Abstract
For more than 40 years, metformin has been used before and during pregnancy. However, it is important to note that metformin can cross the placenta and circulate in the developing foetus. Recent studies reported that the concentration of metformin in foetal cord blood ranges from half to nearly the same concentration as in the maternal plasma. Since metformin has anti-cell growth and pro-apoptotic effects, there are persistent concerns over the use of metformin in early pregnancy. Current human studies are limited by sample size, lack of controls or, short follow-up durations. In this review, we examine the settings in which metformin can be passed on from mother to child during pregnancy and address the current controversies relating to the cellular and molecular mechanisms of metformin. Our efforts highlight the need for more data on the effects of metformin on general offspring health as well as further scrutiny into foetal development upon exposure to metformin.
Keywords: Metformin, Pregnancy, Gestational diabetes, GDM, Polycystic ovary syndrome, PCOS, Development
Highlights
-
•
Metformin is readily transferred from the mother to the foetus, with a wide dynamic range reported clinically
-
•
Though metformin exhibits conflicting positive and negative effects on cell biology, it is increasingly used during pregnancy
-
•
More trials and experimental studies, especially based on human models, are required to assess the safety of metformin usage
1. Introduction
The oral anti-hyperglycaemia agent metformin has had a long and colourful history, from being a component of herbal drugs used in Europe several centuries ago to its discovery and synthesis in the 1920s [1]. Initially, the use of metformin during pregnancy was not widespread due to the absence of data on its safety and, metformin's ability to cross the placenta and circulate in the foetus. There were also safety concerns over phenformin, another anti-diabetic biguanide, and metformin's gastrointestinal side effects [2]. However, due to its relatively lower cost and ease of administration as compared to insulin – the other common treatment for glucose intolerance during pregnancy – metformin is increasingly used before and during pregnancy.
Early reports on the use of metformin during pregnancy were recorded in the Aberdeen International Colloquia on Carbohydrate Metabolism in Pregnancy and the Newborn in 1975 [3]. Pioneering observational studies on its safety and efficacy in South Africa were then published by Coetzee and his colleagues [4]. Over time, the literature on the use of metformin during pregnancy became more reassuring with increasing evidence for a lack of adverse effects on the rate of miscarriage and congenital anomalies at birth in both animal and human studies. However, at the molecular level, the subtle effects of metformin on human foetal tissue development during pregnancy and after birth are only beginning to emerge in recent studies, and these findings leave many questions unanswered about its safety for the unborn child and their later life. In this review, we discuss the clinical settings in which the offspring could be exposed to metformin during pregnancy, raise existing concerns and relate them to the current experimental findings.
2. The use of metformin during pregnancy
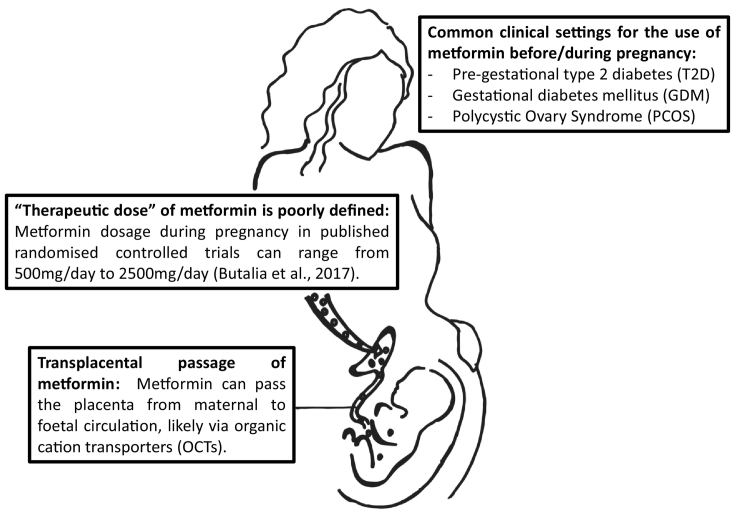
It is commonly known that maternal insulin resistance and hyperglycaemia can have adverse effects on the conceptus/foetus and progression of pregnancy [5], including congenital anomalies, miscarriage, preterm delivery, hypertensive disorders of pregnancy, intrauterine death and, birth trauma. As an anti-hyperglycaemic oral agent, metformin has the distinct advantages of ease of administration, lack of hypoglycaemic risk and lower cost as compared to insulin, which can only be administered through injection. Thus, the use of metformin before and during pregnancy is increasingly prevalent in cases whereby maternal hyperglycaemia and/or insulin resistance cannot be easily controlled by lifestyle changes. Three typical clinical settings in which metformin has been prescribed during pregnancy are (Fig. 1):
-
-
Pre-gestational type 2 diabetes (T2D): Metformin is a common first-line of treatment for T2D and the continued use of metformin before and during early pregnancy is inevitable in many cases. The decision on whether to continue with metformin treatment or not once pregnancy is confirmed is still a matter of debate. One concern for clinicians is to introduce an alternative treatment regimen of insulin for diabetes early in the pregnancy without worsening glycaemic control. Such treatments require much healthcare resources encompassing patient education, close follow-ups and, huge commitments and intensive glucose self-monitoring on the patient's part.
-
-
Gestational diabetes mellitus (GDM): GDM is defined as “carbohydrate intolerance of varying degrees of severity with onset or first recognition during pregnancy” [6]. Glucose intolerance is often diagnosed from the second trimester onwards but can also be detected in a small proportion of women in the first trimester of pregnancy, which may be representative of pre-existing pre-pregnancy glucose intolerance. If it cannot be remedied by lifestyle changes, insulin or metformin may be used to achieve euglycaemia. As a result, metformin is likely to be used after the first trimester until the end of the pregnancy.
-
-
Polycystic ovary syndrome (PCOS): Women with PCOS have dysfunctional ovaries and exhibit at least two out of these three conditions: 1) ovaries that contain more than 12 small cystic follicles each on ultrasonography, 2) oligomenorrhoea/amenorrhoea secondary to anovulation or, 3) hyperandrogenism. PCOS is frequently associated with systemic insulin resistance which has been suggested to underlie infertility or difficulty in conceiving in women with this condition. Therefore, as an insulin sensitizer/anti-hyperglycaemic agent, metformin has been used to promote ovulation and hence fertility, and maintain pregnancy progression in these women. Consumption of metformin inevitably begins from before conception and can extend to the point of birth.
Fig. 1.
The passage of metformin from mother to offspring. Metformin is becoming a popular agent to control insulin resistance before and during pregnancy. However, unlike insulin, metformin can pass through the placenta, likely via the organic cation transporters (OCTs). The foetus is exposed to at least half to the same concentration of metformin in maternal plasma, which can reach approximately 100 μM (Eyal et al., 2010). It is possible that there are mechanisms of counter-transport which might account for the difference in metformin concentrations between maternal and foetal circulation.
3. The pharmacokinetics and mechanisms of action of metformin
3.1. The pharmacokinetics of metformin
Metformin is an oral anti-hyperglycaemia agent absorbed via the duodenum and jejunum. The absorbed metformin is not metabolised, and is excreted unchanged via the kidney and the bile, with a circulating half-life of approximately 6 h [7]. The renal clearance of metformin increases during the second and third trimesters of pregnancy owing to the physiological increase in glomerular filtration, then returns to pre-pregnancy levels following delivery [8]. Therefore, metformin doses often require adjustment with changes in the glomerular filtration rate [7].
Interestingly, an issue rarely addressed in the context of metformin usage and pharmacokinetics is its therapeutic concentration. A recent meta-analysis by Kajbaf et al. found that within 120 publications they have looked at, there are 65 different “therapeutic” plasma metformin concentrations or ranges [9]. The average values range from 0.129 to 90 mg/L. The lowest and highest boundaries found were 0 and 1800 mg/L respectively. Even amongst studies on metformin use during pregnancy, the administered doses varies from study to study, ranging from 500 mg/day to 2500 mg/day [10]. As previously mentioned, foetal metformin concentrations, as assessed in umbilical venous blood at delivery, can range from half to the same level as the concentration in maternal plasma [8,11]. This presents a challenge in predicting the level of metformin that could be present in embryonic and foetal tissues, which requires the consideration of multiple parameters such as metformin dosage, time point during pregnancy, renal clearance and efficiency of transplacental transfer.
