Summary
Immunoglobulin (Ig) G‐ and IgM‐class anti‐cardiolipin antibodies (aCL) and lupus anti‐coagulant (LA) are included in the 1997 update of the American College of Rheumatology (ACR‐97) systemic lupus erythematosus (SLE) criteria. Despite limited evidence, IgA‐aCL and IgA anti‐β2‐glycoprotein‐I (anti‐β2GPI) were included in the 2012 Systemic Lupus International Collaborating Clinics criteria. The present study aimed to evaluate IgG‐/IgA‐/IgM‐aCL and anti‐β2GPI occurrence in relation to disease phenotype, smoking habits, pharmacotherapy, anti‐phospholipid syndrome (APS) and organ damage among 526 Swedish SLE patients meeting ACR‐97. Patients with rheumatoid arthritis (n = 100), primary Sjögren’s syndrome (n = 50) and blood donors (n = 507) served as controls. Anti‐phospholipid antibodies (aPL) were analysed by fluoroenzyme‐immunoassays detecting aCL/anti‐β2GPI. Seventy‐six (14%) SLE cases fulfilled the Sydney APS‐criteria, and ≥ 1 aCL/anti‐β2GPI isotype (IgG/IgA/IgM) occurred in 138 SLE patients (26%). Forty‐five (9%) of the SLE cases had IgA‐aCL, 20 of whom (4%) lacked IgG‐/IgM‐aCL. Seventy‐four (14%) tested positive for IgA anti‐β2GPI, 34 (6%) being seronegative regarding IgG/IgM anti‐β2GPI. Six (1%) had APS manifestations but were seropositive regarding IgA‐aCL and/or IgA anti‐β2GPI in the absence of IgG/IgM‐aPL and LA. Positive LA and IgG‐aPL tests were associated with most APS‐related events and organ damage. Exclusive IgA anti‐β2GPI occurrence associated inversely with Caucasian ethnicity [odds ratio (OR) = 0·21, 95% confidence interval (CI) = 0·06–0·72) and photosensitivity (OR = 0·19, 95% CI = 0·05–0·72). Nephritis, smoking, LA‐positivity and statin/corticosteroid‐medication associated strongly with organ damage, whereas hydroxychloroquine‐medication was protective. In conclusion, IgA‐aPL is not rare in SLE (16%) and IgA‐aPL analysis may have additional value among SLE cases with suspected APS testing negative for other isotypes of aPL and LA.
Keywords: anti‐phospholipid antibodies, anti‐phospholipid syndrome, autoantibodies, immunoglobulin A, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a potentially severe autoimmune condition with an unpredictable disease course, often with fluctuations in disease activity over time 1. Long‐term inflammation and drug‐related side effects may lead subsequently to irreversible organ damage, a consequence which is associated intimately with decreased quality of life and increased mortality 2, 3, 4. In SLE, accrual of organ damage and prognosis has been linked consistently to the presence of anti‐phospholipid antibodies (aPL), with or without clinical events related to the anti‐phospholipid syndrome (APS) 5, 6, 7. Presence of the lupus anti‐coagulant (LA) has been identified as the laboratory finding with the highest predictive value regarding future organ damage in SLE 8.
The 1997 update of the American College of Rheumatology (ACR) classification criteria for SLE incorporated the presence of anti‐cardiolipin antibodies (aCL) of immunoglobulin (Ig)G/IgM isotype and/or a positive LA test and/or a persistent false‐positive serological test for syphilis 9. Recent reviews conclude that 30–40% of all SLE cases display elevated levels of any aPL at some point during the disease course, yet only approximately half of these SLE cases will fulfil the APS classification criteria 10, 11, 12. According to the Sydney classification criteria 13, APS is defined by vascular thrombosis and/or pregnancy morbidity and repeated raised defined levels of IgG and/or IgM isotype aCL and/or anti‐β2 glycoprotein‐I (anti‐β2GPI) antibodies and/or a positive LA test.
Based on the results from some studies, it has been claimed that the assessment of IgA isotype aCL and/or anti‐β2GPI provides additional clinical value and identify IgG/IgM aPL and LA negative cases of APS in SLE 14, 15, 16, 17. Accordingly, the International Consensus Task Force on aPL antibodies recommends IgA isotype testing for both aCL and anti‐β2GPI when results of all other tests are negative and APS is still suspected 18. Recently, it has been suggested that the presence of IgA anti‐β2GPI in people with no history of APS‐related events constitute an important independent risk factor for the development of such events 19. Conversely, other studies found that analysis of IgA aPL did not contribute to the recognition of APS in SLE patients 20, 21, 22, 23. Nevertheless, in addition to the IgG and IgM isotypes, IgA aPL was included in the most recent set of SLE classification criteria proposed by the Systemic Lupus International Collaborating Clinics (SLICC) group in 2012. In their validation set of the SLICC‐12 criteria, a greater sensitivity (97 versus 83%) but a slightly lower specificity (84 versus 96%) compared with the 1997 ACR classification criteria was demonstrated 24. However, it remains to be elucidated whether or not this update helps to identify SLE cases prone to develop APS‐related events and future organ damage 23, 25. In Scandinavia, systematic assessment of IgA aCL and anti‐β2GPI in suspected or newly diagnosed cases of SLE is currently not a part of the general clinical routine. Furthermore, the importance of other aPLs, such as anti‐phosphatidylserine/prothrombin complex IgG and anti‐β2GPI domain 1 IgG, in relation to APS in SLE has been evaluated recently 26.
As the presence of IgA aPLs is of uncertain clinical significance 12, the overall goal of this study was to evaluate IgA aCL and anti‐β2GPI antibodies in serum samples of 526 well‐characterized Swedish SLE patients in relation to controls, other aPL isotypes, disease phenotypes, smoking habits, ongoing pharmacotherapy, APS‐related events as well as the association with damage accrual in each domain of the SLICC/ACR damage index (SDI) 27.
