Abstract
Introduction
The objective of the study is to validate attention and memory tasks that elicit event-related potentials (ERPs) for utility as sensitive biomarkers for early dementia.
Methods
A 3-choice vigilance task designed to evaluate sustained attention and standard image recognition memory task designed to evaluate attention, encoding, and image recognition memory were administered with concurrent electroencephalography acquisition to elicit ERPs in mild cognitive impairment (MCI) and healthy cohorts. ERPs were averaged, and mean or maximum amplitude of components was measured and compared between and within cohorts.
Results
There was significant suppression of the amplitude of the late positive potential in the MCI cohort compared with the healthy controls during 3-choice vigilance task, predominantly over occipital and right temporal-parietal region, and standard image recognition memory task over all regions. During standard image recognition memory task, diminished performance showed strong correlation with electroencephalography measurements. The old/new effects observed in the healthy controls cohort correlated with performance and were lost in MCI.
Discussion
ERPs obtained during cognitive tasks may provide a powerful tool for assessing MCI and have strong potential as sensitive and robust biomarkers for tracking disease progression and evaluating response to investigative therapeutics.
Keywords: Mild cognitive impairment, Neurophysiology, Electroencephalography, Event-related potential, Biomarker
Highlights
-
•
Significant suppression of event-related potential components and diminished performance during sustained attention and memory tasks are correlated in mild cognitive impairment patients compared with healthy, age-matched controls.
-
•
Mild cognitive impairment patients lose the recognition effect observed in healthy controls that reflects activation of neural circuits associated with memory.
-
•
The data support use of cognitive tasks with concurrent electroencephalography as a biomarker for disease progression and as a pharmacodynamic end point for neurodegeneration clinical trials.
1. Introduction
Alzheimer's disease (AD) and other dementias can be particularly difficult to distinguish in the early stages, when cognitive and/or motor impairments are subtle and often subclinical in presentation although critically important differences exist in the underlying pathophysiological processes of these diseases [1]. Early characterization is critical to initiate effective interventions, particularly treatments with purported “disease-modifying” effects [2]. Investigators of novel treatment options for AD and other neurodegenerative diseases urgently need sensitive, reliable, cost-effective, noninvasive tools to quantify cognitive deficits associated with neurodegeneration, preferably in the earliest detectable stages of the underlying pathophysiological process. Neurophysiological metrics including quantitative electroencephalography (EEG) and event-related potentials reliably measure the neural circuits associated with cognitive processes and may provide sensitive metrics for early diagnosis, tracking disease progression, and assessing efficacy of novel interventions.
Event-related potentials (ERPs) are reflections of summated postsynaptic inhibitory and excitatory membrane potentials primarily generated by cortical pyramidal cells. Characteristic time-locked ERP waveforms are elicited in response to sensory, motor, and cognitive events [3]. Components of the waveforms can be used to differentiate cognitive conditions, making ERP methods ideal for quantifying cognitive decline in patients with dementia [4], [5]. ERPs track the flow of information from sensory processing and analysis to response. Early components (50–200 ms poststimulus) reflect sensory processing of the characteristics of the stimuli but can be influenced to some extent by arousal and attention [6], [7]. The late ERP components (P300, N400, P600, and late positive potential [LPP]) reflect feature evaluation, memory matching, and processing speed [5], [8]. Multiple reports suggest that abnormal amplitude and latency of the LPP are associated with cognitive decline [9], [10], [11]. The LPP is believed to reflect memory encoding and retrieval with possible sources located in the parahippocampal gyrus, medial temporal lobe, and posterior cingulate regions known to be affected during the progression of dementia [12].
There exists a strong foundation suggesting the utility of ERPs as quantitative biomarkers of cognitive processes [4], [5], [9], [10], [11], [13], [14], [15]. ERPs reveal abnormal neuronal activity in AD beginning in the very early stages of the disease [16]. Word recognition–elicited ERPs show promise as biomarkers of disease progression and subsequent conversion to dementia in individuals with mild cognitive impairment (MCI) [10], [11]. During memory tasks, activation of the LPP during target trials compared with nontarget trials is indicative of an old/new effect that reflects memory activation [17]. Thus, the target trials typically have an increased LPP compared with nontarget that is predominate over the parietal region [18]. In MCI patients, ERPs elicited in response to images had diminished old/new effects in MCI patients versus healthy controls [19]. Using this image recognition paradigm, ERPs acquired from presymptomatic carriers of the genetic mutations in presenilin-1 showed significant changes in ERP patterns years before the onset of symptoms [20].