Unlike insulin which requires an insulin-antibody complex to cross the placental barrier [12], metformin can freely traverse the placenta from the mother to the unborn child and circulate in the embryo/foetus [12,13]. Recent studies have shown that the level of metformin in foetal circulation ranges from half to similar levels as that in the mother [8,11] (Fig. 1). As a hydrophilic compound, passive cellular uptake is minimal. Most of the cellular uptake of metformin occurs via the organic cation transporter proteins (OCTs), multi drug and toxin extrusion transporters 1 and 2 (MATE1/2), serotonin transporter (SERT), choline high affinity transporter and, plasma membrane monoamine transporter (PMAT) [14]. Even though there are rare variants of OCT1 which can decrease or increase metformin uptake, generally, the structural variants of OCTs and other transporters have minimal effects on the kinetics of metformin [7]. Even though mouse embryonic stem cells (mESCs) do express OCTs, mouse embryos express OCT1 at almost negligible levels and OCT3, MATE1/2 and PMAT at a much lower level than maternal liver [15]. SERT expression was found to be present in mouse placental and yolk sac tissues but also with diffused expression [16]. Additionally, mESCs have significantly fewer mitochondria with immature cristae [17]. As a result, mESCs are less likely to be affected by metformin exposure. However, as the embryo develops, the cellular energy production starts to favour aerobic metabolism with more mature cristae morphology [17] and the expression levels of OCTs on the cell membrane also increase [15], which may increase the amount of metformin being transported into the cells via these membrane proteins. As a result, the differentiating cells in the embryo are exposed to a higher level of metformin and, consequently, are more vulnerable to its impact. Human placental tissues do express isoforms of OCT1, OCT2 and OCT3 [18], which can account for the transplacental passage of metformin into the foetus. However, there is currently no data on the expression of OCTs, MATE1/2 and PMAT in human embryonic and foetal tissues. SERT expression was found in human placental tissues but no data is available on human foetal tissues [16]. Therefore, the extent of metformin uptake and exposure in embryonic and foetal tissues is still unclear. These gaps in knowledge argue for further scrutiny on metformin's “therapeutic” concentration especially in the context of pregnancy.
3.2. Mechanistic actions of metformin
The exact mechanism of how metformin works remains unclear and controversial in some instances. The general consensus is that metformin activates the AMPK signalling pathway. This was first demonstrated by Zhou et al. nearly two decades ago, showing that metformin activates the AMPK signalling pathway by increasing phosphorylation of the Thr172 site in AMPK alpha subunit in primary hepatocytes [19]. The AMPK signalling pathway is a regulator of cellular energy homeostasis. It is known to be activated by falling cellular energy status – increasing ratio of ADP/ATP and AMP/ATP – and has been hypothesised to be a possible glucose sensing mechanism [20].
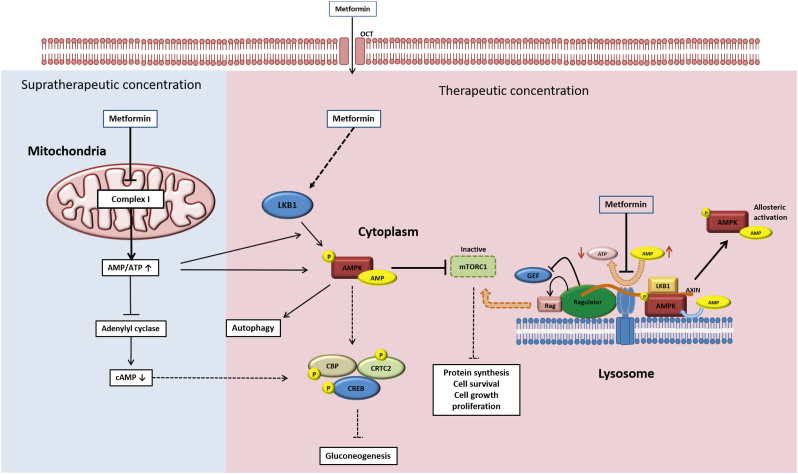
However, as He and Wondisford noted in their review, metformin can have variable working mechanisms depending on its bioavailable concentration [21]. Many in vitro studies use metformin at supratherapeutic concentrations (~2-10 mM). High concentrations of metformin (up to 1800 mg/L, or approximately 13 mM) in the circulation can also occur in the treatment of patients with T2D [9]. It is proposed that complex I of the mitochondrial electron transport chain (ETC) is inhibited when there is a high concentration of metformin, leading to an increase in both ADP/ATP and AMP/ATP ratios [22,23]. The drop in ATP/ADP and ATP/AMP ratios activates AMPK signalling, a cellular energy sensing pathway [24]. Recent studies have also showed that metformin can directly inhibit purified complex I of the ETC [25] (Fig. 2). Foretz et al. proposed that this decreases cAMP levels, which in turn inhibits protein production [23]. However, it is not clear if these mechanisms apply similarly in vivo.
Fig. 2.
Schematic of the mechanisms of action of metformin (Adapted from He and Wondisford, 2015). The working mechanisms of metformin have been shown to vary depending on the concentration of the drug. There are currently three different models of metformin actions. Mode 1 (Left): At supratherapeutic concentration, metformin inhibits complex I of the mitochondrial electron transport chain (ETC). Thus, AMP concentration increases and inhibits the cAMP/PKA pathway, supressing gluconeogenesis. The elevated AMP:ATP ratio also leads to activation of the AMPK pathway by allosteric activation of the AMPK protein. Activated AMPK inhibits the actions of mTORC1 complex actions and therefore, downregulates pathways involving protein, synthesis, cell survival, cell growth and, proliferation. Mode 2 (Middle): This is the classical model of metformin's working mechanism. At therapeutic concentration, metformin acts via the cytoplasmic serine-threonine kinase LKB1, which phosphorylates the protein AMPK and directly activates the AMPK pathway. Activation of the AMPK pathway results in upregulation of the autophagy pathways and inhibition of glucose production via phosphorylation of the proteins CBP and CRTC2. Mode 3 (Right): At therapeutic concentration, metformin inhibits the transmembrane protein vATPase on the surface of the lysosome and increases the AMP/ATP ratio, and activates AMPK via allosteric activation as mentioned above. Simultaneously, metformin also promotes formation of the AXIN-LKB1-vATPase on the lysosomal surface and allosterically activates AMPK proteins attached to the lysosome surface. Formation of the vATPase-AXIN complex also leads to inhibition of the mTORC1 complex anchored on the lysosomal surface, which also leads to the decrease of cell survival, cell growth and proliferation.
Importantly, these mechanisms are not significantly activated at therapeutically-relevant bioavailable concentrations in vivo. At 70 μM of metformin, close to the level of metformin measured in the hepatic portal vein, AMP levels failed to increase in primary hepatocytes in vitro despite efficient activation of the AMPK pathway [21]. Concentrations of the variants of the AMPK subunits and LKB genes, which are serine/threonine kinases upstream of AMPK in the signalling pathway in all cells, are associated with clinical response to metformin, confirming that the AMPK pathway activation is involved in metformin action at therapeutically-relevant concentrations [26]. A recent study demonstrated that at therapeutically-relevant concentrations, metformin directly acts on the lysosomal v-ATPase and promotes AXIN-LKB1 complex translocation to form an in vitro complex with v-ATPase-Ragulator, which is a lysosomal protein complex [27]. Ultimately, the formation of this complex phosphorylates and activates AMPK alpha subunit independently of mitochondrial ETC inhibition. Simultaneously, this complex on the lysosomal membrane also inactivates mTORC1 [20] (Fig. 3).
Fig. 3.
