Abstract
Background:
Alzheimer’s Disease (AD) can be conceptualized as a continuum: patients progress from normal cognition to mild cognitive impairment (MCI) due to AD, followed by increasing severity of AD dementia. Prior research has measured transition probabilities among later stages of AD, but not for the complete spectrum.
Objective:
To estimate annual progression rates across the AD continuum and evaluate the impact of a delay in MCI due to AD on the trajectory of AD dementia and clinical outcomes.
Methods:
Patient-level longitudinal data from the National Alzheimer’s Coordinating Center for n=18,103 patients with multiple visits over the age of 65 were used to estimate annual, age-specific transitional probabilities between normal cognition, MCI due to AD, and AD severity states (defined by Clinical Dementia Rating score). Multivariate models predicted the likelihood of death and institutionalization for each health state, conditional on age and time from the previous evaluation. These probabilities were used to populate a transition matrix describing the likelihood of progressing to a particular disease state or death for any given current state and age. Finally, a health state model was developed to estimate the expected effect of a reduction in the risk of transitioning from normal cognition to MCI due to AD on disease progression rates for a cohort of 65-year-old patients over a 35-year time horizon.
Results:
Annual transition probabilities to more severe states were 8%, 22%, 25%, 36%, and 16% for normal cognition, MCI due to AD, and mild/moderate/severe AD, respectively, at age 65, and increased as a function of age. Progression rates from normal cognition to MCI due to AD ranged from 4% to 10% annually. Severity of cognitive impairment and age both increased the likelihood of institutionalization and death. For a cohort of 100 patients with normal cognition at age 65, a 20% reduction in the annual progression rate to MCI due to AD avoided 5.7 and 5.6 cases of MCI due to AD and AD, respectively. This reduction led to less time spent in severe AD dementia health states and institutionalized, and increased life expectancy.
Conclusion:
Transition probabilities from normal cognition through AD severity states are important for understanding patient progression across the AD spectrum. These estimates can be used to evaluate the clinical benefits of reducing progression from normal cognition to MCI due to AD on lifetime health outcomes.
Keywords: Mild cognitive impairment, lewy bodies, apolipoprotein E, dementia, Alzheimer’s disease, aging
1. INTRODUCTION
Alzheimer’s Disease (AD) is an irreversible and degenerative brain disorder, in which symptoms progress from short-term memory lapses to loss of bodily function and death [1]. Both the underlying pathophysiological process of AD and its clinical symptomatology are best conceptualized as a continuum: patients progress from normal cognition to Mild Cognitive Impairment (MCI) due to AD, followed by increasing severity of AD dementia (mild, moderate, and severe) [2]. AD dementia is the most common form of dementia, accounting for approximately 60–80% of cases [1]. AD is estimated to affect >5 million individuals in the United States (US), and is currently the sixth leading cause of death in the US, with survival times averaging 8.3 years for patients diagnosed with AD at age 65 years [1, 3]. The incidence and prevalence of AD are expected to increase dramatically over the next several decades with the growth of the elderly population and extended life spans [4]. While progression in the AD continuum is not fully understood, it is believed that the pathophysiological processes of the illness begin a decade or more before the clinical signs of dementia are detectable [5].
MCI due to AD is a pre-dementia phase of AD, characterized by the development of noticeable memory problems (amnestic) or impaired judgment or decision-making (nonamnestic), which does not affect independence of functional abilities, does not meet the criteria for dementia, and has AD as a suspected etiology [6, 7]. A meta-analysis of 41 cohort studies found annual conversion rates from MCI to AD of 8.1% and 6.8% in specialist and community settings, respectively [8].
The failure of currently available therapies to prevent AD or to slow disease progression has re-focused research on interventions aimed at preventing progression in pre-symptomatic, high-risk individuals. Therapies that delay the onset of MCI due to AD could have significant implications regarding reducing the number of patients with AD who require institutionalization. However, the impact of a delay in MCI due to AD on the trajectory of AD dementia incidence and prevalence has not been established.
The primary objective of this study was to estimate transitional progression rates from normal cognition to MCI due to AD and to mild/moderate/severe AD dementia, including the age-specific likelihood of institutionalization and death from each health state using a well-defined US population. Prior research has examined transition rates within AD dementia states or from normal cognition to MCI and AD without severity differentiation, but not across the full AD continuum [9–13]. To understand the impact of transitioning from normal cognition to MCI due to AD on progression to AD dementia, institutionalization, and death, the secondary objective was to develop a health state model, using the estimated progression rates, to predict the expected effect of a hypothetical disease-modifying treatment that delays onset of MCI due to AD on lifetime outcomes for a US patient cohort. The results illustrate how the progression rates could be used to direct discussions on the benefits that may be expected from new therapies, inform investment decisions related to the development of therapies that work to delay MCI due to AD, and advise medical services planning efforts to meet the needs of the expanding AD population.
2. MATERIALS AND METHODS
2.1. Study Design
This study was conducted in two phases. Phase I used longitudinal, cognitive evaluation data in multivariate models to estimate transition probabilities between normal cognition, MCI due to AD, various AD dementia severity levels, and non-AD cognitive impairment. The age- and state-specific likelihood of institutionalization (defined as a skilled nursing facility or nursing home) and death for each health state was estimated from the same data. Phase II used the estimated probabilities in a health state transition model to evaluate disease progression along the AD continuum and the clinical outcomes of a closed cohort over a fixed time horizon, assessing disease incidence and prevalence by health state, as well as rates of institutionalization and death.
A counterfactual, delayed onset scenario was also modeled using a slower progression rate from normal cognition to MCI due to AD.