The introduction of novel therapies for AD, MCI, and Parkinson's disease dementia will be more efficient if sensitive biomarkers can be identified for early diagnoses and be suitable for frequent repetition to assess disease progression. Amyloid positron emission tomography imaging is available to quantify the accumulation of β-amyloid known to be associated with AD pathology, but these measurements are made with limited frequency due to the limited access, expense, and cumulative radiation dose. ERPs may provide convenient inexpensive, noninvasive neurophysiological biomarker for early detection of dementia pathology. ERP methods could provide sensitive biomarkers independently, or alternatively, be used as a low-cost accessible prescreening procedure to identify individuals likely to be positive for a secondary, more costly and invasive imaging or other biomarker. Several studies suggest that ERPs are sensitive to the effects of approved pharmacological treatments for MCI and AD; ERP measures reliably reflect improvements in cognition following administration of cholinesterase inhibitors [21] and the selective N-methyl-D-aspartate antagonist memantine [22].
The present study compared a cohort of patients diagnosed with MCI to age-matched healthy control cohort (HCs) using tasks designed to activate the neural circuitry underlying the cognitive processes associated with sustained attention, visual recognition memory, and working memory. Patients with MCI are particularly interesting as potential targets for early intervention as MCI is often a transitional state between normal aging and dementia. However, although many patients with MCI progress rapidly to dementia, the rate of decline is highly variable, and a significant number remains stable or even return to age-appropriate levels of cognitive capabilities [23].
The participants were administered an ERP test battery with concurrently recorded EEG consisting of a 3-choice vigilance task (3CVT) [24], [25] designed to evaluate sustained attention and standard image recognition memory task (SIR) designed to evaluate attention, encoding, and image recognition memory. In the SIR, images were chosen as stimuli to distinguish short term from semantic memory loss and extend previous results of image recognition ERP effects [19], [20]. The sustained attention and working memory tasks are designed to assess neurocognitive functions that may be compromised as a result of disease, drugs, or behavior (e.g., sleep loss). The combination of EEG and performance metrics [26], [27], [28], [29], [30], [31] is highly sensitive and specific in quantifying daytime drowsiness (associated either with sleep deprivation in healthy participants [29], [31], [32] or in sleep disordered patients), predicting susceptibility to sleep deprivation [32], and assessing neurocognitive deficits in patients with sleep disorders and benzodiazepine-related driving impairment [31], [32], [33]. The present study extends these results to a MCI cohort to evaluate early, mild dementia. The objective of the study is to validate the ERP tasks as sensitive biomarkers for early dementia with potential utility as a pharmacodynamic end point in assessment of investigational disease-modifying therapeutics. The investigators hypothesized that patients with MCI will show suppressed LPP for each of the tasks, with more significant suppression in the memory recognition task.
2. Methods
2.1. Participants
Thirty-five individuals (Table 1) were enrolled in this study through the Brain Aging and Dementia Laboratory at Massachusetts General Hospital. Participants were referred for the study through the Massachusetts General Hospital Alzheimer's Disease Research Center or were enrolled from a local longitudinal cohort. All participants were nondemented with Mini–Mental State Examination scores greater than 24. Neuropsychological testing was used to determine clinical status using operational criteria for MCI as defined previously [34], [35], [36], [37]. MCI designation was based on objective criteria of at least two performances within a cognitive domain falling one standard deviation or more below published normative values. Participants were excluded for significant health concerns outside of the domains of study including major neurological or psychiatric disorders (e.g., Parkinson's disease, Huntington's disease, vascular dementia, clinical stroke, brain surgery, psychosis, severe major depression, and moderate-to-severe traumatic brain injury) as well as any substantial systemic illness that would prevent participation or would be likely to confound study procedures and results. All individuals had at least a high school education (12 years). The Partners Healthcare Institutional Review Board approved this work, and informed consent was obtained from each participant. Demographic and clinical data for the groups, including age, sex distribution, years of education, and Mini–Mental State Examination and Montreal Cognitive Assessment scores, are provided in Table 1.
Table 1.
Demographics
| Variable | Total sample (Mean ± SEM) | HC (Mean ± SEM) | MCI (Mean ± SEM) | P value |
|---|---|---|---|---|
| Sample size (N) | 35 | 17 | 18 | - |
| Women | 21 | 12 | 8 | - |
| Men | 15 | 5 | 10 | - |
| Age (years) | 68.18 ± 1.03 | 67.34 ± 1.61 | 69.02 ± 0.35 | .52 |
| Education (years) | 15.71 ± 0.42 | 16.53 ± 0.70 | 14.94 ± 0.45 | .06 |
| MMSE Score | 28.54 ± 0.285 | 29.18 ± 0.20 | 27.94 ± 0.48 | .03 |
| MoCA Score | 25.63 ± 0.49 | 27.35 ± 0.49 | 24.00 ± 0.66 | .001 |
Abbreviations: HC, healthy controls; MCI, mild cognitive impairment; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment; SEM, standard error of the mean.
