Summary
Vitamin D receptor (VDR) mediates various biochemical activities between the cytoplasm and the nucleus in the cell. The nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) protein is involved in the T helper type 2 (Th2) response. This study tests a hypothesis that VDR interacts with NLRP3 to restrict the Th2‐biased response. In this study, VDR–/– mice and WT (WT) mice were used. Th2 cell differentiation between VDR–/– mice and WT mice was observed. We observed that CD4+ T cell activation was higher in VDR–/– mice. The VDR–/–CD4+ T cells were prone to Th2 polarization. VDR–/– mice produced more immunoglobulin (Ig)E. VDR bound NLRP3 to prevent Th2 differentiation by restricting IL4 gene transcription. Th2 biased inflammation spontaneously developed in the intestine of VDR–/– mice. In conclusion, VDR binds NLRP3 to restrict IL4 gene transcription and prevent biased Th2 polarization.
Keywords: allergy, T lymphocyte, T helper cell, Th2 polarization, vitamin D receptor
Introduction
Allergy includes a group of diseases, such as allergic asthma, allergic dermatitis and food allergy, caused by the hypersensitivity of the immune system to antigens in the living environment; these antigens usually do no harm to healthy people 1. For example, food allergy is characterized as an over‐reaction to the innocent food proteins by the immune system in the intestine. The prevalence of allergic disease has reached 20–30% in the general population; it has kept rising in the past several decades [2]. Although there are many remedies for the treatment of allergic diseases, the therapeutic efficacy is currently unsatisfactory [3]. The T helper type 2 (Th2)‐biased immune response plays a critical role in the pathogenesis of allergy [4]. Th2 cells are an important fraction of immune cells characterized by producing the Th2 cytokines, such as interleukin (IL)‐4, IL‐5 and IL‐13 [5]. The physiological functions of Th2 cytokines play important roles in the humoral immunity that facilitate the production of immunoglobulins (Ig) [6], while the over‐production of Th2 cytokine may initiate the Th2‐biased inflammation such as allergic disorders [5]. However, initiation of the Th2‐biased response is not yet understood fully.
Published data indicate that vitamin D‐deficiency is associated with the pathogenesis of allergic diseases [7] and intestinal inflammation [8]. Vitamin D is one of the fat‐soluble vitamins. The essential role of vitamin D is to facilitate the absorption and metabolism of calcium. Recent studies indicate that vitamin D is involved in the immune regulation by activating the vitamin D receptor (VDR) [9], such as VDR binding to the Cyp11a1 promoter in CD8+ Tc2 cells to prevent asthma development [10]. Vitamin D also regulates the expression of a number of genes in CD4+ T cells [11], although the role of VDR in initiation of the Th2‐biased response remains to be investigated further.
It is reported that nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) protein interacts with the Th2 transcription factor interferon regulatory factor 4 (IRF4) to transactivate the IL4 promoter and initiates the Th2 programme [12]. NLRP3 is produced mainly by macrophages; it is a component of the inflammasomes [13]. The activated NLRP3 may trigger biased immune responses. The NLRP3 gene mutation is associated with a number of autoimmune diseases [14]. It is reported that NLRP3 facilitates Th2 cell differentiation [12]. Both NLRP3 and VDR are associated with regulation of the Th2 response; whether VDR interacts with NLRP3 in the initiation of Th2‐biased response is unclear. Based on the information above, we hypothesize that VDR plays a critical role in the regulation of Th2 response via restricting the activities of NLRP3. Thus, we performed this study. The results showed that VDR formed a complex with NLRP3 to restrict the initiation of Th2‐biased response in the intestine.
Materials and methods
Reagents
The VDR shRNA kit, antibodies of CD3, CD28, VDR, NLRP3, IRF4 and GATA binding protein 3 (GATA3), were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). The fluorochrome‐labelled antibodies of CD4, CD8, CD25, CD69, IL‐4 and IFN‐ were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The enzyme‐linked immunosorbent assay (ELISA) kits of IL‐2, IFN‐, IL‐4, IL‐5, IL‐13, immunoglobulin (Ig)A, IgG1, IgG2a, IgG2b, IgG3 and IgM were purchased from R&D Systems (Minneapolis, MN, USA). The ovalbumin (OVA)‐specific IgE kit was purchased from Biocompare (South San Francisco, CA, USA). The reagents and materials for real‐time–quantitative polymerase chain reaction (RT–qPCR) and Western blotting were purchased from Invitrogen (Carlsbad, CA, USA). The calcitriol, phorbol myristate acetate (PMA), OVA, bovine serum albumin (BSA) and chromatin immunoprecipitation (ChIP) kit were purchased from Sigma Aldrich (St Louis, MO, USA). The immune cell isolation kits were purchased from Miltenyi Biotech (San Diego, CA, USA).
Patients
Food allergy (FA) patients were recruited to the present study in the clinic of gastroenterology at the Fifth Hospital of Zhengzhou University (Zhengzhou, China). The diagnosis and management of FA was performed by our physicians following the routine procedures [15]. The characteristics of FA patients are presented in Table 1. The using human tissue in the present study was approved by the Human Ethics Committee at Zhengzhou University. A written informed consent was obtained from each human subject.
