Abstract
The brain is, by weight, only 2% the volume of the body and yet it consumes about 20% of the total glucose, suggesting that the energy requirements of the brain are high and that glucose is the primary energy source for the nervous system. Due to this dependence on glucose, brain physiology critically depends on the tight regulation of glucose transport and its metabolism. Glucose transporters ensure efficient glucose uptake by neural cells and contribute to the physiology and pathology of the nervous system. Despite this, a growing body of evidence demonstrates that for the maintenance of several neuronal functions, lactate, rather than glucose, is the preferred energy metabolite in the nervous system. Monocarboxylate transporters play a crucial role in providing metabolic support to axons by functioning as the principal transporters for lactate in the nervous system. Monocarboxylate transporters are also critical for axonal myelination and regeneration. Most importantly, recent studies have demonstrated the central role of glial cells in brain energy metabolism. A close and regulated metabolic conversation between neurons and both astrocytes and oligodendroglia in the central nervous system, or Schwann cells in the peripheral nervous system, has recently been shown to be an important determinant of the metabolism and function of the nervous system. This article reviews the current understanding of the long existing controversies regarding energy substrate and utilization in the nervous system and discusses the role of metabolic transporters in health and diseases of the nervous system.
Keywords: Glia, Axon, Energy metabolism, Glucose, Lactate, Acetate, Metabolic transporters, Glucose transporters, Monocarboxylate transporters
1. Introduction
Neurons and glia are the primary cellular components that perform the functions of the central and peripheral nervous system (CNS and PNS, respectively). Glia maintain tissue homeostasis, form myelin, regulate development, and contribute to diverse neuropathophysiologies in the CNS and the PNS. Besides providing structural and metabolic support to neurons, glia also contribute to recovery following neuronal injuries. Neurons transmit signals over long distances through their axons; and these axons require an enormous energy supply to maintain their function. Axons are closely associated with glial cells that support their function and prevent degeneration.
Research over the last decades has shown that the brain is an organ of unusually high metabolic demand that utilizes 20% of the total glucose and 20% of the total oxygen in the human body (Magistretti and Allaman, 2015). Studies have reported glucose as the obligatory energy substrate for brain, where it is almost fully oxidized (Kety and Schmidt, 1948; Sokoloff, 1960). Similarly, further studies at the whole organ level have provided some refinements to this view, suggesting that ketone bodies fulfill the energy requirements of the brain under particular conditions, including fasting, uncontrolled diabetes and breast-fed newborn babies (Magistretti, 1999). Additionally, several studies over the last few years have illustrated the significance of lactate as an energy substrate for the brain (Baltan, 2015; Castillo et al., 2015; Machler et al., 2016; Matsui et al., 2017; Magistretti and Allaman, 2018). Specifically, findings from in vitro and in vivo studies demonstrate that lactate sustains neuronal activity during glucose deprivation (Wyss et al., 2011; Sobieski et al., 2018). The astrocyte-neuron lactate shuttle hypothesis (ANLSH) suggests that astrocyte-derived L-lactate is taken up by neurons via monocarboxylate transporters (MCTs), metabolic transporters for monocarboxylates, and used as an energy substrate, possibly in preference to glucose. Though it was proposed over twenty years ago (Magistretti et al., 1993; Pellerin and Magistretti, 1994), ANLSH remains controversial and not fully accepted. A recent study claims that during fasting conditions, glucose contributes indirectly (via circulating lactate) to tissue TCA metabolism in all tissues except the brain (Hui et al., 2017). Additionally, a study modeling the kinetic characteristics and cellular concentrations of the neuronal glucose and lactate transporters opposes the ANLSH primarily due to the fact that neuronal glucose transporter, GLUT3, has higher affinity for glucose than the astrocytic counterpart, GLUT1, an, indicating that glucose may be primarily transported to and consumed by neurons (Simpson et al., 2007). Finally, studies suggest that neurons have the capacity to boost their own glycolysis and potentially export rather than import lactate during brain activation or in response to stimulation (Diaz-Garcia et al., 2017; Yellen, 2018). This article addresses these controversies and reviews different aspects of glia-axon energy metabolism in health and diseases of the nervous system focusing on neural energy substrates consumption and metabolism, and their transporters.
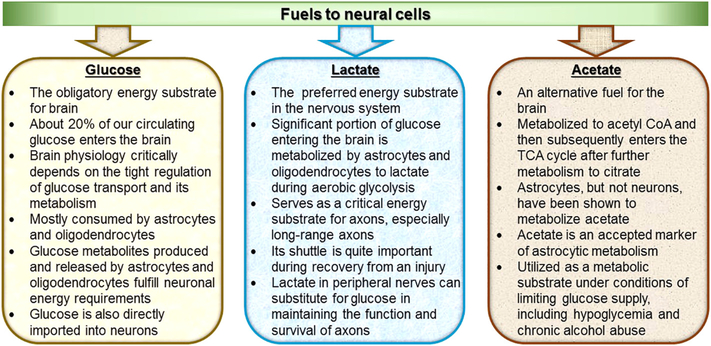
2. Fuels to neural cells: glucose, its “by-product” lactate, and occasionally acetate too!
About 20% of our circulating glucose enters the brain, suggesting that glucose is the primary energy source for the brain. For some time, it had been accepted without reservation that all brain metabolic pathways are subsequent to glucose until the proposition of the ANLSH (Magistretti, 2008, Pellerin and Magistretti, 2012). The ANLSH challenged this precept, stating that activity-dependent uptake of glucose takes place in astrocytes that subsequently metabolize the glucose anaerobically to lactate and then transport lactate to neighboring neurons where it serves as the primary metabolic fuel. The ANLSH was proposed almost 25 years ago (Magistretti et al., 1993; Pellerin and Magistretti, 1994) and is still quite controversial (Simpson et al., 2007; Dienel, 2012; Diaz-Garcia et al., 2017; Yellen, 2018); as such, the exact metabolic fuel to neural cells remains highly debatable. At the same time, a recent study has also indicated acetate as an occasional energy substrate for the brain. This section provides an updated understanding about the fuels to neural cells (Fig. 1).
Fig. 1.
Fuels to neural cells. Glucose, its “by-product” lactate, and occasionally acetate are fuels to neural cells.
2.1. Glucose
Elegant and pioneering in vivo studies in humans by Kety, Schmidt and Sokoloff conducted almost six decades ago identified glucose as the obligatory energy substrate for brain (Kety and Schmidt, 1948; Sokoloff, 1960). Glucose is the main source of energy for the brain, and its physiology is determined by tight regulation of glucose metabolism, suggesting the requirement for continuous delivery of glucose from blood (Mergenthaler et al., 2013). Despite the presumption that neurons would thus preferentially use glucose as their primary energy metabolite, several recent reports suggest that brain glucose is mostly consumed by astrocytes and oligodendrocytes and that neurons depend on glucose metabolites produced and released by these glial cells for their energy requirements (Chuquet et al., 2010; Funfschilling et al., 2012; Lee et al., 2012; Amaral et al., 2016; Saab et al., 2016). Despite the important role of glial cells, neurons clearly express GLUT3 (Simpson et al., 2008), which is a glucose transporter that allows the direct import of glucose into neurons. Glucose supply to the brain is regulated by neurovascular coupling, and it enters the brain from the blood by crossing the blood brain barrier through glucose transporters (primarily GLUT1). The rapid distribution of glucose in the brain is assisted by a highly coupled metabolic network of glial and neuronal cells interconnected by gap junctions (Froes et al., 1999; Parpura et al., 2012). Physiologic functions of the brain are fueled by ATP generated primarily by glucose metabolism. Glucose metabolism disturbances underlie many diverse neurological diseases, including neuroinflammation, neurodegenerative disorders, psychiatric disorders, and peripheral neuropathies. Glucose supply to brain is also crucial due to its potential conversion to lactate.