2.2. Neurocognitive tests
The 3CVT incorporates common measures of sustained attention, as in continuous performance task, Wilkinson reaction time, and psychomotor vigilance task [24], [25]. The 3CVT required participants to discriminate between three geometric shapes: a primary target (triangle) and two secondary targets (downside triangle and diamond). During this 20-minute task, each shape was presented one at a time for 0.2 seconds across varying interstimulus intervals. On presentation of the primary stimulus (target presented 70% of the time), the participant responded by pressing the left arrow key. For all other secondary (nontarget presented 30% of the time), the participant responded by pressing the right arrow key. Training provided before the start minimizes practice effects [32], [38].
The SIR was designed to evaluate attention, encoding, and image recognition memory. This task starts with a training phase, in which participants are presented with a series of 20 images on the screen for 1.25 seconds with a 1.5-second interstimulus interval. These images were shown twice, and participants were instructed to memorize each image to the best of their ability. In the testing phase, the participant views images and is instructed to indicate whether each image was one of the original 20. A total of 100 images were presented, where target stimuli (20 original images) were randomly interspersed with nontarget stimuli (80 new images). During the testing period, each of the 100 images is presented for 100 ms with a 2.1-second interstimulus interval. This task was approximately 7 minutes in duration and contained a practice session to ensure participants understood the instructions, and all were trained to the same criterion. Images used during the practice session were not used in the testing session.
2.3. EEG data acquisition
The B-Alert® X24 wireless EEG system (Advanced Brain Monitoring, Carlsbad, CA) was used for EEG data acquisition. The B-Alert X24 combines battery-powered hardware with a preconfigured sensor strip to provide a lightweight and easy-to-apply system for recording high-quality EEG. In accordance with the International 10-20 system, the B-Alert X24 provides the following 19 EEG channels: Fz, F3, F4, Cz, C3, C4, P3, P4, Pz, O1, O2, T5, T3, F7, Fp1, Fp2, F8, T4, and T6—plus POz. Data was collected at a sampling rate of 256 Hz, with a common mode rejection ratio of 105 dB, and the following band pass characteristics: 0.1 Hz high-pass filter, 100 Hz fifth order low-pass filter.
2.4. Metrics of performance derived from the cognitive tasks
Standard measurements for assessment of sustained attention, target detection, and visual short-term recognition memory were derived from the 3CVT and SIR tests, including response reaction time and accuracy (percentage of correct responses to stimuli) [39], [40]. The F-Measure metric provides a combined measure of processing speed and accuracy [27]. The F-Measure was applied to both the 3CVT and SIR data to provide a single performance measure for each participant:
Scaled reaction time (RTs) and scaled percent of the correct responses (PCs) that are used for F-Measure estimation was adjusted using the following formulas:
where RT and PC represent average reaction time and percent of the correct responses.
2.5. ERP extraction and processing methods
Participants were excluded from SIR if the data had less than 10 epochs and from 3CVT if there were less than 30 epochs. Thirty of the 36 individuals were included in SIR analysis (mean age 68.19; standard deviation 6.57; 53.33% female), and 34 of the 36 individuals were included in 3CVT (mean age 68.31; standard deviation 6.30; 58.82% female). Raw EEG signals were filtered between 0.1 and 50 Hz using a Hamming windowed Sinc finite impulse response filter (8449 point filtering with a 0.1 Hz transition band width). For each event type, EEG data were epoched from 1 second before until 2 seconds after the stimulus onset. Baseline was removed using data from 100 ms before the stimulus onset. Trials were rejected if the absolute value of EEG amplitude in any channel during a window of −50 ms to +750 ms (compared with the stimulus onset) was larger than a threshold level of 150 μV. Independent component analysis was then applied to epochs of EEG signals, and independent components were computed using EEGLAB software (MathWorks, Natick, MA). ADJUST algorithm (NeuroImaging Tools and Resources Clearinghouse) was used as an unsupervised method to isolate independent component analysis components due to artifacts such as blinks and eye movements using stereotyped spatial and temporal features of such artifacts. EEG data were then cleaned by removing the isolated components. Trials (EEG epochs) with abnormally distributed data, improbable data, or with abnormal spectra were also removed using EEGLAB as follows: trials with high kurtosis or low probability of occurrence were excluded using a threshold of 6 z-score per component and 5 z-score per average of components. EEG trials with spectrum 35 dB higher or lower than the baseline in the frequency range of 20–30 Hz were also excluded. The remaining trials were used to calculate the average ERP for subjects in MCI and HC cohorts. For each participant, average ERPs were calculated and measured by computing (a) the P200 defined as the maximum amplitude in the time window 140-200 ms post stimulus and (b) the LPP defined as the average amplitude in the time window 430-600 ms post stimulus. Average ERPs with less than 10 clean trials were excluded. Subsequently, a two-sample two-tailed t-test was used to compare the group means and detect significant differences between HC and MCI cohorts. Given the sample size of (n = 35), t-test was determined to be a sufficiently appropriate statistical test to detect significant differences between the two cohorts. With respect to the analysis of old/new effect, a paired t-test was used to compare the average ERP measures of target trials and nontarget trials for each cohort. A bivariate correlation analysis was performed by computing Pearson's correlation coefficient to determine if EEG measures are associated with behavioral measures of performance in the corresponding neurocognitive test.
