Abstract
Fungi are ubiquitous in indoor and outdoor environments and have been associated with respiratory disease including childhood and adult asthma. A growing body of evidence from human and animal studies has revealed a link between fungal exposure, especially indoor fungal exposure, with asthma initiation, persistence, and exacerbation. Despite the overwhelming evidence linking mold exposure and asthma, the mechanistic basis for the association has remained elusive. It is now clear that fungi need not be intact to impart negative health effects. Fungal components and fungal fragments are biologically active and contribute to asthma development and severity. Recent mechanistic studies have demonstrated that fungi are potent immunomodulators and have powerful effects on asthma independent of their potential to act as antigens. This paper will review the connection between fungal exposure and asthma with a focus on the immunological mechanisms underlying this relationship.
Keywords: Fungi, Mold, Exposure, Asthma, Childhood asthma
Introduction
Asthma is clinically characterized by recurrent episodes of wheezing, breathlessness, coughing, and chest tightness. It is a chronic inflammatory disease of the conducting airways in which many cells of the innate and adaptive immune systems act together with epithelial cells to cause airway hyperresponsiveness (AHR), inflammation, mucus overproduction, airway wall remodeling, and airway narrowing. It is prevalent in more than 10% of the population in many industrialized countries and up to 300 million people are affected worldwide [1, 2]. Asthma has long been characterized as a disease of dysregulated TH2 immune responses to environmental allergens, but accumulating evidence suggests a role for TH17 cells, especially severe steroid resistant asthma [3, 4]. Immunohistochemistry on bronchial biopsies from asthmatics reveals increased IL-17A+ cells in patients with severe asthma compared to mild asthma or controls [5]. In both adults and children, serum IL-17A is significantly higher in severe asthmatics compared to mild asthmatics or controls [6–8].
It is well accepted that IL-17 has an important proactive role against infections with molds [9–11]. Thus, fungal exposure may modify asthma morbidity by promoting TH17 immune responses. Indeed, children exposed to high levels of mold exposure have increased levels of IL-17A and have more frequent asthma symptoms [12••]. Recent data demonstrate that fungal components are potent immunomodulators and promote development of severe steroid-resistant asthma independent of their potential to act as allergens.
Fungal exposure has been strongly implicated in the development and prevalence of asthma. In 2007, nearly half of the weekly requests received by the National Institute for Occupation Safety and Health concerned work-related asthma and fungal exposure [13]. In the Cincinnati Allergy and Air Pollution Study (CCAAPS) longitudinal birth cohort, fungal exposure was associated with increased incidence of wheeze in infants [14], increased risk of developing asthma at age 3 [15•], and was a predictor of asthma development at age 7 [16••]. The identification of fungi as an important component of the environmental contribution to the asthma phenotype leads to questions about possible interventions to prevent and/or attenuate fungi-related health effects. Herein, we will review the connection between fungal exposure and asthma with a focus on the immunological mechanisms underlying this relationship.
Fungal Biology and Structure
Life Cycle of Fungi
Fungi are eukaryotic organisms that lack chlorophyll and obtain their nutrients from the growth media by the use of enzymes. It is estimated that there are at least 1.5 million species, although only about 80,000 have been described [17]. Most fungi are saprobic as they can grow in non-living growth media, such as dead plants, soil, or moist building materials. Parasitic fungi require a specific living host, and symbiotic fungi grow in close association with another living organism. Some fungi can switch from one mode of life to another in order to adapt to environmental conditions [18]. For example, a soil saprophytic fungus, Aspergillus fumigatus, can become pathogenic upon inhalation into the lungs of an immunocompromised subject.
Fungi may be unicellular or multicellular. Yeasts are unicellular organisms, which reproduce mainly by budding (e.g., Candida). Multicellular fungi are formed of filaments (hyphae) that infiltrate the material they feed on and develop into complex networks known as mycelium. The filamentous microfungi found indoors are often called molds (e.g., Aspergillus and Penicillium). They can form visible colonies that commonly appear as green, black, or brownish spots. “Mildew” is a layman’s term referring to fungi growing on windowsills or bathroom tile. Mushrooms, puffballs, bracket fungi are examples of common outdoor fungi that form large fruiting bodies.
Spores are specialized microscopic cells that are actively or passively dispersed from fungal colonies in order to colonize new and suitable environments. These spores can be formed asexually or sexually. The most common spore types encountered in indoor environments are asexual spores, either conidia or sporangiospores. Conidia are formed externally to the spore-producing cells, conidiophores (e.g., Aspergillus and Penicilium). Sporangiospores are formed inside receptacles called sporangia (e.g., Rhizopus and Mucor).
Fungal Spore Components
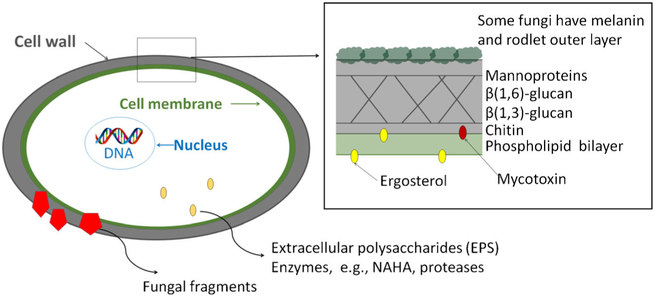
Fungal spores are covered by a rigid cell wall, which consists of three major layers: mannoproteins, glucans, and chitin (Fig. 1). Mannoproteins consists of mannans, which are long chains of mannose residues. They are connected to fungal proteins via N- and O-linkages [19]. There are two major types of glucans on the fungal cell wall: β−1,3-and β−1,6-glucans. β−1,3-glucans form the backbone of the cell wall and account up to 60% of the dry weight of the fungal cell wall. They are connected to varying amounts of β−1,6-glucan branches [20]. Chitin, a fibrous substance consisting of polysaccharides, adds to the structural strength of the cell wall.
Fig. 1.
Health relevant structures in fungal spores
β-D-glucans are commonly used as a marker of mold exposure in the environment [21] and are important for recognition of molds by the immune system. Alterations in exposure of beta-glucans on mold spores can change the host response to molds. Surface availability of beta-glucans on mold spores is more important in determining the immune response than the total beta-glucan content of spores [22]. Some fungi, e.g., Aspergillus, have additional components on the outer layer, e.g., α-glucans, melanin, and rodlets. These can cover the immunologically active cell wall components on the cell wall. For example, when α-glucans cover β-glucans, the recognition by Dectin-1 is prevented [19, 22]. Melanins are amorphous phenolic compounds that protect cells from UV-stress and other oxidative killing mechanisms. An example of a melanin compounds is the green fungal pigment, dihydroxynaphtalene [19]. Rodlets consists of highly hydrophobic proteins. The rodlet layer is fragmented during spore swelling and germination resulting in the exposure of underlying layers [20]. Removal of rodA, either genetically or chemically, from A. fumigatus spores increases activation of dendritic cells and increases binding of Dectin-1 [23]. Several studies have examined the direct relationship between beta-glucans and health effects with inconsistent results [24]. The inconsistencies in the literature may be explained by the fact that it is not the amount of beta-glucans present, but rather the surface availability of beta-glucans on the mold spores in the samples taken. Heat killing of mold spores exposes the beta-glucans and alters the immune response [25, 26].
