained significant until 12 months post-transplantation in both groups, and no significant interactions between the time points and the groups were observed (Fig. 5D).
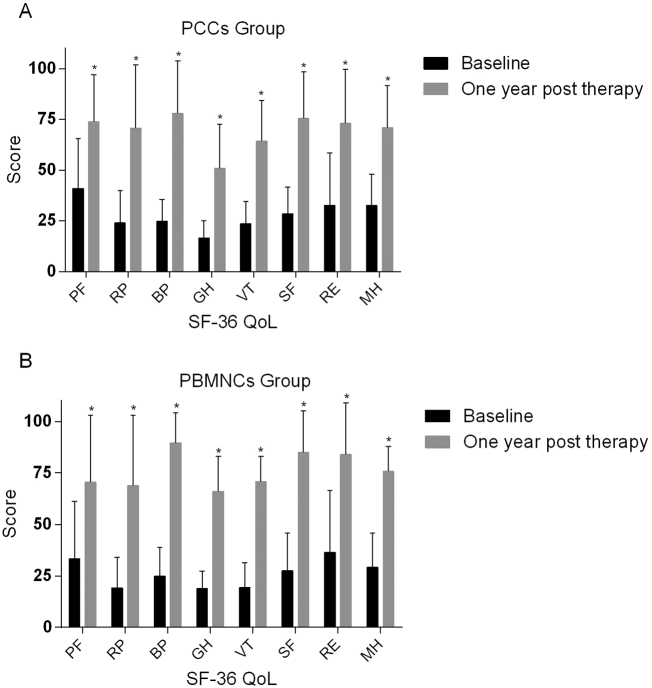
3.8. Quality of life
At 12 months post-transplantation, significant improvements in all 8 dimensions of the patients' QoL scores were observed compared with those at baseline in both groups (Fig. 6). Furthermore, 20/25 patients (80.0%) in the PCCs group and 20/25 patients (80.0%) in the PBMNCs group had resumed work and participated in social activities by the 12-month follow-up assessment. Regarding the TAO patients, 38/47 patients (80.8%) had resumed work by the 12-month follow-up assessment.
Fig. 6.
Quality of life at baseline and at 1-year post-transplantation. The quality of life was assessed using the Short Form-36 scoring system (version 2) in the (A) purified CD34+ cellsgroup and (B) peripheral blood mononuclear cellsgroup. *representsp<0.05 (intra-group comparison with baseline, based on the Wilcoxon signed-rank test).PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells;QoL, quality of life; PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health; VT, vitality, SF, social functioning, RE, role-emotional, MH, mental health.
3.9. Neovascularisation potential in vivo
The groups' BPIs were matched at baseline and at the times of the injections. The PCCs group showed significant improvements in their BPIs on days 8, 16, and 24 compared with those in the control group. The PBMNCs group's mean BPIs were significantly higher than those in the control group on days 8 and 16, but there was no difference between the groups on day 24. There were no significant differences between the CD34− mononuclear cells (MNCs) group and the control group at any time point (Fig. 7A, B). The mean (SD) MVDs were significantly higher on day 8 post-injection in the PCCs group (1.15[0.23]) and the PBMNCs group (0.98[0.21]) compared with the mean MVD in the control group (0.61[0.11])(both p < 0.001).The mean (SD) MVD did not differ significantly between the CD34−MNCs group (0.68[0.10]) and the control group (p = 0.442) on day 8 post-injection(Fig. 7 C, D).
Fig. 7.
In vivo neovascularization potential of the transplants using a non-obese diabetic/severe combined immunodeficiency mouse limb ischaemia model.The longitudinal changes in blood perfusion restoration in the purified CD34+ cells, peripheral blood mononuclear cells, CD34− cells, and the control groupsare presented as (A) laser speckle contrast images and(B) linear graphs of the blood perfusion indexesin which the data are presented as the means and standard deviations. Microvascular density comparisons among groups are presented as (C) immunostaining for mouse-specific CD31 in the ischaemic gastrocnemius muscle on day 8 post-transplantation and (D) bar graphsshowing the mean and standard deviation values. *representsp<0.05 (inter-group comparison with the control group at specific time points, based on Dunnett's t-test). PCCs, purified CD34+ cells; PBMNCs, peripheral blood mononuclear cells.