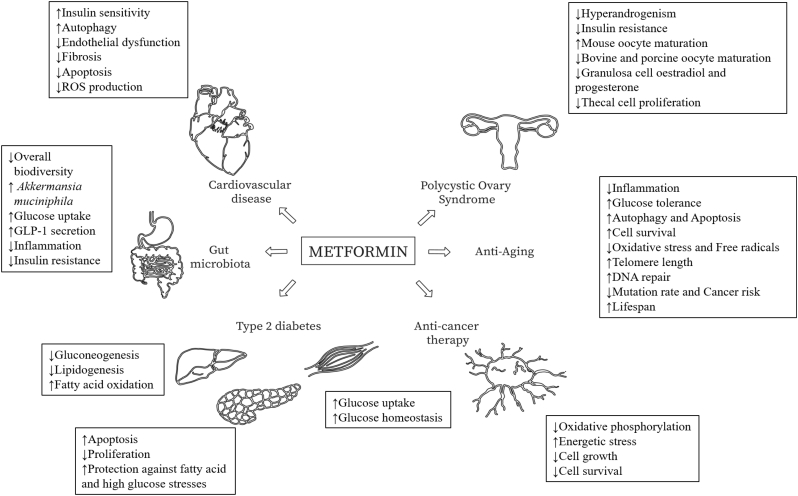
Various actions of metformin in human tissues. Aside from its classic application in controlling hyperglycaemia, the use of metformin is being expanded significantly, from anti-cancer therapy to regulation of gut microbiota. However, the various applications of metformin also highlight the vast range of different possible actions of metformin in different cells/tissues.
4. The varied actions of metformin in different human tissues can have implications on embryonic/foetal development
Metformin has proved to be a multi-functional player with application ranges from hyperglycaemia control and ovary follicle stimulation in patients with PCOS, to protection against metabolic stress in the pancreas and adjuvant use in anti-cancer therapy (Fig. 3). These varied mechanistic actions of metformin can be a cause of concern as they highlight the potential impact of metformin on human embryonic cells and foetal tissues.
4.1. The effects of metformin on pancreatic beta cells remain controversial
Pancreatic beta cells act as the controller of glucose homeostasis in the human body by secreting insulin in response to glucose or nutrient stimulation. In humans, these pancreatic beta cells are fully differentiated by the end of the first trimester and the eventual total beta cell mass is generated via the proliferation of existing beta cells through the second and third trimesters as well as very shortly after birth [28]. In the previously mentioned clinical scenario where metformin is administered in pregnancy, both progenitors and mature pancreatic beta cells may be exposed to metformin (Fig. 1). Gregg et al. showed that in utero exposure to metformin leads to more cells expressing Pdx1 (a pancreatic progenitor marker), but less cells expressing Ngn3 (a later pancreatic endocrine progenitor marker) in mouse embryos [29]. As the endocrine progenitors are the precursors of mature pancreatic beta cells, the decrease in endocrine progenitor population will likely cause a decrease in the eventual proportion of mature beta cells.
Metformin has recently been shown to confer protective effects on mouse pancreatic beta cells exposed to fatty-acid induced stress [30] and chronic high glucose exposure [31]. The studies proposed that metformin preserves glucose-stimulated insulin secretion (GSIS) by maintaining the ATP/ADP ratio within the beta cells, and prevents beta cell failure by activating the AMPK signalling pathway, supressing C/EBPβ expression and ameliorating ER stress [32,33]. Currently, the mechanism(s) by which metformin protects pancreatic beta cell under metabolic stress remain rudimentary.
Converse to these positive effects, when beta cells are exposed to metformin without metabolic challenges, beta cell proliferation is known to be suppressed and apoptosis is promoted [30,34,35]. Prolonged exposure results in apoptosis either via c-JNK activation and caspase-3 cascade [34] or via increasing AMPK-dependent autophagy [30]. Metformin exposure also impairs insulin secretion in primary human islets, mouse islets and, mouse and rat pancreatic beta cell lines in a normoglycaemic environment [34,35]. Therefore, metformin overdosing or exposure without metabolic challenges might result in potential beta cell toxicity. However, it is conceivable that immature foetal pancreatic cells may respond differently to adult cells and there could be additional or detrimental effects instead, although this remains to be determined.
Collectively, even though metformin can provide protection for pancreatic beta cells against metabolic challenges, foetal exposure to metformin might have anti-growth or restrictive effects on pancreatic beta cells, potentially resulting in a smaller beta cell mass formed during foetal development. This may reduce the individual's ability to withstand life-long glucose or nutrient challenges, giving rise to a higher risk of earlier beta cell dysfunction which translates to the development of T2D. Nonetheless, it is important to note that these limited recent studies have only been conducted in mouse models, which may not accurately reflect the biology in human cells and organs. Hence, the effects of metformin on metabolic risks in human pancreatic beta cells remain to be determined.
4.2. Metformin suppresses cell survival and proliferation in tumours
Besides being known as a first-line T2D drug, metformin is also being considered as an adjuvant therapy in cancer treatment. The anti-cancer potential of metformin was evaluated when the risk of developing cancer in T2D patients was found to be lower with metformin treatment [36]. An early study showed that metformin inhibited cell growth in vitro via an AMPK-dependent manner [37]. Metformin was shown to induce oxidative stress in cancer cells by activating AMPK signalling and triggering low-energy state responses [38]. Additionally, AMPK activation inhibits the expression of many genes and mTOR-regulated protein synthesis [19,24]. Since embryogenesis and organogenesis are processes that involve significant cell growth and high energy demands [39], the activation of AMPK by metformin in replicating and differentiating cells might have restrictive consequences on foetal cell growth and differentiation.
Metformin has also been shown to behave in a similar manner as anti-folate chemotherapy drugs [40]. However, because supraphysiological concentrations of 1 mM and 10 mM metformin were used on breast cancer cells in vitro [40], its impact on embryonic and foetal tissues could be different. Another study in C. elegans also showed that metformin inhibits folate-related metabolic pathway, mimicking the ‘methyl folate trap” observed in vitamin B12 deficiency [41]. The anti-folate action of metformin can subsequently lead to methionine deprivation and decreased synthesis of purines and pyrimidines [41]. Hence, the above findings raise the troubling possibility that prenatal metformin exposure can result in the inhibition of cell growth and cause nutrient restriction. Additionally, based on the well-known DOHAD (Developmental Origin of Health and Diseases) hypothesis, adverse in utero environments such as nutrient restriction and hyperglycaemia have been shown to influence foetal epigenetic programming by disrupting DNA methylation [42]. Therefore, excessive use of metformin in mothers during pregnancy might lead to an increased risk of their children developing metabolic diseases later in life.
4.3. The effects of metformin on reproductive tissues
Since insulin resistance is highly associated with PCOS, metformin has been used to ameliorate PCOS symptoms such as weight gain, menstrual disturbances and glucose intolerance. Women with PCOS also exhibit hyperandrogenism that leads to their abnormal or absent menstruation. Interestingly, metformin treatment is found to also reduce the serum level of androgen, the precursor of oestrogen in the female body, in ovarian thecal and granulosa cells of women having PCOS [43,44]. Thus, the inhibitory actions of metformin on androgen production and insulin resistance are thought to increase the chance of conception and lower the risk of miscarriages in women with PCOS.
It is unclear whether metformin acts directly or indirectly on the ovary. Many studies have focused on the direct actions of metformin on AMPK activation in ovarian cell types. It is thought that metformin encourages oocyte maturation in many different species including mice, rats, cows, pigs and, humans [45,46]. However, the detailed mechanisms are still unclear due to differences in oocyte maturation process amongst these different species [45]. In a female body without PCOS, a decrease in androgen production and subsequent decrease in oestrogen from ovarian cells can have adverse effects on the female offspring's reproductive system. In human female development, all egg cells are generated and differentiated before birth and do not proliferate any further throughout the lifespan of a female human. Prenatal exposure to metformin might affect the behaviour and functions of these cells. To date, the detailed effect of metformin on human follicular cells and their possible impact on the fertility of the female offspring remains unclear.