2.2. Data
Patient-level, longitudinal data used to estimate transition probabilities between health states, institutionalization, and death were obtained from the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS). The UDS contains prospectively gathered data on all patients enrolled in Alzheimer’s Disease Centers across the US, including annual cognitive evaluations and interviews with subjects and caregivers [14]. The UDS was created to provide longitudinal data on aging across the continuum of normal cognition, MCI, and AD dementia [15]. Information was available on over 30,000 individuals with varying degrees of cognitive impairment from 2005 through 2014. Data are gathered prospectively by clinicians, neuropsychologists, and other research personnel, and include information on demographics, dementia history, neurological exam findings, functional status, neuropsychological test results, clinical diagnosis, and apolipoprotein E (APOE). The NACC database is funded by National Institute on Aging/National Institutes of Health Grant U01 AG016976.
2.3. Analyses
2.3.1. Phase I: Estimation of Transition Probabilities
This phase of the analysis included all patients in the NACC UDS with more than one visit while aged ≥65 years, so that transitions could be evaluated. Baseline characteristics, including demographics (age, gender, race, ethnicity, education, marital status), living situation (lives alone, able to live independently, residence type), health history (family history of dementia, comorbidities), behavioral issues (agitation, irritability, nighttime behaviors), and clinical measures (APOE genotype), were assessed for all patients at their initial visit in the study.
Each patient-visit was categorized to a health state based on clinical diagnosis (normal cognition, non-dementia Cognitive Impairment (CI), dementia), the global Clinical Dementia Rating (CDR) scale (a composite index of cognitive function designed to measure dementia severity), and history of primary etiologic diagnosis of cognitive impairment (e.g., AD, dementia with Lewy bodies, vascular dementia) (Table 1). Patient counts and characteristics were compared across starting health states in the model, using chi-squared tests for categorical variables. Age-specific transition probabilities to each health state were estimated using a multivariate ordered probit model, controlling for the patient’s health state at the prior visit and their current age. A covariate for days elapsed since the prior visit was included to adjust for variation in visit frequency. This approach assumes that a latent, continuous metric (e.g., CI) underlies the ordinal observations (e.g., clinical diagnosis of disease severity).
Table 1.
Definition of health states.
| Health State | Definition |
|---|---|
| Normal cognition | Diagnosis of normal cognition at visit |
| MCI due to AD | Diagnosis of non-dementia CI AND diagnosis of MCI due to AD at visit or primary etiologic diagnosis of AD at any time |
| Mild AD dementia | Diagnosis of dementia at visit AND primary etiologic diagnosis of AD at any time AND CDR<2 |
| Moderate AD dementia | Diagnosis of dementia at visit AND primary etiologic diagnosis of AD at any time AND CDR=2 |
| Severe AD dementia | Diagnosis of dementia at visit AND primary etiologic diagnosis of AD at any time AND CDR=3 |
| Non-AD cognitive impairment | Diagnosis of CI or dementia at visit AND no primary etiologic diagnosis of AD at any time |
AD, Alzheimer’s disease; CDR, Clinical Dementia Rating; CI, cognitive impairment; MCI, mild cognitive impairment.
Separate multivariate regression models were used to assess the likelihood of institutionalization and death, conditional on patient health state and age. This approach models the likelihood of the binary outcome conditional on individual risk factors to estimate age-state specific probabilities of institutionalization and death. Institutionalization was not considered a distinct health state, but was modeled separately to predict the likelihood of institutionalization for patients within each health state, as has been done in prior studies [9, 11, 13]. Age- and state-specific likelihood of progressing to death was estimated separately from the health state transitions, given the non-ordinal nature of transitioning from CI severity levels to death. For patients who did not progress to AD, a multivariate model of transitioning to non-AD CI (MCI not due to AD or non-AD dementia) was estimated conditional on age to produce age-specific probabilities.
Transition matrices were developed for each age and state. Predicted probabilities from the regression coefficient estimates were generated for each row of the age-specific transition matrices using Stata software (version 13.1, Stata-Corp LP, College Station, TX 77845, US), with rates proportionally adjusted to sum to 100%. To account for diagnostic or measurement error, patients observed to transition from CI back to normal cognition and from AD-related dementia back to normal cognition or MCI due to AD were assumed to remain in their prior health state. Studies suggest that observed reversion in cognitive decline based on tests of cognitive performance is likely due to measurement error, and is not reflective of changes to the neurodegenerative process [16]. Patients who appear to improve from MCI to normal cognition remain at increased risk of transitioning to dementia [17], and patients with a previous CDR of 0.5 (which has been used to define MCI) often have AD pathology at autopsy [5]. Reversion or improvement within AD dementia (e.g., from severe to moderate AD dementia) was estimated based on the results of prior studies of AD progression [9,11].
2.3.2. Phase II: Simulation of Delayed Onset of MCI due to AD
In Phase II, a health state transition model based on the age-specific transition probabilities estimated in Phase I was used to assess disease progression and lifetime outcomes for a hypothetical cohort of patients with and without a reduction in the annual risk of AD progression from normal cognition. The structure of the health state transition model is shown in (Fig. 1). Patients begin the model at age 65 in the state of normal cognition, and are assumed to have characteristics and risk factors of the average patient in the NACC UDS data. In each year, they have a probability of transitioning along the AD continuum, developing non-AD CI, or remaining in the same state, based on their current state and age. All health states have an age-specific probability of transitioning to death. Patients also have a risk of requiring institutionalization, conditional on their health state and age.
Fig. (1).
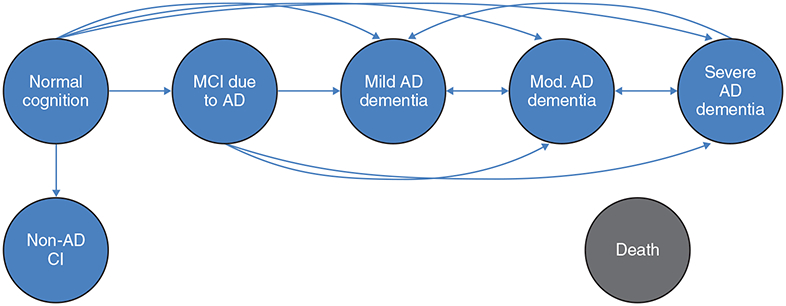
Schematic of health state transition model. Patients begin the model at age 65 years in the state of normal cognition. Patients are at risk of transitioning along the indicated paths if they have a primary etiologic diagnosis of AD at any visit. Patients are at risk of transitioning to the non-AD CI state, which includes MCI not due to AD and non-AD dementia if they do not receive a primary etiologic diagnosis of AD. All health states have a probability of transitioning to death and institutionalization.