The fungal cell membrane consists of two layers of phosholipid molecules. An essential component of the cell membrane is ergosterol that regulates membrane fluidity, reproduction, and function [27]. The cell wall also contains enzymes, such as β-N-acetylhexosaminidase (NAHA), which catalyzes the digestion of chitin and cellulose. While growing, fungi emit various components to the surrounding environment. Proteases (or peptidases) are both intracellular and extracellular, i.e., emitted outside the fungal cell. Proteases play an important role in fungal nutrition by digesting proteincontaining substrates. Proteases are also involved in the fungal reproduction facilitating germination and conidial discharge. Seven major types of proteases have been identified: asparagine, aspartic, cysteine, glutamic, metallo, serine, and threonine. Most clinically relevant proteases belong to serine protease family or the aspartic protease or metalloproteinase groups [28]. Serine proteases are the major allergenic proteins in many Penicillium and Aspergillus species [28]. Extracellular polysaccharides (EPS) are stable carbohydrates that are excreted during fungal growth to facilitate the attachment of the fungus to the surface on which it is growing. EPS have antigenic specificity at genus level.
Fungi produce secondary metabolites during their growth, for example, antibiotics and mycotoxins. Mycotoxins are nonvolatile, low-molecular weight compounds, such as aflatoxins, satratoxins, and ochratoxin. The types and amounts of toxin produced by fungi depend on the species, the fungal strain as well as on the substrate and growth conditions [29].
As a eukaryotic organism, fungal cells have one or more well-defined membrane-bound nuclei. Each nucleus can contain more than one chromosome where the DNA is surrounded by histone proteins. In addition to intact spores, fragments, i.e., pieces of spores and mycelium, can be aerosolized from fungal growth [30]. Fragments have been shown to contain many of the immunologically active components, such as fungal antigens, mycotoxins, β−1,3-glucans, and NAHA enzyme [30, 31]. In a recent study, fragment concentration was assessed by measuring β−13-glucan concentration in particle size fraction below 1 μm. Higher fragment concentrations were found in homes of asthmatic children compared to homes of non-asthmatic children [32].
Role of Fungal Exposure in Asthma Health
Exposure Assessment
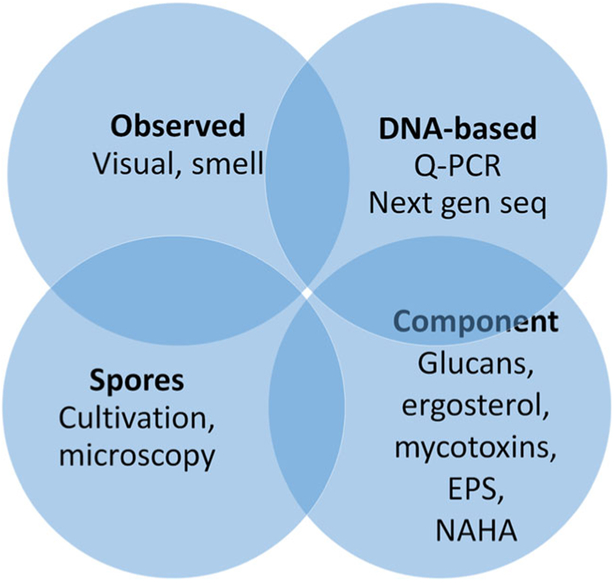
Exposure to fungi can be assessed with qualitative and/or quantitative methods (Fig. 2). Qualitative assessment includes visual observations of water damage, signs of moisture/dampness, or mold as well as olfactory observation of moldy smell [33]. Quantitative assessment can be based on culturable or microscopic count of fungal spores or quantification of fungal components. Glucans, ergosterol, EPS, and NAHA have been used as markers of total fungal biomass [34]. Glucans can be analyzed by using limulus amebocyte lysate (LAL) assay or with enzyme immunoassays (EIAs). EIAs can also be used for the analysis of fungal allergens and EPS, whereas ergosterol analysis involves specific mass spectroscopy-based methodology. NAHA enzyme activity can be measured by a fluorescence-based Mycometer method [31]. Mycotoxins can be analyzed by high-performance liquid chromatography or tandem mass spectrometry or a combination of these two [35].
Fig. 2.
Most common analysis methods for assessment of mold exposure
Quantitative mold-specific PCR targets the DNA-sequence of predetermined fungal species. The recently developed Environmental Relative Moldiness Index (ERMI) is based on the analysis of 36 fungal species whose concentrations are used in calculating the moldiness index [36]. An emerging method is next-generation sequencing, which gives semiquantitative data on the diversity of fungal communities. It produces hundreds to thousands of exposure variables (operational taxonomic units, OTUs) per sample, which are typically used as relative abundance data [34].
Studies that have used several methods for the assessment of mold exposure have shown only moderate or weak correlation between them [37–40]. This inconsistency can be explained by the fungal biology: spore release dynamics and the variation in the characteristics of spores of different species. Fungal spores are not released into the air continuously. Therefore, even when there is visible mold in the room, the airborne fungal concentrations can be low [41]. On the other hand, mold can be hidden behind walls or other surfaces, but spores could still be released into the indoor air through cracks. This causes inconsistencies between observed and measured mold.
Each of the quantitative mold assessment methods provides a different perspective on the quantity of mold. Cultivation accounts only for those spores and cells that can grow on the culture media. Lee et al. [42] reported that in air samples collected from homes, the culturable count was only 2–38% of the respective microscopic count, depending on the species. The concentration of mold components per spore is affected by the growth media and by the fungal species. For example, β−1,3-glucan content for Aspergillus vericolor is 0.08 pg/spores, whereas respective concentration is 8.66 pg/spore for Cladosporium herbarum and 241 pg/spore for Epicoccum nigrum [40, 43].
The sampling method also has an important effect on the measured concentration. Samples for the quantitative analysis can be collected with active or passive air samplers, by vacuuming dust or collecting samples of contaminated materials. Active air sampling can be conducted by using filter samplers, impactors, impingers, or electrostatic samplers. Passive air samples have been collected with standard sampling surfaces, e.g., simple cardboard boxes or electrostatic cloths that have been positioned at a specified height above the floor. Vacuuming is typically done from the floor, bed, or surfaces that are not frequently cleaned. Low correlations have been reported when the same exposure metric has been used for multiple sample types (e.g., floor vs. air) [40, 44]. More detailed reviews of bioaerosol exposure assessments can be found in Reponen et al. [45] and Macher et al. [46].
Summary of Relevant Epidemiological Studies
Numerous epidemiological studies worldwide have established an association between observed mold or water damage and asthma [33, 47]. Evidence supporting the association between measured fungal concentrations and asthma is increasing [16••, 48]. Only a small fraction of these studies, however, has included the atopic status of the study subject in their study design, and these studies are summarized in Table 1.
Table 1.