On the other hand, in human males, sperm cells arise from gonocytes (primitive germ cells) which are formed within the first trimester of gestation [47]. When human and mouse foetal testicular cultures are exposed to metformin, testosterone secretion is significantly decreased [48]. Foetal androgen production is crucial for masculinisation of the foetal reproductive system and maintaining the integrity of gonocytes. Any interference during foetal development could result in disorders of sexual dimorphism, cryptorchidism and hypospadias [47]. To our knowledge so far, overt sex reversal has never been documented with metformin use. Even though the number of gonocytes does not change, the amount of supporting Sertoli cells is decreased at both foetal and neonatal periods [48] with metformin exposure in vitro. These findings suggest that metformin exposure during foetal development may result in the male offspring having lower sperm count, subfertility or higher risk of testicular cancer later in life.
Current data on metformin's effect on the offspring's reproductive tissues pose an important question relating to the use of metformin during pregnancy: Can prenatal metformin exposure lead to changes in the long-term reproductive health of the offspring? This will certainly implicate a trans-generational impact of metformin exposure in pregnancy.
5. Current studies and models of prenatal metformin exposure
As previously stated, the earliest observation of metformin safety and efficacy through the 1970s and 1980s in South Africa was reported by Coetzee et al. [4]. Since then, there have been numerous in vivo and in vitro studies performed on both animal and human models evaluating the effects of metformin exposure in pregnancy.
5.1. Animal studies
Most of the animal studies on the impact of metformin on embryonic and foetal development are performed in rodent models. An early study by Denno and Sadler showed that metformin does not result in major malformations or significant changes in embryonic growth [49]. Since then, there have been many studies using rodent models to shed light on the impact of the use of metformin during pregnancy (Table 1).
Table 1.
Animal studies on the safety and efficacy of metformin use during pregnancy.
| Model | Reference | Maternal condition | Dose | Key findings | |
|---|---|---|---|---|---|
| Rodent In vitro | Mouse whole embryos | Denno and Sadler, 1994 [49] | N/A | 500-2550 mg (3.9–15.8 mmol) per day by oral intake | No alterations in embryonic growth and no major anomalies |
| Mouse embryos and blastocyst | Eng et al., 2007 [66] | 3 week old B6xSJL F1 | 25μg/ml (151μM) in culture media | Metformin increased glucose uptake, decreases apoptosis and improves blastocyst implantation rate in embryos exposed to high IGF-I concentration | |
| Mouse iPSCs | Vazquez-Martin et al., 2012 [59] | N/A | 1–10 mM in culture media | Metformin exposure decreases expression levels of pluripotency factors Oct4, Sox2 and Nanog but does not affect mouse stem cells' pluripotency | |
| Rat prenatal cortical | Ullah et al., 2012 [50] | Sprague-Dawley female rats | 10 mM in culture media | Meformin treatment inhibited neural cell apoptosis and reduced ethanol induced neurodegeneration in primary cultured cortical neurons | |
| Rat cardiac fibroblasts | Bai et al., 2013 [39] | N/A | 10-200 μM (12.9-258 mg) in culture media | Metformin suppresses generation of reactive oxygen species, and inhibits differentiation of cardiac fibroblasts into myofibroblasts | |
| Mouse foetal pancreas | Gregg et al., 2014 [29] | Virgin B57B16 mice | 2 mM (2580 mg) in culture media | Pancreas islets are enlarged Pancreatic progenitor number increases while endocrine progenitor number decreases |
|
| Mouse foetal testicular cells | Tartarin et al., 2014 [48] | N/A | 500 μM (646 mg) | The number of germ cells do not change Androgen secretion and number of supporting Sertoli cells significantly decreased in metformin exposed foetal testicular cells |
|
| Mouse | Louden et al., 2014 [54] | Mouse embryos from TallyHO obese mice | 25 μg/ml (20μM) in culture media | Metformin exposure reverses abnormal blastocyst metabolism in obese female mice, improves insulin-stimulated glucose uptake and normalises lipid accumulation | |
| Rodent In vivo |
Mouse | Solano et al., 2006 [65] | BALB/c virgin female mice treated with/without DHEA | 50 mg/kg (0.39 mmol/kg) in subcutaneous injection | Metformin prevents embryo resorption caused by hyperandrogenisation |
| Mouse | Luchetti et al., 2008 [53] | BALB/c virgin female mice | 240 mg/kg (1.87 mmol/kg) in subcutaneous injection | Metformin inhibits pro-inflammatory effects induced by hyperandrogenisation in early pregnancy | |
| Mouse | Tong et al., 2011 [55] | C57BL/6 J weanling female on control or High Fat diet (HFD) | 2 mg/ml (1.5 mM) in drinking water | Maternal metformin uptake improved glucose uptake via AMPK signalling activation, and prevent adverse maternal obesity-related effects on the development of offspring skeletal muscles Metformin treatment increased number of mitochondria DNA copy but did not change muscle composition in offspring |
|
| Rat | Desai et al., 2013 [63] | 6–7 week old female Wistar rats on NORM or HCAL diets | 300 mg/kg (2.34 mmol/kg) by daily oral intake | Maternal metformin decreases diet induced TNF-α and chemokine ligand 2 in foetal plasma | |
| Mouse | Salomaki et al., 2013 [58] | HFD | 300 mg/kg (2.34 mmol/kg) daily oral intake | At E18.5, metformin exposed foetuses are lighter After birth, metformin exposed mice gain more weight and mesenteric fat Prenatal metformin exposure causes long-term metabolic programming effects |
|
| Mouse | Salomaki et al., 2014 [73] | C57/BL6N Hsd mice on regular diet or HFD | 300 mg/kg (2.34 mmol/kg) daily oral intake | Metformin exposed offspring gain less body weight and adipose tissue Metformin treatment caused effects similar to the “browning” process of white adipose tissues The obesity related gene Ces3m was downregulated by metformin treatment The metformin group also shows improved glucose tolerance |
|
| Mouse | Lee et al., 2014 [15] | ICR mice | 50 mg/kg (0.39 mmol/kg) daily oral intake | Metformin do not activate AMPK signalling pathway in mouse embryo | |
| Mouse | Anisimov et al., 2015 [52] | Sv129 mice | 100 mg/kg (0.78 mmol/kg) daily oral intake | Injection of metformin into new-born Sv129 mice slowed down aging and prolong life span in male mice, but not in female ones. Metformin exposure did not result in hormonal and metabolic changes in blood serum of both male and female mice |
|
| Mouse | Wu et al., 2015 [51] | 4–5 week old C57BL/6 J Type 2 diabetes model female mice | 200 mg/kg (1.56 mmol/kg) daily oral intake | Metformin treatment reduced maternal diabetes-related resorption, as well as the incidence of neural tube defects, cellular stress and, apoptosis in the embryos | |
| Rat | Harris et al., 2016 [56] | 6–7 week old female Wistar rats on HCAL diet | 300 mg/kg (2.34 mmol/kg) daily oral intake | Metformin treatment during pregnancy reduces diet induced inflammation Foetal liver IFN-γ level was significantly lower than in non-treated HCAL diet foetuses |
|
| Mouse | Sun et al., 2018 [64] | Faah −/− mice with higher endocann-abinoid level | 1 mg/kg (7.8 mmol/kg) by oral intake on days 8, 10, and 12 of the pregnancy |
Metformin treatment reduced the risk of preterm births induced by inflammation in mice with high endocannabinoid levels Metformin treatment did not alter placentation or birth weight |
|
| Human in vitro | Endothelial and smooth muscle cells | Bellin et al., 2006 [76] | N/A | 1 mM | Metformin inhibited production of reactive oxygen intermediates in response to high-glucose challenge and fatty acid stress |
| Placental apical membrane | Hemauer et al., 2010 [74] | N/A | 100 nM | Placental P-gp and BCRP proteins contributed to metformin efflux in the foetal-maternal direction and might have been accountable for the difference between foetal and maternal metformin concentration | |
| First trimester trophoblast | Han et al., 2015 [57] | N/A | 0.5 mM | Glucose-induced inflammation in trophoblast cell line was partially reversed by metformin | |
| Chronic villous mesenchymal stem cell (CV-MSCs) | Gu et al., 2017 [77] | N/A | 50μM | Metformin exposure enhanced mineralisation, eNOS expression and osteogenesis in CV-MSCs Metformin also supressed adipocyte formation from CV-MSCs |
|
| Umbilical cord mesenchymal stromal cells (UC-MSCs) | Al Jofi et al., 2018 [75] | N/A | 50μM | Metformin uptake was OCTs-dependent and could activate AMPK signalling pathway in UC-MSCs Metformin actions induced osteogenic effect in UC-MSCs at therapeutically relevant concentration |
Many in vitro and in vivo studies have highlighted the positive effects of metformin on pregnancy outcomes under metabolic challenges. Metformin has been shown to protect neural cells against apoptosis and neural tube defects induced by high glucose challenge [50,51]. Interestingly, when metformin is injected into new-born mice, whose organ maturation processes are still on-going, male mice seemed to have a prolonged lifespan [52]. Additionally, other in vitro rodent studies also showed that metformin can inhibit inflammatory reactions induced by various metabolic challenges such as: Angiotensin II (Ang-II) [39], lipopolysaccharides (LPS)51, cyclooxygenase (COX2) [53], maternal obesity [54,55] or cytokines [53,56]. Han et al. showed that systemic inflammation induced by maternal hyperglycaemia can inhibit angiogenesis and blastocyst implantation, but metformin treatment can ameliorate these adverse effects [57].