Table 1.
Demographic data of food allergy (FA) patients
| Items | Data |
|---|---|
| Number of subjects | 20 |
| Age (years, median) | 29·5 ± 3·6 |
| Male | 10 (50) |
| Female | 10 (50) |
| SPT (diameter)* | |
| < 3 mm | 0 |
| 10–15 mm | 12 (60) |
| > 15 mm | 8 (40) |
| Serum sIgE† | |
| 17·5–50 KU/l | 5(25) |
| 50–100 KU/l | 10 (50) |
| > 100 KU/l | 5 (25) |
*A wheal diameter greater than 3 mm of the negative saline control was considered standard penetration test (SPT)‐positive; the serum immunoglobulin (sIg)E to specific antigens was measured by the ImmunoCap test and a value of more than 0·35 kUA/l was considered a positive response.
Mice
The C57B/6 VDR knock‐out (KO) mice (B6.129S4‐Vdrtm1Mbd/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The WT C57B/6 mice were purchased from the Guangzhou Experimental Animal Centre (Guangzhou, China). The mice were maintained in a special pathogen‐free facility with accessing water and food freely. The experimental procedures were approved by the Animal Ethic Committee at Shenzhen University.
CD4+ T cell isolation and culture
Single cells were isolated from the mouse spleen. CD3+CD4+ T cells were isolated from the single cells by magnetic activated cell sorting (MACS) with commercial reagent kits following the manufacturer’s instructions. The cell purity was checked by flow cytometry. If the purity was less than 95%, the purification procedures were repeated. The cells were cultured in RPMI‐1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 0·1 mg/ml streptomycin and 2 mM L‐glutamine. The medium was changed in 2–3 days. The cell viability was greater than 99%, as checked by Trypan blue exclusion assay.
Flow cytometry
In the surface staining, cells were incubated with the primary antibodies of interest or isotype IgG (labelled with fluorochromes) for 30 min at 4°C. In the intracellular staining, the cells were fixed with 1% paraformaldehyde (containing 0·1% Triton X‐100 to increase the membrane permeability) for 1 h at room temperature, washed with phosphate‐buffered saline (PBS), and then incubated with antibodies or isotype IgG (labelled with fluorochromes) for 30 min. After washing with PBS, the cells were analysed with a flow cytometer (FACSCanto II; BD Biosciences). The data were analysed with FlowJo software (TreeStar Inc., Ashland, OR, USA). The data of isotype IgG staining were used as a gating reference.
ELISA
Cytokine or Ig levels in the sera or culture supernatant were determined by ELISA with commercial reagent kits following the manufacturer’s instructions.
RT–qPCR
Cells were collected at the end of relevant experiments. The total RNA was extracted from the cells with TRIzol reagents. The cDNA was synthesized with the RNA and a reverse‐transcription kit following the manufacturer’s instructions. The samples were amplified in a qPCR device with the SYBR Green Master Mix. The primers used in the study include VDR (TATGACCTGTGAAGGCTGCA and ATCATCTCCCGCTTCCTCTG) and IL‐4 (GCAGTTCTACAGCCACCATG and ACTCTGGTTGGCTTCCTTCA). The results were presented as fold change against the housekeeping gene β‐actin (human: CATGGAATCCTGTGGCATCC and CACACAGAGTACTTGCGCTC; mouse: GGAAATCGTGCGTGACATCA and GCCACAGGATTCCATACCCA).
Preparation of cell extracts
Cells were collected from relevant experiments and treated with a lysing buffer for 30 min. The samples were centrifuged for 10 min at 10 000 g. The supernatant was collected as the cytosolic extracts. The pellet was resuspended in a nuclear lysing buffer for 30 min. The samples were centrifuged for 10 min at 10 000 g. The supernatant was collected as the nuclear extracts. All the procedures were performed at 4°C.
Western blotting
Total proteins were extracted from the cells, quantified with the bicinchoninic acid (BCA) method, fractioned by sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% skimmed milk for 30 min, the membrane was incubated with the primary antibodies of interest or isotype IgG overnight at 4°C, washed with Tris‐buffered saline Tween 20 (TBST) three times and incubated with the second antibodies (labelled with peroxidase) for 1 h at room temperature. The plots on the membrane were developed with the enhanced chemiluminescence. The results were photographed with an imaging device.
RNA interference (RNAi)
The VDR gene was knocked down in T cells by transducing the VDR shRNA‐carrying lentivirus or scramble shRNA‐carrying lentivirus following the manufacturer’s instructions. Two days later, the effects of RNAi were assessed by Western blotting.
CD4+ T cell isolation and differentiation
CD3+CD4+ T cells were isolated from spleen cells by MACS with a reagent kit (Miltenyi Biotech) following the manufacturer’s instructions. The purity of isolated CD3+CD4+ T cells was greater than 96%, as verified by flow cytometry.