Recent epidemiological studies on the association between mold exposure and children’s asthma that included assessment of atopic wheezing and/or asthma
| Source | Study design | Exposure measurement | Outcome | Risk estimate (% prevalence or OR)* |
|---|---|---|---|---|
| Rylander et al., 1998a [49] | 347 children in two schools (problem versus control school) | Visually observed mold | Atopic wheeze Non-atopic wheeze |
13.5 vs. 2.8% (p = 0.014) 36.4 vs. 13.3% (p >0.05) |
| Ronmark et al., 1999b [50] | Cohort of 3431 children age 7–8 in Sweden | Parent reported dampness in home | Any asthma Atopic asthma Non-atopic asthma |
1.54 (1.10–2.14) 1.40 (0.81–2.42) 1.78 (1.10–2.89) |
| Schram-Bijkerk et al., 2005c [51] | Cohort of 899 children age 5–13 (168 current atopic wheezers and 441 controls) | EPS in dust β−1,3-glucan in dust |
Atopic wheeze Atopic wheeze |
0.95 (0.70–1.30) 0.83 (0.56–2.11) |
| Bernstein et al., 2006d [52] | UV-HVAC intervention in homes of 19 mold-sensitized asthmatic children age 5–17 | UV-HVAC vs. placebo | PEFR variability Severity scores of shortness of breath and chest tightness |
9.6±13.6% vs. 9.8±3.8% (p <0.05) 75% reduction vs. 33% reduction (p < 0.05) |
| Inal et al., 2007e[53] | Prospective cohort of 19 mold-sensitized asthmatic children age 4–13; | Culturable airborne fungi | Daily asthma score PEF morning PEF evening |
r = −0.021 (p = 0.932) r = −0.056 (p = 0.475) r = −0.057 (p = 0.471) |
| Pongracic et al., 2010f [54] | Prospective cohort of 469 mold-sensitized asthmatic children age 5–11 | Culturable airborne total fungi indoors Culturable airborne Penicillium indoors |
Unscheduled visits to emergency room Unscheduled visits to emergency room |
1.16 (1.02–1.33) 1.13 (1.04–1.24) |
| Gent et al., 2012g [55] | Prospective cohort of 1233 asthmatic children age 5–10 | Culturable airborne Penicillium Culturable airborne Cladosporium |
Wheeze among Penicillium sensitized Asthma severity score among Penicillium sensitized Wheeze among Cladosporium sensitized Asthma severity score amongCladosporium sensitized |
2.12 (1.12–4.04) 1.99 (1.06–3.72) 1.22 (0.66–2.26) 1.58 (0.88–2.83) |
| Choi et al., 2014h [37] | Nested case-control of 198 symptomatic (asthma, rhinitis and/or eczema) and 202 non-symptomatic children | Culturable fungi in dust (including most common genera and yeasts) β−1,3-glucan in dust Ergosterol in dust |
Asthma, rhinitis, eczema Allergen sensitization |
No differences in measured fungal concentration between cases and controls No differences in measured fungal concentration between sensitized and non-sensitized cases |
| Maheswaran et al., 2014i [56] | Longitudinal birth cohort of 422 children | β−1,3-glucan in dust at age 7–10 (models adjusted for endotoxin) | Prevalent asthma Incident asthma Prevalent atopic asthma Incident atopic asthma Incident non-atopic asthma |
1.62 (1.06–2.47) 2.09 (0.89–4.90) 1.53 (1.02–2.30) 2.81 (0.82–9.56) 1.01 (0.60–1.69) |
| Sharpe et al., 2015j [57] | Cross-sectional on 2849 children age 6–17 in NHANES study | Self-reported presence of mildew/musty odor Alternaria alternata allergen in house dust Aspergillus fumigatusallergen in house dust |
Any asthma Atopic asthma Non-atopic asthma Any asthma Atopic asthma Non-atopic asthma Any asthma Atopic asthma Non-atopic asthma |
1.59 (1.04–2.42) 1.81 (1.01–3.25) 1.47 (0.79–2.72) 0.35 (0.14–0.87) 0.39 (0.10–1.55) 0.25 (0.07–0.86) 1.03 (0.82–1.41) 0.84 (0.41–1.72) 0.94 (0.51–1.77) |
| Dannemiller et al., 2016k[58•] | Cohort of 196 asthmatic children age 5–10 years Longitudinal birth cohort study of 640 children2 |
Fungi in dust determined by qPCR Allergenic species in dust by NGS and qPCR β−13-glucan in dust determined by LAL assay |
Asthma severity: Any asthma Atopic asthma Non-atopic asthma Any asthma Atopic asthma Non-atopic asthma Any asthma Atopic asthma Non-atopic asthma Wheeze at age 1: Any recurrent wheeze |
2.02 (1.14–3.56) 1.69 (0.77–3.75) 2.40 (1.06–5.44) 2.53 (1.28–5.00) 2.71 (0.99–7.39) 2.38 (0.94–6.01) 0.55 (0.24–1.26) 0.47(0.13–1.68) 0.60 (0.20–1.83) 2.1 (1.2–3.6) |
| Cho et al. [14] 2006l | Visually observed mold (high vs. none) in home at age 1 | Atopic recurrent wheeze Atopic recurrent wheeze with mold sensitization |
4.7 (2.1–10.5) 0.6 (0.1–4.0) |
|
| Iossifova et al. 20071 [59] | Sub-set of 574 from Cho et al. 2006 | Visually observed mold (high vs. none) in home at age 1 Low β−1,3-glucan in dust determined by LAL assay High β−1,3-glucan in dust determined by LAL assay |
Wheeze at age 3: Any recurrent wheeze Atopic wheeze vs. no wheeze and no atopy Any recurrent wheeze Atopic wheeze vs. no wheeze and no atopy Any recurrent wheeze Atopic wheeze vs. no wheeze and no atopy |
4.44 (1.63–12.05) 9.51 (2.34–38.63) 3.04 (1.25–7.38) 4.89 (1.02–23.57) 0.39 (0.16–0.93) 0.13 (0.03–0.61) |
| Reponen et al. 20111 [16••] | Sub-set of 176 children from Cho et al. 2006 | Visually observed mold: -Age 1 - Age 7 ERMI in dust: -Age 1 - Age 7 |
Asthma at age 7: Any asthma Any asthma Any asthma Any asthma |
0.3 (0.03–2.04) 1.1 (0.29–4.52) 3.1 (1.38–6.93) 0.7 (0.25–1.71) |
| Zhang et al. 20161 [12••] | Sub-set of 288 children from Cho et al. 2006 | ERMI in dust at age 1 | Asthma at age 7: Any asthma Atopic asthma Atopic asthma without mold sensitization |
3.54 (1.85–6.81) 4.38 (2.08–9.22) 7.36 (2.13–25.43) |
All the values in italics are statistically significant (p > 0.05).
Rylander skin prick test for house dust mire, fungi, animal dander, and pollens
Ronmark: skin prick test for birch, timothy, mugwort, dog, cat, horse, two mites (Dermatophagoides farinae and D. pteronyssinus), and two molds (Cladosporium and Alternaria)
Schram-Bijkerk: atopy = Phadiatop positive (?)