AD, Alzheimer’s disease; CI, cognitive impairment; MCI, mild cognitive impairment.
The annual incidence and prevalence of MCI due to AD and AD dementia were estimated, and the number of patient-years spent in each health state were totaled over the time horizon of 35 years. Time spent in institutionalized settings and dead, as well as years of survival, were evaluated. To simulate a delay in the onset of MCI due to AD, counterfactual progression was modeled assuming a 20% reduction in the annual risk of transitioning from normal cognition to MCI due to AD. Differences in incidence, prevalence, mortality, and institutionalization between the two scenarios represent the effect of delays in the onset of MCI due to AD. A sensitivity analysis was also conducted assuming a 2-year fixed delay in the risk of progressing from normal cognition to MCI due to AD, rather than a reduction in the annual transition probability.
3. RESULTS
3.1. Study population
This study included 18,103 patients from the NACC UDS with ≥1 visit while ≥65 years old. Baseline characteristics of the patients included in Phase I of the study are shown in Table 2. Overall, patients had a mean age of 75.7 years (standard deviation=7.3, median=75), 57.1% were female, 81.3% were White, 12.3% were Black, and 6.9% were Hispanic/Latino ethnicity. Based on initial visit, 42.0% of patients had normal cognition, 18.6% had MCI due to AD, and 20.9%, 3.8%, and 1.6% had mild, moderate, and severe AD dementia, respectively.
Table 2.
Baseline characteristics of study population.
| Characteristic, n (%) | All Patients (n=18,103) |
|---|---|
| Age in years, mean (SD) [median] | 75.7 (7.3) [75] |
| 65–74 | 8,383 (46.3%) |
| 75–84 | 7,382 (40.8%) |
| 85–94 | 2,236 (12.4%) |
| 95+ | 102 (0.6%) |
| Female | 10,336 (57.1%) |
| Race/ethnicity | - |
| White | 14,713 (81.3%) |
| Black | 2,221 (12.3%) |
| Other/unknown | 1,169 (6.5%) |
| Hispanic* | 1,251 (6.9%) |
| Education Level | - |
| High school or less | 4,955 (27.4%) |
| College | 7,273 (40.2%) |
| Graduate school | 5,793 (32.0%) |
| Unknown | 82 (0.5%) |
| Marital status | - |
| Married | 11,327 (62.6%) |
| Widowed/divorced/separated | 5,876 (32.5%) |
| Never married | 758 (4.2%) |
| Other/unknown | 142 (0.8%) |
| Living situation | - |
| Lives alone | 4,856 (26.8%) |
| Able to live independently | 12,255 (67.7%) |
| Residence | - |
| Single family residence | 15,669 (86.6%) |
| Retirement community | 1,328 (7.3%) |
| Assisted living boarding home | 381 (2.1%) |
| Skilled nursing facility /nursing home | 189 (1.0%) |
| Other/unknown | 536 (3.0%) |
| Health history | - |
| First-degree family member with dementia | 9,484 (52.4%) |
| Congestive heart failure | 328 (1.8%) |
| Cerebrovascular disease | 2,981 (16.5%) |
| Depression | 4,913 (27.1%) |
| Diabetes | 2,246 (12.4%) |
| Hypercholesterolemia | 8,981 (49.6%) |
| Hypertension | 11,378 (62.9%) |
| Incontinence | 3,002 (16.6%) |
| Ischemic attack | 329 (1.8%) |
| Parkinson’s disease | 366 (2.0%) |
| Psychiatric disorders | 644 (3.6%) |
| Seizures | 124 (0.7%) |
| Smoking | 652 (3.6%) |
| Stroke | 709 (3.9%) |
| Thyroid disease | 2,887 (15.9%) |
| Behavioral disturbances | - |
| Agitation | 3,373 (18.6%) |
| Irritability | 4,889 (27.0%) |
| Nighttime behaviors | 3,316 (18.3%) |
| Drug use | - |
| Anxiolytic, sedative, or hypnotic agent | 1,854 (10.2%) |
| Antidepressant | 4,500 (24.9%) |
| Medication for AD symptoms | 4,924 (27.2%) |
| Antiparkinson agent | 684 (3.8%) |
| Antipsychotic agent | 620 (3.4%) |
| Number of APOE e4 alleles | - |
| Zero | 8,514 (47.0%) |
| One | 4,758 (26.3%) |
| Two | 861 (4.8%) |
| Unknown | 3,970 (21.9%) |
| Cognitive state | - |
| Normal cognition | 7,612 (42.0%) |
| MCI due to AD | 3,370 (18.6%) |
| Mild AD dementia | 3,775 (20.9%) |
| Moderate AD dementia | 681 (3.8%) |
| Severe AD dementia | 292 (1.6%) |
| Non-AD CI | 2,373 (13.1%) |
Ethnicity assessed separately from race, thus does not add to 100%.
AD, Alzheimer’s disease; APOE, apolipoprotein E; CI; cognitive impairment; MCI, mild cognitive impairment; SD. standard deviation.
Relative to normal cognition, patients with MCI due to AD were older (p<0.0001), married (p<0.0001), displayed behavioral disturbances (p<0.0001), took medication for AD symptoms (p<0.0001), and possessed APOE e4 alleles (p<0.0001). In addition, they had an elevated prevalence of diabetes (p<0.01), hypercholesterolemia (p<0.0001), and hypertension (p<0.0001), as well as nearly double the frequency of depression (p<0.0001). Finally, males accounted for a higher proportion of patients with MCI due to AD versus those with normal cognition (p<0.0001). These trends were generally similar across the AD dementia spectrum (Table 3).
Table 3.