Materials and Methods
SLE
SLE patients (n = 526) diagnosed at the rheumatology clinics at the Linköping (n = 231) and Karolinska (Stockholm) University hospitals (n = 295) were included. All cases were classified as SLE according to the 1997 ACR criteria update 9, and both cohorts have been described in detail previously 28, 29. Altogether, 461 (88%) were prevalent cases and 65 (12%) had a newly diagnosed SLE (≤ 12 months’ disease duration) at the time of sampling. Data on smoking habits (past/present/never) were recorded at the time‐point of blood collection. A total of 476 of 526 (90%) cases were of Caucasian ethnicity, whereas the majority of non‐Caucasian SLE patients had Asian or Hispanic origin. Detailed information regarding organ damage at the time of sampling in each separate domain of SDI was obtained by chart review for each patient 27. SDI covers 12 organ systems and measures accumulated organ damage that has occurred since the disease onset, and is scored regardless of whether the damage can be attributed to SLE or to other causes 27. In addition, data on APS classification including pregnancy morbidities and other APS‐related events were collected 13. SLE patient characteristics are detailed in Table 1.
Table 1.
Detailed characteristics of the 526 systemic lupus erythematosus (SLE) cases
| Background variables | |
|---|---|
| Females, n (%) | 475 (90·3) |
| Age at blood sampling, mean years (range, years) | 48·1 (18–88) |
| Caucasian ethnicity, n (%) | 476 (90·5) |
| Body mass index, mean (range) | 25·2 (14·2–59·1) |
| Ever smoker (former or current), n (%) | 263 (50·2) |
| Daily dose of prednisolone at blood sampling, mean (range, mg) | 5·4 (0–60) |
| Disease variables | |
| Age at diagnosis, mean years (range, years) | 35·1 (3–85) |
| Disease duration at blood sampling, mean years (range, years) | 15·0 (0–58) |
| Established disease at time for blood sampling, n (%) | 461 (87·6) |
| Meeting ACR‐97 criteria, n (%) | 526 (100) |
| Number of fulfilled ACR‐97 criteria, mean (range) | 5·8 (4–10) |
| SLEDAI‐2K at blood sampling, mean (range) | 3·9 (0–28) |
| SLICC/ACR damage index, mean (range) | 1·7 (0–11) |
| Clinical SLE phenotypes (ACR‐97 defined), n (%) | |
| (1) Malar rash | 260 (49·4) |
| (2) Discoid rash | 98 (18·6) |
| (3) Photosensitivity | 327 (62·2) |
| (4) Oral ulcers | 129 (24·5) |
| (5) Arthritis | 424 (80·6) |
| (6) Serositis | 210 (39·9) |
| Pleuritis | 189 (35·9) |
| Pericarditis | 84 (16·0) |
| (7) Renal disorder | 181 (34·4) |
| (8) Neurological disorder | 45 (8·6) |
| Seizures | 39 (7·4) |
| Psychosis | 11 (2·1) |
| (9) Haematological disorder | 354 (67·3) |
| Haemolytic anaemia | 26 (4·9) |
| Leucocytopenia | 228 (43·3) |
| Lymphopenia | 235 (44·7) |
| Thrombocytopenia | 90 (17·1) |
| Raynaud | 181 (34·4) |
| Immunological features (ACR‐97 defined), n (%) | |
| (10) Immunological disorder | 338 (64·3) |
| Anti‐dsDNA antibody (anti‐dsDNA) | 310 (58·9) |
| Anti‐Smith antibody (anti‐Sm) | 89 (16·9) |
| (11) Anti‐nuclear antibody (ANA)a | 519 (98·7) |
| Anti‐Sjögren’s syndrome A (Ro52) | 155 (29·5) |
| Anti‐Sjögren’s syndrome A (Ro60) | 213 (40·7) |
| Anti‐Sjögren’s syndrome B (La) | 131 (24·9) |
| Lupus anti‐coagulant (LA) test positiveb | 128 (25·7) |
| Clinical APS phenotypes, n (%) | |
| Anti‐phospholipid syndrome (clinical diagnosis) | 98 (18·6) |
| Anti‐phospholipid syndrome (defined by classification)c | 76 (14·4) |
| Any arterial event (MI, all cerebrovascular lesions) | 77 (14·6) |
| Myocardial infarction (MI) | 38 (7·2) |
| Angina pectoris | 25 (4·8) |
| Coronary bypass | 14 (2·7) |
| Valvular disease | 44 (8·4) |
| Valvular surgery | 8 (1·5) |
| Arterial embolism (MI, ischaemic stroke) | 73 (13·9) |
| Cerebrovascular lesions (ischaemic stroke, cerebral haemorrhage, TIA) | 61 (11·6) |
| Ischaemic stroke | 47 (8·9) |
| Cerebral haemorrhage | 9 (1·7) |
| Transient ischaemic attack (TIA) | 19 (3·6) |
| Venous thromboembolism (DVT and/or PE) | 72 (13·7) |
| Deep vein thrombosis (DVT) | 64 (12·2) |
| Pulmonary embolism (PE) | 23 (4·4) |
| Intermittent claudication | 7 (1·3) |
| Any miscarriage | 78 (16·4) |
| ≥ 3 miscarriages before the 10th week of gestation | 6 (1·3) |
| ≥ 1 miscarriage beyond the 10th week of gestation | 46 (9·7) |
Positive by immunofluorescence microscopy (IF‐ANA).
Data available in 499 of 526 cases.
According to Miyakis et al . 13
ACR = American College of Rheumatology; SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; SLICC = Systemic Lupus International Collaborating Clinics.
Disease controls and blood donors
Patients with primary Sjögren’s syndrome (pSS) and patients with rheumatoid arthritis (RA) served as disease controls. None of these patients had a concomitant APS diagnosis. Sera from 50 patients with established pSS (94% women; mean age = 62 years) meeting the American–European consensus criteria was collected 30. Forty‐nine per cent of the pSS patients had a history of extra glandular disease; 90% were positive for anti‐SSA antibodies (± anti‐SSB). 51% received prednisolone, 53% were treated with hydroxychloroquine (HCQ) and 27% were prescribed other disease‐modifying anti‐rheumatic drugs (DMARDs), of which methotrexate was the most common (14%).
Sera from 100 patients with early RA included in TIRA‐2 (Swedish acronym for ‘timely interventions in RA’) were collected. The patients were diagnosed with recent‐onset RA by the ACR 1987 criteria (≤ 12 months since the first joint swelling) and included in Linköping’s TIRA‐2 cohort between 2006 and 2009 31, 32. At sampling, 83% of the patients received DMARDs. The mean age was 55 years, 69% were women, 64% were anti‐cyclic citrullinated peptide‐2 antibody (anti‐CCP2)‐positive and 60% were IgM rheumatoid factor (RF)‐positive. During 8 years of follow‐up, none of them developed SLE.