Bernstein: skin prick test, Aspergillus fumigatus, Penicillium notatum, Cladosporium herbarum, and Alternaria alternate (also for dust mite, cat, dog, cockroach were tested)
Inal: skin prick test for mites (D. pteronyssinus and D. farinae), grass mix, tree mix, mold mix, Alternaria, Cladosporium, Penicillium, Aspergillus (also eucalyptus pollen, olive pollen, cat and dog, and dust mites were included)
Poncratic: skin prick test for Alternaria alternata, Cladosporium herbarum, Penicillium chrysogenum, Aspergillus mix (A. flavus, A. fumigatus, A. glaucus, A. nidulans, A. niger) (also included Dermatophagoides farinae, Dermatophagoides pteronyssinus, cockroach mix (German and American), rat, mouse, cat, and dog)
Gent: total IgE and IgE antibodies for 10 allergens: Penicillium notatum [properly known as P. chrysogenum], Cladosporium herbarum), dust mite (Der p 1, Der f 1), cat (Fel d 1), dog (Can f 1), cockroach (Bla g 1), meadow grass (Kentucky blue, Poa pratensis), ragweed (Ambrosia elatior), and egg
Choi: IgE antibodies to 10 allergens: timothy, mugworth, birch, cat, horse, dog, house dust mites, Penicillium, and Cladosporium
Maheswaran: skin prick test to 16 allergens: including tree pollen mix, weed pollen mix, ragweed, grass pollen mix, Alternaria, Cladosporium, Penicillium, house dust mites, cockroach, cat, dog, feathers, and peanut
Sharpe: total IgE0
Dannemiller: total IgE
Cincinnati Childhood Allergy and Air Pollution Study: skin prick test for 15 aeroallergens: meadow fescue, timothy, white oak, maple, American elm, red cedar, short ragweed, Alternaria spp., Aspergillus fumigatus, Penicillium spp., Cladosporium spp., cat, dog, German cockroach, and house dust mite
Several studies have focused on the respiratory health of asthmatic children sensitized to fungi. Pongratic et al. [54] and Gent et al. [55] reported a positive association between respiratory health and culturable airborne total fungi and culturable airborne Penicillium. The association between culturable airborne Pencillium became stronger when the model was adjusted for outdoor concentration of culturable fungi [54]. Wheeze and asthma severity score among Penicillium-sensitized children was associated with the concentration of culturable airborne Penicillium spores; however, wheeze and asthma among Cladoporium-senitized children was not associated with the concentration of culturable airborne Cladosporium [55].
Choi et al. [37] compared concentrations of β−1,3-glucan, ergosterol, and culturable fungi, including most common genera and yeast, in dust samples collected from homes of cases (homes that had a child with asthma, rhinitis, and/or eczema) and controls (non-symptomatic children). No difference was found in any of the measured concentrations between cases and controls. Similarly, no difference was found in any of the concentrations between sensitized and non-sensitized cases. In contrast, Maheswaran et al. [56] found that increased β−1,3-glucan concentration was significantly associated with increased prevalence of atopic asthma. No association was found between β−1,3-glucan and non-atopic asthma. This nicely aligns with recent mechanistic data (discussed below) demonstrating that β-glucan exposure exacerbates atopic asthma [12••]. Sharpe et al. [60] also found an association between self-reported presence of mildew/musty odor and atopic asthma, but not with non-atopic asthma.
Dannemiller et al. [58•] used quantitative PCR (qPCR) and next-generation sequencing (NGS) to analyze archived dust samples collected from the homes of asthmatic children. Asthma severity was associated with the total concentration of fungi analyzed by the qPCR. The concentration of allergenic fungal species was associated with severity of any asthma. Fungal richness assessed by NGS was not associated with asthma severity.
Our group conducted a longitudinal birth cohort study, the CCAAPS. Among CCAAPS children, visually observed mold in child’s home at age 1 was associated with wheezing at ages 1 and 3 [14, 59] but not with asthma at age 7 [16••]. Furthermore, visually observed mold in home at age 7 was not associated with asthma at age 7 [16••]. The association with wheeze was stronger among atopic than among non-atopic children [14, 15•]. The association of β−1,3-glucan exposure with wheezing was also assessed among the CCAAPS children [59]. Among the children with a low β−1,3-glucan level in the dust, the exposure was a risk factor, but among the children with a high β−1,3-glucan level in the dust, the exposure was protective. This observation supports that exposure dose or load of β−1,3-glucan may be an important factor in determining health outcome. This may be relevant to mouse studies discussed in the next section. A significant positive association was found between asthma at age 7 and fungal exposure measured using qPCR-based ERMI: children who had high ERMI in their home at age 1 were more likely to have developed asthma at age 7 than children who had low ERMI in their home [16••]. In order to determine whether fungal sensitization was required for the observed effect, we stratified the data by aeroallergen sensitization (skin prick test (SPT) result) and we found that high ERMI exposure was associated with increased asthma prevalence at age 7 only in the SPT positive or atopic group [12••]. Further, this association was not dependent on fungal sensitization. These data demonstrate that fungal exposure impacts asthma independent of the ability of fungus to act as an allergen. The same study showed that β-glucans have immunomodulatory effects on the immune response induced by other allergens, which co-exist in the environment with fungi.
Measurements of chitin and proteases, which are highly relevant in the mechanism of allergy development, have not been included in epidemiological studies. Studies that included more than one mold exposure metric, typically reported differing, sometimes even opposing results between the various matrices. This underscores the difficulty in untangling the complex roles of various mold components in the development of allergic respiratory diseases.
Mechanistic Underpinnings of Health Impact of Fungal Exposure on Asthma
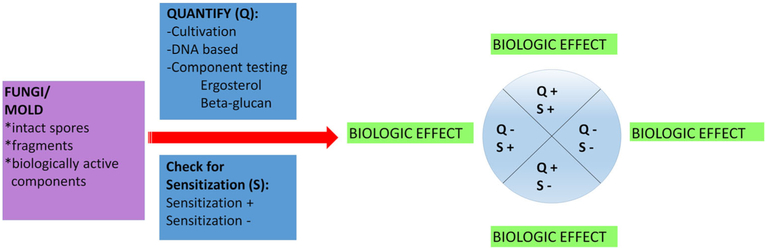
Fungal components can serve as allergens and trigger an IgE response that contributes to asthma development and asthma severity. In clinical practice, allergen-specific IgE, demonstrated by skin testing or in vitro assays, is generally used as a guide for environmental modification and immunotherapy. However, it is important to recognize that biologically relevant fungal exposure does not require sensitization. While sensitization is indicative of exposure, it does not provide any information regarding the level of exposure. Similarly, a lack of sensitization does not indicate an absence of exposure or low exposure. In fact, as discussed above, many mold components, which lack allergenic potential, are biologically active and have been shown to contribute to asthma severity [12••]. Thus, it is important to quantify fungal exposure in addition to assessing fungal sensitization (Fig. 3). A biologic effect may be observed even in the absence of measured exposure because the current assessment methods are not very sensitive and often do not correlate with each other.
Fig. 3.