Baseline characteristics by cognitive state.
| Characteristic, n (%) | Normal cognition (n=7,612) | MCI due to AD (n=3,370) |
Mild AD dementia (n = 3,775) |
Mod. AD dementia (n = 681) | Severe AD dementia (n = 292) | Non-AD CI (n = 2,373) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||||||||
| Age in years | |||||||||||||||||
| 65–74 | 3,953 | (51.9%) | 1,371 | (40.7%) | *** | 1,352 | (35.8%) | *** | 197 | (28.9%) | *** | 89 | (30.5%) | *** | 1,421 | (59.9%) | *** |
| 75–84 | 2,709 | (35.6%) | 1,560 | (46.3%) | *** | 1,855 | (49.1%) | *** | 318 | (46.7%) | *** | 136 | (46.6%) | ** | 804 | (33.9%) | |
| 85–94 | 910 | (12.0%) | 417 | (12.4%) | 545 | (14.4%) | ** | 158 | (23.2%) | *** | 61 | (20.9%) | *** | 145 | (6.1%) | *** | |
| 95+ | 40 | (0.5%) | 22 | (0.7%) | 23 | (0.6%) | 8 | (1.2%) | * | 6 | (2.1%) | ** | 3 | (0.1%) | ** | ||
| Female | 4,962 | (65.2%) | 1,684 | (50.0%) | *** | 2,001 | (53.0%) | *** | 418 | (61.4%) | * | 183 | (62.7%) | 1,088 | (45.9%) | *** | |
| Race | |||||||||||||||||
| White | 6,173 | (81.1%) | 2,805 | (83.2%) | ** | 3,085 | (81.7%) | 493 | (72.4%) | *** | 231 | (79.1%) | 1,926 | (81.2%) | |||
| Black | 1,038 | (13.6%) | 351 | (10.4%) | *** | 416 | (11.0%) | *** | 114 | (16.7%) | * | 34 | (11.6%) | 268 | (11.3%) | ** | |
| Other/unknown | 401 | (5.3%) | 214 | (6.4%) | * | 274 | (7.3%) | *** | 74 | (10.9%) | *** | 27 | (9.3%) | ** | 179 | (7.5%) | *** |
| Hispanic | 394 | (5.2%) | 215 | (6.4%) | * | 292 | (7.7%) | *** | 110 | (16.2%) | *** | 49 | (16.8%) | *** | 191 | (8.1%) | *** |
| Education level | |||||||||||||||||
| High school or less | 1,523 | (20.0%) | 853 | (25.3%) | *** | 1,416 | (37.5%) | *** | 323 | (47.4%) | *** | 133 | (45.6%) | *** | 707 | (29.8%) | *** |
| College | 3,220 | (42.3%) | 1,376 | (40.8%) | 1,409 | (37.3%) | *** | 217 | (31.9%) | *** | 95 | (32.5%) | ** | 956 | (40.3%) | ||
| Graduate school | 2,837 | (37.3%) | 1,135 | (33.7%) | ** | 933 | (24.7%) | *** | 135 | (19.8%) | *** | 57 | (19.5%) | *** | 696 | (29.3%) | *** |
| Unknown | 32 | (0.4%) | 6 | (0.2%) | * | 17 | (0.5%) | 6 | (0.9%) | 7 | (2.4%) | *** | 14 | (0.6%) | |||
| Marital status | |||||||||||||||||
| Married | 4,359 | (57.3%) | 2,223 | (66.0%) | *** | 2,552 | (67.6%) | *** | 368 | (54.0%) | 169 | (57.9%) | 1,656 | (69.8%) | *** | ||
| Widowed/divorced/separated | 2,773 | (36.4%) | 980 | (29.1%) | *** | 1,113 | (29.5%) | *** | 285 | (41.9%) | **> | 111 | (38.0%) | 614 | (25.9%) | *** | |
| Never married | 411 | (5.4%) | 140 | (4.2%) | ** | 99 | (2.6%) | *** | 19 | (2.8%) | **> | 10 | (3.4%) | 79 | (3.3%) | *** | |
| Other/unknown | 69 | (0.9%) | 27 | (0.8%) | 11 | (0.3%) | ** | 9 | (1.3%) | 2 | (0.7%) | 24 | (1.0%) | ||||
| Living situation | |||||||||||||||||
| Lives alone | 2,747 | (36.1%) | 895 | (26.6%) | *** | 653 | (17.3%) | *** | 72 | (10.6%) | *** | 4 | (1.4%) | *** | 485 | (20.4%) | *** |
| Able to live independently | 7,404 | (97.3%) | 2,570 | (76.3%) | *** | 938 | (24.9%) | *** | 13 | (1.9%) | *** | 1 | (0.3%) | *** | 1,329 | (56.0%) | *** |
| Residence | |||||||||||||||||
| Single family residence | 6,639 | (87.2%) | 2,926 | (86.8%) | 3,315 | (87.8%) | 562 | (82.5%) | ** | 160 | (54.8%) | *** | 2,067 | (87.1%) | |||
| Retirement community | 737 | (9.7%) | 233 | (6.9%) | *** | 219 | (5.8%) | *** | 25 | (3.7%) | *** | 2 | (0.7%) | *** | 112 | (4.7%) | *** |
| Assisted living/boarding home | 37 | (0.5%) | 53 | (1.6%) | *** | 138 | (3.7%) | *** | 56 | (8.2%) | *** | 29 | (9.9%) | *** | 68 | (2.9%) | *** |
| Skilled nursing facility/nursing home | 3 | (0.0%) | 1 | (0.0%) | 21 | (0.6%) | *** | 21 | (3.1%) | *** | 94 | (32.2%) | *** | 49 | (2.1%) | *** | |
| Other/unknown | 196 | (2.6%) | 157 | (4.7%) | *** | 82 | (2.2%) | 17 | (2.5%) | 7 | (2.4%) | 77 | (3.2%) | ||||
| Health history | |||||||||||||||||
| First-degree family member with dementia | 3,846 | (50.5%) | 1,822 | (54.1%) | ** | 2,146 | (56.9%) | *** | 370 | (54.3%) | 180 | (61.6%) | ** | 1,120 | (47.2%) | ** | |