The Sydney criteria for APS require cut‐off levels corresponding to the ≥ 99th percentile of the levels in controls 13. This was determined for each aPL isotype using 507 control sera (75% female). Of these, 212 were healthy blood donors from Linköping University hospital (mean age = 44 years) and 295 were controls from the general population, Karolinska University hospital (mean age = 48 years) without any history of thrombosis or pregnancy morbidity, as defined in the APS criteria.
aPL and anti‐coagulant assays
IgG, IgA and IgM aCL and anti‐β2GPI were analysed in the accredited clinical immunology laboratories at Linköping, Uppsala and Karolinska University hospitals using fluoroenzyme‐immunoassays (Phadia‐250 instrument; Thermo‐Fisher Scientific Phadia AB, Uppsala, Sweden). The defined cut‐off level for each isotype was for aCL, IgG/IgA/IgM 26 GPL‐U/ml, 17 APL‐U/ml and 34 MPL‐U/ml, and for anti‐β2GPI, IgG/IgA/IgM 31, 13 and 7·2 U/ml, respectively. LA was determined by the dilute Russell’s viper venom time (dRVVT) method (Siemens Healthcare Diagnostics, Erlangen, Germany) in Linköping, and by a modified dRVVT (Biopool, Umeå, Sweden) using Bioclot LA at Karolinska.
Definitions
aPL‐positive cases were categorized as being ‘independently positive’ for an isotype (i.e. regardless of being positive for other isotypes or LA) or ‘exclusively positive’ for an isotype (i.e. isolated positive for the specific antibody, meaning absence of other aPL isotypes and LA) or ‘triple‐positive’ (i.e. at least one positive isotype of aCL combined with any anti‐β2GPI isotype plus a positive LA test). In order to evaluate the potential additive value of IgA aPL analysis, we also studied cases categorized as being positive for at least one IgA isotype in the absence of other isotypes and LA.
Statistical analyses
Comparisons of aPL levels between groups were performed using the Mann–Whitney U‐test. Furthermore, the Mann–Whitney U‐test was used to establish potential differences in aPL levels within blood donors and SLE cases. Correlation analyses between aPL levels and age in SLE, disease controls and blood donors were calculated using Spearman’s rho.
Associations between (a) aPL antibody positivity and (b) SLE phenotypes, APS‐related events, pharmacotherapy and damage accrual were examined by χ2 or Fisher’s exact test when numbers were ≤ 5.
Poisson regression was used to establish the empirical relationship between damage accrual (global SDI score) and each of the isotypes, age, disease duration, smoking habits, hypertension, lupus nephritis, ongoing treatments with HCQ and statins, a daily dose of prednisolone of ≥ 7·5 mg and LA positivity (univariate model). Thereafter, all variables significant in the univariate model were combined and a stepwise procedure eliminating non‐significant (P ≥ 0·05) variables at each step was performed until a multiple model with only significant variables remained (the model with highest pseudo‐R 2 with only significant predictors). Two‐tailed P‐values < 0·05 were considered significant. Statistical analyses were performed with spss Statistics version 23.0 (IBM, Armonk, NY, USA) or GraphPad Prism, version 6.07 (GraphPad Software, La Jolla, CA, USA).
Ethical approval
Oral and written informed consent was obtained from all participants. The study protocols were approved by the regional ethics review boards in Linköping regarding SLE (M75‐08/2008) and early RA (M168‐05), in Stockholm regarding SLE (03‐556/031216), and in Uppsala regarding pSS (2006/217/2).
Results
aPL levels among disease controls and blood donors
Fourteen (14%) of the RA patients and six (12%) of the pSS cases (without APS diagnosis) tested positive for at least one aPL isotype. The distribution of aPL levels in RA and pSS controls are demonstrated in Fig. 1a,b. No differences were found in aPL levels with regard to age or sex among disease controls. There were no significant differences regarding the levels of any aCL/anti‐β2GPI isotype between blood donors and population‐based donors, but the population‐based donors were slightly older (P < 0·02; the difference between medians was 2 years). IgG aCL was the only isotype which correlated significantly with age (rho = −0·10; P < 0·05). No differences were identified comparing aPL levels in women and men.
Figure 1.
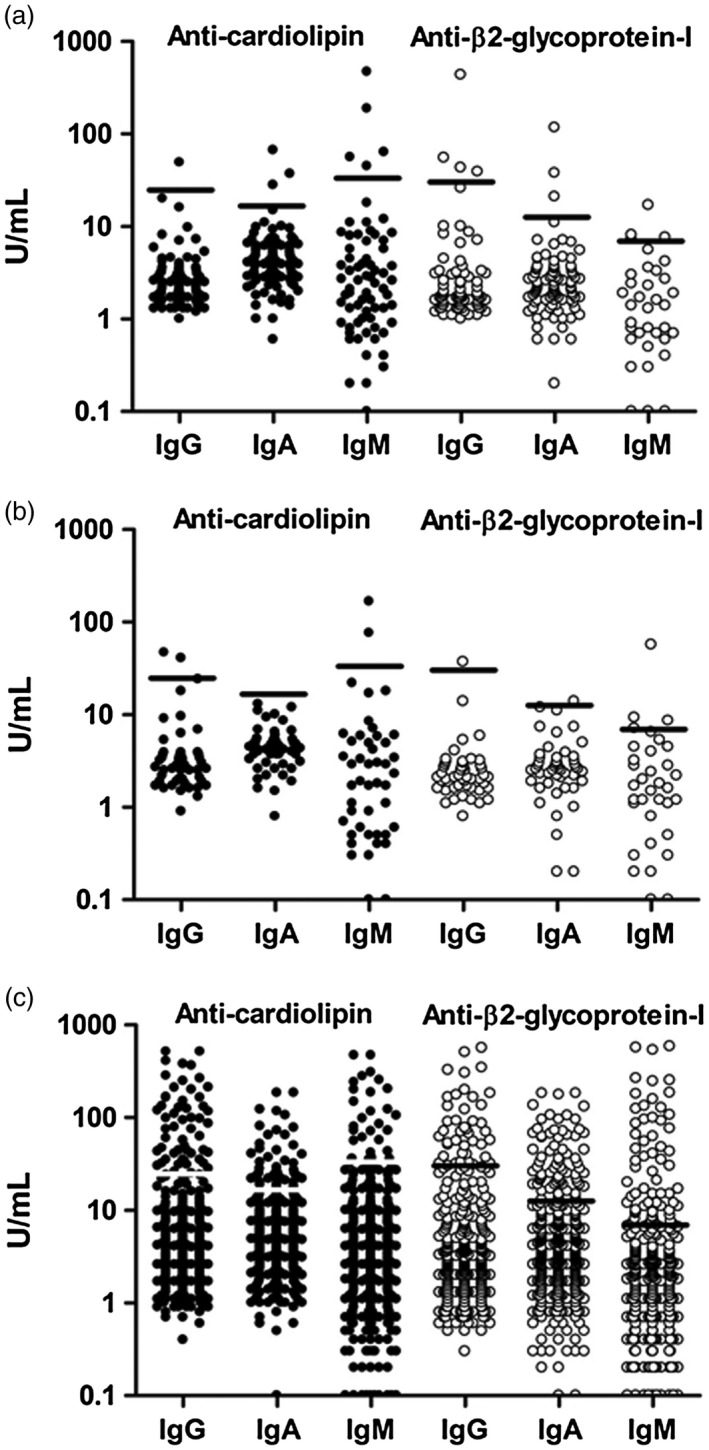
(a–c) Serum levels of anti‐phospholipid antibody (aPL) isotypes were determined by fluoroenzyme‐immunoassays. Cut‐off level corresponded to the 99th percentile of the levels of healthy controls. Closed circles represent anti‐cardiolipin antibodies (aCL), while open circles represent anti‐β2‐glycoprotein‐I (anti‐β2GPI). (a) Serum levels of aCL and anti‐β2GPI isotypes in 100 rheumatoid arthritis (RA) controls. (b) Serum levels of aCL and anti‐β2GPI isotypes in 50 primary Sjögren’s syndrome (pSS controls. (c) Serum levels of aCL and anti‐β2GPI isotypes in 526 systemic lupus erythematosus (SLE) cases.