Proposed algorithm for fungal exposure assessment and related biologic effect
Because fungal cell wall components are widely conserved across the fungal kingdom but absent in humans, they are convenient targets for immune recognition by airway epithelial cells or other cells of the immune system. These cells express a broad repertoire of pattern recognition receptors (PRRs), which can sense both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) produced by local cellular death or stress. The components of fungal cell wall are the main source of PAMPs recognized by PRRs on the host cells [61, 62]. In normal environments, individuals are exposed to numerous factors (viruses, bacteria, dust mites, pets, traffic pollution, etc.) simultaneously, not only mold. Most studies have examined the impact of one or two exposures without considering the total environment. Obviously, these studies are difficult to do, but biologically, the immunomodulatory effect of mold may be modified in the presence of other TLR ligands, PAMPs, and/ or DAMPs. We will review the mechanistic underpinnings of non-allergenic fungal components (β-glucans, fungal chitin, et al.) on asthma health, i.e., the non-IgE-related mechanisms by which fungi contribute to asthma.
β-glucans
β-(1,3)-glucans are non-allergenic water-insoluble cell wall components of most fungi, some bacteria, most higher plants, and many lower plants. β-(1,3)-glucans are recognized by the Dectin-1 receptor which is expressed on macrophages, monocytes, neutrophils, and dendritic cells. Dectin-1 is a type II transmembrane C-type lectin-like receptor, and signaling through Dectin-1 promotes fungal immunity by inducing dendritic cells to polarize T cells toward Th17 cells [63]. Several studies have explored the potential effects of β-glucans on cultured inflammatory cells. Kataoka et al. [64] reported that curdlan, a (1,3)-β-glucan, can activate macrophages and induces expression of inducible nitric-oxide synthase, TNFα, and MIP-2. Treatment of RAW 264.7 cells, a macrophage cell line, with curdlan induces dose- and time-dependent changes in gene transcription, especially in genes involved in the NFκB, TGF-β, p53, JAK/STAT, P13/AKT, phospholipase C, and stress signaling pathways [65]. These effects were Dectin-1 dependent. β-glucans also have effects on other cells including epithelial cells. Exposure to β-glucans induces IL-6, IL-8, and CCL-20 from airway epithelial cells in vitro and in vivo through Dectin-1 [66–69]. Exposure of mice to curdlan intratracheally results in the induction of numerous genes including CCL3, CCL11, CCL17, IFNγ, IL1α, IL-20, and TNFα [70]. Although β-glucan exposure induces an inflammatory response in vivo, by itself, it is not enough to induce an asthma phenotype such as AHR or lung eosinophilia in mice. This is consistent with epidemiologic studies that have shown that the impact of β-glucan exposure is most evident in the context of atopic or allergic asthma. The impact of β-glucan is best studied in the context of other exposures especially allergens which are often highly prevalent and coexisting in the same environments as the fungi.
Several studies have examined the effect of co-exposures to fungi and allergen. In one study, investigators exposed mice to Candida albicans plus ovalbumin (OVA) allergen [71]. The co-exposed mice had exacerbated eosinophilic airway inflammation, TH2, and TH17 cytokines, chemokines (KC, MIP-1α, and eotaxin) compared to mice exposed to OVA alone. In a more recent study [72], A. fumigatus conidia plus OVA was used to induce asthma in rats, and increased airway eosinphilia and AHR were observed in the combination treatment group compared to the OVA alone group. These studies indicated that exposure to intact fungi in combination with allergen resulted in enhanced AHR and inflammation, but the relevant components of fungi that were responsible for the health effects remained unknown. More recently, the role of β-glucans has been directly examined. One study found that exposure to curdlan plus OVA allergen resulted in a modest increase in lung inflammation and neutrophilia in mice [73]. Recently, our group directly compared exposure to fungal spores combined with house dust mite (HDM) allergen and curdlan plus HDM in a mouse model of asthma [12••]. We found that coexposure to Avicularia versicolor spores and HDM allergen resulted in a much more severe asthma phenotype with augmented AHR, eosinophilia, neutrophilia, and lung tissue inflammation compared to exposure to either fungi or HDM alone. The nature of the immune response was also altered. Exposure to HDM caused a TH2 response, but little or no TH17 response. In contrast, exposure to A. versicolor spores plus HDM resulted in a mixed TH2 and TH17 response. The fungal exposure significantly enhanced the Th2 response and induced a strong TH17 response as well. Further, this severe asthma is resistant to steroids and characterized by mixed TH2 and TH17 responses, including IL-13+IL17+CD4+ double-producing T effector cells. These cells have been linked to asthma severity and steroid resistance [74], and transfer of antigen-specific, IL-17A, and IL-13 double-producing CD4+ T cells into naïve mice triggered more severe inflammation upon allergen challenge compared to conventional TH2 or Th17 cells [75]. We found that steroid resistance is dependent on fungal-induced TH17 responses as steroid sensitivity was restored in IL-17RC−/− mice [12••]. Although fungal sensitization occurred in the co-exposure model, we found that the impact of fungal exposure on allergen-induced asthma was not dependent of sensitization based on the following. First, all of the observations made with fungal spores in the HDM co-exposure model were also see with curdlan plus HDM exposure, and curdlan cannot induce sensitization. Second, consistent with the mouse findings, children exposed to high levels of fungus (quantified by ERMI) had increased asthma severity and increased IL-17A and this was independent of fungal sensitization. Furthermore, these changes were confirmed to be Dectin-1/TH17 signaling dependent through dentin-1 null mice and anti-IL17A neutralizing antibodies.
In addition to the mounting data supporting that β-glucan exposure contributes to asthma development and severity, there are several studies that support that β-glucan exposure is protective in asthma development [76–79]. Unfortunately, these studies did not examine the role of Dectin 1 or IL-17A. The conflicting results may be due to differences in the route of β-glucan exposure, the β-glucan dose or load, and/or the immunologic activity of the β-glucan used (different β-glucans have different affinities for Dectin-1). In these studies, the β-glucan was delivered orally, by intraperitoneal injection or applied to the skin, which may yield very different results than inhalation or lung delivery. Also the doses of β-glucan used were greater (6 to 125 mg/kg) than those used in asthma models (less than 1 mg/kg) [12••]. Collectively, these findings imply β-glucans may have different health effects depending on the dose, different exposure route, and the timing of exposure. Additional co-exposures undoubtedly also impact the biologic processes and the ultimate health outcome.
Chitins
Chitin, a polymer of N-acetylglucosamine and the second most abundant polysaccharide in nature following cellulose, is an essential component of fungi, house dust mites, exoskeletons of crabs, shrimp and insects, parasitic nematodes, and digestive tracts of many insects. In 2008, chitin was identified as a PAMP, which has important immunologic effects in vitro and in vivo. Chitin binds to different receptors in a size-dependent manner. Thus, chitin may have sizedependent effects on immune cell functions. Large chitin particles (>70 μm) induce no known response; medium-sized particles (40–70 μm) induce inflammatory responses characterized by IL-17 and TNFα; and small particles (<40 μm) have been reported to induce an anti-inflammatory response characterized by IL-10 [80, 81]. Chitins can activate macrophages and natural killer cells to release a number of proinflammatory cytokines, and in vivo induce eosinophilic lung inflammation and enhance the recruitment of innate lymphoid cells (ILC) to the lung [82–84]. Chitins have been reported to induce the expression of IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), which activate ILC2 cells to express IL-5 and IL-13, leading to eosinophilia [85•]. Another recent publication reported that exposure to chitins derived from Mucor rouxii rapidly induced the expression of IL-25, IL-33, and TSLP in BEAS-2B, human bronchial epithelial cells, A549, and H292 lung carcinoma cells [86].