4. Discussion
Although the mechanism underlying the improvements associated with cell therapy has not yet been completely established, collectively, the vasculogenesis induced by endothelial progenitor cells (EPCs) and the angiogenesis stimulated via the paracrine effects of transplanted cells are major contributors to perfusion improvements and limb salvage [11,12,[19], [20], [21], [22]].CD34+ cells are a key component of the EPC-enriched fraction of BMMNCs and PBMNCs, and the number of CD34+ cells is important for quality control in cell therapy [[5], [6], [7], [8], [9], [10]].The purity of CD34+ cells is directly proportional to their angiogenic efficacy because a higher level of purity promotes angiogenesis and reduces the inflammatory reaction induced by the non-EPC fraction [12,23].The paracrine effects are derived from CD34+ and CD34− cells [11,21,22].The current study's results did not demonstrate statistically significant differences between the groups with respect to most of the primary and secondary outcomes. The results from the animal experiment, which was carried out inparallel, demonstrated the significant advantages of the PCCs group and the PBMNCs group, rather than the CD34- cell group, over the EBM-2 group in relation to the BPI or MVD. However, no significant differences between the PBMNCs and PCCs groups were observed. Hence, one could infer that CD34+ cells might play a predominant role in cell therapy and that they are capable of salvaging limbs and improving patients' QoL scores alone, even in the absence of CD34− cells. The significantly higher purity of CD34+ cells in the PCCs group may have offset the potential negative impact of the removal of CD34− cells.
Although the PCCs and PBMNCs showed similar efficacies, each of the two cell types had its own clinical advantage. At 2 weeks post-transplantation, a significantly higher percentage of the patients had achieved rest pain relief in the PCCs group than in the PBMNCs group (68% vs. 32%; p = 0.011), which suggests that the PCCs improved perfusion earlier than the PBMNCs and that treatment with PCCs could be preferred for patients with more critical and progressive ischaemia. The PCCs transplant may have been free of non-EPC components, which might induce an inflammatory reaction in the ischaemic tissue, increase oxygen consumption and delay perfusion improvements [12]. Furthermore, pain at the injection site was much more common in the PBMNCs group than in the PCCs group (56% vs 8%; p < 0.001), and this may also have been associated with the removal of the non-EPC components from the PCCs. Another possible advantage of PCCs over PBMNCs might relate to their higher level of potential suitability for future allotransplantation. From May 2009 to December 2017, 108NO-CLI patients underwent cell therapy at our centre, 62 of whom were transplanted with PCCs and 46of whom were transplanted with PBMNCs. However, 5 patients were excluded because they were ineligible for rhG-CSF mobilisation, and the limbs of 3patients who had atherosclerosis obliterans (ASO) were not saved, possibly because of the insufficient angiogenic potency of their cells. As an alternative, injecting cells from young donors into patients in these subgroups might be possible. Compared with the process associated with obtaining the PBMNCs, the process associated with acquiring the PCCs removed a large number of mixed cells and minimised the source of immunological rejection. Regarding the advantages of using PBMNCs, almost all of the CD34+cells obtained by apheresis were preserved, which is in contrast to the isolation of the PCCs during which around 73% of the CD34+ cells were lost. Indeed, the number of the transplanted CD34+ cells in the PBMNCs group was almost two-fold as that in the PCCs group in clinical practice because we generally used all of the collected PCC transplants to exceed the lower limit of the required dosage(no <105/kg), while in the PBMNC group, we regularly reduced the volume of the transplant to bring the CD34+ count below the upper limit of the required dosage (no >106/kg).Therefore, the use of PBMNCs would be preferred for patients with relatively or absolutely inadequate CD34+ cells, where the relative inadequacy of the cells suggests that patientswith2 or more critically ischaemic limbs require higher numbers of CD34+ cells to guarantee sufficient doses of cells for each limb. Absolute inadequacy indicates that based on the number of CD34+ cells in the apheresis product and the usual acquisition rate after their isolation, the number of CD34+ cells injected is estimated to be less than the minimal dose required for a single limb (105/kg), whichoccurred in 3 patients in the current study, namely,2in the PCCs group and 1in the PBMNCs group.
