Abstract
Objectives:
We aimed to assess the asymptomatic C. difficile carriage rates following FMT.
Methods:
All patients who underwent FMT for recurrent CDI via colonoscopy or sigmoidoscopy between June 2013 and April 2015 and had a minimum of 8-week follow-up post-FMT at two tertiary care referral centers were included in the study. Patients were prospectively followed both clinically and with stool assessments for 8 weeks post FMT. Assessments occurred at 1 week and 4 weeks post-FMT to assess for failure. Failure was defined as presence of diarrheal symptoms and a positive CDI stool test by polymerase chain reaction for toxin gene (PCR) at any time point during the 8-week follow up period. CDI stool testing using PCR was performed at weeks 1 and 4 post-FMT in asymptomatic patients as well.
Results:
167 patients were included. Twenty-eight patients (16.7% [28/167]) were FMT failures throughout the 8-week period. At week 1, 7 patients had already failed the FMT. Of the remaining 160 patients, 144 were asymptomatic, and among these, 141 were negative for c. difficile toxin gene by PCR. This resulted in an asymptomatic carriage rate of 2.1% (3/144). At week 4, 143 patients had not yet failed FMT. Of these patients 129 patients were asymptomatic and among those, 125 were negative by PCR, resulting in an asymptomatic carriage rate of 3% (3/129).
Conclusions:
Asymptomatic carriage after FMT is rare. This suggests testing for cure after FMT in asymptomatic patients is not necessary.
Introduction:
The rates of asymptomatic C. difficile carriage following fecal microbiota transplantation (FMT) are unknown. This is due to the paucity of reports of C. difficile testing after FMT, as current FMT practice embraces C. difficile treatment guidelines recommending against testing for cure [1]. This guidance, however, relies on data from clinical trials using antimicrobial agents. Studies suggested that molecular tests such as polymerase chain reaction (PCR), which detect the presence of toxin gene in the stool, were shown to remain present for up to 30 days despite clinically successful treatment with antibiotics due to their bacteriostatic nature [2,3,4]. Specifically, vancomycin is ineffective at eradicating C. difficile carriage, and although asymptomatic, patients remain persistent vectors to spread C. difficile, a major public health threat [5]. Unlike antimicrobial agents, FMT is not bacteriostatic and likely has a novel mechanism to treat C. difficile infection (CDI) by establishing a newly enriched microbiota that outcompetes C. difficile for nutritional and colonization resources. [2,3,6] However, little data exists about the rates of asymptomatic C. difficile carriage post-FMT due to a lack of testing. Given this, we aimed to assess the asymptomatic C. difficile carriage rate following FMT.
Methods:
We conducted a prospective observational cohort study. All outpatients who underwent FMT for recurrent CDI between June 2013 and April 2015 and had a minimum of 8-week follow-up post-FMT at two tertiary care referral centers, Brigham and Women’s Hospital and Indiana University, were included in the study [7]. Recurrent CDI was defined as three or more confirmed CDI episodes. Donor selection, screening for relevant communicable and microbiome-mediated diseases, and stool processing were performed as outlined by the Fecal Microbiota Transplantation Working Group and FMT best practices [8]. All patients discontinued anti-CDI therapy 24–48 hours prior to FMT and underwent a standard bowel preparation. All FMTs were delivered via colonoscopy or sigmoidoscopy at the discretion of the endoscopist. The stool source was either a directed donor (e.g. spouse) or unrelated healthy volunteer from a universal stool bank (OpenBiome, Somerville) [9].
Patients were prospectively followed both clinically and with stool assessments for 8 weeks post FMT. Assessments occurred at 1 week and 4 weeks post-FMT to assess for failure. Specifically, patients were assessed for diarrhea, defined as three or more unformed stools (Bristol Stool Type 6 or 7) within a 24-hour period [10]. Failure was defined as presence of diarrheal symptoms and a positive CDI stool test using polymerase chain reaction (PCR) to detect the presence of clostridium difficile toxin gene, based on hospital availability, (Cepheid, Sunnyvale CA) at any time point during the 8-week follow up period. CDI stool testing using PCR only was performed at weeks 1 and 4 post-FMT in asymptomatic patients as well. The study protocol was approved by the institutional review boards at both hospitals and all patients were consented for participation.
Results:
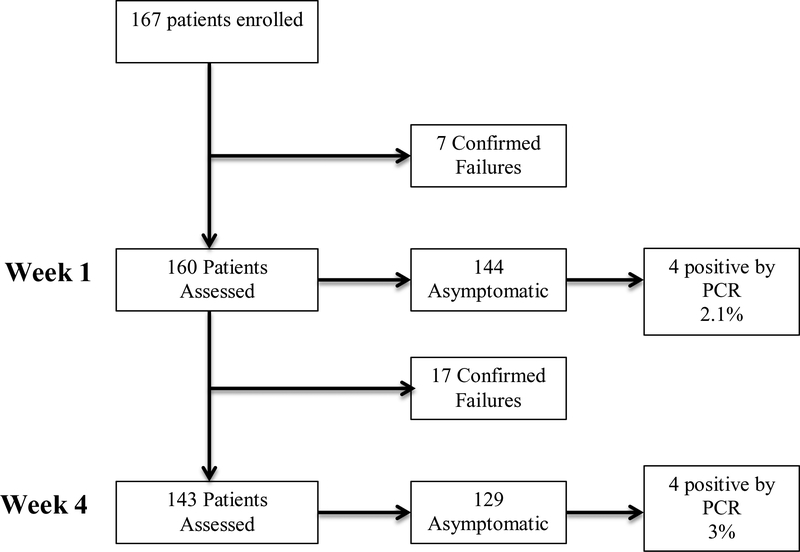
Overall, 167 patients were included in the study (Figure 1). Twenty-eight patients (16.7% [28/167]) were deemed FMT failures throughout the 8-week follow up period.