3. Results
3.1. Neurocognitive test performance results
For the SIR task, statistically significant difference between HC and MCI were observed in percent correct (two-sample two-tailed t-test, P = .015, t = 2.56, df = 34) as well as F-Measure (two-sample two-tailed t-test, P = .011, t = 2.67, df = 34) (Table 2). No significant differences were observed in performance measures for 3CVT.
Table 2.
Average performance measures for HC and MCI cohorts
| Task | Variable | HC | MCI | P value |
|---|---|---|---|---|
| SIR | RT (s) | 0.77 ± 0.03 | 0.77 ± 0.03 | .01 |
| PC | 87.50 ± 2.63 | 73.63 ± 4.7 | .01 | |
| F-Measure | 0.787 ± 0.02 | 0.70 ± 0.02 | .01 | |
| 3CVT | RT (s) | 0.69 ± 0.01 | 0.72 ± 0.02 | .33 |
| PC | 95.7 ± 1.4 | 92.2 ± 1.7 | .12 | |
| F-Measure | 0.79 ± 0.01 | 0.76 ± 0.01 | .13 |
Abbreviations: 3CVT, 3-choice vigilance task; HC, healthy controls; MCI, mild cognitive impairment; PC, percent correct; RT, reaction time; SIR, standard image recognition memory task.
3.2. EEG results
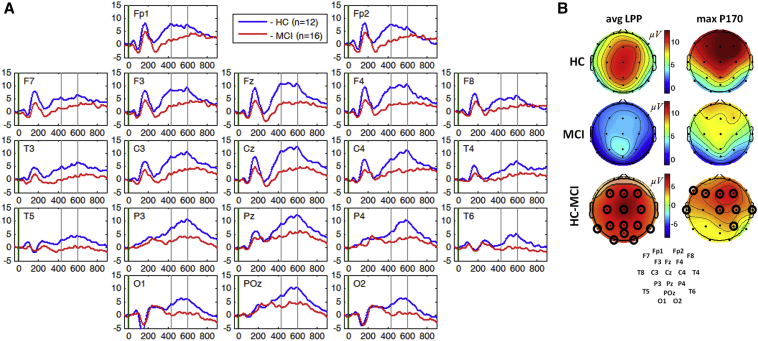
Grand average mean of the ERPs of MCI and HC cohorts were compared for SIR (Fig. 1) and 3CVT (Fig. 2). In SIR, both early (P200) and late component (LPP) were suppressed in the MCI cohort compared with HC (Fig. 1). Comparison of mean amplitude of the LPP in target trials indicates significantly suppressed amplitude for the MCI cohort during SIR in all regions of the brain (two-sample two-tailed t-test P < .05, t > 2.13, df = 26) (Fig. 1B) with the most significant difference at channel Cz (P = .007, t = 2.91). Comparison of maximum amplitude of the P200 indicates significantly suppressed amplitude for the MCI cohort (two-sample two-tailed t-test P < .05, t > 2.16, df = 26), predominately over the fronto-central regions (Fig. 1B).
Fig. 1.
Panel A: Grand average ERPs for correct response to target trials in SIR task at each channel for HC (blue) and MCI cohorts (red). Panel B: Topographical maps of the mean amplitude of the LPP component for HC and MCI cohorts and the difference between the two cohorts. Channels with significant differences (P < .05) in mean LPP are circled. Abbreviations: EPRs, event-related potentials; HC, healthy controls; LPP, late positive potential.