| Congestive heart failure | 129 | (1.7%) | 60 | (1.8%) | 71 | (1.9%) | 21 | (3.1%) | 7 | (2.4%) | 40 | (1.7%) | |||||
| Cerebrovascular disease | 1,198 | (15.7%) | 580 | (17.2%) | 633 | (16.8%) | 120 | (17.6%) | 36 | (12.3%) | 414 | (17.5%) | * | ||||
| Depression | 1,183 | (15.5%) | 1,007 | (29.9%) | 1,397 | (37.0%) | *** | 273 | (40.1%) | *** | 114 | (39.0%) | *** | 939 | (39.6%) | *** | |
| Diabetes | 846 | (11.1%) | 436 | (12.9%) | ** | 458 | (12.1%) | 116 | (17.0%) | *** | 36 | (12.3%) | 354 | (14.9%) | *** | ||
| Hypercholesterolemia | 3,619 | (47.5%) | 1,790 | (53.1%) | 1,922 | (50.9%) | 324 | (47.6%) | 92 | (31.5%) | *** | 1,234 | (52.0%) | ||||
| Hypertension | 4,661 | (61.2%) | 2,225 | (66.0%) | *** | 2,427 | (64.3%) | ** | 429 | (63.0%) | 137 | (46.9%) | *** | 1,499 | (63.2%) | ||
| Incontinence | 912 | (12.0%) | 495 | (14.7%) | *** | 639 | (16.9%) | *** | 219 | (32.2%) | *** | 210 | (71.9%) | *** | 527 | (22.2%) | *** |
| Ischemic attack | 107 | (1.4%) | 57 | (1.7%) | 88 | (2.3%) | 15 | (2.2%) | 8 | (2.7%) | 54 | (2.3%) | |||||
| Parkinson’s disease | 86 | (1.1%) | 9 | (0.3%) | *** | 32 | (0.9%) | 4 | (0.6%) | 5 | (1.7%) | 230 | (9.7%) | *** | |||
| Psychiatric disorders | 187 | (2.5%) | 144 | (4.3%) | *** | 140 | (3.7%) | ** | 30 | (4.4%) | ** | 10 | (3.4%) | 133 | (5.6%) | *** | |
| Seizures | 27 | (0.4%) | 20 | (0.6%) | 27 | (0.7%) | ** | 8 | (1.2%) | ** | 14 | (4.8%) | *** | 28 | (1.2%) | *** | |
| Smoking | 280 | (3.7%) | 108 | (3.2%) | 133 | (3.5%) | 30 | (4.4%) | 3 | (1.0%) | * | 98 | (4.1%) | ||||
| Stroke | 171 | (2.3%) | 126 | (3.7%) | *** | 187 | (5.0%) | *** | 49 | (7.2%) | *** | 21 | (7.2%) | *** | 155 | (6.5%) | *** |
| Thyroid disease | 1,327 | (17.4%) | 529 | (15.7%) | * | 526 | (13.9%) | *** | 124 | (18.2%) | 41 | (14.0%) | 340 | (14.3%) | ** | ||
| Behavioral disturbances | |||||||||||||||||
| Agitation | 371 | (4.9%) | 593 | (17.6%) | *** | 1,232 | (32.6%) | *** | 342 | (50.2%) | *** | 183 | (62.7%) | *** | 652 | (27.5%) | *** |
| Irritability | 762 | (10.0%) | 1,044 | (31.0%) | *** | 1,646 | (43.6%) | *** | 374 | (54.9%) | *** | 160 | (54.8%) | *** | 903 | (38.1%) | *** |
| Nighttime behaviors | 653 | (8.6%) | 658 | (19.5%) | *** | 963 | (25.5%) | *** | 219 | (32.2%) | *** | 94 | (32.2%) | *** | 729 | (30.7%) | *** |
| Drug use | |||||||||||||||||
| Anxiolytic, sedative, or hypnotic agent | 735 | (9.7%) | 344 | (10.2%) | 304 | (8.1%) | ** | 55 | (8.1%) | 50 | (17.1%) | *** | 366 | (15.4%) | *** | ||
| Antidepressant | 1,117 | (14.7%) | 824 | (24.5%) | *** | 1,263 | (33.5%) | *** | 269 | (39.5%) | *** | 145 | (49.7%) | *** | 882 | (37.2%) | *** |
| Medication for AD symptoms | 103 | (1.4%) | 855 | (25.4%) | *** | 2,551 | (67.6%) | *** | 524 | (77.0%) | *** | 197 | (67.5%) | *** | 694 | (29.3%) | *** |
| Antiparkinson agent | 193 | (2.5%) | 70 | (2.1%) | 84 | (2.2%) | 7 | (1.0%) | * | 9 | (3.1%) | 321 | (13.5%) | *** | |||
| Antipsychotic agent | 35 | (0.5%) | 40 | (1.2%) | *** | 161 | (4.3%) | *** | 95 | (14.0%) | *** | 110 | (37.7%) | *** | 179 | (7.5%) | *** |
| Number ofAPOE e4 alleles | |||||||||||||||||
| Zero | 4,413 | (58.0%) | 1,475 | (43.8%) | *** | 1,219 | (32.3%) | *** | 190 | (27.9%) | *** | 79 | (27.1%) | *** | 1,138 | (48.0%) | *** |
| One | 1,596 | (21.0%) | 1,017 | (30.2%) | *** | 1,326 | (35.1%) | *** | 222 | (32.6%) | *** | 89 | (30.5%) | *** | 508 | (21.4%) | |
| Two | 123 | (1.6%) | 186 | (5.5%) | *** | 403 | (10.7%) | *** | 55 | (8.1%) | *** | 24 | (8.2%) | *** | 70 | (3.0%) | *** |
| Unknown | 1,480 | (19.4%) | 692 | (20.5%) | 827 | (21.9%) | ** | 214 | (31.4%) | *** | 100 | (34.3%) | *** | 657 | (27.7%) | *** | |
*=p<0.05; **=p<0.01; ***=p<0.0001; all comparisons relative to normal cognition
Notes:
[1] Baseline characteristics assessed at the first visit when age 65 or older. Includes all patients with ≥2 visits while age 65 or older.