Assessment of Th2‐pattern inflammation in the intestine of VDR–/– mice
WT C57B/6 mice and VDR–/– mice were fed with OVA (2 mg/mouse; without adjuvant) daily for 2 weeks. The mice were killed the next day after the last feeding with OVA. The systemic allergic response (change of the core temperature and diarrhoea), serum Th2 status (Th2 cytokines and OVA‐specific IgE) and the allergic inflammation in the intestine (including assessing the OVA‐specific CD4+ T cells and infiltration of mast cell in the intestinal mucosa) were evaluated with the routine procedures in our laboratory [16].
Immunoprecipitation (IP)
The cells were lysed with a lysing buffer. The lysates were precleared by incubating with protein G agarose for 2 h at 4°C. The samples were centrifuged; the supernatant was collected to be incubated with antibodies of interest or isotype IgG overnight at 4°C. The immune complexes were precipitated by protein G agarose for 2 h at 4°C. The protein complexes on the agarose beads were eluted with an eluting buffer. The proteins were analysed further by Western blotting.
Chromatin immunoprecipitation (ChIP)
The cells were fixed with 1% formalin for 15 min. After washing with PBS, the cells were sonicated to shear the DNA into small pieces [200–500 base pairs (bp)]. The samples were precleared by incubation with protein G agarose for 2 h at 4°C and centrifuged to collect the supernatant. Antibodies of interest or isotype IgG was added to the samples and incubated overnight at 4°C. The immune complexes were precipitated by incubating with protein G agarose for 2 h at 4°C. The precipitated DNA was recovered with a reagent kit following the manufacturer’s instructions. The DNA was analysed by qPCR with the primers of the IL‐4 promoter (GGCCTCTCCCTTCTATGCAA and GATTGTCAGTCACTTGGGGC). The results were presented as fold change against the input.
Over‐expression of VDR in CD4+ T cells
The customized VDR‐expression plasmids were constructed by Genescript (Nanjing, China). CD4+ T cells were prepared as described above. The cells were activated by incubation in the presence of anti‐CD3 antibody (1 µg/ml) and anti‐CD28 antibody (1 µg/ml) for 48 h. The cells were seeded in a six‐well plate (1 × 106 cells/well). Upon reaching 90% confluence, transient transfection was conducted with the Lipofectamine™ 2000 reagent kit (Invitrogen) following the manufacturer’s instructions. The cells were collected 48 h later to be used in further experiments. The effects of the transduction were assessed by Western blotting.
In‐vitro T cell differentiation
Naive CD4+ T cells (CD4+CD62Lhi) were negatively selected from spleen cells of wild‐type (WT) CD57B/6 mice using reagent kits purchased from Miltenyi Biotech. The purity of the isolated cells was greater than 95%, as assessed by flow cytometry. For generating Th0 cells, the cells were then cultured in the presence of anti‐CD3 (2 μg/ml), anti‐CD28 (2 μg/ml), anti‐IFN‐γ (10 μg/ml) and anti‐IL‐4 (10 μg/ml) for 72 h. For generating Th2 cells, anti‐IFN‐γ (10 μg/ml) and IL‐4 (10 ng/ml) were added to the culture.
Formation of the VDR/NLRP3 complex in vitro
The VDR‐expression plasmids (labelled with FLAG) and NLRP3‐expression plasmids (labelled with His) were constructed by Genescript (Nanjing, China). Following the manufacturer’s instructions, the plasmids were transfected into Escherihcia coli, respectively, to produce the recombinant VDR‐FLAG and NLRP3‐His. The recombinant proteins were purified with affinity chromatography with anti‐FLAG or anti‐His antibody in the columns. The two proteins were mixed in an Eppendorf tube at a ratio of 1 : 1 and incubated at 37°C for 30 min and then analysed using the immunoprecipitation (IP).
Statistics
Statistical analysis of the data was performed using an unpaired Student’s t‐test for two groups or analysis of variance (anova) followed by the Student–Newman–Keuls test for comparing more than two groups. The correlation test was performed with Pearson’s correlation assay. P ˂ 0·05 was set as a significant criterion. Samples from individual mice were analysed separately. Each experiment was repeated at least three times.
Results
Expression of VDR correlates negatively with Th2 cytokines in CD4+ T cells from FA patients
To define the role of VDR in the pathogenesis of FA, we assessed the expression of VDR and Th2 cytokines in CD4+ T cells isolated from the peripheral blood samples collected from FA patients and healthy subjects. The results showed that, compared to the healthy group, CD4+ T cells from the FA group showed lower levels of VDR and higher levels of Th2 cytokines (including IL‐4, IL‐5 and IL‐13) (Fig. 1a,b). The expression of VDR was correlated negatively with the levels of Th2 cytokines, including IL‐4, IL‐5 and IL‐13 (Fig. 1c). These results imply that the lower expression of VDR may associate with the Th2 polarization in FA patients.
Figure 1.