aPL levels among cases with SLE
The levels of each separate aPL isotype among cases with SLE are shown in Fig. 1c. In total, 138 (26%) were positive for at least one antibody isotype. As demonstrated in Fig. 2, IgA aCL and/or anti‐β2GPI were found in 82 (16%) cases. Figure 2 illustrates the 45 (9%) IgA aCL‐positive cases, 20 (4%) of whom were positive in the absence of IgG/IgM isotypes; Fig. 2 shows that 74 (14%) of the SLE cases were IgA anti‐β2GPI‐positive, 34 (6%) of whom were positive in the absence of IgG/IgM isotypes. Figure 2 demonstrates the overlap between exclusively IgA aPL‐positive SLE cases.
Figure 2.
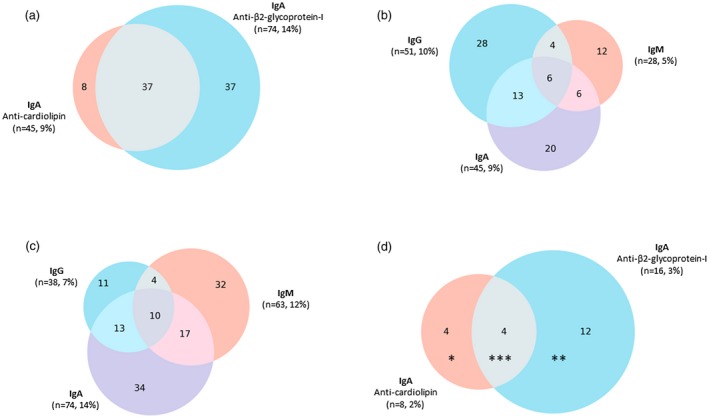
(a) Distribution of immunoglobulin (Ig)A anti‐cardiolipin antibody (aCL) and IgA anti‐β2‐glycoprotein‐I (anti‐β2GPI)‐positive cases in the full systemic lupus erythematosus (SLE) cohort; 82 (16%) of the SLE cases had IgA positivity, 45 (9%) of aCL and 74 (14%) of anti‐β2GPI type. (b) Distribution of IgG/A/M isotypes of aCL in the SLE cohort; 90 (17%) SLE cases were positive for at least one aCL isotype. (c) Distribution of IgG/A/M isotypes of anti‐β2GPI in the SLE cohort. 121 (23%) SLE cases were positive for at least one anti‐β2GPI isotype. (d) Distribution of exclusively IgA aCL and IgA anti‐β2GPI‐positive cases in the SLE cohort; 20 (4%) of the SLE cases had IgA positivity, eight (2%) of aCL and 16 (3%) of anti‐β2GPI type. Each asterisk (*) indicates one patient with an anti‐phospholipid syndrome (APS)‐related event.
aPL levels versus age, smoking habits and ethnicity in SLE
The levels of IgG‐ and IgA‐class aPL antibodies were correlated inversely with age among SLE cases (IgG aCL rho = –0·09, IgA aCL rho = –0·09, IgG anti‐β2GPI rho = –0·10, IgA anti‐β2GPI rho = –0·09; P < 0·05 for each comparison). A positive LA test and/or IgG anti‐β2GPI positivity were associated with being a past or present tobacco smoker (Table 2). Regardless of seropositivity for other aPL isotypes, IgG anti‐β2GPI seemed to associate with Caucasian ethnicity (Table 2). Conversely, non‐Caucasian ethnicity was associated significantly with exclusive positivity for IgA anti‐β2GPI (Table 3).
Table 2.