[2] Normal cognition defined as a diagnosis of normal cognition (i.e., absence of impairment and dementia).
[3] MCI due to AD defined as a diagnosis of non-dementia cognitive impairment and identification of AD as the cause or a primary etiologic
diagnosis of AD at any time.
[4] AD dementia defined as a diagnosis of dementia and a primary etiologic diagnosis of AD at any time. Severity determined by global CDR
score: <2 for mild, 2 for moderate, and 3 for severe.
[5] Non-AD CI defined as a diagnosis of cognitive impairment or dementia and no primary etiologic diagnosis of AD at any time.
[6] Statistical significance calculated relative to normal cognition using Fisher’s exact test for categorical variables.
AD, Alzheimer’s disease; APOE, apolipoprotein E; CI, cognitive impairment; MCI, mild cognitive impairment.
3.2. Phase I: Estimation of Transition Probabilities
In the estimation of health state transitions, all prior health states and age were significant predictors of future health states (all p<0.001). The estimated transition matrices indicated that a normal-cognition age 65 patient has a 92% likelihood of remaining in normal cognition at age 66 years, a 4% chance of transitioning to MCI due to AD, a 3% probability of being diagnosed with non-AD cognitive impairment, and a 1% chance of death. These risks increased with age, so that at age 75 years, a normal-cognition patient had a 5% chance of developing MCI due to AD ( Table 4).
Table 4.
Estimated transition matrices. A: Age 65; B: Age 75.
| Age 66 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Normal | MCI | Mild AD | Mod. AD | Severe AD | Non-AD CI | Death | ||
| Age 65 | Normal | 92% | 4% | 0% | 0% | 0% | 3% | 1% |
| MCI | - | 78% | 21% | 0% | 0% | - | 1% | |
| Mild AD | - | - | 75% | 19% | 1% | - | 4% | |
| Mod. AD | - | - | 15% | 49% | 27% | - | 9% | |
| Severe AD | - | - | 0% | 5% | 79% | - | 16% | |
| Non-AD CI | - | - | - | - | - | 93% | 7% | |
| Death | - | - | - | - | - | - | 100% | |
| Age 76 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Normal | MCI | Mild AD | Mod. AD | Severe AD | Non-AD CI | Death | ||
| Age 75 | Normal | 90% | 5% | 0% | 0% | 0% | 3% | 1% |
| MCI | - | 75% | 23% | 0% | 0% | - | 2% | |
| Mild AD | - | - | 72% | 21% | 1% | - | 6% | |
| Mod. AD | - | - | 13% | 46% | 29% | - | 12% | |
| Severe AD | - | - | 0% | 4% | 76% | - | 20% | |
| Non-AD CI | - | - | - | - | - | 90% | 10% | |
| Death | - | - | - | - | - | - | 100% | |
AD, Alzheimer’s disease; CI, cognitive impairment; MCI, mild cognitive impairment
Estimated using a multivariate ordered probit of health state conditional on age and health state in the prior year; transitions from normal cognition to non-AD CI estimated in a separate probit controlling for age and age-squared; death may be underreported in the NACC data. Rates adjusted to sum to 100%.
AD, Alzheimer’s disease; CI, cognitive impairment; MCI, mild cognitive impairment.
The estimated risk of institutionalization and death increased with age and severity state. Rates of institutionalization at age 65 years ranged from 0% for normal cognition through mild AD to 1% for moderate AD, and 30% for severe AD patients. Mortality rates at age 65 were 1% for normal cognition, 1% for MCI due to AD, and 4%, 9%, and 16% for mild, moderate, and severe AD, respectively.
3.3. Phase II: Simulation of Delayed Onset of MCI due to AD
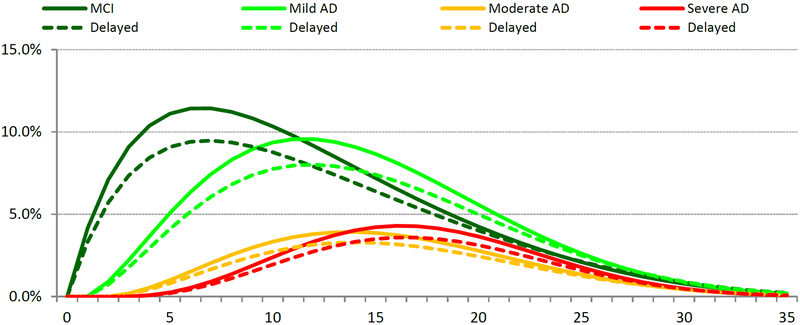
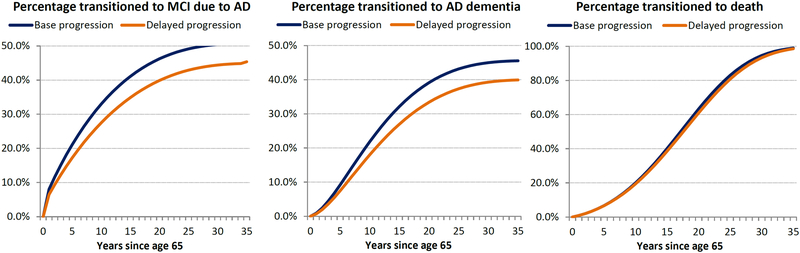
For a person with normal cognition at age 65, the estimated transition probabilities predict an average age of onset of MCI due to AD of 74.0 years and of AD dementia of 77.1 years, with an average lifespan of 81.6 years. A 20% reduction in the onset risk of MCI due to AD slowed the progression to AD-related dementia and extended time to death (Fig.2). For a cohort of 100 normal cognition patients at age 65 years, reducing annual progression to MCI due to AD by 20% avoided 5.73 cases of MCI due to AD and 5.60 cases of AD dementia over the 35-year time horizon. In addition, the reduction increased time with normal cognition by 0.91 years, delayed average time to AD onset by 0.57 years, reduced time spent institutionalized by 0.03 years, and increased survival by 0.42 years. Overall, relative to the base case, the reduction increased time spent in normal cognition (+9% cumulative years) and reduced time spent in more severe AD states (−13%, −14%, −14%, and −15% for MCI due to AD and mild, moderate, and severe AD, respectively) or dead (−2.3%).