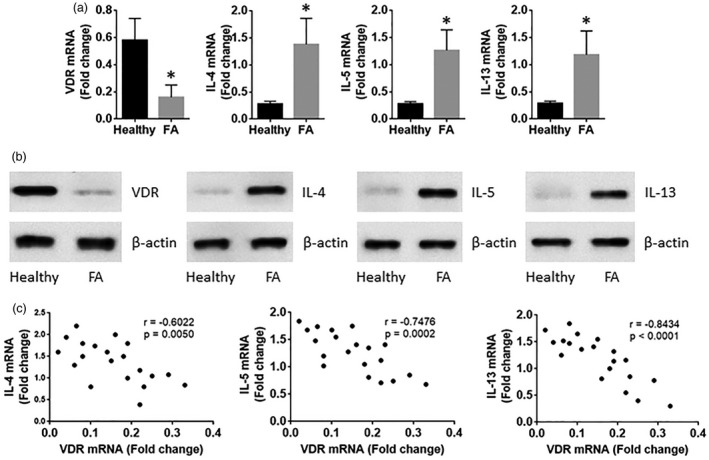
Vitamin D receptor (VDR) expression negatively correlates with T helper type 2 (Th2) cytokines in CD4+ T cells of food allergy (FA) patients. Peripheral blood samples were collected from healthy subjects (n = 20) and FA patients (n = 20). CD4+CD25+CD127+ T cells were isolated from the blood samples by magnetic cell sorting (MACS). The T cells were analysed by real‐time–quantitative polymerase chain reaction (RT–qPCR) and Western blotting. (a,b) The bars indicate the mRNA levels (a) and protein levels (a) of VDR, interleukin (IL‐4), IL‐5 and IL‐13 in the T cells. (c) The dot‐plots show the correlation between VDR and Th2 cytokines. The data of bars are presented as mean ± standared deviation (s.d.). *P < 0·01, compared with the healthy subjects. The samples from individual subjects were analysed separately. Each sample was analysed in duplicate.
CD4+ T cells from FA patients show hyperresponsiveness to external stimuli
CD4+ T cells were isolated from the blood samples collected from FA patients and healthy subjects. The cells were cultured for 3 days in the presence of T cell activators, PMA/ionomycin or saline. The results showed higher cell proliferation and Th2 cytokine production in the FA group than that in the healthy group. The results indicate that the CD4+ T cells from FA patients are hyperresponsive to exogenous stimuli. As the insufficient expression of VDR is associated with Th2 cytokine expression in FA CD4+ T cells, as shown by Fig. 1c, vitamin D3 can up‐regulate the expression of VDR [17], calcitriol was added to the culture of CD4+ T cells of FA patients. Indeed, the cell proliferation and Th2 cytokine production of the CD4+ T cells were reduced to the levels of the healthy group (Fig. 2).
Figure 2.
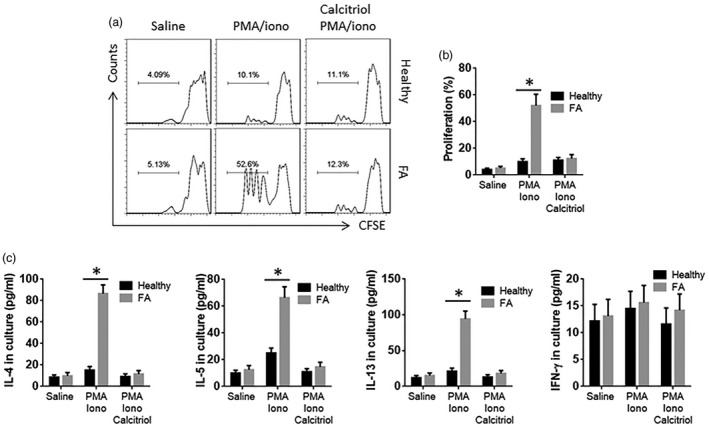
CD4+ T cells from food allergy (FA) patients show hyperreaction to external stimuli. CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMC) of healthy subjects (n = 6) and FA patients (n = 6). The cells were cultured in the presence of saline, phorbol myristate acetate (PMA) (10 ng/ml)/ionomycin (iono; 100 ng/ml) or PMA/ionomycin/calcitriol (10 nM) for 3 days. (a) The cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) before the culture. The histograms show the proliferation of CD4+ T cells. (b) The bars show the summarized data of panel (a). (c) The bars show the cytokines in the culture supernatant [by enzyme‐linked immunosorbent assay (ELISA)]; *P < 0·01 (t‐test). The samples from individual subjects were analysed separately. Each sample was analysed in duplicate.