On the other hand, other animal studies do raise legitimate concerns over the use of metformin during pregnancy. Salomaki et al. raised the concern that metformin exposure during pregnancy can lead to long-term modifications in metabolic reprogramming. They found that metformin-exposed foetuses are lighter and the resulting mice gained more weight and mesenteric fat after birth [58]. Male offspring exposed to prenatal metformin treatment were found to have impaired glucose tolerance and higher fasting glucose when subjected to a high fat diet (HFD), similar to offspring with maternal malnutrition. The results imply that prenatal exposure of metformin can affect long-term metabolic reprogramming in offspring. These concerns were also echoed in in vitro studies by Gregg et al. [29] and Vazquez-Martin et al. [59]. When metformin is introduced to mESCs and developing mouse pancreata, both showed significant changes in expression levels of markers reflective of their functions and specific characteristics: pluripotency factors in mESCs [59] and pancreatic progenitor markers in prenatal pancreatic islets [29]. Additionally, metformin exposure is shown to inhibit differentiation of cardiac myofibroblasts [39] and reduce the number of reproductive supporting Sertoli cells [48]. While the exact mechanisms of these actions are still unclear, the body of evidence suggests that metformin use during pregnancy should be put under more scrutiny.
Even though rodent models have shed light on several potential aspects of prenatal metformin treatment, it is essential to confirm these findings in human models and trials due to species-specific differences between the developmental processes in mouse and human. The most obvious difference between mouse and human development would be their time of birth in respect to organogenesis and maturation. In mice, the time of birth occurs right after organogenesis is completed and the organs continue to mature in the neonatal period. By contrast, human foetuses remain in the uterus and proceed to a more advanced stage of development with major organ maturation nearing completion at the time of delivery. Thus, the use of rodent models to study metformin exposure during pregnancy cannot fully capture its full impact on organ maturation and all the developmental processes occurring in the human foetus. Hence, human clinical data supplemented by in vitro models can provide precious information on the action of this anti-hyperglycaemia agent.
5.2. Clinical trials of metformin exposure during pregnancy
More than 40 years after the initial use of metformin during pregnancy, there have been numerous studies on pregnancy outcomes. A large randomised controlled trial (RCT) of 751 women raised concerns that metformin might increase the rate of premature delivery but the study did not find any other significant difference in pregnancy outcomes between the metformin and control groups [60]. In 2016, Butalia et al. systematically reviewed all public trials involving the impact of metformin on pregnancy outcomes. Amongst 41 relevant studies, the authors concluded that there was no increased risk of preterm labour, pre-eclampsia, small for gestational age/large for gestational age babies, neonatal ICU admission, perinatal morbidity or mortality in RCTs with a short-term follow-up period [10].
In a retrospective study on the use of metformin during pregnancy, Jakubowicz et al. reported that metformin treatment reduces first trimester pregnancy loss in women with PCOS, compared to those who did not take metformin [61]. As previously demonstrated in animal studies, this positive impact of metformin might be due to the anti-inflammation effects of metformin. Maternal hyperglycaemia and metabolic diseases can induce systemic inflammation and result in adverse pregnancy outcomes [62,63]. Women who received metformin treatment during pregnancy were found to have lower concentrations of the pro-inflammatory interleukin-6 (IL-6) [62]. A decrease in inflammation can increase in the chance of a successful pregnancy by reducing preterm birth risks [61,64], decreasing rates of embryonic resorption and, increasing the rates of blastocyst implantation [65,66]. Therefore, the anti-inflammatory effects of metformin might result in higher rates of successful blastocyst implantation and decrease the risk of pre-eclampsia and pregnancy loss as described in human studies [61,67].
However, besides the studies and meta-analyses on the teratogenicity of metformin and its impact at the time of birth, there have been very few long-term follow-up studies evaluating the impact of metformin exposure on the offspring. Currently, there are five published RCTs and a prospective study by Wouldes et al. on metformin effects in children of various ages exposed to either metformin or insulin/placebo during pregnancy (Table 2). The results in the first 3 RCT studies are either inconclusive [68], limited by small population sizes [68,69] or have conflicting results. The prospective study from Wouldes et al. focused on the neurodevelopment and behaviour of children exposed to either metformin or insulin during GDM pregnancy and did not find any significant differences between the two cohorts [70]. Notably, in early 2018, Hanem et al. published a large follow-up study for children 2 years and older with a total of 182 children exposed to either metformin or placebos prenatally. The study is a follow-up of two randomised, controlled and double-blinded trials and documented for height, weight, BMI and overweight/obesity at 4 years of age and head circumference at 1 year of age [71]. The children exposed to metformin prenatally were found to have higher BMI and higher prevalence of obesity at 4 years of age even though factors such as baseline maternal characteristics at the point of inclusion, maternal weight gain, pregnancy complications and, duration of breast feeding were comparable [71]. Similarly, another group in Australia, Rowan and colleagues, also published another big follow-up study documenting the impact of in utero metformin exposure on children 7–9 years of age [72]. At 9 years of age, the children in this study also showed higher readings for weight, BMI, abdominal fat volume, arm and waist circumferences and, triceps skin fold [72]. However, the study did not find any differences in body fat and abdominal fat percentages and other metabolic measures [72]. It is uncertain what the increased BMI at 4 years and 9 years of age in children exposed to metformin prenatally may imply. However, it is certainly noteworthy when we consider that the data from Salomaki et al. [58,73] demonstrated that mice exposed to prenatal metformin showed body composition and weight gain similar to models of offspring with maternal malnourishment.
Table 2.