Figure 1.
Study Flow Chart
At the week 1 assessment, 7 patients had already failed the FMT. Of the remaining 160 patients, 144 were asymptomatic, and among these, 141 were negative by PCR. This resulted in an asymptomatic carriage rate of 2.1% (3/144).
At the week 4 assessment, there were 143 patients remaining that had not yet failed FMT. Of these patients 129 patients were asymptomatic and among those, 125 were negative by PCR, resulting in an asymptomatic carriage rate of 3% (4/129).
Discussion:
In this data set, we found that the majority of asymptomatic patients who were tested in this cohort at both week 1 and 4 were negative by PCR, translating into a C. difficile carriage rate of 3% or less. This suggests that FMT has a much lower C. difficile carriage rate than the ~15% established by Wenisch and colleagues following metronidazole, teicoplanin, vancomycin, or fusidic acid therapy [3]. Thus, asymptomatic carriage after FMT is rare.
Our study should be interpreted within the context of its limitations. Stool PCR testing was carried out at multiple laboratories using different commercial assays with variable performance characteristics though all validated and FDA approved. Additionally given laboratory availability we were only able to use PCR testing, which cannot distinguish between colonization and true infection in symptomatic patients, therefor all symptomatic patients with a positive test were deemed failures and retreated. This is due to the fact that molecular tests, such as PCR, target toxin genes, and therefor detect bacteria but do not detect the actual presence of toxin, making it unclear if a positive test represents clinical disease or merely colonization with toxigenic organisms when symptoms are present [11]. Testing using enzyme immune assays for toxin A/B in the post-FMT setting is preferable for assessing FMT failure but was not possible to assess in this study.
This data supports current clinical guidelines, which suggest testing for cure after FMT in asymptomatic patients is not necessary. However, the rationale here is distinct from post-antibiotic treatment. FMT nearly eliminates asymptomatic toxigenic C. difficile carriage whereas vancomycin commonly results in asymptomatic carriage. Additionally it has been recently shown that FMT leads to sustained microbial engraftment, which may account for its high efficacy [12]. Importantly, this highlights that patients successfully treated with FMT are not likely vectors of infection; either to others or re-infection in themselves [13,14]. Infection control practitioners have had limited tools in their armamentarium beyond antimicrobial stewardship, hand washing practices and isolation, and FMT may have the potential to decrease the public health burden of C. difficile. Given the continued rise of CDI and difficulty with outbreaks in hospitals and long care facilities where standard practice has failed to prevent the spread of disease, FMT may represent a possible novel therapy to eradicate these vectors and lesson the burden of CDI. Further microbiome analysis in this patient population is needed to understand the possible public health implication further.
Acknowledgments
Funding:
No external funding was received
Footnotes
Authorship:
JA, MF: study concept and design, analysis and interpretation of data, critical revision of the manuscript. EP: acquisition of data. HX, AA: study and design analysis and revision of manuscript. All authors approved the final version of this manuscript
Conflicts of Interest:
Allegretti has served on scientific advisory boards for Finch Therapeutics.
References
- 1.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 2.Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, Elmer GW. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 2000; 31: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 3.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis 1996; 22: 813–818. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky GS, Jump RL, Donskey CJ. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 2008; 47: 56–62. [DOI] [PubMed] [Google Scholar]
- 5.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med 2006; 73: 187–197. [DOI] [PubMed] [Google Scholar]
- 6.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Hogenauer C, Malfertheiner P, Mattila E, Milosavljevic T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A, European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, Moore TA, Russell G, Surawicz C, Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith M, Kassam Z, Edelstein C, Burgess J, Alm E. OpenBiome remains open to serve the medical community. Nat Biotechnol 2014; 32: 867. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31: 431–455. [DOI] [PubMed] [Google Scholar]
- 11.Polage CR, Gyorke CE, Kennedy MA, Leslie JL, Chin DL, Wang S, Nguyen HH, Huang B, Tang YW, Lee LW, Kim K, Taylor S, Romano PS, Panacek EA, Goodell PB, Solnick JV, Cohen SH. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern Med 2015; 175: 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalanka J, Mattila E, Jouhten H, Hartman J, de Vos WM, Arkkila P, Satokari R. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med 2016; 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes S, van Nood E, Tims S, Heikamp-de Jong I, ter Braak CJ, Keller JJ, Zoetendal EG, de Vos WM. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J 2014; 8: 1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]