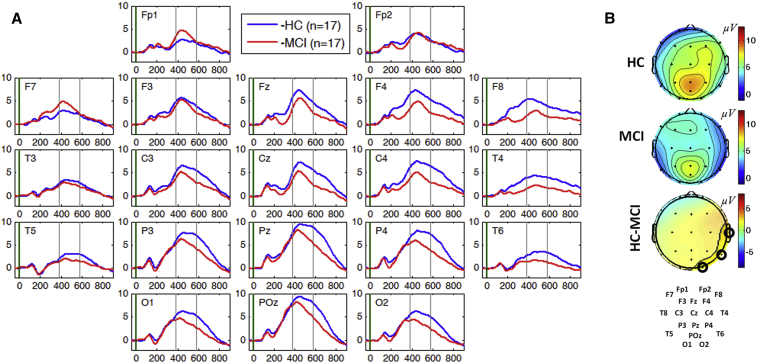
Fig. 2.
Panel A: Grand average ERPs for correct response to target trials in 3CVT task plotted for all channels in both HC (blue) and MCI cohort (red). Panel B: Topographical maps of the mean amplitude of the LPP component for HC and MCI cohorts and the difference between the two cohorts. Channels with significant differences (P < .05) in mean LPP are circled. Abbreviations: 3CVT, 3-choice vigilance task; ERPs, event-related potentials; HC, healthy controls; LPP, late positive potential.
For 3CVT, significant suppression in the LPP mean amplitude was observed over the right occipital and temporal areas (two-sample two-tailed t-test P < .05, t > 2.14, df = 32) with the most significant difference at channel T4 (P = .009, t = 2.76) (Fig. 2B).
3.3. Correlation between performance and LPP measures
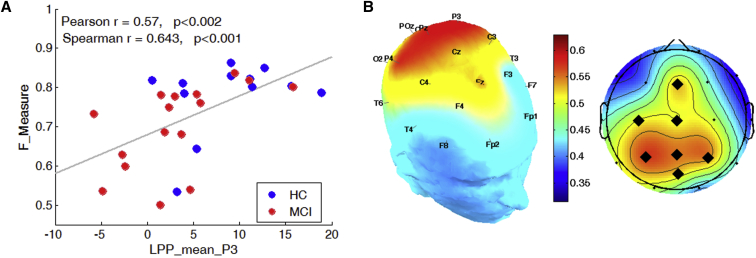
Significant correlations were observed between F-Measure and LPP mean amplitude during SIR (Fig. 3). The strongest and most significant correlations (r > 0.5 and P < .01) were observed in the parietal region at P3, PZ, and P4 channels. A scatter plot of the average values of F-Measure and LPP mean amplitude for channel P3 is shown in Fig. 3A with the corresponding Pearson's linear correlation coefficient (r). The topological map in Fig. 3B highlights significantly correlated channels with black diamonds.
Fig. 3.
Panel A: Scatter plots of performance (F-Measure) and mean amplitude of LPP in channel P3 during SIR task. Panel B: Topographical maps of the Pearson's correlation coefficient r on 2D and 3D head maps. Channels with significant (P < .01) correlation values greater than 0.5 are marked with black diamonds. Abbreviations: HC, healthy controls; LPP, late positive potential; SIR, standard image recognition memory task.
3.4. Old/new effect correlation with performance
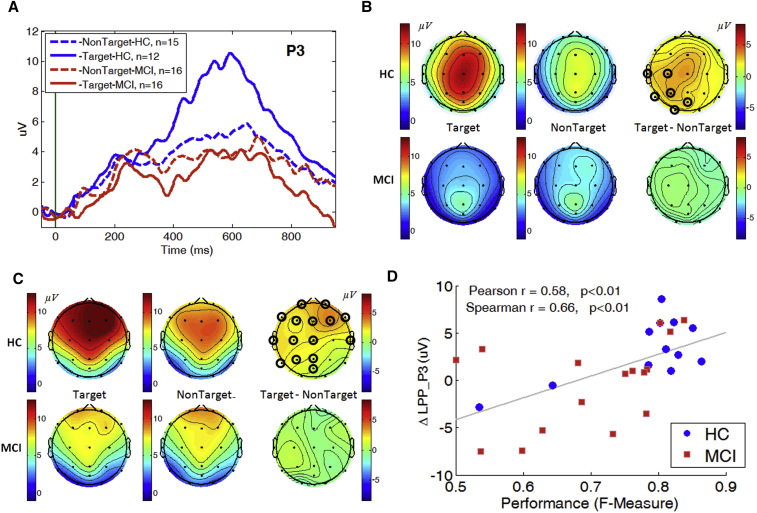
The old/new effect was evaluated by comparing the maximum amplitude of P200 and mean amplitude of the LPP of target trials vs. nontarget trials for both HC and MCI cohorts (Fig. 4). The LPP of the target ERPs was significantly enhanced over the left parietal region (Fig. 4B) and the P200 significantly enhanced over frontal, central, temporal, and occipital regions (Fig. 4C) compared with nontarget in the HC cohort. In contrast, there were no significant differences between the target and nontarget components in the MCI cohort at any channel. Correlations were observed between the old/new effect and performance as measure by F-measure (Fig. 4D) with high significance and correlation coefficient (Pearson r = 0.58, P < .01, Spearman r = 0.66, P < .01).