Chitin content is an important determinant of the impact of fungal exposure on lung inflammation. O’Dea EM et al. [87] compared lung immune responses to conidia from two fungal isolates that expressed different levels of chitin in their cell walls and found that repeated aspirations of the high-chitinexpressing isolate induced increased airway eosinophilia in the lungs of recipient mice compared to that induced by the low-chitin-expressing isolate. Collectively, these findings imply that the health impact of chitin exposure depending on the dose and size of the chitin.
Proteases
Fungi contain and release proteases, and many fungal allergens possess highly proteolytic activities. Fungal proteases induce inflammatory responses by compromising mucociliary clearance, altering the permeability of epithelial barrier, and activating innate immune responses. The main protease sensing mechanism involves activation of protease-activated receptors (PARs). Cleavage of PARs by proteases leads to the activation of NF-κB and mitogen-activated protein kinases [28]. Recently, two papers provided evidence supporting the role of fungal protease in asthma development. Balenga et al. [88•] showed that a major AF allergen, asp f13, which is a serine protease, promotes AHR by infiltrating the bronchial submucosa and disrupting airway smooth muscle cell-extracellular matrix interactions. In another study, Millien et al. [89•] showed that a protease from A. oryzae induces allergic inflammation in the airway through cleavage of the clotting protein fibrinogen, which yields cleavage products that can act as TLR4 ligands on airway epithelial cells and macrophages.
Conclusion
Rapid and significant progress is being made to understand the molecular mechanisms by which fungi promote allergic asthma. Recent findings highlight immune responses induced by fungal components (β-glucans, chitins, and proteases) that directly activate innate immune responses through PRRs and PARs. As such, the impact of fungi is independent of fungal sensitization. Further, fungi do not need to be intact or viable to have a significant health impact. Fungal fragments and components are sufficient. Recent mechanistic data have significant implications on how we measure or define fungal exposure and the clinical significance of fungal sensitization.
Acknowledgments
Funding 2U19AI70235 (GKKH) and P30 ES006096 (TR)
Footnotes
Conflict of Interest Drs. Zhang, Reponen, and Hershey declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2008. Vital and health statistics Series 10, Data from the National Health Survey. 2009(244):1–81. [PubMed] [Google Scholar]
- 2.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital and health statistics Series 10, Data from the National Health Survey. 2009(242):1–157. [PubMed] [Google Scholar]
- 3.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181 (6): 4089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–101. doi: 10.1164/rccm.201405-0859P. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H) 17-associated cytokines (IL-17A and IL- 17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–7. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD4+ T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 2013;43(9):1018–26. doi: 10.1111/cea.12119. [DOI] [PubMed] [Google Scholar]
- 8.Alyasin S, Karimi MH, Amin R, Babaei M, Darougar S. Interleukin-17 gene expression and serum levels in children with severe asthma. Iran J Immunol: IJI 2013;10(3):177–85. [PubMed] [Google Scholar]
- 9.Dennehy KM, Willment JA, Williams DL, Brown GD. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur J Immunol 2009;39(5):1379–86. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem 2007;282(17):12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/ TH17 polarization. Eur J Immunol 2009;39(5):1369–78. doi: 10.1002/eji.200839313. [DOI] [PubMed] [Google Scholar]
- 12.••.Zhang Z, Biagini Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, et al. Beta-glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J Allergy Clin Immunol 2016. doi: 10.1016/j.jaci.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper integrated epidemiologic and experimental asthma models to explore the effect of fungal exposure on asthma development and severity. Fungal exposure enhances allergen-driven TH2 responses, promoting severe allergic asthma. This effect is independent of fungal sensitization and can be reconstituted with beta-glucan and abrogated by neutralization of IL-17A. Similarly, in children with asthma, fungal exposure was associated with increased serum IL-17A levels and asthma severity. This paper demonstrated that fungi are potent immunomodulators and have powerful effects on asthma independent of their potential to act as antigens.
- 13.Sahakian NM, Park JH, Cox-Ganser JM. Dampness and mold in the indoor environment: implications for asthma. Immunol Allergy Clin North Am 2008;28(3):485–505. doi: 10.1016/j.iac.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Cho SH, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, et al. Mold damage in homes and wheezing in infants. AnnAllergy, Asthma Immunol: Off Publ Am Coll Allergy, Asthma, Immunol 2006;97(4):539–45. doi: 10.1016/S1081-1206(10)60947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•.Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a predictor of potential asthma development. AnnAllergy, Asthma Immunol: Off Publ Am Coll Allergy, Asthma, Immunol 2009;102(2):131–7. doi: 10.1016/S1081-1206(10)60243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, visible mold was evaluated by means of home inspection. (1–3)-Beta-D-glucan levels were measured in settled dust, and its association with the risk for asthma at later ages was assessed. The study indicated that the presence of high visible mold and mother’s smoking during infancy were the strongest risk factors for a positive API at the age of 3 years, suggesting an increased risk of asthma. High (1–3)-beta-D-glucan exposure seems to have an opposite effect on API than does visible mold.
- 16.••.Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, et al. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. AnnAllergy, Asthma Immunol: Off Publ Am Coll Allergy, Asthma, Immunol 2011;107(2):120–6. doi: 10.1016/j.anai.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study followed up a high-risk birth cohort from infancy to 7 years of age. Mold was assessed by a DNA-based analysis for the 36 molds that make up the Environmental Relative Moldiness Index at the ages of 1 and 7 years. The study indicated that early exposure to molds as measured by ERMI at 1 year of age, but not 7 years of age, significantly increased the risk for asthma at 7 years of age.