Significant associations between disease phenotypes/serologies/damage/pharmacotherapy and each independent anti‐cardiolipin antibodies (aCL)/anti‐β2‐glycoprotein‐I (anti‐β2GPI) isotype and lupus anti‐coagulant (LA) (pos/neg) expressed by odds ratios (OR) with 95% confidence intervals
| aCL | anti‐β2GPI | LA | Triple‐positiveb | |||||
|---|---|---|---|---|---|---|---|---|
| IgG (51/526) | IgA (45/526) | IgM (28/526) | IgG (38/526) | IgA (74/526) | IgM (63/526) | (128/499) | (49/499) | |
| Caucasian ethnicity (n = 476) | 8%a, c | |||||||
| Ever smoker (n = 263) | 2·39 (1·18–4·83) | 1·73 (1·15–2·60) | ||||||
| Malar rash (n = 260) | 0·48 (0·26–0·88) | 0·26 (0·10–0·66) | 0·49 (0·29–0·85) | 0·50 (0·33–0·76) | 0·46 (0·25–0·86) | |||
| Discoid rash (n = 98) | 0·42 (0·17–0·99) | |||||||
| Photosensitivity (n = 327) | 0·35 (0·20–0·64) | 0·53 (0·29–0·96) | 0·49 (0·26–0·95) | 0·46 (0·28–0·76) | 0·42 (0·25–0·72) | 0·62 (0·41–0·93) | 0·50 (0·28–0·91) | |
| Thrombocytopenia (n = 90) | 1·99 (1·02–3·85) | |||||||
| Raynaud (n = 181) | 0·45 (0·24–0·84) | |||||||
| Anti‐dsDNA antibody (n = 310) | 2·23 (1·13–4·40) | 1·78 (1·04–3·03) | ||||||
| Anti‐SSA/Ro52 (n = 155) | 0·25 (0·09–0·73) | 0·26 (0·12–0·59) | 0·60 (0·37–0·96) | |||||
| Anti‐SSA/Ro60 (n = 213) | 0·48 (0·25–0·93) | 0·33 (0·17–0·62) | 0·61 (0·40–0·93) | 0·51 (0·26–0·99) | ||||
| Anti‐SSB (n = 131) | 0·30 (0·12–0·77) | 0·15 (0·04–0·63) | 0·33 (0·15–0·75) | 0·53 (0·32–0·89) | 0·39 (0·16–0·95) | |||
| APS, clinical (n = 98) | 4·82 (2·64–8·80) | 6·03 (3·25–11·20) | 3·59 (1·64–7·87) | 6·21 (3·16–12·20) | 4·46 (2·63–7·56) | 4·02 (2·30–7·01) | 12·55 (7·51–20·98) | 7·75 (4·17–14·41) |
| APS, classification (n = 76) | 5·35 (2·86–9·98) | 6·64 (3·52–12·53) | 4·31 (1·93–9·61)a | 7·17 (3·61–14·23) | 4·72 (2·70–8·26) | 3·96 (2·20–7·13) | 10·51 (6·02–18·35) | 9·65 (5·13–18·18) |
| Any arterial event (n = 77) | 2·16 (1·01–4·64) | 3·01 (1·78–5·09) | 2·33 (1·17–4·63) | |||||
| Valvular disease (n = 44) | 4·22 (2·01–8·85)a | 2·48 (1·29–4·76) | 2·37 (1·03–5·43)a | |||||
| Arterial embolism (myocardial infarction or ischaemic stroke) (n = 73) | 2·09 (1·04–4·21) | 2·61 (1·52–4·48) | 2·21 (1·09–4·47) | |||||
| Cerebrovascular lesion (n = 61) | 2·34 (1·13–4·84) | 3·27 (1·85–5·81) | 2·14 (1·01–4·55) | |||||
| Ischaemic stroke (n = 47) | 3·35 (1·59–7·09)a | 2·44 (1·01–5·89)a | 2·31 (1·14–4·68) | 4·53 (2·36–8·66) | 3·05 (1·41–6·60)a | |||
| Transient ischaemic attack (n = 19) | 3·76 (1·29–10·92)a | |||||||
| Venous thrombo‐embolism (deep vein thrombosis or pulmonary embolism) (n = 72) | 2·32 (1·14–4·71) | 2·36 (1·10–5·09) | 1·95 (1·01–3·75) | 2·88 (1·70–4·89) | 2·56 (1·28–5·10) | |||
| Deep vein thrombosis (n = 64) | 2·73 (1·34–5·58) | 2·27 (1·28–5·99)a | 2·31 (1·19–4·47) | 3·00 (1·74–5·18) | 3·02 (1·50–6·06) | |||
| Pulmonary embolism (n = 23) | 13%a, d | |||||||
| Intermittent claudication (n = 7) | 7·36 (1·60–33·86)a | 8·55 (1·87–39·03)a | 7·50 (1·44–39·15)a | 7·71 (1·67–35·52)a | ||||
| Neuropsychiatric damage (n = 129) | 2·38 (1·31–4·32) | 2·03 (1·30–3·17) | 1·91 (1·03–3·55) | |||||
| Cardiovascular damage (n = 73) | 3·00 (1·55–5·81) | 2·19 (1·26–3·81) | 2·21 (1·09–4·47) | |||||
| Peripheral vascular damage (n = 49) | 2·96 (1·37–6·40)a | 2·89 (1·11–7·50)a | 2·81 (1·21–6·51)a | 2·17 (1·07–4·39) | 2·65 (1·30–5·40) | 2·87 (1·56–5·30) | 3·34 (1·58–7·07)a | |
| Any miscarriage (n = 78) | 2·51 (1·26–4·97) | 2·22 (1·01–4·85) | 1·71 (1·01–2·91) | 2·29 (1·14–4·61) | ||||
| ≥1 miscarriage (beyond the 10th week of gestation) (n = 46) | 3·04 (1·39–6·64)a | 3·17 (1·34–7·50)a | 2·18 (1·15–4·12) | 2·66 (1·19–5·96)a | ||||
| Warfarin (ongoing) (n = 103) | 2·76 (1·49–5·11) | 3·07 (1·65–5·74) | 4·60 (2·12–9·98) | 3·20 (1·62–6·31) | 2·84 (1·67–4·85) | 2·91 (1·66–5·10) | 6·83 (4·22–11·06) | 4·39 (2·38–8·08) |
| Salicylic acid (ongoing) (n = 114) | 2·15 (1·16–3·98) | 1·93 (1·02–3·67) | 2·08 (1·22–3·54) | 1·84 (1·16–2·93) |
APS = anti‐phospholipid syndrome.
Fisher’s exact test.
At least one positive isotype of aCL combined with any anti‐β2GPI isotype plus a positive LA test.
OR not possible to calculate as none of 50 (0%) non‐Caucasian patients have anti‐β2GPI immunoglobulin (Ig)G; 38 of 476 (8%) Caucasian patients have anti‐β2GPI IgG.
OR not possible to calculate as none of 23 (0%) patients with pulmonary embolism do not have anti‐β2GPI IgM; 64 of 503 (13%) of patients with pulmonary embolism have anti‐β2GPI IgM.
Table 3.