VDR–/–CD4+ T cells are prone to Th2 polarization
To elucidate the phenotyping of the VDR–/– T cells, we isolated spleen CD4+ T cells from WT mice and VDR–/– mice. The cells were cultured in the presence of T cell activators (PMA/ionomycin) in the culture for 72 h. The exposure to activators resulted in more Th2 cells and fewer Th1 cells in the VDR–/– CD4+ T cells compared to the WT CD4+ T cells (Fig. 3a,b). The levels of Th2 cytokines (IL‐4, IL‐5 and IL‐13) and Th1 cytokine (IFN‐) in the culture supernatant (Fig. 3c) were in parallel to the frequency of Th2 and Th1 cells. To corroborate the results, CD4+ T cells were isolated from human peripheral blood cells and treated with VDR RNAi (to be VDR–/–CD4+ T cells) or control RNAi (Fig. 3d). The cells were cultured in the presence of T cell activators (PMA/ionomycin) in the culture for 72 h. The exposure to activators resulted in more Th2 cells and fewer Th1 cells in the VDR–/–CD4+ T cells compared to the CD4+ T cells treated with control RNAi (Fig. 3e,f). The levels of Th2 cytokines (IL‐4, IL‐5 and IL‐13) and Th1 cytokine (IFN‐) in the culture supernatant (Fig. 3g) were in parallel to the frequency of Th2 and Th1 cells. The results demonstrate that the VDR–/– CD4+ T cells are prone to differentiate into Th2 cells.
Figure 3.
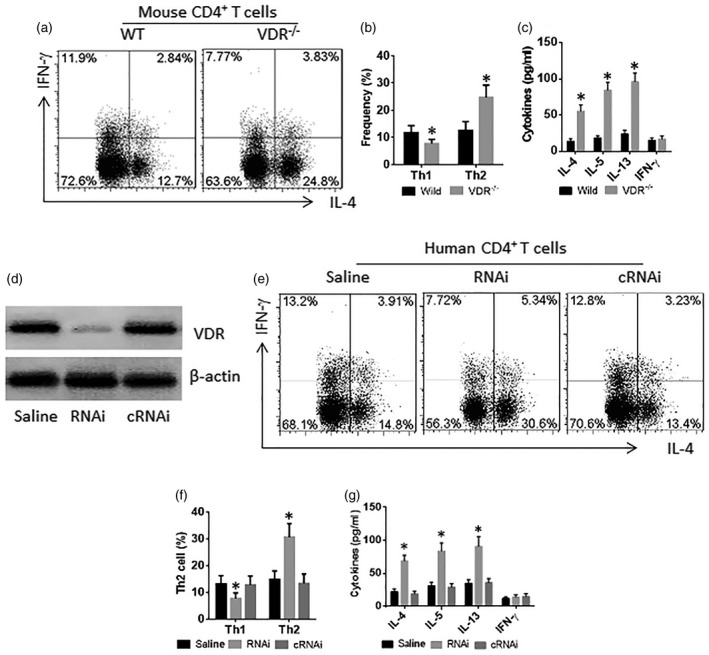
Vitamin D receptor (VDR)–/–CD4+ T cells are prone to differentiating to T helper type 2 (Th2) cells. (a–c) Spleen cells were prepared from wild‐type (WT) mice and VDR–/– mice. The cells were exposed to phorbol myristate acetate (PMA) (10 ng/ml)/ionomycin (100 ng/ml) (c) in the culture for 72 h. (a) Cells were analysed by flow cytometry. The CD3+CD4+ T cells were gated first (data not shown); the histograms show the frequency of CD4+interferon (IFN)‐+ T cells and CD4+ interleukin (IL)‐4+ T cells. (b) The bars show the summarized data of the gated histograms. (c) The bars show the cytokine levels in the culture supernatant. (d–g) CD3+CD4+ T cells were isolated from healthy subject peripheral blood cells (data not shown); treated with VDR RNAi (RNAi) or control RNAi (cRNAi) (d), and cultured for 72 h in the presence of PMA/ionomycin. (e) The cells were analysed by flow cytometry. The gated histograms show the frequency of IL‐4+ T cells (Th2 cells) and IFN‐γ+ T cells (Th1 cells). (f) The bars indicate the summarized data of gated cells in panel (e). (g) The bars indicate the cytokine levels in the culture supernatant [by enzyme‐linked immunosorbent assay (ELISA)]. The data of bars are presented as mean ± standard deviation (s.d.); *P < 0·01 (t‐test), compared with the wild‐type group. The data represent three independent experiments.
VDR binds NLRP3 to restrict IL‐4 expression in CD4+ T cells
NLRP3 is a critical transcription factor in the Th2 response [12]. We wondered if VDR regulated the NLRP3‐induced Th2 differentiation. To test this, we performed a co‐immunoprecipitation (IP) assay with the nuclear extracts of WT CD4+ T cells. A VDR–NLRP3 complex was detected in nuclear extracts (Fig. 4a). To corroborate the results, a VDR‐FLAG plasmid and an NLRP3‐His plasmid were transfected into human embryonic kidney (HEK)293 cells. A complex of VDR and NLRP3 was also detected in the HEK293 cells, as revealed by co‐IP with antibodies of FLAG and His as the precipitation antibodies (Fig. 4b); the complex was also formed in vitro (Fig. 4c). To test if the physical contact between VDR and NLRP3 alter the NLRP3’s activities, the cell extracts were analysed by ChIP. The results showed that the levels of NLRP3 and RNA polymerase II (Pol II) were much higher in the IL4 promoter locus of VDR–/–CD4+ T cells than that in WT cells, which were suppressed after the reconstitution of the VDR expression (Fig. 4d–f). The expression of IL‐4 was also altered in parallel to the changes of NLRP3 in the CD4+ T cells (Fig. 4g,h). The results demonstrate that VDR restricts the expression of IL‐4 in CD4+ T cells.