Fig. (2).
Modeling the effects of a 20% reduction in the onset of MCI due to AD in a cohort of 100 normal cognition patients at age 65 years. A: Progression with and without a 20% risk reduction in onset of MCI due to AD; B: Transitions with and without a 20% risk reduction in onset of MCI due to AD; C: Epidemiology results for 20% risk reduction in onset of MCI due to AD.
AD, Alzheimer’s disease; MCI, mild cognitive impairment.
A sensitivity analysis was conducted assuming a 2-year fixed delay in the onset of MCI due to AD instead of a 20% annual risk reduction (i.e., patients faced a risk of transitioning beginning at age 67). The impact on patient outcomes was similar, with the delay slowing progression to AD-related dementia and extending time to death (Fig. S1). For a cohort of 100 normal cognition patients at age 65 years, a 2-year fixed delay in the risk of transitioning from normal cognition to MCI due to AD increased time with normal cognition by 0.83 years, avoided 4.00 cases of AD dementia, delayed average time to AD onset by 1.47 years, reduced time spent institutionalized by 0.03 years, and increased survival by 0.42 years.
4. DISCUSSION
This study used real-world longitudinal data to estimate age-specific disease progression rates for the full AD continuum, from normal cognition to MCI due to AD to mild, moderate, and severe AD dementia. Annual transition probabilities to more severe states at age 65 were 8% for normal cognition, 22% for MCI due to AD, and 25%, 36%, and 16% for mild, moderate, and severe AD, respectively. The likelihood of progression increased with age for each health state. In addition, rates of institutionalization and death increased with age and AD severity. Given the estimated progression rates, a health state transition model was developed to simulate outcomes for a cohort of 100 patients at age 65 with normal cognition who face a risk of eventually developing AD-related or non-AD CI. To demonstrate how the model and underlying transition probabilities could be used to evaluate the impact of a hypothetical disease-modifying treatment that slows CI progression and improves on the current standard of care, a simulation was conducted assuming a 20% reduction in progression from normal cognition to MCI due to AD to assess the impact of delaying the onset of MCI due to AD on time spent in severe AD disease states, life expectancy, and AD dementia-related institutionalization and death in the US.
Results showed that higher AD severity states were associated with elevated risks of institutionalization and death. Delaying the onset of MCI due to AD delayed the progression to AD dementia, increased life expectancy, and reduced time spent in severe AD dementia health states and in a nursing home setting. These data suggest that delays in the onset of MCI due to AD change the trajectory of AD dementia, increasing time spent with normal cognition. Importantly, the study demonstrates that even modest delays in the onset of MCI due to AD have beneficial outcomes. To the authors’ knowledge this is the first study to address the full cognitive decline continuum in AD. Such research is in accordance with The National Institute on Aging/Alzheimer’s Association Diagnostic Guidelines for Alzheimer’s disease, which were updated in 2011 in recognition of the need to address the full spectrum of AD rather than only the later stages when symptoms of dementia were already apparent [2, 6, 18,19].
The transition probabilities estimated here update and expand on previous studies assessing progression between stages of AD, institutionalization, and death. Several studies used an earlier version of the NACC UDS data to evaluate AD progression across more limited sets of cognitive states, with differences primarily driven by mortality rate estimates. Spackman et al. [9] estimated comparable progression rates at age 77 for mild and moderate AD (77% vs. 71% likelihood of remaining in mild AD and 50% vs. 45% for moderate AD), but much higher mortality for severe AD (48% vs. 22%), and did not include patients with normal cognition or MCI. Bloudek et al. [13] also estimated a much higher transition probability to death for severe AD (90%) in a study restricted to AD dementia patients. Both studies evaluated the odds of death in a multinomial regression along with the other health state transitions, which may have increased their mortality estimates. The mortality rates found here are very similar to those from studies relying on survival analyses to produce death transition probabilities. Neumann et al. [11] evaluated progression among AD patients using data from the Consortium to Establish a Registry for Alzheimer’s Disease, finding annual transition probabilities to death of 15% for severe AD, compared with 16% at baseline in this study. Using the NACC UDS, Olchanski et al. [10] similarly estimated age-specific mortality rates for MCI (3% vs. 2% in this study), mild AD (6% vs. 6%), moderate AD (15% vs. 12%), and severe AD (23% vs. 21%) at age 75 in a study of patients with AD-related CI. Finally, Hubbard and Zhou [12] assessed risk factors in transitioning from normal cognition to MCI and AD, but did not separate AD severity levels, nor produce annual progression rate estimates. Thus, the current study is the most comprehensive in estimating the full transition matrix across the AD spectrum (including normal cognition, MCI due to AD, mild AD, moderate AD, and severe AD), the likelihood of institutionalization and death for each disease state, and modeling age-related progression.
The modeled cohort outcomes are supported by observations from previous reports. Using a stochastic, multistate model in conjunction with the United Nations’ worldwide population forecasts and data from epidemiological studies of the risks of AD, Brookmeyer et al. [4] showed that even small delays in the onset and progression of AD could sig nificantly reduce the global burden of AD. A delay in both disease onset and progression by one year was predicted to reduce the number of AD cases in 2050 by 9.2 million, with nearly the entire decline attributable to decreases in persons needing a high level of care [4]. A report by the Alzheimer’s Association concluded that a hypothetical treatment introduced in 2025 that delays AD onset by five years could reduce the proportion of the US population aged ≥65 years living with AD to 8% versus 11% under the current trajectory in 2030, and to 9% versus 16% under the current trajectory in 2050 [1]. Budd et al. [20] used Markov models to simulate transitions of AD patient cohorts beginning in predementia, and followed for ten years. Treatment with hypothetical disease-modifying therapies that reduced the annual risk of progression by 25% increased life-years in predementia/mild states from 3.2 to 4.2 and decreased life-years spent in moderate/severe AD from 2.6 to 2.2. Average time in the community increased from 4.4 to 5.4 years, and time in long-term care decreased from 1.3 to 0.9 years [20].