Of the 50 study participants, 47 had TAO, and 3 had other angiitis. Thus, the2groups were markedly similar and had comparable baseline conditions, which minimised the potential for statistical bias. Such an optimal enrolment was attributed to the factors that are detailed next. First, the results from our pilot study and those from studies undertaken by other investigators have shown that cell therapy is associated with significantly better outcomes in patients with TAO than in patients with ASO. Notably, the patients enrolled in our study were characterised by much lower frequencies of cardiovascular risk factors and were far younger than the patients with ASO, which might explain the high survival rates following cell therapy. In addition, the age of human donors has been demonstrated to correlate inversely with the angiogenic potency of mononuclear cells in preclinical experiments [24]. Furthermore, cell transplants from elderly patients could not induce sufficient angiogenesis or vasculogenesis due to the impaired survival, migration, differentiation and paracrine ability of the pro-angiogenic cell fraction [10,[25], [26], [27], [28]]. This might also explain the variation in the effects of cell therapy in treating TAO- and ASO-induced CLI. The results from the Intraarterial Progenitor Cell Transplantation of Bone Marrow Mononuclear Cells for Induction of Neovascularization in Patients With Peripheral Arterial Occlusive Disease (PROVASA) and the Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-Arterial Supplementation (JUVENTAS)placebo-controlled trials showed no significant difference between the BMMNCs and the control groups in relation to the major amputation rates, but the results from the PROVASA trial demonstrated that cell therapy was associated with significant improvements in ulcer healing and reductions in rest pain compared with the group that received a placebo [29,30]. The investigators regarded the aetiology of ASO and the advanced ages of most of the participants as 2 causes of the negative results. All of the subjects in the JUVENTAS trial and most of the subjects in the PROVASA trial (32/40) were elderly patients with ASO whose mean ages were 67 years and 64 years, respectively [29,30].The PROVASA trial included 8patients with TAO, and its results revealed a significant difference between the TAO and ASO subgroups with respect to treatment effectiveness (100% [8/8] vs 56% [18/32]; p = 0.02) [28]. Matoba et al. compared the 3-year outcomes of 41 patients with TAO and 74 patients with ASO following BMMNCs transplantation. The 3-year amputation-free rate was significantly higher in the TAO group than in the ASO group (91% vs 60%) [25]. At our centre, MAFS rates of 88.89% at 5 years were observed after injecting PCCsinto23 patients with TAO. More importantly, 65.38% of these patients had satisfactory recoveries and were able to return to their previous jobs [31]. Therefore, we prefer cell therapy as the first-line treatment for TAO patients. Second, the prevalence of TAO in Asia appears to be higher than that in western countries. Indeed, among the patients with peripheral arterial diseases, the proportions of patients with TAOare16–66% in eastern Asia and 0.5–5.6% in western Europe [[32], [33], [34], [35], [36], [37], [38]]. Third, our department is one of the foremost centres for vascular treatment in China, and many complicated patients are referred to us from several other provinces. To our knowledge, the present study is the first prospective randomised trial to compare the efficacies of PBMNCs and PCCs. Of the 47 patients with TAO in the current study, similar satisfactory results were achieved at 12 months post-transplantation, namely, a major amputation rate of 4% and labour competence recovery rate of 80.8%(38/47).These results have encouraged us to further explore cell therapy as the first-line treatment for TAO-induced NO-CLI. Given their much younger ages, patients with TAO expect much more from treatment, namely, long-term limb salvage, an improved QoL, and regained labour competence, than patients with ASO.