- 17.Levetin E An atlas of fungal spores. J Allergy Clin Immunol 2004;113(2):366–8. doi: 10.1016/j.jaci.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 18.Walker GM WN. Introduction to fungal physiology In: Kavanagh K, editor. Fungi: Biology and applications. John Wiley and Sons, Ltd.; 2005. 1–34. [Google Scholar]
- 19.Erwig LP, Gow NA. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 2016;14(3):163–76. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 20.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog 2010;6(4), e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tischer C, Chen CM, Heinrich J. Association between domestic mould and mould components, and asthma and allergy in children: a systematic review. Eur Respir J 2011;38(4):812–24. doi: 10.1183/09031936.00184010. [DOI] [PubMed] [Google Scholar]
- 22.Mintz-Cole RA, Brandt EB, Bass SA, Gibson AM, Reponen T, Khurana Hershey GK. Surface availability of beta-glucans is critical determinant of host immune response to Cladosporium cladosporioides. J Allergy Clin Immunol 2013;132(1):159–69. doi: 10.1016/j.jaci.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–21. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 24.Douwes J (1–3)-Beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15(3):160–9. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 25.Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis 2007;196(10):1565–71. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005;24(6):1277–86. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Tong J, Lee CW, Ha S, Eom SH, Im YJ. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat Commun 2015;6:6129. doi: 10.1038/ncomms7129. [DOI] [PubMed] [Google Scholar]
- 28.Yike I Fungal proteases and their pathophysiological effects. Mycopathologia. 2011;171(5):299–323. doi: 10.1007/s11046-010-9386-2. [DOI] [PubMed] [Google Scholar]
- 29.H. A Inhalation exposure and toxic effects of mycotoxins In: Li D-W, editor. Biology of Microfungi, Fungal Biology. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 30.Reponen T, Seo SC, Grimsley F, Lee T, Crawford C, Grinshpun SA. Fungal fragments in moldy houses: a field study in homes in New Orleans and Southern Ohio. Atmos Environ. 2007;41(37): 8140–9. doi: 10.1016/j.atmosenv.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rylander R Fungi in homes—how do we measure? Indoor Air. 2014;24(2):221–2. doi: 10.1111/ina.12075. [DOI] [PubMed] [Google Scholar]
- 32.Seo S, Choung JT, Chen BT, Lindsley WG, Kim KY. The level of submicron fungal fragments in homes with asthmatic children. Environ Res. 2014;131:71–6. doi: 10.1016/j.envres.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119(6):748–56. doi: 10.1289/ehp.1002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casas L, Tischer C, Taubel M. Pediatric asthma and the indoor microbial environment. Curr Environ Health Rep 2016;3(3):238–49. doi: 10.1007/s40572-016-0095-y. [DOI] [PubMed] [Google Scholar]
- 35.Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 2009;395(5):1355–72. doi: 10.1007/s00216-009-2995-2. [DOI] [PubMed] [Google Scholar]
- 36.Vesper S, Wymer L. The relationship between environmental relative moldiness index values and asthma. Int J Hyg Environ Health. 2016;219(3):233–8. doi: 10.1016/j.ijheh.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Choi H, Byrne S, Larsen LS, Sigsgaard T, Thorne PS, Larsson L, et al. Residential culturable fungi, (1–3, 1–6)-beta-d-glucan, and ergosterol concentrations in dust are not associated with asthma, rhinitis, or eczema diagnoses in children. Indoor Air. 2014;24(2): 158–70. doi: 10.1111/ina.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karvonen AM, Hyvarinen A, Rintala H, Korppi M, Taubel M, Doekes G, et al. Quantity and diversity of environmental microbial exposure and development of asthma: a birth cohort study. Allergy. 2014;69(8):1092–101. doi: 10.1111/aH.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mensah-Attipoe J, Reponen T, Veijalainen AM, Rintala H, Taubel M, Rantakokko P, et al. Comparison of methods for assessing temporal variation of growth of fungi on building materials. Microbiology. 2016. doi: 10.1099/mic.0.000372. [DOI] [PubMed] [Google Scholar]
- 40.Reponen T, Singh U, Schaffer C, Vesper S, Johansson E, Adhikari A, et al. Visually observed mold and moldy odor versus quantitatively measured microbial exposure in homes. Sci Total Environ 2010;408(22):5565–74. doi: 10.1016/j.scitotenv.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivasubramani SK, Niemeier RT, Reponen T, Grinshpun SA. Assessment of the aerosolization potential for fungal spores in moldy homes. Indoor Air. 2004;14(6):405–12. doi: 10.1111/j.1600-0668.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee T, Grinshpun SA, Martuzevicius D, Adhikari A, Crawford CM, Reponen T. Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmos Environ 2006;40(16):2902–10. doi: 10.1016/j.atmosenv.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iossifova Y, Reponen T, Sucharew H, Succop P, Vesper S. Use of (1–3)-beta-d-glucan concentrations in dust as a surrogate method for estimating specific fungal exposures. Indoor Air. 2008;18(3): 225–32. doi: 10.1111/j.1600-0668.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- 44.Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58(1):13–20. [DOI] [PubMed] [Google Scholar]
- 45.Reponen T, Willeke K, Grinshpun S, Nevalainen A. Biological particle sampling In: Kulkarni P, Baron P, Willeke K, editor. Aerosol Measurement, Principles, Techniques, and Applications, 3rd edition. John Wiley & Johns, Inc.; 2011. 549–70. [Google Scholar]
- 46.Macher J, Douwes J, Prezant B, Reponen T. Bioaerosols In: Ruzer LaHNM, editor. Aerosol Handbook. CRC press; 2013. p. 285–343. [Google Scholar]
- 47.WHO Guidelines for Indoor Air Quality: Dampness and Mould WHO Guidelines Approved by the Guidelines Review Committee. Geneva: 2009. [PubMed] [Google Scholar]
- 48.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect 2015;123(1):6–20. doi: 10.1289/ehp.1307922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rylander R, Norrhall M, Engdahl U, Tunsater A, Holt PG. Airways inflammation, atopy, and (1–3)-beta-D-glucan exposures in two schools. Am J Respir Crit Care Med 1998;158(5 Pt 1):1685–7. doi: 10.1164/ajrccm.158.5.9712139. [DOI] [PubMed] [Google Scholar]
- 50.Ronmark E, Jonsson E, Platts-Mills T, Lundback B. Different pattern of risk factors for atopic and nonatopic asthma among children—report from the obstructive lung disease in Northern Sweden Study. Allergy. 1999;54(9):926–35. [DOI] [PubMed] [Google Scholar]
- 51.Schram-Bijkerk D, Doekes G, Douwes J, Boeve M, Riedler J, Ublagger E, et al. Bacterial and fungal agents in house dust and wheeze in children: the PARSIFAL study. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 2005;35(10):1272–8. doi: 10.1111/j.1365-2222.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 52.Bernstein JA, Bobbitt RC, Levin L, Floyd R, Crandall MS, Shalwitz RA, et al. Health effects of ultraviolet irradiation in asthmatic children’s homes. J Asthma: Off J Assoc Care Asthma. 2006;43(4):255–62. doi: 10.1080/02770900600616887. [DOI] [PubMed] [Google Scholar]
- 53.Inal A, Karakoc GB, Altintas DU, Guvenmez HK, Aka Y, Gelisken R, et al. Effect of indoor mold concentrations on daily symptom severity of children with asthma and/or rhinitis monosensitized to molds. J Asthma: Off J Assoc Care Asthma. 2007;44(7):543–6. doi: 10.1080/02770900701496130. [DOI] [PubMed] [Google Scholar]
- 54.Pongracic JA, O’Connor GT, Muilenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol 2010;125(3):593–9. doi: 10.1016/j.jaci.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gent JF, Kezik JM, Hill ME, Tsai E, Li DW, Leaderer BP. Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res 2012;118:86–93. doi: 10.1016/j.envres.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maheswaran D, Zeng Y, Chan-Yeung M, Scott J, Osornio-Vargas A, Becker AB, et al. Exposure to Beta-(1,3)-D-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLoS One. 2014;9(6), e98878. doi: 10.1371/journal.pone.0098878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J Allergy Clin Immunol 2015;135(1):110–22. doi: 10.1016/j.jaci.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 58.•.Dannemiller KC, Gent JF, Leaderer BP, Peccia J. Indoor microbial communities: influence on asthma severity in atopic and nonatopic children. J Allergy Clin Immunol. 2016;138(1):76–83 e1. doi: 10.1016/j.jaci.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; The associations between exposures to household microbes and childhood asthma severity were assessed and stratified by atopic status. The study indicated that asthma severity in atopic children was associated with fungal community composition.