Fig. 4.
Old/new effect on ERP measures in SIR task at channel P3. Panel A: Grand average ERP waveforms for both target and nontarget trials. Panel B and C: Topographical maps indicate mean amplitude of LPP (B) and maximum amplitude of P200 (C) for target, nontarget and the difference between target and nontarget. Channels with significant differences between target and nontarget are highlighted with black circles. D: Correlation between old/new effect and performance (F-measure). Abbreviations: ERP, event-related potential; LPP, late positive potential; SIR, standard image recognition memory task.
4. Discussion
To summarize, an MCI cohort had significantly different EEG patterns elicited during cognitive tasks that measure sustained attention or encoding and image recognition memory compared with a HC cohort. It is worth noting that the participants in this study were all referred to the Memory Clinic at Massachusetts General Hospital due to concerns regarding memory loss. Following administration of neuropsychological examinations, each participant was assigned to either MCI or HC cohorts based on their scores. Thus, the population is very homogeneous yet significant electrophysiologic differences were observed during both cognitive tasks administered. The most striking difference between MCI and HC cohorts was observed during comparison of amplitude of the early and late components during the SIR task. The P200 maximum amplitude was significantly suppressed in the MCI cohort predominately over the fronto-central regions confirming prior reports suggesting the source of the early component [41]. Early components are reported to index stimulus evaluation and reflect detection of features in task-relevant stimuli. Diminished amplitudes of early components may reflect abnormal early visual integration during memory processes. LPP mean amplitude was significantly suppressed in all regions of the brain in MCI patients compared with HC. Patients with MCI also exhibited suppressed LPP during 3CVT at right occipital and temporal channels (O2, T4, and T6). While 3CVT is a measure of sustained attention only, SIR also incorporates a memory component and evaluates encoding and image recognition as well as attention. The cognitive mechanisms supporting the memory function appear to be severely attenuated along relevant processing stages in MCI patients, particularly as reflected in the LPP, an ERP component associated with memory matching, stimulus evaluation, and decision-making [5], [8]. The MCI cohort also differed on performance during SIR as evaluated using processing speed, accuracy, and F-Measure, a combined metric incorporating both processing speed and accuracy [27]. Patients with MCI showed significant suppression of the F-measure for SIR task and correlations between the performance and neurophysiological measures were significant and strong, particularly over the parietal region of the brain, suggesting interdependence between the performance and strength of LPP during SIR. The significant differences in the SIR ERP components and performance are particularly striking considering the homogeneity of the two cohorts, with very close Mini–Mental State Examination scores and similar subjective memory complaints.
Differences between target and nontarget trial LPP mean amplitude were compared for each cohort and summarized in Fig. 4. The results show that only the HC cohort shows significant differences between target and nontarget stimuli (i.e., old/new effect) for both LPP and P200; the MCI cohort does not show difference for either component at any of the channels. The loss of the old/new effect at LPP is correlated with performance as measured by F-measure. The data suggest that the engagement of the neural circuitry during the neurocognitive task has decreased or is absent in MCI patients such that the neural patterns normally associated with attention, memory, and cognition have been significantly altered paralleling a loss of cognition reflected in the performance. The results complement previous observations of loss of the old/new effect in patients with MCI compared with healthy controls in ERPs elicited during an image old/new task [19]. Ally et al. [19] observed old/new effect in HC at 500–800 ms with loss in MCI patients. However, no old/new effect was observed at an earlier component (300–500 ms). The present results indicate loss of the old/new effect in both early and late components in the MCI cohort. The difference in results may be attributed to differences in stimuli presentation and instructions for response in the test bed. In a recognition task using words as a stimulus, Olichney et al. [10] observed that loss of the old/new effect was strongly associated with conversion of MCI patients to dementia in a longitudinal study. Measurement of the differences between target and nontarget in the SIR has potential to be indicative of cognitive impairment on an individual basis and serve as a biomarker for detecting and staging early dementia. Longitudinal follow-up with the cohorts studied here is warranted to explore association of the loss of old/new effect with conversion to dementia.
The results of the study comparing MCI and closely matched controls suggests that the ERP tasks administered concurrently with EEG are sensitive and reliable measures for cognitive decline and mild dementia. In particular, the ERPs elicited in response to the SIR represent a robust dementia biomarker that reflects the memory deficits in early dementia and has strong potential for utility in monitoring the efficacy of investigational, disease-modifying interventions.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Although event-related potentials show promise as biomarkers for neurodegenerative disease, further evaluation is necessary to implement electroencephalography (EEG) biomarkers as pharmacodynamic end points in interventional clinical trials. Relevant citations are appropriately cited.