Significant associations between disease phenotypes/serologies/damage/pharmacotherapy and each exclusively positive anti‐cardiolipin antibodies (aCL)/anti‐β2‐glycoprotein‐I (anti‐β2GPI) isotype or lupus anti‐coagulant (LA), as well as for cases positive for ≥ 1 immunogobulin (Ig)A aPL in the absence of other isotypes or LA, expressed by odds ratios (OR) with 95% confidence intervals
| aCL | anti‐β2GPI | LA | ≥1 IgA isotype (aCL/anti‐β2GPI) | |||||
|---|---|---|---|---|---|---|---|---|
| IgG (51/526) | IgA (45/526) | IgM (28/526) | IgG (38/526) | IgA (74/526) | IgM (63/526) | (128/499) | (82/526) | |
| Male sex (n = 51) | 2·17 (1·02–4·64) | |||||||
| Caucasian ethnicity (n = 476) | 0·21 (0·06–0·72)a | |||||||
| Non‐Caucasian ethnicity (n = 50) | 4·76 (1·39–16·67)a | |||||||
| Photosensitivity (n = 327) | 0·19 (0·05–0·72)a | |||||||
| Serositis (n = 210) | 1·88 (1·09–3·23) | |||||||
| Anti‐dsDNA antibody (n = 310) | 4%a, b | |||||||
| Anti‐SSA/Ro52 (n = 155) | 3·51 (1·09–11·23)a | |||||||
| Anti‐SSA/Ro60 (n = 213) | 4·41 (1·18–16·49)a | 2·79 (1·16–6·72) | ||||||
| APS, clinical (n = 98) | 4·10 (2·32–7·26) | |||||||
| APS, classification (n = 76) | 2·99 (1·61–5·56) | |||||||
| Any arterial event (n = 77) | 2·65 (1·40–5·02) | |||||||
| Valvular disease (n = 44) | 2·27 (1·03–5·04) | |||||||
| Arterial embolism (myocardial infarction OR ischaemic stroke) (n = 73) | 2·10 (1·07–4·15) | |||||||
| Cerebrovascular lesion (n = 61) | 2·95 (1·50–5·83) | |||||||
| Ischaemic stroke (n = 47) | 2·95 (1·40–6·25) | |||||||
| Ocular damage (n = 88) | 3%a, c | |||||||
| Pulmonary damage (n = 32) | 2·63 (1·07–6·48)a | 3·96 (1·25–12·57)a | ||||||
| Musculoskeletal damage (n = 95) | 1·98 (1·07–3·67) | |||||||
| Ciclosporin/ sirolimus (ongoing) (n = 13) | 48·40 (2·82–829·82)a | 8·72 (2·15–35·37)a | ||||||
| Warfarin (ongoing) (n = 103) | 3·40 (1·91–6·04) | |||||||
| Salicylic acid (ongoing) (n = 114) | 1·92 (1·06–3·48) | 3·12 (1·33–7·33)a |
APS = Anti‐phospholipid syndrome.
Fisher’s exact test.
OR not possible to calculate as none of 203 (0%) patients without anti‐dsDNA have isolated anti‐β2GPI immunoglobulin (Ig)M. Eleven of 296 (4%) patients with anti‐dsDNA have isolated anti‐β2GPI IgM.
OR not possible to calculate as none of 419 (0%) patients without ocular damage have isolated IgM aCL. Two of 80 (3%) patients with ocular damage have isolated IgM aCL.
aPL isotypes versus APS‐related events and pharmacotherapy
In total, 76 SLE patients (14%) fulfilled the APS classification criteria. Table 2 presents the significant associations between antibody specificities and SLE phenotypes, APS‐related events, positivity for other autoantibodies, pharmacotherapy and damage accrual regardless of the number of positive aCL/anti‐β2GPI isotypes and/or LA. Triple‐positive cases as well as cases with a positive LA test and/or IgG aPL were associated with most APS events and damage in several organ domains of the SDI.
Table 3 shows significant associations regarding exclusive occurrence of individual aPL isotypes and LA, as well as one column with ≥ 1 IgA isotype demonstrating the potential additive value of introducing analysis of IgA aPLs. LA showed significant associations with several types of damage and APS‐related events. Cases exclusively positive for ≥ 1 IgA isotype associated with presence of anti‐SSA/Ro60 antibodies, organ damage of the pulmonary domain, use of cyclosporin/sirolimus and salicylic acid.
APS‐related events in exclusively IgA‐positive cases
As demonstrated in Fig. 2, we identified eight cases (2%) who were exclusively IgA aCL‐positive, whereas 16 (3%) were exclusively IgA anti‐β2GPI‐positive. Of the 20 cases with exclusively positive IgA aCL and/or anti‐β2GPI, six (1% of all SLE cases) had manifestations compatible with APS. Thus, given that IgA aPLs were included in the APS criteria, another six cases would have been classified as APS (provided testing above defined levels after ≥ 12 weeks), in addition to the 76 identified previously.
Factors associated with damage accrual
Table 4 illustrates factors and manifestations that were associated significantly with damage accrual. In the univariate model several factors were identified. However, in the multiple model disease duration [odds ratio (OR) = 1·020], age (OR = 1·034), past/present) smoking (OR = 1·175), meeting the ACR‐defined nephritis criterion (OR = 1·498), LA positivity (OR = 1·268), daily treatment with ≥ 7·5 mg prednisolone (OR = 1·727), ongoing use of statins (OR = 1·249) and ongoing treatment with HCQ (OR = 0·851) remained in the model. The overall pseudo‐R 2 was 0·471, indicating that almost 50% of the total variation of global SDI scores could be explained by the significant factors included in the multiple model (Table 4).
Table 4.