Long-term follow-up studies on metformin exposure during pregnancy.
| Study | Maternal condition | Treatment groups | Age at follow-up | Key findings |
|---|---|---|---|---|
| Randomised controlled trials | ||||
| Rowan et al., 2011 [60] | GDM | 154 metformin 164 insulin |
2 years | Children exposed to metformin had more subcutaneous fat. Total fat mass and percentage of body fat were not different |
| Ro et al., 2012 [68] | PCOS | 12 metformin 13 placebos |
7–9 years | No differences in height, weight and body composition. Children exposed to metformin had slightly higher fasting glucose There was a possible trend towards higher systolic blood pressure and lower LDL cholesterol level |
| Ijas et al., 2014 [69] | GDM | 47 metformin 50 insulin |
12 months and 18 months |
Children exposed to metformin were heavier. No difference observed in motor, linguistic and social skills at 18 months of age. |
| Hanem et al., 2018 [71] | PCOS | 92 metformin 90 placebos |
4 years | Metformin exposed children had higher BMI and increased prevalence of overweight/ obesity |
| Rowan et al., 2018 [72] | GDM | 103 metformin 105 insulin |
7–9 years | At 9 years of age, metformin exposed children have higher readings for measures such as weight, arms and waist circumferences, BMI, triceps skinfold and abdominal fat volume No significant difference was observed in body fat percentage, abdominal fat percentage and metabolic measures |
| Prospective observational studies | ||||
| Wouldes et al., 2016 [70] | GDM | 103 metformin 113 insulin |
2 years | No significant differences were found in neurodevelopment outcomes between children of mothers taking insulin and metformin |
| Retrospective cohort studies not available | ||||
Unfortunately, metabolic diseases associated with maternal hyperglycaemia such as T2D, obesity or, cardiac diseases often manifest much later in life. Organising and conducting long-term follow-up on large prospective cohorts is expensive and challenging; hence, the limited body of literature. Currently, there are no retrospective studies which might negate the loss of individuals in follow-up studies (Table 2). Overall, there is too little data at this moment to fully evaluate the long-term effects of metformin on children exposed to the drug in utero.
5.3. Human in vitro studies
Even though RCTs can demonstrate the impact of metformin on the risk of teratogenicity and aberrant postnatal development, they have limited ability in shedding light on the underlying mechanisms of these effects. While non-invasive functional MRIs have been used to conduct studies on drug use during pregnancy, we do not yet have a non-invasive, easily accessible method to study the underlying mechanisms of metformin exposure on offspring tissues at cellular and molecular levels. Human in vitro models can hence be useful in understanding what is going on inside different tissue types of the embryo and foetus with/without metformin (Table 1).
The most obvious source of tissues which reflect metformin exposure in children whose mothers took metformin during pregnancy is their primary cells. At the point of delivery, the placenta and umbilical cord can be collected to isolate various cell lines such as human umbilical vein endothelial cells (HUVECs), Wharton's jelly mesenchymal stem cells (MSCs), placental brush border inside-out vesicles (IOVs), placental trophoblasts and, arterial smooth muscle. These cells arise from the same zygote and were exposed to both maternal hyperglycaemia and metformin in a similar manner as the tissues in the offspring. Many studies have demonstrated the passage of metformin into the foetus through placental tissues using isolated placental syncytiotrophoblast apical membranes [74], Wharton's jelly MSCs [75]; as well as its positive impacts on cellular functions such as MSCs' osteogenic capacity [75] and, HUVECs' metabolic control [76].
However, these cells have some major drawbacks: their inability to expand beyond a certain number of passages. In addition, the variation in genetic backgrounds and other environmental factors between primary cells can introduce confounding factors into the studies. As a result, many researchers have turned to commercially available immortalized cell lines although they may be less representative of the in vivo tissue. In the same line of thought as those who utilised primary placental and umbilical cells, Han et al. demonstrated that in first trimester trophoblast cell lines, metformin can have a mild ameliorating effect on hyperglycaemia-induced inflammatory response [57]. However, metformin exposure could not rescue the trophoblast cells from anti-angiogenic and anti-migratory responses under metabolic challenges, even though these processes are highly influenced by changes in inflammatory factors. MSC lines were also used to study the impact of metformin on stem cell functions. Gu et al. demonstrated that metformin augmented the hyperglycaemia-induced anti-osteogenic response in MSCs via activation of the eNOS and AMPK pathway. Overall, metformin seems to act through various signalling pathways and can have very different impacts on different cell types [77].
All the aforementioned in vitro models represent partially or fully differentiated cells. They can demonstrate the impact of metformin on specific cell types during development or at birth. However, they cannot show the impact of metformin on much earlier processes occurring during embryogenesis and organogenesis, a time when metformin exposure commonly occurs clinically. This calls for alternative models that reflect the earlier processes of human tissue and organ development [78]. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) fit these requirements perfectly. Their pluripotency and limitless self-renewal potential allow them to bypass the disadvantages of current in vitro models [79]. With the recent interest in stem cell biology, there are now many differentiation protocols that can recapitulate the differentiation processes of various cell types or the development of many organs [80]. These cells can shed light on how metformin could impact on a range of different tissue differentiation processes and the underlying mechanisms involved. We suggest that hESCs and iPSCs might hold some of the answers to the many unaddressed questions in the current literature.
6. Outstanding questions and concluding remarks
The use of metformin during pregnancy is becoming more common worldwide. Currently, there is relatively little known about the extent and full implications of metformin exposure in utero, particularly how it can affect embryonic and foetal development. There have been insufficient long-term follow-up studies conducted to date that can uncover the possible adverse effects of prenatal metformin exposure on later offspring health. Another troubling issue is the emerging evidence of possible anti-growth, nutritionally-restrictive and energetically-restrictive actions of metformin in certain cell types. Thus, we do not as yet have a well-defined therapeutic plasma concentration range for metformin which is optimal for maintaining a low risk pregnancy – such that potential harms to the foetus can be limited whilst extracting maximal therapeutic benefit from metformin. Further data is certainly needed to determine the appropriate dosage and whether specific clinical characteristics should also be considered. In conclusion, we need much more data on how cellular processes and foetal tissue maturation during development could potentially be disrupted in pregnancies exposed to metformin therapy and map out the long-term impact on the offspring. We suggest that until more data is available, we should consider setting stricter parameters for metformin use during pregnancy.
Search strategy and selection criteria.
Data for this review were identified by searching PubMed using the following search terms: “metformin, “metformin working mechanisms”, “metformin pharmacokinetics”; “metformin pregnancy”; “metformin GDM”, “metformin PCOS”, “metformin dose”, “AMPK signalling metformin”, “metformin proliferation”, “metformin growth”. Only articles published in English were included.
Acknowledgments
Acknowledgements
We thank Stephanie Ler and members of the Teo laboratory for the critical reading of this manuscript. L.N. is supported by the Singapore International Graduate Award (SINGA), A*STAR. A.K.K.T. is supported by the Institute of Molecular and Cell Biology (IMCB), A*STAR, NHG-KTPH SIG/14033, the NUHS-CG Metabolic In-Vitro Core Seed Funding, the JCO Career Development Award (CDA) 15302FG148, A*STAR, the NMRC Open Fund-Young Individual Research Grant (OF-YIRG) (OFYIRG16may014), A*STAR ETPL Gap Funding (ETPL/18-GAP005-R20H) and the Lee Foundation Grant (SHTX/LFG/002/2018). S.-Y.C. and her work is supported by grants administered by the Singapore National Research Foundation and Singapore Ministry of Health's National Medical Research Council (NMRC) - Clinician Scientist Award (NMRC/CSA-INV/0010/2016), Translational and Clinical Research (TCR) Flagship Programme (NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014), the National University of Singapore, Singapore Institute for Clinical Sciences and A*STAR. S.-Y.C. also declare grants and non-financial support from industry funding received by the Epigen Academic Consortium, outside the submitted work. Sponsors had no involvement in the conduct of the research and/or preparation of the article.
Authors' contributions
Conceptualisation: L.N., S.-Y.C. and A.K.K.T. Investigation: L.N. Writing – Original Draft: L.N. Writing – Review & Editing: L.N., S.-Y.C. and A.K.K.T. Supervision: A.K.K.T. Project Administration: A.K.K.T. Funding Acquisition: S.-Y.C. and A.K.K.T.
References
- 1.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.J., Path M.R.C., Metformin Turner R.C. N Engl J Med. 1996;334(9):575. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland HW, Stowers JM. Carbohydrate Metabolism in Pregnancy and the Newborn: Incorporating the Proceedings of the International Colloquium at Aberdeen, Scotland, July, 19731975.