2.2. Lactate
A significant portion of glucose entering the brain is metabolized to lactate during aerobic glycolysis (Vaishnavi et al., 2010). The aerobic glycolysis accounts for up to 30% of brain glucose metabolism during development and 25% glucose metabolism in specific regions of the adult brain, such as the dorsolateral prefrontal cortex, the superior and medial frontal gyrus, or the precuneus and posterior cingulate cortex (Goyal et al., 2014; Magistretti and Allaman, 2015). At the cellular level, astrocytes are better equipped for aerobic glycolysis and lactate production than neurons (Belanger et al., 2011). Emerging evidence establishes astrocytes as naturally glycolytic cells (Supplie et al., 2017). Molecularly, GLUT1 expressed by both endothelial cells and astrocytes facilitates glucose uptake from the circulation. LDH5, which is primarily expressed by astrocytes in the CNS, converts pyruvate derived from glycolysis to lactate, which can then be shuttled to neurons through monocarboxylate transporters (primarily MCT1 and MCT4 in astrocytes and MCT2 in neurons). Neurons express LDH1 that facilitates the utilization of lactate as neuronal energy substrate by converting it to pyruvate. Neurons also have the capacity to take up glucose directly, however, since the glucose transporter, GLUT3, is expressed in neurons (Belanger et al., 2011). Due to the location of astrocytic endfeet surrounding blood vessels, however, it is likely that most of the glucose that enters the brain does so through astrocytes (Kacem et al., 1998; Belanger et al., 2011; Mergenthaler et al., 2013).
Glial cells were proposed to contribute to neuronal energy metabolism almost 50 years ago, and about two decades ago it was established that metabolite transfer occurs from glial cells to neuronal cells at least in honeybee retina (Tsacopoulos et al., 1994). Refined versions of the ANLSH assert that lactate is produced by astrocytes, and possibly active neurons, and is released into a lactate pool that is eventually used as energy substrate by neurons at rest or during active state (Baltan, 2015). A functional intercellular lactate shuttle, commonly called ANLSH in the CNS, exists in multiple CNS regions (Magistretti et al., 1994; Wender et al., 2000; Baltan, 2015), along with muscle (Gladden, 2004) and potentially peripheral nerve (Vega et al., 1998; Brown et al., 2012; Evans et al., 2013; Stecker and Stevenson, 2015; Hu et al., 2017). Lactate serves as a critical energy substrate for axons, especially long axons, to meet the metabolic needs of transport and signal transduction. Astrocyte glycogen and oligodendrocyte glycolysis are potentially the major sources of lactate to support axon function in the CNS (Baltan, 2015). As suggested by the ANLSH, astrocytes take up the capillary glucose and store it as glycogen, which can then be metabolized to lactate and delivered to axons when needed, usually to fulfill the axonal metabolic demand under higher functional activity. Observations from adult mouse optic nerve, a favorable white matter preparation, suggest that CNS glycogen, contained predominantly in astrocytes, remains under dynamic control and that glycogen is used to support the CNS axons’ energy need via converting to lactate during both physiological and pathological processes (Brown et al., 2003). These findings demonstrate the significance of a functioning lactate shuttle in white matter, which is metabolically challenged even under physiological condition due to higher energy demand, substantial glial maintenance, presence of hydrophobic myelin forming a barrier around axons, and unique vascular network; and suggest that glycogen is converted to lactate to support axon function under relative aglycemic conditions (Brown et al., 2003). Besides supporting a well-defined lactate shuttle system between astrocytes and neurons, these results collectively suggest that lactate is the preferred substrate to support axon function (Tekkok et al., 2005) and the primary energy metabolite utilized during increased physiological activity (Brown et al., 2003). This lactate shuttle is quite important during recovery from an injury as well as recruitment of synapses upon high frequency stimulation of axons during learning and memory.
More recently, the ANLSH has been expanded to also include oligodendrocytes. Publications from our lab and others showed that oligodendrocyte-derived lactate is important for supporting axons (Nave, 2010; Funfschilling et al., 2012; Lee et al., 2012; Meyer et al., 2018). In Dr. Nave’s publication, disrupting oligodendrocyte mitochondria led to increased extracellular lactate in the presence of anesthesia that quickly fell after reversal of anesthesia, presumably due to uptake of lactate into metabolically active neurons (Funfschilling et al., 2012). In our publication, which will be discussed in greater detail below in section on transporters, axon degeneration or neuron loss was observed in mice and spinal cord organotypic cultures with global or oligodendrocytespecific knockdown of lactate transporters (Lee et al., 2012). A recent study demonstrates that combined blockade of oligodendroglial lactate and glucose export via inhibition of lactate transporters MCT1/2 and glucose transporter GLUT1 completely abolishes oligodendroglial support to axons during aglycaemia (Meyer et al., 2018). Additionally, satellite gliocytes surrounding neurons in cranial cervical sympathetic ganglion (CCSG) are reported to produce significantly more lactate than neurons, suggesting that CCSG, like the brain, develops a gradient for lactate shuttle between sympathetic neurons and surrounding satellite gliocytes (Gorelikov and Savel’ev, 2008).
Growing evidence suggests that lactate is an effective energy source for the peripheral nerves as well, and the lactate shuttle between glial and neurons has been demonstrated in the peripheral nervous system (Vega et al., 1998; Brown et al., 2012; Evans et al., 2013; Stecker and Stevenson, 2015; Hu et al., 2017). These studies have primarily used intact ex-vivo preparations of peripheral nerves. In this paradigm, peripheral or cranial nerves are removed from animals and nerve function quantified in the presence or absence of energy metabolites or inhibitors to specific enzymes or transporters in the system. These studies have shown that Schwann cells in peripheral nerves contain glycogen, have the capacity to metabolize glycogen to lactate, and that lactate can substitute for glucose in maintaining the function and survival of axons (Brown et al., 2012; Evans et al., 2013). Unlike the CNS, however, there are no published studies demonstrating the critical nature of lactate to peripheral nerve function in vivo under normal physiologic conditions. As discussed in greater detail below, we have shown that lactate transporters are important for the response of peripheral nerves to injury (Morrison et al., 2015).
2.3. Acetate
Acetate, an alcohol by-product formed in liver, can also be an alternative fuel for the brain. Acetate can be metabolized to acetyl CoA by acetyl-CoA synthase (Starai and Escalante-Semerena, 2004) and then subsequently enter the TCA cycle after further metabolism to citrate (Kiviluoma et al., 1989; Diamond et al., 1991; Kiselevski et al., 2003; Mailliard and Diamond, 2004; Williams and O’Neill, 2018). Astrocytes, but not neurons, have been shown to metabolize acetate. In fact, acetate, as a two carbon intermediate in metabolism, is an accepted marker of astrocytic metabolism (Muir et al., 1986; Sonnewald et al., 1993; Rae et al., 2012). The exclusivity of astrocyte acetate metabolism is controversial, however, since earlier in vitro studies suggest that extracellularly supplied acetate is metabolized by neurons to glutamate and aspartate, while astrocytes metabolize acetate to glutamine (Brand et al., 1997). Acetate is taken up and metabolized by hippocampal nerve terminals (Carroll, 1997) and in whole rat brain (Chapa et al., 2000). In vivo, radioactive acetate is rapidly incorporated into glutamine, GABA, glutamate and aspartate, with the greatest proportion being metabolized to glutamine (Muir et al., 1986; Badar-Goffer et al., 1990; Rae et al., 2003; Rae et al., 2012). Acetate is a good substrate for glial MCT1 and neuronal MCT2 but a poor substrate for glial MCT4, due to very low affinity with this transporter (Rae et al., 2012). Moreover, neurons also express sodium-dependent MCT1 (SMCT1) that has an affinity for acetate comparable to that of MCT1 and MCT2 (Rae et al., 2012). Circulating acetate levels under normal resting condition can contribute to 10%−15% of the basal energy demand of brain astrocytes (Dienel and Cruz, 2006). Emerging evidence suggests that astrocytes are able to support neuronal function by utilizing acetate as a metabolic substrate under conditions of limiting glucose supply, including hypoglycemia and chronic alcohol abuse (Jiang et al., 2013; Cloughesy et al., 2014).