Poisson regression models to establish empirical relations with damage accrual (global SDI score)
| Univariate model | Multiple model | ||||
|---|---|---|---|---|---|
| ORa | 95% CI | Pseudo‐R 2 | OR | 95% CI | |
| Disease duration | 1·035 | 1·029−1·040 | 0·124 | 1·020 | 1·014−1·026 |
| Age | 1·036 | 1·031−1·040 | 0·230 | 1·034 | 1·029−1·040 |
| Ever smoker | 1·422 | 1·244−1·626 | 0·017 | 1·175 | 1·019−1·355 |
| Lupus nephritis | 1·232 | 1·076−1·410 | 0·003 | 1·498 | 1·289−1·742 |
| Daily prednisolone dose ≥ 7·5 mg (ongoing) | 1·325 | 1·151−1·526 | 0·008 | 1·727 | 1·485−2·008 |
| Statins (ongoing) | 1·822 | 1·488−2·230 | 0·023 | 1·249 | 1·013−1·539 |
| LA positivity | 1·521 | 1·315−1·760 | 0·171 | 1·268 | 1·092−1·471 |
| HCQ (ongoing) | 0·686 | 0·598−0·787 | 0·021 | 0·851 | 0·732−0·989 |
| Hypertension | 1·367 | 1·175−1·589 | 0·024 | ||
| Triple positivity | 1·298 | 1·058−1·593 | <0·001 | ||
| Total pseudo‐R 2 (multiple model) | 0·471 | ||||
Pseudo‐R 2 is different from the R 2 used in ordinary least‐squares regression models. However, it will give an approximation of how well the independent variables are related with the outcome [sum of global SLICC/ACR damage index (SDI)]. CI = confidence intervals.
The odds ratios (OR) can be interpreted as follows: an increase of 1 year of disease duration is associated with 3·5% higher score (OR = 1·035) in the number of SDI points, and ongoing treatment with hydroxychloroquine (HCQ) is associated with 31·4% less score (OR = 0·686, 1–0·686 = 0·314) in the sum of global SDI score compared to those not having ongoing treatment with HCQ.
Discussion
The main objective of this study was to evaluate the frequencies of IgA aCL and anti‐β2GPI in well‐characterized patients with SLE, the majority of whom had established disease, in relation to disease phenotypes, vascular events, smoking habits and accrual of organ damage. We identified a subgroup of patients with IgA aPL antibodies (16%), in some cases even in the absence of IgG and IgM isotypes (4%). The presence of IgA anti‐β2GPI positivity without other isotypes was found to be associated with non‐Caucasian ethnicity (representing fewer than 10% of cases in the study). Apart from ethnicity, exclusive positivity of ≥ 1 IgA aPL antibody showed significant associations with anti‐Ro60 positivity, pulmonary damage and ongoing use of cyclosporin/sirolimus or salicylic acid.
In previous studies of SLE, exclusive occurrence of IgA aCL has been demonstrated in 4−17%, but reports regarding its association with clinical APS are inconsistent 15, 20, 23. In two studies, no associations were found between IgA aCL occurrence and clinical APS‐related events 20, 21, whereas other studies observed associations between IgA aCL and deep vein thrombosis and/or pregnancy loss 15, 23. In the study by Samarkos et al., the occurrence of IgA aCL did not improve sensitivity, specificity or the positive predictive value for APS diagnosis 23. In contrast, some reports have indicated that a positive IgA anti‐β2GPI test is associated with clinical manifestations of APS 15, 16, 17, whereas other studies have been inconclusive 20, 23, 33. Thus, the clinical relevance of IgA aPLs in APS‐related events of SLE cases remains obscure. According to Meijide et al., there is not yet enough evidence to recommend routine analysis of IgA aCL and/or IgA anti‐β2GPI in order to increase the diagnostic accuracy of APS 34. However, comparisons of different studies may be hampered by differences in study populations and lack of diagnostic gold standards regarding methodology, including definition of cut‐off levels for positive results 33, 35, 36, 37. In our study, the overlap between IgA aCL and IgA anti‐β2GPI (Fig. 2) was surprisingly limited. However, we feel confident with the results, as cut‐off levels for both assays were based on samples from more than 500 blood donors.
In the review by Andreoli et al., it was concluded that IgA anti‐β2GPI is of clinical importance regarding APS in patients with SLE, whereas the importance of IgA aCL is less clear 25. This conclusion is supported by other studies showing that exclusive occurrence of IgA anti‐β2GPI antibodies associates with thromboembolic events 38, especially on the arterial side 39. In addition, Tortosa et al. demonstrated recently an annual predictive value for APS events among isolated IgA anti‐β2GPI‐positive asymptomatic individuals of 3.1% over 5 years 19. Similarly, studies of primary APS indicate larger clinical relevance of IgA anti‐β2GPI compared to IgA aCL 40, 41. However, in the present study we found that the overall occurrence of at least one aCL isotype (including IgA) is indeed associated with APS‐related events and vascular damage. Being exclusively IgA aPL‐positive, however, was not associated significantly with APS events or organ damage. IgA anti‐β2GPI was more frequent than IgA aCL, and associated with non‐Caucasian ethnicity. The latter is partly in line with the study by Cucurull et al., who reported higher prevalence rates of IgA aCL and anti‐β2GPI in an African American population with SLE compared to other ethnicities 15. However, in our hands, the exclusive occurrence of IgA aCL did not contribute further with clinically useful information. Exclusive occurrence of IgA anti‐β2GPI associated significantly with photosensitivity and anti‐SSA antibodies (Ro52 as well as Ro60), but these associations were based on only 12 cases.
Our observation that IgG aPLs, LA, as well as triple‐positive patients, had the largest number of significant associations with APS‐related events and damage accrual is extremely consistent with earlier studies, including a review of primary APS 11, 42. Cigarette smoking has been found previously to associate with aPLs 29. Herein, we identified significant associations between past or present tobacco smoking and positive LA test as well as with IgG anti‐β2GPI.
Development of organ damage, defined according to SDI, is highly predictive of prognosis and mortality in SLE 27, 43, 44. The presence of aPLs, as well as manifest APS, is associated with increased morbidity and mortality, as well as a lower quality of life 5, 6, 7, 45. Hence, it is of major importance to analyse these antibody specificities and identify new SLE cases with significant risks of future pregnancy morbidity, other APS‐related events as well as damage accrual.
The prevalence rates of aPLs in SLE studies deviate considerably, possibly due in part to differences regarding disease severity and ethnicity, but most probably also to different methodological issues. Consensus guidelines and proposals for aPL testing have been published during the last two decades and resulted in improvements. However, methodological standardization has not yet been reached. Developments regarding the definition of international units and reference materials for anti‐β2‐GPI testing are ongoing, and may lessen the discordance in prevalence 18, 35, 46.