A clearer understanding of the relationship between a delay in MCI onset due to AD and AD disease trajectory will have important implications for payers. In 2015, the cost of care for people living with AD and other dementias in the US is expected to total $226 billion [1]. Based on the current trajectory of AD, costs are projected to increase to over $1.1 trillion in 2050 [1]. A hypothetical treatment in 2025 that delays the onset of AD by five years could reduce the total costs of AD care in 2030 from $451 billion under the current trajectory to $368 billion. In 2050, total costs could decrease from $1.101 trillion under the current trajectory to $734 billion, a savings of $367 billion [21]. Further research is required to substantiate these predictions and to quantity the benefits of delaying entry into more severe AD states and long-term care, as well as reducing time spent in the more severe and costly AD health states.
This study had several limitations. First, because patients voluntarily enter the data or are referral-based, they do not necessarily comprise a representative sample of the US population. As such, transitional probability estimates may not be generalizable outside of the NACC UDS. Second, progression/staging was modeled based on CDR and does not consider changes to specific symptoms or mechanisms of the disease. Third, data are collected annually, which introduces measurement error in the timing of transitions, and multiple transitions between visits will not be observed. Fourth, the NACC UDS has potentially incomplete institutionalization and death data; both are expected to be under-reported, which may downwardly bias the estimated benefits of delaying disease progression to more severe health states. Finally, estimates are for a closed cohort, and do not account for dynamics such as incidence, diagnosis, treatment, and mortality rates, which would be included in an open cohort epidemiological model.
CONCLUSION
In conclusion, this study produced age-specific disease progression rates across the full AD continuum, including normal cognition through MCI due to AD and severity levels of AD dementia. A model based on these rates demonstrated that delaying the onset of MCI due to AD delayed in turn the progression to AD dementia, reduced time spent in severe AD dementia health states and long-term care, and increased life expectancy. This study suggests that therapies with the potential to delay the onset of MCI due to AD could have significant implications for rates of AD dementia and AD-associated institutionalization and death.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge the medical writing support of Jane Kondejewski, PhD of SNELL Medical Communication, Inc.
Footnotes
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol and manuscript underwent rigorous internal review at Takeda by a cross function review committee.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All humans research procedures followed were in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
CONSENT FOR PUBLICATION
This study used data provided by NACC from the UDS which ensures the privacy of all patient level data.
CONFLICT OF INTEREST
MD, TC, and SJ were employees of Medicus Economics and received funding from Takeda Pharmaceuticals International for the study.
SC, EM, and FM were employees of Takeda Pharmaceuticals at the time of the study completion and manuscript development.
KS has received funding from Takeda Pharmaceuticals International for research unrelated to this study.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- [1].Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11(3): 332–84 (2015a). [DOI] [PubMed] [Google Scholar]
- [2].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease:recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 280–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol 59(11): 1764–7 (2002). [DOI] [PubMed] [Google Scholar]
- [4].Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Fore casting the global burden of Alzheimer’s disease. Alzheimers Dement 3(3): 186–91 (2007). [DOI] [PubMed] [Google Scholar]
- [5].Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord 19(3): 163–65 (2005). [DOI] [PubMed] [Google Scholar]
- [6].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 270–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312(23): 2551–61 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica 119(4): 252–65(2009). [DOI] [PubMed] [Google Scholar]
- [9].Spackman DE, Kadiyala S, Neumann PJ, Veenstra DL, Sullivan SD. Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res 9(9):1050–58 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Olchanski N, Lin PJ, Cohen JT, Neumann PJ. Alzheimer’s disease progression rates: new data from the National Alzheimer’s Coordinating Center. American Neurological Association: 2012 Annual Meeting. Poster Presentation. [Google Scholar]
- [11].Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KS, et al. Measuring Alzheimer’s disease progression with transition probabilities: estimates from CERAD. Neurology 57(6):957–64 (2001). [DOI] [PubMed] [Google Scholar]
- [12].Hubbard RA, Zhou XH. A comparison of non-homogeneous Markov regression models with application to Alzheimer’s disease progression. J App Stat 38(10): 2313–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bloudek LM, Spackman DE, Veenstra DL, Sullivan SD. CDR state transition probabilities in Alzheimer’s disease with and without cholinesterase inhibitor intervention in an observational cohort. J Alzheimers Dis 24(3): 599–607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J,et al. The national Alzheimer’s coordinating center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord 21(3): 249–58 (2007). [DOI] [PubMed] [Google Scholar]
- [15].Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR,Chui H, et al. The Alzheimer’s disease centers’ uniform data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 23(2): 91–101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abner EL, Kryscio RJ, Cooper GE, Fardo DW, Jicha GA, Mendiondo MS, et al. Mild cognitive impairment: statistical models of transition using longitudinal clinical data. Intern J Alzheimer’s Dis 2012(1): 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology 79(15): 1591–98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 257–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association Workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3): 263–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Budd D, Burns LC, Guo Z, L’italien G, Lapuerta P. Impact of early intervention and disease modification in patients with predementia Alzheimer’s disease: a Markov model simulation. Clin Eco Outcomes Res 3: 189–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alzheimer’s Association. Changing the trajectory of Alzheimer’s disease: a national imperative. 2015b. Available from: http://www.cdph.ca.gov/programs/alzheimers/Documents/Changing%20the%20Trajectory%20of%20AD.pdf. Accessed March 15,2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.