The present study had limitations. First, the number of patients was relatively small, despite the sample size being calculated using the non-inferiority test. Besides, the observed incidence of the major amputation was extremely low, which might decrease the statistical power of the conclusion that the PCCs transplantation is not inferior to the PBMNCs. Second, a placebo-treated group was not set as a blank control, which might neglect a placebo effect of the cell therapy. Several randomised, double-blinded, placebo-controlled trials (e.g. the PROVASA and JUVENTAS studies) revealed that bone marrow-derived mononuclear cell therapy resulted in a moderate limb salvage rate that was similar to that of the placebo control group, and recent meta-analyses have suggested that a placebo arm might be essential for cell therapy [39,40]. However, we considered placebo use to be unethical because the enrolled patients were at the CLI stage and had barely benefited from previous conservative treatments. Third, since the patients had a high smoking cessation rate of over 80% in the follow-up, a “quite smoking” effect might also partly explain the observed CLI improvement, given the pathogenesis of TAO which accounted for a majority of the patient's etiologies. However, since all of the patients were treated conservatively with smoking cessation before cell therapy and achieved no remission of rest pain or wound healing, we believe the PBMNCs and the PCCs transplantation might mainly contribute to short-term CLI improvement. On the other hand, the smoking cessation might contribute to the long-term remission of the TAO induced NO-CLI., which could be expected in the future result of our study.
In summary, the results of this study indicate that the use of PCCs to treat NO-CLI is not inferior to the use of PBMNCs, and PCCs even seem to achieve earlier ischaemia relief. Each of the 2 cell types has its own advantages in addition to being similarly effective at limb salvage and QoL improvements. These outcomes require verification with corroborating evidence from longer-term studies that involvelarger numbers of patients.
Contributors
DZH designed the trial; recruited, randomised and allocated patients; oversaw all aspects of the transplantation procedures and follow-up assessment; and drafted and revised the manuscript.
PTY designed the trial; designed and performed the parallel animal experiment; collected data; performed statistical analyses; and drafted and revised the manuscript.
FY designed the trial, managed patients' follow-up assessments, collected data and revised the manuscript.
WZ and GSY designed the trial, performed the procedures before transplantation and revised the manuscript.
FG and LYF designed the trial, performed the surgery of cell transplantation solely and revised the manuscript.
LY and LH executed the patients' follow-up assessments, collected the data and revised the manuscript.
ZTJ interpreted the data, performed and improved the statistical analyses of the data, and revised the manuscript.
HMY designed and performed the parallel animal experiment and revised the manuscript.
GDQ, XX, CB, JJH, YJ, SZY, ZT and SY recruited the patients, collected data, and revised the manuscript.
LP and FWG designed the trial, oversaw all aspects of the transplantation procedures and follow-up assessment, and revised the manuscript.
DZH and PTY contributed equally to this work.
Funding
Training program for outstanding academic leaders of Shanghai health and family planning system (Hundred Talent Program,Grant No. 2018BR40); China National Natural Science Funds (Grant No. 30801122); The excellent core member training programme at Zhongshan Hospital, Fudan University, China (Grant No. 2015ZSYXGG02); and Zhongshan Funds for the Institute of Vascular Surgery, Fudan University, China.
Declaration of interests
All of the authors declare no conflicts of interest.
Roles of the funding sources
Staff at the organisations that provided funding did not participate in the study's design; the collection, analysis, or interpretation of the data; or the writing of the report. All of the authors had full access to all of the data during the study, and the corresponding author had final responsibility for the decision to submit the manuscript for publication.