-
2.
Interpretation: The data suggest event-related potentials acquired during our sustained attention and memory tasks are sensitive to mild cognitive impairment and promising candidates as EEG biomarkers for early dementia.
-
3.
Future directions: Follow-up studies should include the following: (1) a larger, longitudinal study using our battery of cognitive tasks and concurrent EEG to further validate the tasks and demonstrate sensitivity to disease progression, (2) multimodal studies to benchmark EEG biomarkers against neuroimaging including structural and functional magnetic resonance imaging, FDG, and amyloid PET, and (3) implementation of EEG biomarkers in interventional clinical trials to validate use as a pharmacodynamic endpoint.
Footnotes
S.W., A.M., and M.S.K. are employees of Advanced Brain Monitoring. C.B. is an employee and shareholder of Advanced Brain Monitoring. D.H.S., K.S., C.A. and T.S., are employees at Harvard Medical School. A.V. is an employee of United Neuroscience.
Funding: This work was funded by Biogen.
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picton T.W., Bentin S., Berg P., Donchin E., Hillyard S.A., Johnson R., Jr. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- 4.Polich J., Ehlers C.L., Otis S., Mandell A.J., Bloom F.E. P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr Clin Neurophysiol. 1986;63:138–144. doi: 10.1016/0013-4694(86)90007-6. [DOI] [PubMed] [Google Scholar]
- 5.Polich J., Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- 6.Hillyard S.A., Hink R.F., Schwent V.L., Picton T.W. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- 7.Coles M.G., Scheffers M.K., Fournier L. Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol (Amst) 1995;90:129–144. doi: 10.1016/0001-6918(95)00020-u. [DOI] [PubMed] [Google Scholar]
- 8.Hillyard S.A., Kutas M. Electrophysiology of cognitive processing. Annu Rev Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- 9.Olichney J.M., Morris S.K., Ochoa C., Salmon D.P., Thal L.J., Kutas M. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olichney J.M., Taylor J.R., Gatherwright J., Salmon D.P., Bressler A.J., Kutas M. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olichney J.M., Riggins B.R., Hillert D.G., Nowacki R., Tecoma E., Kutas M. Reduced sensitivity of the N400 and late positive component to semantic congruity and word repetition in left temporal lobe epilepsy. Clin Electroencephalogr. 2002;33:111–118. doi: 10.1177/155005940203300307. [DOI] [PubMed] [Google Scholar]
- 12.Guillem F., Rougier A., Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. J Cogn Neurosci. 1999;11:437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- 13.Bennys K., Rondouin G., Benattar E., Gabelle A., Touchon J. Can event-related potential predict the progression of mild cognitive impairment? J Clin Neurophysiol. 2011;28:625–632. doi: 10.1097/WNP.0b013e31823cc2d3. [DOI] [PubMed] [Google Scholar]
- 14.Kimiskidis V.K., Papaliagkas V.T. Event-related potentials for the diagnosis of mild cognitive impairment and Alzheimer's disease. Expert Opin Med Diagn. 2012;6:15–26. doi: 10.1517/17530059.2012.634795. [DOI] [PubMed] [Google Scholar]
- 15.Papaliagkas V.T., Kimiskidis V.K., Tsolaki M.N., Anogianakis G. Cognitive event-related potentials: longitudinal changes in mild cognitive impairment. Clin Neurophysiol. 2011;122:1322–1326. doi: 10.1016/j.clinph.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Quiroz Y.T., Lopera F., Budson A.E. Charting the path for early diagnosis and prevention of Alzheimer's disease. Expert Rev Neurother. 2011;11:1665–1667. doi: 10.1586/ern.11.162. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi S., Knight R.T. Effects of temporal-parietal lesions on the somatosensory P3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol. 1992;84:139–148. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 18.Wolk D.A., Manning K., Kliot D., Arnold S.E. Recognition memory in amnestic-mild cognitive impairment: insights from event-related potentials. Front Aging Neurosci. 2013;5:89. doi: 10.3389/fnagi.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ally B.A., McKeever J.D., Waring J.D., Budson A.E. Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. Neuropsychologia. 2009;47:2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quiroz Y.T., Ally B.A., Celone K., McKeever J., Ruiz-Rizzo A.L., Lopera F. Event-related potential markers of brain changes in preclinical familial Alzheimer disease. Neurology. 2011;77:469–475. doi: 10.1212/WNL.0b013e318227b1b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katada E., Sato K., Sawaki A., Dohi Y., Ueda R., Ojika K. Long-term effects of donepezil on P300 auditory event-related potentials in patients with Alzheimer's disease. J Geriatr Psychiatry Neurol. 2003;16:39–43. doi: 10.1177/0891988702250561. [DOI] [PubMed] [Google Scholar]