As detailed above, several energy metabolites, particularly glucose, lactate and acetate, are used by both the CNS and PNS. In general, glucose is likely the major metabolite utilized during resting physiology, while lactate and acetate are more prominently required during times of increased energy demand, such as during neuronal hyperactivity, or when either oxygen or glucose supply are limiting. This conclusion is not universally agreed upon and recent studies claim that brain activation or neuronal stimulation increases direct glucose consumption by neurons by triggering neuronal glycolysis and not lactate uptake (Diaz-Garcia et al., 2017; Yellen, 2018).
3. Glucose transporters
3.1. Glucose transporters ensure efficient glucose uptake by neural cells
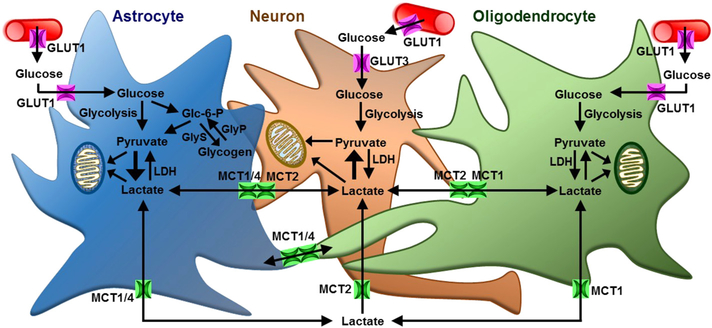
Glucose is transported across the cell membrane by facilitative diffusion mediated by members of the GLUT family, which belongs to the major facilitator superfamily of membrane transporters (Pao et al., 1998; Thorens and Mueckler, 2010). Most of the GLUTs catalyze the ATP-dependent bidirectional transfer of glucose across membranes. GLUT 1–4 are the well-studied/established glucose transporters and have distinct regulatory and/or kinetic properties, suggesting their cellspecific role. GLUT1 and GLUT3 are the major glucose transporters expressed in the brain (Fig. 2), with GLUT1 being the key glucose transporter that catalyzes the rate-limiting step in supplying glucose to cells of the CNS (Yu et al., 2008). GLUT1 is expressed both at the luminal and abluminal sides of the brain capillaries (Farrell and Pardridge, 1991) and also in CNS astrocytes (McEwen and Reagan, 2004; Allen and Messier, 2013). GLUT3 is the major neuronal glucose transporter, which is expressed in both dendrites and axons, and its expression level in different brain regions is correlated with local cerebral glucose utilization (Simpson et al., 2008). GLUT3 has a high affinity for glucose and greater transport capacity for glucose than astrocytic/endothelial GLUT1 (Simpson et al., 2008), ensuring efficient glucose transport to neurons from the extracellular space. The expression and function of both GLUT1 and GLUT3 are highly regulated in the brain by both transcriptional and post-transcriptional processes. GLUT1 and GLUT3 are both upregulated by circulating glucose (McGowan et al., 1995) and hypoxic conditions through hypoxia-inducible factor 1α (HIF-1α)-mediated transcriptional activation (Huang et al., 2012). Interestingly, the response to glutamate appears to differ between the two transporters, with GLUT1 activity in astrocytes increasing (Pellerin et al., 2007; Porras et al., 2008) and GLUT3 activity decreasing following exposure to glutamate (Barros and Deitmer, 2010). The attenuation of GLUT3 activity in neurons following glutamate has been proposed to reduce glycolytic flux and promote lactate uptake in highly active neuron populations (Barrow and Deitmer, 2010).
Fig. 2.
Glia-neuron metabolic interactions in the CNS are most likely mediated by monocarboxylate and glucose transporters (MCTs and GLUTs, respectively). Astrocytes and oligodendrocytes take up glucose from blood circulation through GLUT1. Glycolysis breaks down glucose to pyruvate, which can either be converted to lactate or metabolized further in mitochondria via oxidative metabolism. Astrocytes can also store the capillary glucose as glycogen, which can then be metabolized to lactate. Astrocyte or oligodendrocyte-derived intracellular lactate can exit the cell through MCT1 and/or MCT4. Neurons take up the extracellular lactate through MCT2. Neuron can also take up glucose from blood circulation or extracellular space through GLUT3. Glc-6-P; glucose-6-phosphate, GlyS; glycogen synthase, GlyP; glycogen phosphorylase, LDH; lactate dehydrogenase.
Though GLUT1 and GLUT3 are the predominant glucose transporters in the brain, other glucose transporters are also expressed though their function has been less well studied. GLUT2, which facilitates the first step in glucose-induced insulin secretion by transporting glucose into the pancreatic β-cell (Marty et al., 2007), is expressed in brain, primarily in regions of the hypothalamus that regulate food intake, suggesting a homologous role to the glucose sensor in the pancreas (Arluison et al., 2004; Arluison et al., 2004; Roncero et al., 2004; Eny et al., 2008). GLUT4 is present in specific neuronal cell populations of the brain that also express insulin receptors and are primarily associated with control of motor system. GLUT5 and GLUT7 are present at very low levels in the brain and appear to function primarily as fructose transporters (Douard and Ferraris, 2008). GLUT5 is detected in human and rat brain microglia (Payne et al., 1997). GLUT6, which has very low affinity to glucose, is also expressed in the brain, but its function is unknown. GLUT8 is reported to play important role in hippocampal neuronal proliferation (Schmidt et al., 2009). GLUT13, a myoinositol transporter, is expressed predominantly in the brain (Uldry et al., 2001), however its functional role has yet to be established.
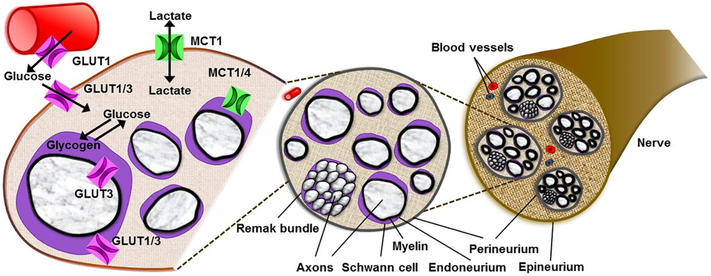
Similar to the CNS, GLUT1 and GLUT3 are the major glucose transporters in the PNS (Fig. 3) (Muona et al., 1992; Magnani et al., 1996; Choeiri et al., 2002). GLUT1, which is expressed in sciatic nerves, is localized to the perineurium (Allt and Lawrenson, 2000) and to some endo- and epi-neurial capillaries (Muona et al., 1992). Similarly, both rat Schwann cells in vivo (Magnani et al., 1996) and in vitro (Muona et al., 1992) express GLUT1, primarily in the paranodal region and Schmidt-Lanterman incisures. Likewise, GLUT3 is expressed in the perineurium, endoneurial capillaries, and paranodal Schwann cells and axolemma in aged, but not in young, rats (Magnani et al., 1996). As in the CNS, GLUT3 is the primary neuronal GLUT in the PNS (Muona et al., 1993; Magnani et al., 1996; Stuart et al., 2000). The expression pattern of GLUT1 and GLUT3 in the peripheral nerve suggests their crucial involvement in the glucose transport into the metabolically active nodal apparatus of the peripheral myelinated axon.
Fig. 3.
Differential expression of monocarboxylate and glucose transporters (MCTs and GLUTs, respectively) in peripheral nerve. MCT1, MCT2, and MCT4 are the major MCTs in peripheral nerves, and they each have distinct patterns of expression. MCT1 is highly expressed in perineurial cells within the perineurium. MCT1 and MCT4 are expressed in Schwann cells. GLUT1 and GLUT3 are the major GLUTs in peripheral nerves, and they each have distinct patterns of expression. GLUT1, which is highly expressed in sciatic nerves, is localized to the perineurium and to some endo- and epineurial capillaries. Schwann cells express GLUT1, primarily in the paranodal region and Schmidt-Lanterman incisures. GLUT3 is expressed in neurons, perineurium, endoneurial capillaries, and paranodal Schwann cells. The expression pattern of MCTs and GLUTs in peripheral nerves suggest their crucial involvement in metabolite transport in the peripheral nervous system.