Acknowledgments
We would like to thank Lan Huang from the Institute of Vascular Surgery, Fudan University (Shanghai, China) and the Department of Vascular Surgery, Zhongshan hospital, Fudan University (Shanghai, China) for her technical assistance and longstanding contributions to patients' follow-up assessments. We would like to thank Huaigen Jing and Longbiao Wei from the Department of Cardiology, Zhongshan hospital, Fudan University (Shanghai, China) for his technical assistance in performing treadmill test. We would also like to thankDr.Farrell Owen Mendelsohn fromthe Center for TherapeuticAngiogenesis,Birmingham (Alabama, USA), Dr.Atsuhiko Kawamoto from Division of Vascular Regeneration Therapy, Laboratory for Stem Cell Translational Research,Institute of Biomedical Research and Innovation, Kobe, Japan, Dr.SatoakiMatoba fromDepartment of Cardiovascular Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan, and Dr.Douglas W. Losordo from Northwestern University, Chicago, U.S.A for theirsincere help during the early years when we started PCCs transplantation.
Contributor Information
Zhihui Dong, Email: dong.zhihui@zs-hospital.sh.cn.
Weiguo Fu, Email: fu.weiguo@zs-hospital.sh.cn.
References
- 1.Dormandy J., Heeck L., Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 2.Lawall H., Bramlage P., Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. J Vasc Surg. 2011;53:445–453. doi: 10.1016/j.jvs.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L., Hiatt W.R., Dormandy J.A. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl S):5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Powell R.J. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264–266. doi: 10.1016/j.jvs.2012.03.255. [DOI] [PubMed] [Google Scholar]
- 5.Minamino T., Toko H., Tateno K., Nagai T., Komuro I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet. 2002;360:2083–2084. doi: 10.1016/s0140-6736(02)11977-5. [DOI] [PubMed] [Google Scholar]
- 6.Dubsky M., Jirkovska A., Bem R. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab Res Rev. 2013;29:369–376. doi: 10.1002/dmrr.2399. [DOI] [PubMed] [Google Scholar]
- 7.Huang P.P., Yang X.F., Li S.Z., Wen J.C., Zhang Y., Han Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98:1335–1342. doi: 10.1160/th07-02-0137. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A., Katayama M., Handa N. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 9.Losordo D.W., Kibbe M.R., Mendelsohn F. Autologous CD34+ Cell Therapy for critical limb ischemia investigators. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Z.H., Chen B., Fu W.G. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. J Vasc Surg. 2013;5:404–411. doi: 10.1016/j.jvs.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Sahoo S., Klychko E., Thorne T. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamoto A., Iwasaki H., Kusano K. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 13.Kwon S.M., Lee J.H., Lee S.H. Cross talk with hematopoietic cells regulates the endothelial progenitor cell differentiation of CD34 positive cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahara T., Murohara T., Sullivan A. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Fukuhara S., Bito S., Green J., Hsiao A., Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara S., Ware J.E., Jr., Kosinski M., Wada S., Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 health survey. J Clin Epidemiol. 1998;51:1045–1053. doi: 10.1016/s0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 17.Moriya J., Minamino T., Tateno K. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. 2009;2:245–254. doi: 10.1161/CIRCINTERVENTIONS.108.799361. [DOI] [PubMed] [Google Scholar]
- 18.De Angelis B., Gentile P., Orlandi F. Limb rescue: a new autologous-peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue Eng Part C Methods. 2015;21:423–435. doi: 10.1089/ten.TEC.2014.0245. [DOI] [PubMed] [Google Scholar]
- 19.Fujita Y., Kinoshita M., Furukawa Y. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ J. 2014;78:490–501. doi: 10.1253/circj.cj-13-0864. [DOI] [PubMed] [Google Scholar]