Figure 4.
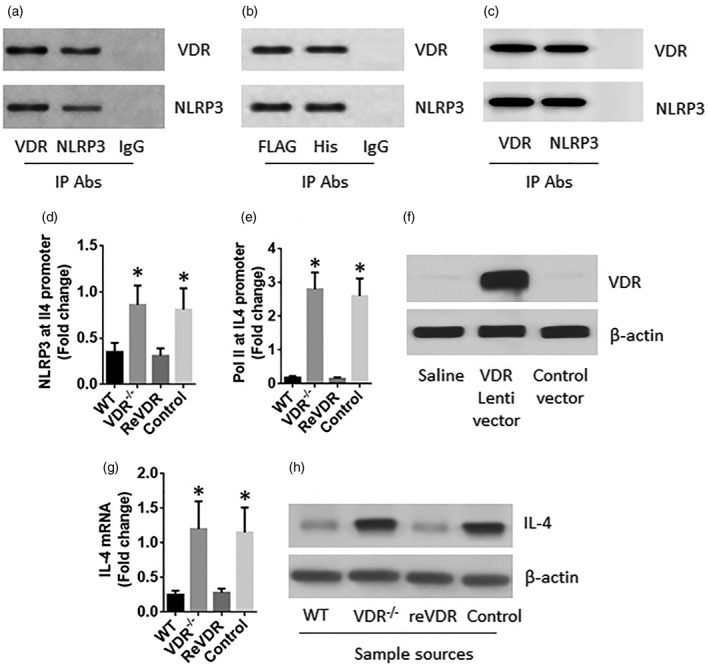
Vitamin D receptor (VDR) restricts interleukin (IL)‐4 expression in CD4+ T cells. (a) A complex of VDR and nucleotide‐binding, oligomerization domain (NOD)‐like receptor family, pyrin domain containing 3 (NLRP3) in wild‐type (WT) CD4+ T cells. (b) A complex of VDR and NLRP3 in human embryonic kidney (HEK)293 cells after transfection of VDR‐FLAG plasmids and NLRP3‐his plasmids. (c) A complex of recombinant VDR and recombinant NLRP3 formed in vitro. (d–h) CD4+ T cells were isolated from the spleen of WT and VDR–/– mice. A portion of the VDR–/–CD4+ T cells were transduced with the VDR gene‐carrying lentivectors or control vectors. (d,e) The nuclear extracts of the cells were analysed by chromatin immunoprecipitation (ChIP). The bars indicate the levels of NLRP3 and RNA polymerase II (Pol II) at the IL4 promoter locus. (f) The results of VDR over expression in CD4+ T cells. (g,h) The expression of IL‐4 in the cells. The data of bars are presented as mean ± standard deviation (s.d.); *P < 0·01 (t‐test), compared with the WT group. The data represent three independent experiments.
VDR–/– mice show Th2‐biased inflammation in the intestine
The data reported above imply that the Th2‐biased inflammation might occur in the intestine of VDR–/– mice. To test this, we prepared protein extracts with the intestinal tissue. The proteins were analysed by ELISA. The results showed higher levels of IL‐4, IL‐5, IL‐13 and IgE, while the levels of IFN‐ were lower in the VDR–/– mice than that in the WT mice (Fig. 5a). The number of lamina propria mononuclear cells (LPMCs) was much greater in the VDR–/– mice than that in the WT mice, indicating more immune cell infiltration into the intestinal tissue in the VDR‐deficient environment (Fig. 5b). The LPMCs were analysed by flow cytometry; there were more CD4+IL‐4+ T cells and fewer CD4+ IFN‐+ cells in the intestine of the VDR–/– mice compared to the WT mice (Fig. 5c). Histology studies were also performed. The results showed a profound infiltration of mast cells in the intestine (Fig. 5d). The results demonstrate that VDR–/– mice have Th2‐biased inflammation in the intestine.
Figure 5.