3.2. Glucose transporters are important contributors to physiology and pathology of the nervous system
GLUT1 and GLUT3 are the glucose transporters that have clearly defined roles in both physiological and pathological conditions of the mammalian brain (Benarroch, 2014). The expression of GLUT1 is much higher than that of GLUT3 in developing and prenatal brain. However, the expression of both GLUT1 and GLUT3 increases with cerebral maturation in parallel with increased cerebral glucose utilization (Nehlig and Pereira de Vasconcelos, 1993; Nehlig, 1996). GLUT1 expression increases steadily throughout cerebral maturation, whereas the GLUT3 expression depends on region and activity. Earlier studies indicate a very low expression of GLUT3 in the prenatal rat brain, but predominant expression of GLUT1 in neural progenitor cells of the germinal matrix (Bondy et al., 1992). However, the substantial expression of GLUT3 in neuronal cells after migration to the cerebral cortex establishes a strong correlation of GLUT3 with neuronal maturation and the establishment of synaptic connections, which drives the largest energy demand of the brain (Attwell and Laughlin, 2001; Simpson et al., 2008). Though expressed at lower levels, other glucose transporter have been shown to be physiologically active in the CNS. GLUT2, which is expressed in hypothalamic neurons as described above, functions as a glucose sensor to regulate food intake, synaptic activity, and neurotransmitter release (Jurcovicova, 2014). GLUT4, which is present at hippocampal nerve terminals, is mobilized by neuronal activity to support the energetic demands of firing synapses (Ashrafi et al., 2017), while GLUT8 plays a role in hippocampal neurogenesis (Membrez et al., 2006). These findings suggest the crucial role of GLUTs in the maintenance of nervous physiology.
As expected for a physiologically important transporter, alterations in GLUT expression can lead to human disease. GLUT1 deficiency, caused by genetic mutations in the GLUT1 gene, leads to several neurologic conditions, most commonly being seizures (epilepsy), ataxia and developmental delay (Coman et al., 2006; Graham, 2012). In most cases, this is a severe disorder that presents soon after birth. Affected newborns frequently have microcephaly that develops after birth and intellectual disability. About 10% of the affected patients have the nonepileptic form of GLUT1 deficiency syndrome (Wang et al., 1993). Alterations in GLUT function can occur in acquired diseases, as well. Diabetic conditions can affect both GLUT localization (Bakirtzi et al., 2009) and activity (for example, GLUT4 activity in the periphery is impaired under type 2 diabetes) (Pearson-Leary and McNay, 2016); however, expression levels of GLUTs in glial or neuronal cells does not appear to be altered by diabetic conditions (de Preux Charles et al.,2010; Hur et al., 2011; Pande et al., 2011). Schwann cells, which metabolically support axons in the PNS, are crucial cell types in the pathogenesis of diabetic neuropathy (Zenker et al., 2013; Mizisin, 2014; Feldman et al., 2017; Goncalves et al., 2017). Schwann cells need to maintain GLUT1 levels to metabolically support axons and potentially maintain their own function and survival. As conjectured in a recent review article (Feldman et al., 2017), Schwann cell GLUT 1 activity may be impeded in diabetes, similar to the effects of GLUT4 in the periphery in type 2 diabetes, potentially impeding their glycolysis, reducing metabolic support to axons, and thereby contributing to axonal degeneration. Future detailed study of GLUTs in peripheral nerve cells are thus important to provide new insight into the bioenergetic mechanism of diabetic neuropathy.
4. Monocarboxylate transporters
4.1. Monocarboxylate transporters are widely expressed metabolic transporters in central and peripheral nervous systems
The existence of glia-axon metabolic interactions in the CNS and PNS is most likely mediated by the monocarboxylate transporters (MCTs) (Fig. 2 and Fig. 3). MCTs are extracellular membrane channels that can transport monocarboxylates (such as lactate, pyruvate and ketone bodies), along with protons, down their concentration gradient across membranes (Garcia et al., 1994). MCTs are vital for metabolic shuttling between glia and neurons and facilitate the functioning of lactate as a preferred energy metabolite to support axon function. MCT1–4 are responsible for bidirectional proton-linked transport of monocarboxylates across the plasma membranes. Lactate is one of the most important metabolites for these transporters, with a stereoselectivity for L-lactate over D-lactate (Halestrap, 2012).
In the CNS, MCT1 is widely expressed in oligodendrocytes (Rinholm et al., 2011; Lee et al., 2012), astrocytes (Broer et al., 1997; Leino et al., 1999; Hanu et al., 2000; Pierre et al., 2000), microglia (Moreira et al., 2009; Nijland et al., 2014), endothelial cells (Gerhart et al., 1997; Pellerin et al., 1998; Mac and Nalecz, 2003), and some specific neurons (Morrison et al., 2013; Perez-Escuredo et al., 2016). MCT1 is also expressed in tanycytes, a glial cell type found in the hypothalamus and this cell type is reported to produce lactate from glucose via glycolytic metabolism (Cortes-Campos et al., 2011). Similar to other MCTs, MCT1 is a bi-directional transporter of monocarboxylates, and it can increase mitochondrial energy metabolism by facilitating the use of lactate as a metabolic substrate for TCA cycles and oxidative phosphorylation. In the CNS, MCT2 is a high affinity MCT isoform expressed primarily in neurons. The ANLSH depends on lactate being produced and released from astrocytes (via MCT1) and taken up by neurons (via MCT2) during times of neuronal activity (Pierre et al., 2000). MCT2 expression has also been seen in endothelial cells (Mac and Nalecz, 2003; BalmacedaAguilera et al., 2012), some rat astrocytes (Gerhart et al., 1998; Hanu et al., 2000), and tanycytes in the hypothalamus (Cortes-Campos et al., 2011). The expression of MCT3 is restricted to retinal pigmented epithelial cells (Yoon et al., 1997; Philp et al., 1998) and choroid plexus epithelium (Philp et al., 2001). Though expressed at low levels in the CNS, MCT4 appears to be exclusively expressed by astrocytes (Marcillac et al., 2011; Lee et al., 2012).
Recent studies have also investigated the expression of MCTs in the PNS. MCT1, MCT2, and MCT4 are the major MCTs expressed in the PNS, and they each have distinct patterns of expression (Takebe et al., 2008; Domenech-Estevez et al., 2015; Morrison et al., 2015). MCT1 is highly expressed in perineurial cells (Tserentsoodol et al., 1999; Takebe et al., 2008). Perineurial cells express tight junction proteins and function as barrier cells separating neurons and Schwann cells in the endoneurium from the blood supply (Peltonen et al., 2013; Kucenas, 2015). They also potentially contribute to peripheral nerve development and regeneration following injury (Kucenas et al., 2008; Binari et al., 2013; Lewis and Kucenas, 2014). In addition, MCT1 is also expressed in the endoneurium, including Schwann cells and DRG neurons (Domenech-Estevez et al., 2015; Morrison et al., 2015). MCT4 is expressed in Schwann cells within the peripheral nerve (DomenechEstevez et al., 2015). Though clearly expressed in peripheral nerve (Domenech-Estevez et al., 2015; Morrison et al., 2015), the cellular localization of MCT2 has not yet been determined.
The widespread distribution of MCTs in the nervous system illustrates the key role of these transporters in intercellular metabolic shuttling in brain. The expression of MCT1 and MCT4 on astrocytes, which are predominantly glycolytic cells (Supplie et al., 2017), and on oligodendrocytes, which play a supportive role for CNS axons (Funfschilling et al., 2012; Lee et al., 2012; Morrison et al., 2013), and MCT2 on neurons, which metabolically depend on oxidative energy substrates, support the proposed glia (both astrocyte and oligodendrocyte)-to-neuron lactate shuttle hypothesis (Morrison et al., 2013). Although Schwann cells are well known for their role in myelinating axons of the peripheral nerves and facilitating saltatory conduction, emerging evidence also suggests that they function as metabolic supporters of axons in peripheral nerves (Fields, 2015; Salzer, 2015). Like astrocytes in the CNS, myelinating Schwann cells in the PNS express MCT1 and MCT4 (Domenech-Estevez et al., 2015; Morrison et al., 2015). They also sustain axon activity through the metabolism of glycogen to lactate (Brown et al., 2012; Evans et al., 2013), suggesting that Schwann cells also participate in the support of axons through lactate shuttling to neurons.