- 4.Coetzee E.J., Jackson W.P.U. Oral hypoglycaemics in the first trimester and fetal outcome. SA Med J. 1984;65:635–637. [PubMed] [Google Scholar]
- 5.Schaefer-Graf U., Napoli A., Nolan C.J. Diabetic Pregnancy Study G. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018;61(5):1012–1021. doi: 10.1007/s00125-018-4545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association AD Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl. 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham G.G., Punt J., Arora M., Day R.O., Doogue M.P., Duong J.K. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Eyal S., Easterling T.R., Carr D., Umans J.G., Miodovnik M., Hankins G.D. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833–840. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajbaf F., De Broe M.E., Lalau J.D. Therapeutic concentrations of metformin: a systematic review. Clin Pharmacokinet. 2016;55(4):439–459. doi: 10.1007/s40262-015-0323-x. [DOI] [PubMed] [Google Scholar]
- 10.Butalia S., Gutierrez L., Lodha A., Aitken E., Zakariasen A., Donovan L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta-analysis. Diabet Med. 2017;34(1):27–36. doi: 10.1111/dme.13150. [DOI] [PubMed] [Google Scholar]
- 11.Charles B., Norris R., Xiao X., Hague W. Population pharmacokinetics of metformin in late pregnancy. Drug Monit. 2006;28(1):67–72. doi: 10.1097/01.ftd.0000184161.52573.0e. [DOI] [PubMed] [Google Scholar]
- 12.Menon R.K., Cohen R.M., Sperling M.A., Cutfield W.S., Mimouni F., Khoury J.C. Transplacental passage of insulin in pregnant women with insulin-dependent diabetes mellitus: its role in fetal macrosomia. N Engl J Med. 1990;323(5):309–315. doi: 10.1056/NEJM199008023230505. [DOI] [PubMed] [Google Scholar]
- 13.Vanky E., Zahlsen K., Spigset O., Carlsen S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83(5):1575–1578. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 14.Han T.K., Proctor W.R., Costales C.L., Cai H., Everett R.S., Thakker D.R. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther. 2015;352(3):519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.Y., Wei D., Loeken M.R. Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 2014;30(1):23–30. doi: 10.1002/dmrr.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kliman H.J., Quaratella S.B., Setaro A.C., Siegman E.C., Subha Z.T., Tal R. Pathway of maternal serotonin to the human embryo and fetus. Endocrinology. 2018;159(4):1609–1629. doi: 10.1210/en.2017-03025. [DOI] [PubMed] [Google Scholar]
- 17.Xu X., Duan S., Yi F., Ocampo A., Liu G.H., Izpisua Belmonte J.C. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18(3):325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadimoghaddam D., Zemankova L., Nachtigal P., Dolezelova E., Neumanova Z., Cerveny L. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol Reprod. 2013;88(3):55. doi: 10.1095/biolreprod.112.105064. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Investig. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C.S., Hawley S.A., Zong Y., Li M., Wang Z., Gray A. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L., Wondisford F.E. Metformin action: concentrations matter. Cell Metab. 2015;21(2):159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 22.El-Mir M., Nogueira V., Fontaine E., Averet N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 23.Foretz M., Hebrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S.C., Hardie D.G.A.M.P.K. Sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Andrzejewski S., Gs P., Poliak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metabol. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jablonski K.A., McAteer J.B., de Bakker P.I., Franks P.W., Pollin T.I., Hanson R.L. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes. 2010;59(10):2672–2681. doi: 10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C.S., Li M., Ma T., Zong Y., Cui J., Feng J.W. Metformin activates AMPK through the lysosomal pathway. Cell Metab. 2016;24(4):521–522. doi: 10.1016/j.cmet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Pan F.C., Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes. 2014;21(2):77–82. doi: 10.1097/MED.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregg B., Elghazi L., Alejandro E.U., Smith M.R., Blandino-Rosano M., El-Gabri D. Exposure of mouse embryonic pancreas to metformin enhances the number of pancreatic progenitors. Diabetologia. 2014;57(12):2566–2575. doi: 10.1007/s00125-014-3379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y., Huang W., Wang J., Xu Z., He J., Lin X. Metformin plays a dual role in MIN6 pancreatic beta cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10(3):268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masini M., Anello M., Bugliani M., Marselli L., Filipponi F., Boggi U. Prevention by metformin of alterations induced by chronic exposure to high glucose in human islet beta cells is associated with preserved ATP/ADP ratio. Diabetes Res Clin Pract. 2014;104(1):163–170. doi: 10.1016/j.diabres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda T., Takahashi H., Mieda Y., Shimizu S., Kawamoto T., Matsuura Y. Regulation of pancreatic beta cell mass by cross-interaction between CCAAT enhancer binding protein beta induced by endoplasmic reticulum stress and AMP-activated protein kinase activity. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon-Szabo L., Kokas M., Mandl J., Keri G., Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0097868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kefas B.A., Cai Y., Kerckhofs K., Ling Z., Martens G., Heimberg H. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem Pharmacol. 2004;68(3):409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Leclerc I., Woltersdorf W.W., Xavier G.D.S., Rowe R.L., Cross S.E., Korbutt G.S. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2004;286 doi: 10.1152/ajpendo.00532.2003. E1023-E31. [DOI] [PubMed] [Google Scholar]
- 36.Gandini S., Puntoni M., Heckman-Stoddard B.M., Dunn B.K., Ford L., Decensi A. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7(9):867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakikhani M., Dowling R., Fantus I.G., Sonenberg N., Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 38.Huang X., Wullschleger S., Shpiro N., McGuire V.A., Sakamoto K., Woods Y.L. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 39.Bai J., Zhang N., Hua Y., Wang B., Ling L., Ferro A. Metformin inhibits angiotensin II-induced differentiation of cardiac fibroblasts into myofibroblasts. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0072120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corominas-Faja B., Quirantes-Pine R., Oliveras-Ferraros C., Vanquez-Martin A., Cufi S., Martin-Castillo B. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate calss of chemotherapy drug. Aging. 2012;4(7):480–498. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cocheme H.M., Noori T. Metformin retards aging in C. Elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen J. The pregnant diabetic and her newborn: Problems and management. Pediatrics. 1967;42(2) [Google Scholar]
- 43.Palomba S., Falbo A., Russo T., Orio F., Tolino A., Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25(4):1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 44.Mansfield R., Galea R., Brincat M., Hole D., Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79(4):956–962. doi: 10.1016/s0015-0282(02)04925-7. [DOI] [PubMed] [Google Scholar]
- 45.Tosca L., Uzbekova S., Chabrolle C., Dupont J. Possible role of 5'AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod. 2007;77(3):452–465. doi: 10.1095/biolreprod.107.060848. [DOI] [PubMed] [Google Scholar]
- 46.Mayes M.A., Laforest M.F., Guillemette C., Gilchrist R.B., Richard F.J. Adenosine 5′-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol Reprod. 2007;76(4):589–597. doi: 10.1095/biolreprod.106.057828. [DOI] [PubMed] [Google Scholar]