- 59.Iossifova YY, Reponen T, Bernstein DI, Levin L, Kalra H, Campo P, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62(5):504–13. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpe RA, Thornton CR, Tyrrell J, Nikolaou V, Osborne NJ. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005–2006. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 2015;45(10):1566–78. doi: 10.1111/cea.12549. [DOI] [PubMed] [Google Scholar]
- 61.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 62.Roy RM, Klein BS. Fungal glycan interactions with epithelial cells in allergic airway disease. Curr Opin Microbiol 2013;16(4):404–8. doi: 10.1016/j.mib.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 2012;13(9):817–22. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kataoka K, Muta T, Yamazaki S, Takeshige K. Activation of macrophages by linear (1right-arrow3)-beta-D-glucans. Impliations for the recognition of fungi by innate immunity. J Biol Chem 2002;277(39):36825–31. doi: 10.1074/jbc.M206756200. [DOI] [PubMed] [Google Scholar]
- 65.Rand TG, Robbins C, Rajaraman D, Sun M, Miller JD. Induction of Dectin-1 and asthma-associated signal transduction pathways in RAW 264.7 cells by a triple-helical (1, 3)-beta-D glucan, curdlan. Arch Toxicol 2013;87(10):1841–50. doi: 10.1007/s00204-013-1042-4. [DOI] [PubMed] [Google Scholar]
- 66.Carmona EM, Lamont JD, Xue A, Wylam M, Limper AH. Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respir Res 2010;11:95. doi: 10.1186/1465-9921-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol 2009;123(3):612–8. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neveu WA, Bernardo E, Allard JL, Nagaleekar V, Wargo MJ, Davis RJ, et al. Fungal allergen beta-glucans trigger p38 mitogen-activated protein kinase-mediated IL-6 translation in lung epithelial cells. Am J Respir Cell Mol Biol 2011. ;45(6):1133–41. doi: 10.1165/rcmb.2011-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol 2013;131(2):549–61. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 70.Rand TG, Sun M, Gilyan A, Downey J, Miller JD. Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-beta-D glucan. Arch Toxicol 2010;84(3):205–20. doi: 10.1007/s00204-009-0481-4. [DOI] [PubMed] [Google Scholar]
- 71.Inoue K, Koike E, Yanagisawa R, Adachi Y, Ishibashi K, Ohno N, et al. Pulmonary exposure to soluble cell wall beta-(1, 3)-glucan of aspergillus induces proinflammatory response in mice. Int J Immunopathol Pharmacol 2009;22(2):287–97. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, Zheng M, Qiao J, Dang Y, Zhang P, Jin X. Role of prostaglandin D2/CRTH2 pathway on asthma exacerbation induced by Aspergillus fumigatus. Immunology. 2014;142(1):78–88. doi: 10.1111/imm.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fakih D, Pilecki B, Schlosser A, Jepsen CS, Thomsen LK, Ormhoj M, et al. Protective effects of surfactant protein D treatment in 1,3- beta-glucan-modulated allergic inflammation. Am J Physiol Lung Cell Mol Physiol 2015;309(11):L1333–43. doi: 10.1152/ajplung.00090.2015. [DOI] [PubMed] [Google Scholar]
- 74.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014;134(5):1175–86. doi: 10.1016/j.jaci.2014.05.038.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burg AR, Quigley L, Jones AV, O’Connor GM, Boelte K, McVicar DW, et al. Orally administered beta-glucan attenuates the Th2 response in a model of airway hypersensitivity. Springer Plus. 2016;5(1):815. doi: 10.1186/s40064-016-2501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawashima S, Hirose K, Iwata A, Takahashi K, Ohkubo A, Tamachi T, et al. beta-glucan curdlan induces IL-10-producing CD4+ T cells and inhibits allergic airway inflammation. J Immunol 2012;189(12):5713–21. doi: 10.4049/jimmunol.1201521. [DOI] [PubMed] [Google Scholar]
- 78.Ku SK, Kim JW, Cho HR, Kim KY, Min YH, Park JH, et al. Effect of beta-glucan originated from Aureobasidium pullulans on asthma induced by ovalbumin in mouse. Arch Pharm Res 2012;35(6): 1073–81. doi: 10.1007/s12272-012-0615-8. [DOI] [PubMed] [Google Scholar]
- 79.Lin JY, Chen JS, Chen PC, Chung MH, Liu CY, Miaw SC, et al. Concurrent exposure to a Dectin-1 agonist suppresses the Th2 response to epicutaneously introduced antigen in mice. J Biomed Sci 2013;20:1. doi: 10.1186/1423-0127-20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brinchmann BC, Bayat M, Brogger T, Muttuvelu DV, Tjonneland A, Sigsgaard T. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med: AAEM. 2011;18(1): 7–12. [PubMed] [Google Scholar]
- 81.Mack I, Hector A, Ballbach M, Kohlhaufl J, Fuchs KJ, Weber A, et al. The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol Cell Pedia 2015;2(1):3. doi: 10.1186/s40348-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447(7140):92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun 1997;65(5):1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, et al. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol 2011;187(5): 2261–7. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.•.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB,Thornton EE, Ziegler SF, et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40(3):414–24. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that inhaled chitin induced expression of three epithelial cytokines, IL-25, IL-33, and TSLP, which nonredundantly activated resident ILC2s to express IL-5 and IL-13 necessary for accumulation of eosinophils and alternatively activated macrophages. Thus, chitin elicited patterns of innate cytokines that targeted distinct populations of resident lymphoid cells, revealing divergent but interacting pathways underlying the tissue accumulation of specific types of inflammatory myeloid cells.
- 86.Khosravi AR, Erle DJ. Chitin-induced airway epithelial cell innate immune responses are inhibited by carvacrol/thymol. PLoS One. 2016;11(7), e0159459. doi: 10.1371/journal.pone.0159459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Dea EM, Amarsaikhan N, Li H, Downey J, Steele E, Van Dyken SJ, et al. Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infect Immun 2014;82(8):3199–205. doi: 10.1128/IAI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.•.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that a major Af allergen, Asp f13, which is a serine protease, promotes airway hyper-responsiveness by infiltrating the bronchial submucosa and disrupting airway smooth muscle cell-extracellular matrix (ECM) interactions. Alp 1-mediated ECM degradation evokes pathophysiological RhoA-dependent Ca(2+) sensitivity and bronchoconstriction. These findings support a pathogenic mechanism in asthma and other lung diseases associated with epithelial barrier impairment, whereby ASM cells respond directly to inhaled environmental allergens to generate airway hyper-responsiveness.
- 89.•.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341(6147):792–6. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated that TLR4 is activated by airway proteinase activity to initiate both allergic airway disease and antifungal immunity. These outcomes were induced by proteinase cleavage of the clotting protein fibrinogen, yielding fibrinogen cleavage products that acted as TLR4 ligands on airway epithelial cells and macrophages.