4.2. Monocarboxylate transporters mediate glia-axon metabolic interactions critical for axonal myelination and regeneration
Studies over the last couple of decades have identified the crucial role of lactate and its transporters in axonal myelination and regeneration. Oligodendrocytes and Schwann cells are the cells that make myelin to ensheath neuronal axons in the CNS and the PNS, respectively. Proper myelination by oligodendrocytes depends on lactate metabolism as a fuel (Sanchez-Abarca et al., 2001). Myelination is a process that requires large energy stores to maintain cell function and produce the lipids and proteins for myelination (Sanchez-Abarca et al., 2001). To fulfill this high metabolic demand, oligodendrocytes use lactate as a source of energy and as a precursor of lipids. In the setting of glucose deprivation, lactate is sufficient alone to myelinate axons in vitro (Rinholm et al., 2011). Lactate uptake by oligodendrocytes is mediated through the expression of MCTs, especially MCT1 (Lee et al., 2012). MCT1 mediates bi-directional transport of lactate and thus may be important both for importing lactate into oligodendrocytes that can ultimately be metabolized in the TCA cycle or exporting lactate to clear this end product of glycolysis and/or to provide metabolic energy to axons and contribute to the maintenance of axonal integrity (Harris and Attwell, 2012; Saab et al., 2016). Our lab, and others, have previously reported higher expression of MCT1 in the CNS myelin than in axons and conversely higher expression of MCT2 in axons than myelin (Rinholm et al., 2011) (Lee et al., 2012). Recent studies have also identified that oligodendrocyte progenitor cells (OPCs) utilize lactate through MCTs and that lactate, produced from glycogen, is crucial to promote cell cycling and differentiation in OPC-rich culture (Ichihara et al., 2017). Surprisingly, an in vitro study using co-cultures of DRG neurons and Schwann cells demonstrated increased myelination following downregulation of MCT1 in Schwann cells by lentiviral shRNAs (Domenech-Estevez et al., 2015); thus the exact role of this transporter in SC myelination in not yet clear. Further studies employing experimental animal models and patient samples will likely provide a more complete explanation for the role of oligodendrocyte or SC MCT1 in myelination. Lactate and MCTs may, however, be an intriguing future target for therapeutics to promote remyelination of demyelinated tissues in diverse neuropathologies, including multiple sclerosis (MS), inherited leukodystrophies, and demyelinating peripheral neuropathies.
In agreement with an earlier study that used sciatic nerve explants to demonstrate the dependence of axons on lactate for metabolic energy (Brown et al., 2012), we have recently demonstrated that proper functioning of peripheral nerves in vivo depends on lactate as an energy substrate, especially during conditions of high energy demands such as nerve regeneration following injury (Morrison et al., 2015). In this study, MCT1 deficiency delayed nerve regeneration following peripheral nerve injury in mice. The widespread distribution of MCT1 in the PNS limits definitive conclusions regarding the mechanism for MCT1-dependent nerve regeneration, and further cell-specific modifications of PNS are necessary for fully defining the role of MCT1 in the PNS.
4.3. Disruption of monocarboxylate transporters contributes to neurodegeneration
A strong correlation exists between metabolic alterations and neurodegeneration. Glia-axon metabolic interactions via MCTs is an important, but not yet fully explored, pathway to understand the pathogenesis of neurodegenerative disorders. We have previously reported that disruption of MCT1, which is abundantly expressed in the CNS and highly enriched within oligodendrocytes, results in axonal damage and neuronal loss in animal and cell culture models (Lee et al., 2012). Expression of MCT1 was found to be reduced in the spinal cord of amyotrophic lateral sclerosis (ALS) patients and ALS rodent models, suggesting that oligodendroglial MCT1 may play a role in the pathogenesis of neurodegeneration (Lee et al., 2012). Similarly, mutant superoxide dismutase (SOD1), which is the second most common cause of familial ALS, directly affects MCT1 expression, and SOD1G93A mice have reduced expression of MCT1 in spinal cord oligodendrocytes (Lee et al., 2012). Furthermore, MCTs may be important for aging and Alzheimer’s disease (AD) pathogenesis, as it has previously been shown that MCT1 expression declines in both aging and AD (Ding et al., 2013).
In addition, reduced MCT2 expression and lactate content in the cerebral cortex and hippocampus have been identified in AD, which also suggest impaired energy metabolism in the brain (Lu et al., 2015). Whether alterations in MCTs is a primary event that contributes to AD neurodegeneration or a secondary event that results from neuron loss or glial changes is not yet known. A clue that alterations in MCTs may be an early event comes from study of young adult carriers of apolipoprotein E ɛ4 allele (APOE4), who are at high risk for developing AD. Young adult APOE4 carriers have increased expression of MCT2 and decreased expression of MCT4, suggesting that brain energy metabolism alterations may contribute to the risk that APOE4 confers for AD (Perkins et al., 2016). This is not a universal mechanism for all neurodegenerative diseases, however, since neither the expression of MCT1 and MCT2, nor the content of lactate, is altered in the substantia nigra and striatum of a mouse model of Parkinson’s disease (Puchades et al., 2013). Another disease in which metabolic transporters appear to be altered is multiple sclerosis (MS). MCT1 expression is increased in infiltrating leukocytes and reactive astrocytes in active MS lesions, and MCT2 expression is decreased in inactive MS lesions (Nijland et al., 2014). The altered expression of MCT2 in MS brains may be due to loss of neurons expressing MCT2, but if this occurs prior to neuron death, it suggests that a deficiency of nutrient supply to hypoxic demyelinated axons may drive ongoing axonal and neuronal degeneration in MS. Further studies in MS patients and particularly animal models of the disease should help clarify this issue. Though not yet definitive, several published studies have clearly shown that MCTs are important for maintaining neuron and axon integrity and may contribute to nerve injury in several neurologic diseases, including ALS, AD, and MS.
5. Conclusions
It is now more than half a century since glia were acknowledged to contribute to neuronal energy metabolism. Glia are now widely recognized as dynamic cells that sense neuronal metabolic changes and regulate metabolism by transferring metabolites from glia to neurons. Both central and peripheral neurons alternate between glucose and lactate as an effective energy source, but prefer lactate during increased energy demand. Astrocytes and oligodendrocytes in the CNS, and potentially Schwann cells in the PNS, take up circulating glucose, metabolize it to lactate, and deliver it to axons and neuron cell bodies when needed, usually to fulfill their metabolic demands under higher functional activity. MCTs and GLUTs are differentially expressed in these nervous system cells to facilitate the metabolite transfer. Functional expression of MCTs and GLUTs are crucial for the maintenance of neurophysiology, and their disruption contributes to diverse neuropathologies, including epilepsy and neurodegenerative diseases.
Further studies on the role of nervous system metabolic transporters in health and diseases are unquestionably required. There has been appreciable progress in the nervous system metabolic transporter research, especially for the CNS. Future research is needed to evaluate the role of these transporters in the PNS. Furthermore, the cell-specific role of metabolic transporters in the CNS and PNS needs to be determined. Many of these transporters are expressed in numerous nervous system cells and careful dissection of their role in individual cell types will better define their mechanisms of action. Genetic ablation of these transporters in cell-specific manner or development of transporterspecific high affinity pharmacological inhibitors are the most awaited tools for further studies of their biology. Additionally, further studies using the genetically-encoded fluorescence resonance energy transfer (FRET) sensor Laconic (San Martin et al., 2013), either alone or in combination with other metabolic FRET reporters, should be completed in vivo by two-photon microscopy to better understand the functional role of nervous system metabolic transporters in glia-glia, neuron-glia, and neuro-immune interactions via transfer of metabolites. Future research focused on functional dissection of metabolic transporters on neural cells will further define the glia-neuron metabolic interactions and broaden our understanding of CNS and PNS physiology and pathology, potentially uncovering new therapeutic options for patients with a number of neurologic diseases.
Acknowledgements
This work was supported by the National Institutes of Health (R01NS086818).