- 47.Bertoldo M.J., Faure M., Dupont J., Froment P. Impact of metformin on reproductive tissues: an overview from gametogenesis to gestation. Ann Transl Med. 2014;2(6):55. doi: 10.3978/j.issn.2305-5839.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tartarin P., Moison D., Guibert E., Dupont J., Habert R., Rouiller-Fabre V. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod. 2012;27(11):3304–3314. doi: 10.1093/humrep/des264. [DOI] [PubMed] [Google Scholar]
- 49.Denno K.M., Sadler T.W. Effects of Biguanide Class of Oral Hypoglycemic Agents on Mouse Embryogenesis. Teratology. 1994;49:260–266. doi: 10.1002/tera.1420490405. [DOI] [PubMed] [Google Scholar]
- 50.Ullah I., Ullah N., Naseer M.I., Lee H.Y., Kim M.O. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y., Wang F., Mao F., Wang C., Quon M.J., Yang P. Cellular stress, Excessive Apoptosis, and the effect of Metformin in a Mouse Model of Type 2 Diabetic Embryophathy. Diabetes. 2015;64:2526–2536. doi: 10.2337/db14-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anisimov V.N., Popovich I.G., Zabezhinski M.A., Egormin P.A., Yurova M.N., Semenchenko A.V. Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle. 2015;14(1):46–55. doi: 10.4161/15384101.2014.973308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luchetti C.G., Miko E., Szekeres-Bartho J., Paz D.A., Motta A.B. Dehydroepiandrosterone and metformin modulate progesterone-induced blocking factor (PIBF), cyclooxygenase 2 (COX2) and cytokines in early pregnant mice. J Steroid Biochem Mol Biol. 2008;111(3–5):200–207. doi: 10.1016/j.jsbmb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Louden E.D., Luzzo K.M., Jimenez P.T., Chi T., Chi M., Moley K.H. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev. 2014;27(1):31–39. doi: 10.1071/RD14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong J.F., Yan X., Zhao J.X., Zhu M.J., Nathanielsz P.W., Du M. Metformin mitigates the impaired development of skeletal muscle in the offspring of obese mice. Nutr Diabetes. 2011;1:e7. doi: 10.1038/nutd.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris K., Desai N., Gupta M., Xue X., Chatterjee P.K., Rochelson B. The effects of prenatal metformin on obesogenic diet-induced alterations in maternal and fetal fatty acid metabolism. Nutr Metab (Lond) 2016;13(1):55. doi: 10.1186/s12986-016-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han C.S., Herrin M.A., Pitruzzello M.C., Mulla M.J., Werner E.F., Pettker C.M. Glucose and metformin modulate human first trimester trophoblast function: a model and potential therapy for diabetes-associated uteroplacental insufficiency. Am J Reprod Immunol. 2015;73(4):362–371. doi: 10.1111/aji.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salomaki H., Vahatalo L.H., Laurila K., Jappinen N.T., Penttinen A.M., Ailanen L. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez-Martin A., Cufi S., Lopez-Bonet E., Corominas-Faja B., Oliveras-Ferraros C., Martin-Castillo B. Metformin limits the tumourigenicity of iPS cells without affecting their pluripotency. Sci Rep. 2012;2:964. doi: 10.1038/srep00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowan J.A., Hague W.M., Gao W., Battin M., Moore P.M. Metformin Versus Insulin for the treatment of Gestational Diabetes. N Engl J Med. 2008;358(19):2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 61.Jakubowicz D.J., Iuorno M.J., Jakubowicz S., Roberts K.A., Nestler J.E. Effects of Metformin on early Pregnancy loss in the Polycystic Ovary Syndrome. J Clin Endocrinol Metabol. 2002;87(2):524–529. doi: 10.1210/jcem.87.2.8207. [DOI] [PubMed] [Google Scholar]
- 62.Chiswick C., Reynolds R.M., Denison F., Drake A.J., Forbes S., Newby D.E. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diab Endocrinol. 2015;3(10):778–786. doi: 10.1016/S2213-8587(15)00219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desai N., Roman A., Rochelson B., Gupta M., Xue X., Chatterjee P.K. Maternal metformin treatment decreases fetal inflammation in a rat model of obesity and metabolic syndrome. Am J Obstet Gynecol. 2013;209(2):136 e1–9. doi: 10.1016/j.ajog.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Sun X., Tavenier A., Deng W., Leishman E., Bradshaw H.B., Dey S.K. Metformin attenuates susceptibility to inflammation-induced preterm birth in mice with higher endocannabinoid levels. Biol Reprod. 2018;98(2):208–217. doi: 10.1093/biolre/iox164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solano M.E., Elia E., Luchetti C.G., Sander V., Di Girolamo G., Gonzalez C. Metformin prevents embryonic resorption induced by hyperandrogenisation with dehydroepiandrogesterone in mice. Reprod Fertil Dev. 2006;18:533–544. doi: 10.1071/rd05099. [DOI] [PubMed] [Google Scholar]
- 66.Eng G.S., Sheridan R.A., Wyman A., Chi M.M., Bibee K.P., Jungheim E.S. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56(9):2228–2234. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- 67.Syngelaki A., Nicolaides K.H., Balani J., Hyer S., Akolekar R., Kotecha R. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N Engl J Med. 2016;374(5):434–443. doi: 10.1056/NEJMoa1509819. [DOI] [PubMed] [Google Scholar]
- 68.Ro T.B., Ludvigsen H.V., Carlsen S.M., Vanky E. Growth, body composition and metabolic profile of 8-year-old children exposed to metformin in utero. Scand J Clin Lab Invest. 2012;72(7):570–575. doi: 10.3109/00365513.2012.712319. [DOI] [PubMed] [Google Scholar]
- 69.Ijas H., Vaarasmaki M., Saarela T., Keravuo R., Raudaskoski T. A follow up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG. 2014;122(7):1001. doi: 10.1111/1471-0528.12964. [DOI] [PubMed] [Google Scholar]
- 70.Wouldes T.A., Battin M., Coat S., Rush E.C., Hague W.M., Rowan J.A. Neurodevelopmental outcome at 2 years in offspring of women randomised to metformin or insulin treatment for gestational diabetes. Arch Dis Child Fetal Neonatal Ed. 2016;101:F488–F493. doi: 10.1136/archdischild-2015-309602. [DOI] [PubMed] [Google Scholar]
- 71.Hanem L.G.E., Stridsklev S., Juliusson P.B., Salvesen O., Roelants M., Carlsen S.M. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab. 2018;103(4):1612–1621. doi: 10.1210/jc.2017-02419. [DOI] [PubMed] [Google Scholar]
- 72.Rowan J.A., Rush E.C., Plank L.D., Lu J., Obolonkin V., Coat S. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res Care. 2018;6(1) doi: 10.1136/bmjdrc-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salomaki H., Heinaniemi M., Vahatalo L.H., Ailanen L., Eerola K., Ruohonen S.T. Prenatal metformin exposure in a maternal high fat diet mouse model alters the transcriptome and modifies the metabolic responses of the offspring. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol 2010;202(4):383 e1–7. [DOI] [PMC free article] [PubMed]
- 75.Al Jofi F.E., Ma T., Guo D., Schneider M.P., Shu Y., Xu H.H.K. Functional organic cation transporters mediate osteogenic response to metformin in human umbilical cord mesenchymal stromal cells. Cytotherapy. 2018;20(5):650–659. doi: 10.1016/j.jcyt.2018.02.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellin C., de Wiza D.H., Wiernsperger N.F., Rosen P. Generation of reactive oxygen species by endothelial and smooth muscle cells: influence of hyperglycemia and metformin. Horm Metab Res. 2006;38(11):732–739. doi: 10.1055/s-2006-955084. [DOI] [PubMed] [Google Scholar]
- 77.Gu Q., Gu Y., Yang H., Shi Q. Metformin enhances osteogenesis and suppresses adipogenesis of human chorionic villous mesenchymal stem cells. Tohoku J Exp Med. 2017;241(1):13–19. doi: 10.1620/tjem.241.13. [DOI] [PubMed] [Google Scholar]
- 78.Santosa M.M., Low B.S., Pek N.M., Teo A.K. Knowledge gaps in rodent pancreas biology: taking human pluripotent stem cell-derived pancreatic beta cells into our own hands. Front Endocrinol (Lausanne) 2015;6:194. doi: 10.3389/fendo.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teo A.K., Gupta M.K., Doria A., Kulkarni R.N. Dissecting diabetes/metabolic disease mechanisms using pluripotent stem cells and genome editing tools. Mol Metab. 2015;4(9):593–604. doi: 10.1016/j.molmet.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loo L.S.W., Lau H.H., Jasmen J.B., Lim C.S., Teo A.K.K. An arduous journey from human pluripotent stem cells to functional pancreatic beta cells. Diabetes Obes Metab. 2018;20(1):3–13. doi: 10.1111/dom.12996. [DOI] [PubMed] [Google Scholar]