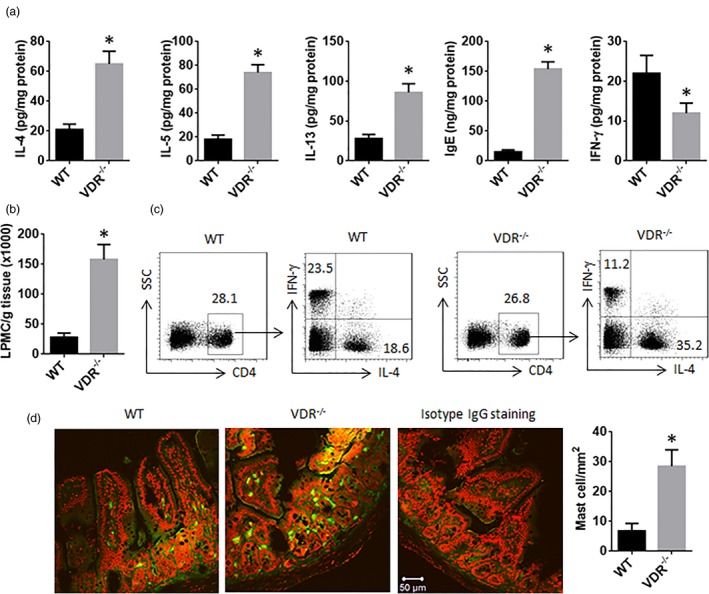
T helper type 2 (Th2) biased inflammation in the intestine of vitamin D receptor (VDR)–/– mice. (a) The protein levels of interleukin (IL)‐4, IL‐5, IL‐13, immunoglobulin (Ig)E and interferon (IFN)‐ in the extracts of the intestinal tissue. (b) The counts of LPMC. (c) Flow cytometry dot‐plots; CD4+ T cells were gated from the LPMCs first; the CD4+ T cells were gated further into IL‐4+ fraction and IFN‐+ fraction. (d) Representative confocal images show mast cells (stained in green) in the intestine. The bars show the counts of mast cells (20 fields were counted from each sample; original magnification 200). The data of bars are presented as mean ± standard deviation (s.d.); *P < 0·01 (t‐test), compared with the wild‐type group. Each group consists of six mice.
Discussion
This study revealed that VDR plays an important role in the development of biased Th2 polarization. The biased Th2 polarization is the signature pathological change in allergic diseases. The underlying mechanism is not currently understood fully. Previous studies mentioned that VDR‐deregulation was associated with the pathogenesis of allergic diseases, such as allergic dermatitis [18], asthma [19] and food allergy [19]. The present data provide mechanistic evidence by showing that VDR plays an important role in restricting the Th2 cell differentiation. We observed that mice with VDR deficiency were prone to develop Th2‐biased inflammation in the intestine. The VDR–/–CD4+ T cells of mouse and human showed the features of Th2‐biased response upon proper stimulation. VDR bound to NLRP3 to form a complex to prevent the role of NLRP3 in initiation of Th2 cell differentiation.
Although the major role of vitamin D is to facilitate calcium absorption and metabolism, cumulative reports indicate that vitamin D plays a crucial role in the regulation of immune responses in the body via activation of the VDR [9]. The deficiency of vitamin D is associated with a number of immune diseases, such as the high prevalence of vitamin D deficiency or insufficiency in schoolchildren, was associated with the prevalence of atopic dermatitis and allergic rhinitis [20]. The relation between vitamin D deficiency and allergic asthma has been demonstrated by published evidence [21]. The Th2‐biased inflammation is associated with vitamin D deficiency [22]. Our study provides mechanistic evidence by showing that the abnormality of VDR links with the pathogenesis of the Th2‐biased inflammation in the intestine. The evidence includes that VDR–/– T cells differentiate spontaneously into Th2 cells and produce higher levels of Th2 cytokines. Mice with VDR deficiency were prone to suffering from Th2‐biased inflammation in the intestine.
VDR is a nuclear receptor; it mediates the cell activities between the cytoplasm and the nucleus. The present data show that VDR formed a complex with NLRP3 in the nuclei of CD4+ T cells. Such a physical contact between VDR and NLRP3 resulted in that NLRP3 could not transactivate the IL‐4 promoter. NLRP3 is found originally in the formation of inflammasome [23]. Recent reports indicate that NLRP3 is also a critical transcription factor in the Th2 programme [12]. The data demonstrate that VDR plays an important role in maintaining the Th2 programme at a given level by restricting NLRP3 from binding the IL4 promoter. Following such a physical interaction between VDR and the IL‐4 transcription factors, inhibition of IL‐4 expression by T cells was observed in the present study. Others also found that vitamin D deficiency reduced the expression of VDR [24]. Whether the VDR agonists [25] modulate Th2‐biased polarization is an interesting topic and is worth being investigated further, as a large body of studies observed that vitamin D deficiency or insufficiency was associated with immune diseases with the Th2‐biased response [20–22]. To up‐regulate VDR can suppress IgE production [26] via binding the ε germline gene promoter [27].
In summary, the present data show that VDR contributes to restricting Th2‐biased response by restricting the activities of IL‐4 transcription factors. The data suggest that VDR deficiency may be necessary to be considered when making therapeutic plan for Th2‐biased inflammation.
Disclosure
None.
Author contributions
H. H., J.‐Y. H., Y.‐J. W., E.‐Y. W., Z.‐Q. L., B.‐H. C. and L. M. performed experiments, analysed data and reviewed the manuscript. P.‐Y. Z., Z.‐G. L. and P.‐C. Y. organized the study and supervised experiments. P.‐C. Y. designed the project and wrote the paper.
Acknowledgements
This study was supported by grants from the National ‘13th‐5’ key programme, Precision medicine research project (2016YFC0905802 and 2016YFC0903700), Guangdong provincial scientific technological research project (2016A020216029) and Shenzhen scientific technological basic research project (JCYJ20160429114659119).