References
- Allen A, Messier C, 2013. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav. Brain Res 240, 95–102. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG, 2000. The blood-nerve barrier: enzymes, transporters and receptors–a comparison with the blood-brain barrier. Brain Res. Bull 52 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- Amaral AI, Hadera MG, Tavares JM, Kotter MR, Sonnewald U, 2016. Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells. Glia 64 (1), 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arluison M, Quignon M, Nguyen P, Thorens B, Leloup C, Penicaud L, 2004. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain—an immunohistochemical study. J. Chem. Neuroanat 28 (3), 117–136. [DOI] [PubMed] [Google Scholar]
- Arluison M, Quignon M, Thorens B, Leloup C, Penicaud L, 2004. Immunocytochemical localization of the glucose transporter 2 (GLUT2) in the adult rat brain. II. Electron microscopic study. J. Chem. Neuroanat 28 (3), 137–146. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Wu Z, Farrell RJ, Ryan TA, 2017. GLUT4 mobilization supports energetic demands of active synapses. Neuron 93 (3), 606–615 e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB, 2001. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab 21 (10), 1133–1145. [DOI] [PubMed] [Google Scholar]
- Badar-Goffer RS, Bachelard HS, Morris PG, 1990. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem. J 266 (1), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakirtzi K, Belfort G, Lopez-Coviella I, Kuruppu D, Cao L, Abel ED, Brownell AL, Kandror KV, 2009. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J. Neurosci 29 (16), 5193–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaceda-Aguilera C, Cortes-Campos C, Cifuentes M, Peruzzo B, Mack L, Tapia JC, Oyarce K, Garcia MA, Nualart F, 2012. Glucose transporter 1 and monocarboxylate transporters 1, 2, and 4 localization within the glial cells of shark bloodbrain-barriers. PLoS One 7 (2), e32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltan S, 2015. Can lactate serve as an energy substrate for axons in good times and in bad, in sickness and in health? Metab. Brain Dis 30 (1), 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF, Deitmer JW, 2010. Glucose and lactate supply to the synapse. Brain Res.Rev 63 (1–2), 149–159. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ, 2011. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14 (6), 724–738. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, 2014. Brain glucose transporters: implications for neurologic disease.Neurology 82 (15), 1374–1379. [DOI] [PubMed] [Google Scholar]
- Binari LA, Lewis GM, Kucenas S, 2013. Perineurial glia require Notch signaling during motor nerve development but not regeneration. J. Neurosci 33 (10), 4241–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy CA, Lee WH, Zhou J, 1992. Ontogeny and cellular distribution of brain glucose transporter gene expression. Mol. Cell. Neurosci 3 (4), 305–314. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D, 1997. Metabolism of acetate in rat brain neurons, astrocytes and cocultures: metabolic interactions between neurons and glia cells, monitored by NMR spectroscopy. Cell Mol. Biol. (Noisy-le-grand) 43 (5), 645–657. [PubMed] [Google Scholar]
- Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ, 1997. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem 272 (48), 30096–30102. [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR, 2012. Schwann cell glycogen selectively supports myelinated axon function. Ann. Neurol 72 (3), 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Tekkok SB, Ransom BR, 2003. Glycogen regulation and functional role in mouse white matter. J. Physiol 549 (Pt 2), 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll PT, 1997. Evidence to suggest that extracellular acetate is accumulated by rat hippocampal cholinergic nerve terminals for acetylcholine formation and release. Brain Res. 753 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- Castillo X, Rosafio K, Wyss MT, Drandarov K, Buck A, Pellerin L, Weber B, Hirt L, 2015. A probable dual mode of action for both L- and D-lactate neuroprotection in cerebral ischemia. J. Cereb. Blood Flow Metab 35 (10), 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapa F, Cruz F, Garcia-Martin ML, Garcia-Espinosa MA, Cerdan S, 2000. Metabolism of (1-(13)C) glucose and (2-(13)C, 2-(2)H(3)) acetate in the neuronal and glial compartments of the adult rat brain as detected by [(13)C, (2)H] NMR spectroscopy. Neurochem. Int 37 (2–3), 217–228. [DOI] [PubMed] [Google Scholar]
- Choeiri C, Staines W, Messier C, 2002. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience 111 (1), 19–34. [DOI] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsaki G, 2010. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J. Neurosci 30 (45), 15298–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Cavenee WK, Mischel PS, 2014. Glioblastoma: from molecular pathology to targeted treatment. Annu. Rev. Pathol 9, 1–25. [DOI] [PubMed] [Google Scholar]
- Coman DJ, Sinclair KG, Burke CJ, Appleton DB, Pelekanos JT, O’Neil CM, Wallace GB, Bowling FG, Wang D, De Vivo DC, McGill JJ, 2006. Seizures, ataxia, developmental delay and the general paediatrician: glucose transporter 1 deficiency syndrome. J. Paediatr. Child Health 42 (5), 263–267. [DOI] [PubMed] [Google Scholar]
- Cortes-Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, Garcia MA, 2011. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One 6 (1), e16411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Nagy L, Mochly-Rosen D, Gordon A, 1991. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. Ann. N. Y. Acad. Sci 625, 473–487. [DOI] [PubMed] [Google Scholar]
- Diaz-Garcia CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G, 2017. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26 (2), 361–374 e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, 2012. Brain lactate metabolism: the discoveries and the controversies. J.Cereb. Blood Flow Metab 32 (7), 1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, 2006. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem. Int 48 (6–7), 586–595. [DOI] [PubMed] [Google Scholar]
- Ding F, Yao J, Rettberg JR, Chen S, Brinton RD, 2013. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One 8 (11), e79977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech-Estevez E, Baloui H, Repond C, Rosafio K, Medard JJ, Tricaud N, Pellerin L, Chrast R, 2015. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci 35 (10), 4151–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard V, Ferraris RP, 2008. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab 295 (2), E227–E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eny KM, Wolever TM, Fontaine-Bisson B, El-Sohemy A, 2008. Genetic variant in the glucose transporter type 2 is associated with higher intakes of sugars in two distinct populations. Physiol. Genomics 33 (3), 355–360. [DOI] [PubMed] [Google Scholar]
- Evans RD, Brown AM, Ransom BR, 2013. Glycogen function in adult central and peripheral nerves. J. Neurosci. Res 91 (8), 1044–1049. [DOI] [PubMed] [Google Scholar]
- Farrell CL, Pardridge WM, 1991. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc. Natl. Acad. Sci. U. S. A 88 (13), 5779–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Nave KA, Jensen TS, Bennett DLH, 2017. New horizons in diabetic neuropathy: Mechanisms, bioenergetics, and pain. Neuron 93 (6), 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, 2015. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci 16 (12), 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froes MM, Correia AH, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Neto MV, 1999. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc. Natl. Acad. Sci. U. S. A 96 (13), 7541–7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA, 2012. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485 (7399), 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS, 1994. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76 (5), 865–873. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR, 1997. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Phys 273 (1 Pt 1), E207–E213. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR, 1998. Expression of the monocarboxylate transporter MCT2 by rat brain glia. Glia 22 (3), 272–281. [PubMed] [Google Scholar]