- 22.Werber A.E., Klein C., Rabey J.M. Evaluation of cholinergic treatment in demented patients by P300 evoked related potentials. Neurol Neurochir Pol. 2001;35(Suppl 3):37–43. [PubMed] [Google Scholar]
- 23.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sateia M.J. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 25.Riccio C.A., Reynolds C.R., Lowe P.A. John Wiley & Sons, Inc.; New York: 2001. Clinical Applications of Continuous Performance Tests: Measuring Attention and Impulsive Responding in Children and Adults. [Google Scholar]
- 26.Stone B.T., Correa K.A., Brown T.L., Spurgin A.L., Stikic M., Johnson R.R. Behavioral and neurophysiological signatures of benzodiazepine-related driving impairments. Front Psychol. 2015;6:1799. doi: 10.3389/fpsyg.2015.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stikic M., Johnson R.R., Levendowski D.J., Popovic D.P., Olmstead R.E., Berka C. EEG-derived estimators of present and future cognitive performance. Front Hum Neurosci. 2011;5:70. doi: 10.3389/fnhum.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa K.A., Stone B.T., Stikic M., Johnson R.R., Berka C. Characterizing donation behavior from psychophysiological indices of narrative experience. Front Neurosci. 2015;9:301. doi: 10.3389/fnins.2015.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berka C., Levendowski D.J., Lumicao M.N., Yau A., Davis G., Zivkovic V.T. EEG correlates of task engagement and mental workload in vigilance, learning, and memory tasks. Aviat Space Environ Med. 2007;78:B231–B244. [PubMed] [Google Scholar]
- 30.Johnson R.R., Stone B.T., Miranda C.M., Vila B., James L., James S.M. Identifying psychophysiological indices of expert vs. novice performance in deadly force judgment and decision making. Front Hum Neurosci. 2014;8:512. doi: 10.3389/fnhum.2014.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R.R., Popovic D.P., Olmstead R.E., Stikic M., Levendowski D.J., Berka C. Drowsiness/alertness algorithm development and validation using synchronized EEG and cognitive performance to individualize a generalized model. Biol Psychol. 2011;87:241–250. doi: 10.1016/j.biopsycho.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levendowski D., Berka C., Olmstead R., Konstantinovic Z.R., Davis G., Lumicao M. Electroencephalographic indices predict future vulnerability to fatigue induced by sleep deprivation. Sleep. 2001;24:A243–A244. [Google Scholar]
- 33.Stone B.T., Correa K.A., Brown T.L., Spurgin A.L., Stikic M., Johnson R.R. Behavioral and Neurophysiological Signatures of Benzodiazepine-Related Driving Impairments. Front Psychol. 2015;6:1799. doi: 10.3389/fpsyg.2015.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jak A.J., Bondi M.W., Delano-Wood L., Wierenga C., Corey-Bloom J., Salmon D.P. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondi M.W., Edmonds E.C., Jak A.J., Clark L.R., Delano-Wood L., McDonald C.R. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bondi M.W., Jak A.J., Delano-Wood L., Jacobson M.W., Delis D.C., Salmon D.P. Neuropsychological contributions to the early identification of Alzheimer's disease. Neuropsychol Rev. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stricker N.H., Salat D.H., Foley J.M., Zink T.A., Kellison I.L., McFarland C.P. Decreased white matter integrity in neuropsychologically defined mild cognitive impairment is independent of cortical thinning. J Int Neuropsychol Soc. 2013;19:925–937. doi: 10.1017/S1355617713000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levendowski D., Olmstead R., Konstantinovic Z.R., Berka C., Westbrook P. Detection of Electroencephalographic Indices of Drowsiness in Real-time using a Multi-Level Discriminant Function Analysis. Sleep. 2000;23:A243–A244. [Google Scholar]
- 39.Esterman M., Reagan A., Liu G., Turner C., DeGutis J. Reward reveals dissociable aspects of sustained attention. J Exp Psychol Gen. 2014;143:2287–2295. doi: 10.1037/xge0000019. [DOI] [PubMed] [Google Scholar]
- 40.Kaestner E.J., Polich J. Affective recognition memory processing and event-related brain potentials. Cogn Affect Behav Neurosci. 2011;11:186–198. doi: 10.3758/s13415-011-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luck S.J., Hillyard S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]