Contributor Information
Z.‐G. Liu, Email: lzg@szu.edu.cn
P.‐C. Yang, Email: pcy2356@szu.edu.cn
P.‐Y. Zheng, Email: medp7123@126.com
References
- 1. Agrawal T, Gupta GK, Agrawal DK. Vitamin D deficiency decreases the expression of VDR and prohibitin in the lungs of mice with allergic airway inflammation. Exp Mol Pathol 2012; 93:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed SZ, Jaleel A, Hameed K, Qazi S, Suleman A. Does vitamin D deficiency contribute to the severity of asthma in children and adults? J Ayub Med Coll Abbottabad 2015; 27:458–63. [PubMed] [Google Scholar]
- 3. Bruchard M, Rebe C, Derangere V et al The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol 2015; 16:859–70. [DOI] [PubMed] [Google Scholar]
- 4. Crescioli C. Vitamin D receptor agonists: suitable candidates as novel therapeutic options in autoimmune inflammatory myopathy. Biomed Res Int 2014; 2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 2015; 265:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannetti MP, Cardet JC. Interleukin‐5 antagonists usher in a new generation of asthma therapy. Curr Allergy Asthma Rep 2016; 16:80. [DOI] [PubMed] [Google Scholar]
- 7. Ginter E, Simko V. Deficiency of vitamin D and vitamin C in the pathogenesis of bronchial asthma. Bratisl Lek Listy 2016; 117:305–7. [DOI] [PubMed] [Google Scholar]
- 8. Hartmann B, Heine G, Babina M et al Targeting the vitamin D receptor inhibits the B cell‐dependent allergic immune response. Allergy 2011; 66:540–8. [DOI] [PubMed] [Google Scholar]
- 9. Hartmann B, Riedel R, Jörß K et al Vitamin D receptor activation improves allergen‐triggered eczema in mice. J Investig Dermatol 2012; 132:330–6. [DOI] [PubMed] [Google Scholar]
- 10. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers 2015; 1:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016; 21:736–44. [DOI] [PubMed] [Google Scholar]
- 12. Licona‐Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol 2013; 14:536–42. [DOI] [PubMed] [Google Scholar]
- 13. Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 2003; 89:922–32. [DOI] [PubMed] [Google Scholar]
- 14. Milovanovic M, Heine G, Hallatschek W, Opitz B, Radbruch A, Worm M. Vitamin D receptor binds to the epsilon germline gene promoter and exhibits transrepressive activity. J Allerg Clin Immunol 2010;126:1016–23, 23.e1–4. [DOI] [PubMed] [Google Scholar]
- 15. Mortimer L, Moreau F, MacDonald JA. NLRP3 inflammasome inhibition is disrupted in a group of auto‐inflammatory disease CAPS mutations. Nat Immunol 2016; 17:1176–86. [DOI] [PubMed] [Google Scholar]
- 16. Olsson K, Saini A, Stromberg A et al Evidence for vitamin D receptor expression and direct effects of 1alpha,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology 2016; 157:98–111. [DOI] [PubMed] [Google Scholar]
- 17. Parulekar AD, Diamant Z, Hanania NA. Role of T2 inflammation biomarkers in severe asthma. Curr Opin Pulm Med 2016; 22:59–68. [DOI] [PubMed] [Google Scholar]
- 18. Schedel M, Jia Y, Michel S. 1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter. Nat Commun 2016; 7:10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suaini NH, Zhang Y, Vuillermin PJ, Allen KJ, Harrison LC. Immune modulation by vitamin D and its relevance to food allergy. Nutrients 2015; 7:6088–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh GM. Biologics targeting IL‐5, IL‐4 or IL‐13 for the treatment of asthma – an update. Expert Rev Clin Immunol 2017; 13:143–7. [DOI] [PubMed] [Google Scholar]
- 21. Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015; 7:8251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor‐deficient mice fail to develop experimental allergic asthma. J Immunol 2004; 173:3432–6. [DOI] [PubMed] [Google Scholar]
- 23. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 2015; 15:271–82. [DOI] [PubMed] [Google Scholar]
- 24. Yang G, Geng X‐R, Liu Z‐Q et al Thrombospondin‐1 (TSP1)‐producing B cells restore antigen (Ag)‐specific immune tolerance in an allergic environment. J Biol Chem 2015; 290:12858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang G, Zhang H, Liu Y et al Alternation of circadian clock modulates forkhead box protein‐3 gene transcription in CD4(+) T cells in the intestine. J Allerg Clin Immunol. 2016;138(1446–9):e10. [DOI] [PubMed] [Google Scholar]
- 26. Yang HK, Choi J, Kim WK et al The association between hypovitaminosis D and pediatric allergic diseases: a Korean nationwide population‐based study. Allergy Asthma Proc 2016; 37:64–9. [DOI] [PubMed] [Google Scholar]
- 27. Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alpha‐alpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci USA. 2008;105:20834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