- Gladden LB, 2004. Lactate metabolism: a new paradigm for the third millennium. J. Physiol 558 (Pt 1), 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS, 2017. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol 13 (3), 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelikov PL, Savel’ev SV, 2008. Lactate dehydrogenase isoenzymes in sympathetic neurons and satellite gliocytes in normal conditions and in blockade of nicotinic cholinoreceptors. Neurosci. Behav. Physiol 38 (8), 817–820. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME, 2014. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 19 (1), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JM Jr., 2012. GLUT1 deficiency syndrome as a cause of encephalopathy that includes cognitive disability, treatment-resistant infantile epilepsy and a complex movement disorder. Eur J Med Genet 55 (5), 332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, 2012. The monocarboxylate transporter family—structure and functional characterization. IUBMB Life 64 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- Hanu R, McKenna M, O’Neill A, Resneck WG, Bloch RJ, 2000. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Phys. Cell Phys 278 (5), C921–C930. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D, 2012. The energetics of CNS white matter. J. Neurosci 32 (1), 356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Hou H, Bastian C, He W, Qiu S, Ge Y, Yin X, Kidd GJ, Brunet S, Trapp BD, Baltan S, Yan R, 2017. BACE1 regulates the proliferation and cellular functions of Schwann cells. Glia 65 (5), 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lei L, Liu D, Jovin I, Russell R, Johnson RS, Di Lorenzo A, Giordano FJ, 2012. Normal glucose uptake in the brain and heart requires an endothelial cellspecific HIF-1alpha-dependent function. Proc. Natl. Acad. Sci. U. S. A 109 (43), 17478–17483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, White E, Rabinowitz JD, 2017. Glucose feeds the TCA cycle via circulating lactate. Nature 551 (7678), 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Sullivan KA, Pande M, Hong Y, Sima AA, Jagadish HV, Kretzler M, Feldman EL, 2011. The identification of gene expression profiles associated with progression of human diabetic neuropathy. Brain 134 (Pt 11), 3222–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara Y, Doi T, Ryu Y, Nagao M, Sawada Y, Ogata T, 2017. Oligodendrocyte Progenitor Cells Directly Utilize Lactate for Promoting Cell Cycling and Differentiation. J. Cell. Physiol 232 (5), 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, Koretski J, Harman S, Petrakis IL, Krystal JH, Mason GF, 2013. Increased brain uptake and oxidation of acetate in heavy drinkers. J. Clin. Invest 123 (4), 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcovicova J, 2014. Glucose transport in brain - effect of inflammation. Endocr. Regul 48 (1), 35–48. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G, 1998. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 23 (1), 1–10. [PubMed] [Google Scholar]
- Kety SS, Schmidt CF, 1948. The Nitrous Oxide Method for the Quantitative Determination of Cerebral Blood Flow in Man: Theory, Procedure and Normal Values. J. Clin. Invest 27 (4), 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselevski Y, Oganesian N, Zimatkin S, Szutowicz A, Angielski S, Niezabitowski P, Uracz W, Gryglewski RJ, 2003. Acetate metabolism in brain mechanisms of adaptation to ethanol. Med. Sci. Monit 9 (5), BR178–182. [PubMed] [Google Scholar]
- Kiviluoma KT, Peuhkurinen KJ, Hassinen IE, 1989. Adenine nucleotide transport and adenosine production in isolated rat heart mitochondria during acetate metabolism. Biochim. Biophys. Acta 974 (3), 274–281. [DOI] [PubMed] [Google Scholar]
- Kucenas S, 2015. Perineurial glia. Cold Spring Harb. Perspect. Biol 7 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B, 2008. CNSderived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci 11 (2), 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR, 2012. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9 (9), 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD, 2012. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487 (7408), 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Drewes LR, 1999. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res. Dev. Brain Res 113 (1–2), 47–54. [DOI] [PubMed] [Google Scholar]
- Lewis GM, Kucenas S, 2014. Perineurial glia are essential for motor axon regrowth following nerve injury. J. Neurosci 34 (38), 12762–12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Huang J, Sun S, Huang S, Gan S, Xu J, Yang M, Xu S, Jiang X, 2015. Changes in lactate content and monocarboxylate transporter 2 expression in Abeta(2) (5)(−)(3)(5)-treated rat model of Alzheimer’s disease. Neurol. Sci 36 (6), 871–876. [DOI] [PubMed] [Google Scholar]
- Mac M, Nalecz KA, 2003. Expression of monocarboxylic acid transporters (MCT) in brain cells. Implication for branched chain alpha-ketoacids transport in neurons. Neurochem. Int 43 (4–5), 305–309. [DOI] [PubMed] [Google Scholar]
- Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A,Kaelin V, Zuend M, San Martin A, Romero-Gomez I, Baeza-Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF, Weber B, 2016. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23 (1), 94–102. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, 1999. Brain energy metabolism In: Fundamental Neuroscience. Academic Press, San Diego, CA, pp. 389–413. [Google Scholar]
- Magistretti PJ, 2008. Brain energy metabolism. In: Squire L, Berg D, Bloom FE (Eds.), Fundamental Neuroscience, 3rd ed. Academic Press, San Diego, CA, pp. 271–293. [Google Scholar]
- Magistretti PJ, Allaman I, 2015. A cellular perspective on brain energy metabolism and functional imaging. Neuron 86 (4), 883–901. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I, 2018. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci 19 (4), 235–249. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Naichen Y, Pellerin L, de Rham S, Martin JL, 1994. Regulation of astrocyte energy metabolism by neurotransmitters. Ren. Physiol. Biochem 17 (3–4), 168–171. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Yu N, Martin JL, Pellerin L, 1993. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev. Neurosci 15 (3–5), 306–312. [DOI] [PubMed] [Google Scholar]
- Magnani P, Cherian PV, Gould GW, Greene DA, Sima AA, Brosius FC 3rd, 1996. Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism 45 (12), 1466–1473. [DOI] [PubMed] [Google Scholar]
- Mailliard WS, Diamond I, 2004. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol. Ther 101 (1), 39–46. [DOI] [PubMed] [Google Scholar]
- Marcillac F, Brix B, Repond C, Johren O, Pellerin L, 2011. Nitric oxide induces the expression of the monocarboxylate transporter MCT4 in cultured astrocytes by a cGMP-independent transcriptional activation. Glia 59 (12), 1987–1995. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Thorens B, 2007. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22, 241–251. [DOI] [PubMed] [Google Scholar]
- Matsui T, Omuro H, Liu YF, Soya M, Shima T, McEwen BS, Soya H, 2017. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc. Natl. Acad. Sci. U. S. A 114 (24), 6358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP, 2004. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol 490 (1–3), 13–24. [DOI] [PubMed] [Google Scholar]
- McGowan KM, Long SD, Pekala PH, 1995. Glucose transporter gene expression: regulation of transcription and mRNA stability. Pharmacol. Ther 66 (3), 465–505. [DOI] [PubMed] [Google Scholar]
- Membrez M, Hummler E, Beermann F, Haefliger JA, Savioz R, Pedrazzini T, Thorens B, 2006. GLUT8 is dispensable for embryonic development but influences hippocampal neurogenesis and heart function. Mol. Cell. Biol 26 (11), 4268–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A, 2013. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36 (10), 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhauser C, Semtner M, Nolte C, Kettenmann H, 2018. Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose. Cell Rep. 22 (9), 2383–2394. [DOI] [PubMed] [Google Scholar]
- Mizisin AP, 2014. Mechanisms of diabetic neuropathy: Schwann cells. Handb. Clin. Neurol 126, 401–428. [DOI] [PubMed] [Google Scholar]
- Moreira TJ, Pierre K, Maekawa F, Repond C, Cebere A, Liljequist S, Pellerin L, 2009. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J. Cereb. Blood Flow Metab 29 (7), 1273–1283. [DOI] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, Rothstein JD, 2013. Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 23 (12), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Tsingalia A, Vidensky S, Lee Y, Jin L, Farah MH, Lengacher S, Magistretti PJ, Pellerin L, Rothstein JD, 2015. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp. Neurol 263, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D, Berl S, Clarke DD, 1986. Acetate and fluoroacetate as possible markers for glial metabolism in vivo. Brain Res. 380 (2), 336–340. [DOI] [PubMed] [Google Scholar]
- Muona P, Jaakkola S, Salonen V, Peltonen J, 1993. Expression of glucose transporter 1 in adult and developing human peripheral nerve. Diabetologia 36 (2), 133–140. [DOI] [PubMed] [Google Scholar]
- Muona P, Sollberg S, Peltonen J, Uitto J, 1992. Glucose transporters of rat peripheral nerve. Differential expression of GLUT1 gene by Schwann cells and perineural cells in vivo and in vitro. Diabetes 41 (12), 1587–1596. [DOI] [PubMed] [Google Scholar]
- Nave KA, 2010. Myelination and support of axonal integrity by glia. Nature 468 (7321), 244–252. [DOI] [PubMed] [Google Scholar]
- Nehlig A, 1996. Respective roles of glucose and ketone bodies as substrates for cerebral energy metabolism in the suckling rat. Dev. Neurosci 18 (5–6), 426–433. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Pereira de Vasconcelos A, 1993. Glucose and ketone body utilization by the brain of neonatal rats. Prog. Neurobiol 40 (2), 163–221. [DOI] [PubMed] [Google Scholar]
- Nijland PG, Michailidou I, Witte ME, Mizee MR, van der Pol SM, van Het Hof B, Reijerkerk A, Pellerin L, van der Valk P, de Vries HE, van Horssen J, 2014. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 62 (7), 1125–1141. [DOI] [PubMed] [Google Scholar]