- 20.You D., Waeckel L., Ebrahimian T.G. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation. 2006;114:328–338. doi: 10.1161/CIRCULATIONAHA.105.589937. [DOI] [PubMed] [Google Scholar]
- 21.Shintani S., Kusano K., Ii M. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl. 1):S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 22.Mathiyalagan P., Liang Y., Kim D. Angiogenicmechanisms of human CD34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120:1466–1476. doi: 10.1161/CIRCRESAHA.116.310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A.H., Caplice N.M. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 24.Li T.S., Kubo M., Ueda K. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120:S255–S261. doi: 10.1161/CIRCULATIONAHA.108.837039. [DOI] [PubMed] [Google Scholar]
- 25.Fadini G.P., Agostini C., Avogaro A. Autologous stem cell therapy for peripheral arterial disease Meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 26.Matoba S., Tatsumi T., Murohara T. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y., Sasaki K., Katsuda Y. Effect of autologous bone marrow cell transplantation on ischemic ulcer in patients with Burger's disease. Circ J. 2007;71:1187–1192. doi: 10.1253/circj.71.1187. [DOI] [PubMed] [Google Scholar]
- 28.Horie T., Onodera R., Akamastu M. Long-term clinical outcomes for patients with lower limb ischemia implanted with G-CSF-mobilized autologous peripheral blood mono- nuclear cells. Atherosclerosis. 2010;208:461–466. doi: 10.1016/j.atherosclerosis.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Walter D.H., Krankenberg H., Balzer J.O. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 30.Teraa M., Sprengers R.W., Schutgens R.E. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled rejuvenating endothelial progenitor cells via transcutaneous intra-arterial supplementation (JUVENTAS) trial. Circulation. 2015;131:851–860. doi: 10.1161/CIRCULATIONAHA.114.012913. [DOI] [PubMed] [Google Scholar]
- 31.Fang Y., Wei Z., Chen Bin. A five-year study of the efficacy of purified CD34+ cell therapy for angiitis-induced no-option critical limb ischemia. Stem Cells Transl Med. 2018 doi: 10.1002/sctm.17-0252. Accepted on 23 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijayakumar A., Tiwari R., Kumar P.V. Thromboangiitis obliterans (Buerger's disease) – current practices. Int J Inflam. 2013:1–9. doi: 10.1155/2013/156905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielubowicz J., Rosnowski A., Pruszynski B., Przetakiewicz Z., Potemkowski A. Natural history of Buerger's disease. J Cardiovasc Surg (Torino) 1980;21:529–540. [PubMed] [Google Scholar]
- 34.Adar R., Papa M.Z., Halpern Z. Cellular sensitivity to collagen in thromboangiitis obliterans. N Engl J Med. 1983;308:1113–1116. doi: 10.1056/NEJM198305123081901. [DOI] [PubMed] [Google Scholar]
- 35.Hagen B., Lohse S. Clinical and radiologic aspects of Buerger's disease. Cardiovasc Intervent Radiol. 1984;7:283–293. doi: 10.1007/BF02625113. [DOI] [PubMed] [Google Scholar]
- 36.Dehaine-Bamberger N., Amar R., Touboul C., Emmerich J., Fiessinger J.N. Buerger disease, clinical prognostic aspects. 83 cases. Presse Med. 1993;22:945–948. [PubMed] [Google Scholar]
- 37.Shionoya S., Ban I., Nakata Y., Matsubara J., Shinjo K. Diagnosis, pathology and treatment of Buerger's disease. Surgery. 1974;75:695–700. [PubMed] [Google Scholar]
- 38.Arkkila P.E. Thromboangiitis obliterans (Buerger's disease) Orphanet J Rare Dis. 2006;1:14. doi: 10.1186/1750-1172-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigato M., Monami M., Fadini G.P. Autologous cell therapy for peripheral arterial disease: systematic review and meta-analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326–1340. doi: 10.1161/CIRCRESAHA.116.309045. [DOI] [PubMed] [Google Scholar]
- 40.Pan T., Wei Z., Fang Y., Dong Z., Fu W. Therapeutic efficacy of CD34(+) cell-involved mononuclear cell therapy for no-option critical limb ischemia: a meta-analysis of randomized controlled clinical trials. Vasc Med. 2018;23:219–231. doi: 10.1177/1358863X17752556. [DOI] [PubMed] [Google Scholar]