- Pande M, Hur J, Hong Y, Backus C, Hayes JM, Oh SS, Kretzler M, Feldman EL, 2011. Transcriptional profiling of diabetic neuropathy in the BKS db/db mouse: a model of type 2 diabetes. Diabetes 60 (7), 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao SS, Paulsen IT, Saier MH Jr., 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev 62 (1), 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr., Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A, 2012. Glial cells in (patho)physiology. J. Neurochem 121 (1), 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J, Maher F, Simpson I, Mattice L, Davies P, 1997. Glucose transporter Glut 5 expression in microglial cells. Glia 21 (3), 327–331. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J, McNay EC, 2016. Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J. Neurosci 36 (47), 11851–11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ, 2007. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55 (12), 1251–1262. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ, 1994. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U. S. A 91 (22), 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ, 2012. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab 32 (7), 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Martin JL, Magistretti PJ, 1998. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc. Natl. Acad. Sci. U. S. A 95 (7), 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen S, Alanne M, Peltonen J, 2013. Barriers of the peripheral nerve. Tissue Barriers 1 (3), e24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Escuredo J, Van Hee VF, Sboarina M, Falces J, Payen VL, Pellerin L, Sonveaux P, 2016. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863 (10), 2481–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, Ballina L, Ly S, Saini A, Jones TB, Vallejo J, Jentarra G, Valla J, 2016. Altered energy metabolism pathways in the posterior cingulate in young adult apolipoprotein E varepsilon4 carriers. J. Alzheimers Dis 53 (1), 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Grollman EF, 1998. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am. J. Phys 274 (6 Pt 2), R1824–R1828. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Lombardi L, 2001. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am. J. Phys. Cell Phys 280 (5), C1319–C1326. [DOI] [PubMed] [Google Scholar]
- Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ, 2000. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100 (3), 617–627. [DOI] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF, 2008. Na(+)-Ca(2+) cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia 56 (1), 59–68. [DOI] [PubMed] [Google Scholar]
- de Preux Charles AS, Verdier V, Zenker J, Peter B, Medard JJ, Kuntzer T, Beckmann JS, Bergmann S, Chrast R, 2010. Global transcriptional programs in peripheral nerve endoneurium and DRG are resistant to the onset of type 1 diabetic neuropathy in Ins2 mice. PLoS One 5 (5), e10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchades M, Sogn CJ, Maehlen J, Bergersen LH, Gundersen V, 2013. Unaltered lactate and glucose transporter levels in the MPTP mouse model of Parkinson’s disease. J Parkinsons Dis 3 (3), 371–385. [DOI] [PubMed] [Google Scholar]
- Rae C, Fekete AD, Kashem MA, Nasrallah FA, Broer S, 2012. Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice. Neurochem. Res. 37 (11), 2541–2553. [DOI] [PubMed] [Google Scholar]
- Rae C, Hare N, Bubb WA, McEwan SR, Broer A, McQuillan JA, Balcar VJ, Conigrave AD, Broer S, 2003. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation. J. Neurochem 85 (2), 503–514. [DOI] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D, 2011. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci 31 (2), 538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero I, Alvarez E, Chowen JA, Sanz C, Rabano A, Vazquez P, Blazquez E, 2004. Expression of glucose transporter isoform GLUT-2 and glucokinase genes in human brain. J. Neurochem 88 (5), 1203–1210. [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Mobius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Perez-Samartin A, Perez-Cerda F, Bakhtiari D, Matute C, Lowel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave KA, 2016. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91 (1), 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, 2015. Schwann cell myelination. Cold Spring Harb. Perspect. Biol 7 (8), a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Martin A, Ceballo S, Ruminot I, Lerchundi R, Frommer WB, Barros LF, 2013. A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS One 8 (2), e57712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Abarca LI, Tabernero A, Medina JM, 2001. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia 36 (3), 321–329. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Joost HG, Schurmann A, 2009. GLUT8, the enigmatic intracellular hexose transporter. Am. J. Physiol. Endocrinol. Metab 296 (4), E614–E618. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ, 2007. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab 27 (11), 1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ, 2008. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab 295 (2), E242–E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieski C, Warikoo N, Shu HJ, Mennerick S, 2018. Ambient but not local lactate underlies neuronal tolerance to prolonged glucose deprivation. PLoS One 13 (4), e0195520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, 1960. Metabolism of the central nervous system in vivo In: Field J, Magoun HW, Hall VE (Eds.), Handbook of Physiology -Neurophysiology. vol. III American Physiological Society, Washington, D. C., pp. 1843–1864. [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB, 1993. Direct demonstration by [13C]NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem. Int 22 (1), 19–29. [DOI] [PubMed] [Google Scholar]
- Starai VJ, Escalante-Semerena JC, 2004. Acetyl-coenzyme A synthetase (AMP forming). Cell. Mol. Life Sci 61 (16), 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker MM, Stevenson MR, 2015. Anoxia-induced changes in optimal substrate for peripheral nerve. Neuroscience 284, 653–667. [DOI] [PubMed] [Google Scholar]
- Stuart CA, Wen G, Peng BH, Popov VL, Hudnall SD, Campbell GA, 2000. GLUT3 expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab 279 (4), E855–E861. [DOI] [PubMed] [Google Scholar]
- Supplie LM, Duking T, Campbell G, Diaz F, Moraes CT, Gotz M, Hamprecht B, Boretius S, Mahad D, Nave KA, 2017. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J. Neurosci 37 (16), 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T, 2008. Histochemical demonstration of a monocarboxylate transporter in the mouse perineurium with special reference to GLUT1. Biomed. Res 29 (6), 297–306. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR, 2005. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res 81 (5), 644–652. [DOI] [PubMed] [Google Scholar]
- Thorens B, Mueckler M, 2010. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab 298 (2), E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G, 1994. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J. Neurosci 14 (3 Pt 1), 1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Shin BC, Koyama H, Suzuki T, Takata K, 1999. Immunolocalization of tight junction proteins, occludin and ZO-1, and glucose transporter GLUT1 in the cells of the blood-nerve barrier. Arch. Histol. Cytol 62 (5), 459–469. [DOI] [PubMed] [Google Scholar]
- Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B, 2001. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 20 (16), 4467–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME, 2010. Regional aerobic glycolysis in the human brain. Proc. Natl. Acad. Sci. U. S. A 107 (41), 17757–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C, Poitry-Yamate CL, Jirounek P, Tsacopoulos M, Coles JA, 1998. Lactate is released and taken up by isolated rabbit vagus nerve during aerobic metabolism. J.Neurochem 71 (1), 330–337. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM & De Vivo D (1993).Glucose transporter type 1 deficiency syndrome. In GeneReviews((R))(Eds Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K and Amemiya A). Seattle (WA). [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR, 2000. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci 20 (18), 6804–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NC, O’Neill LAJ, 2018. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol 9, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B, 2011. In vivo evidence for lactate as a neuronal energy source. J. Neurosci 31 (20), 7477–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G, 2018. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol 217 (7), 2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Fanelli A, Grollman EF, Philp NJ, 1997. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem. Biophys. Res. Commun 234 (1), 90–94. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhao T, Guo M, Fang H, Ma J, Ding A, Wang F, Chan P, Fan M, 2008. Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes. Brain Res. 1211, 22–29. [DOI] [PubMed] [Google Scholar]
- Zenker J, Ziegler D, Chrast R, 2013. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 36 (8), 439–449. [DOI] [PubMed] [Google Scholar]