A limitation of the study is the cross‐sectional design which leaves unanswered the question regarding changes in aPL levels and aPL positivity over time. In addition, the blood donors were healthy at the time of blood sampling, and were not followed over time. Nevertheless, an obvious strength of the present study is the use of disease control groups with a long follow‐up. RA and pSS may both mimic SLE clinically, particularly in early disease. None of the disease controls met the APS classification criteria, although almost 20% had either aPL of at least one isotype and/or a positive LA test. The proportions of positive laboratory tests in the control groups were higher than we expected, as pSS and RA are associated less commonly with APS compared to SLE 47, 48. However, similar frequencies of aPL in pSS have been demonstrated for the IgG/IgM isotypes 47, and in a review by Olech and Merrill a mean prevalence of 28% was reported regarding aPL in RA 48. Cardio‐ and cerebrovascular events are expected to be found, as it is well known that several rheumatological diseases have an increased risk for such events 49, 50, 51, 52. Two of the RA patients (one of whom was positive for both IgA aCL and IgA anti‐β2GPI, and the other positive with regard to IgM aCL only) and one patient with pSS (IgG aCL and LA‐positive) had suffered from cardiovascular or cerebrovascular events.
Disease duration, age, tobacco smoking (past/present), lupus nephritis, use of statins and ≥ 7·5 mg prednisolone daily and a positive LA test were associated with damage accrual in the multiple model, whereas ongoing treatment with HCQ showed a protective effect. Our findings are well in line with the observations by the SLICC group which reported that hypertension, LA positivity and HCQ constitute factors which associate with damage accrual over time 44, and the results are also compatible with data from the Hopkins Lupus Cohort and others 5, 7, 8, 53. In this context, accumulated corticosteroid dose would clearly have been valuable in the regression models, but this was unfortunately not available.
To conclude, the addition of IgA‐class autoantibody analyses, especially IgA anti‐β2GPI, provided some additional clinical correlates, coinciding with non‐Caucasian ethnicity, and was associated inversely with photosensitivity. However, further evaluations of the importance of IgA‐class aPL antibody analyses are required before it is introduced in general clinical routine. Based on the results presented here, we agree with recent consensus documents, suggesting that serum IgA‐class antibody analyses should be restricted to SLE patients with clinically suspected APS, who test negative for IgG and IgM aCL/anti‐β2GPI, and LA 18, 54.
Disclosure
The authors declare that they have no disclosures related to this manuscript.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. M. F. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: M. F., A. V., G. G., J. W., Ö. D., E. S. and C. S. Acquisition of data: M. F., A. V., G. G., T. S., K. E., I. G., A. K., J. R., E. S. and C. S. Analysis and interpretation of data: M. F., A. V., G. G., T. S., K. E., I. G., A. K., Ö. D., J. R., E. S. and C. S.
Acknowledgements
We thank Marianne Peterson and Eva Jemseby for biobank administration and Philip Wallin for construction of Venn diagrams. This work was supported by the Swedish Society for Medical Research, the Swedish Rheumatism Association, the Swedish Society of Medicine, the King Gustaf V’s 80‐year foundation, the King Gustaf V and Queen Victoria’s Freemasons foundation, the Swedish Heart–Lung Foundation, the Swedish Research Council and the County Councils of Stockholm, Uppsala and Östergötland. The views expressed in this paper are the personal views of the authors and may not be understood or quoted as the views of the Swedish Medical Products Agency.
References
- 1. Cervera R, Doria A, Amoura Z et al. Patterns of systemic lupus erythematosus expression in Europe. Autoimmun Rev 2014; 13:621–9. [DOI] [PubMed] [Google Scholar]
- 2. Nuttall A, Isenberg DA. Assessment of disease activity, damage and quality of life in systemic lupus erythematosus: new aspects. Best Pract Res Clin Rheumatol 2013; 27:309–18. [DOI] [PubMed] [Google Scholar]
- 3. Bjork M, Dahlstrom O, Wettero J, Sjowall C. Quality of life and acquired organ damage are intimately related to activity limitations in patients with systemic lupus erythematosus. BMC Musculoskel Dis 2015; 16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nived O, Jonsen A, Bengtsson AA, Bengtsson C, Sturfelt G. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol 2002; 29:1398–400. [PubMed] [Google Scholar]
- 5. Taraborelli M, Leuenberger L, Lazzaroni MG et al. The contribution of antiphospholipid antibodies to organ damage in systemic lupus erythematosus. Lupus 2016; 25:1365–8. [DOI] [PubMed] [Google Scholar]
- 6. Cervera R, Serrano R, Pons‐Estel GJ et al. Morbidity and mortality in the antiphospholipid syndrome during a 10‐year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2015; 74:1011–8. [DOI] [PubMed] [Google Scholar]
- 7. Ruiz‐Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004; 164:77–82. [DOI] [PubMed] [Google Scholar]
- 8. Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. ArthritisRheum 2012; 64:4021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 10. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet 2014; 384:1878–88. [DOI] [PubMed] [Google Scholar]
- 11. Pons‐Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun 2017; 76:10–20. [DOI] [PubMed] [Google Scholar]
- 12. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378:2010–21. [DOI] [PubMed] [Google Scholar]
- 13. Miyakis S, Lockshin MD, Atsumi T et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
- 14. Shen YM, Lee R, Frenkel E, Sarode R. IgA antiphospholipid antibodies are an independent risk factor for thromboses. Lupus 2008; 17:996–1003. [DOI] [PubMed] [Google Scholar]
- 15. Cucurull E, Gharavi AE, Diri E, Mendez E, Kapoor D, Espinoza LR. IgA anticardiolipin and anti‐beta2‐glycoprotein I are the most prevalent isotypes in African American patients with systemic lupus erythematosus. Am J Med Sci 1999; 318:55–60. [DOI] [PubMed] [Google Scholar]
- 16. Sweiss NJ, Bo R, Kapadia R et al. IgA anti‐beta2‐glycoprotein I autoantibodies are associated with an increased risk of thromboembolic events in patients with systemic lupus erythematosus. PLOS ONE 2010; 5:e12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehrani T, Petri M. Association of IgA Anti‐beta2 glycoprotein I with clinical and laboratory manifestations of systemic lupus erythematosus. J Rheumatol 2011; 38:64–8. [DOI] [PubMed] [Google Scholar]
- 18. Lakos G, Favaloro EJ, Harris EN et al. International consensus guidelines on anticardiolipin and anti‐beta2‐glycoprotein I testing: report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum 2012; 64:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Tortosa C, Cabrera‐Marante O, Serrano M et al. Incidence of thromboembolic events in asymptomatic carriers of IgA anti‐B2 glycoprotein‐I antibodies. PLOS ONE 2017; 12:e0178889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertolaccini ML, Atsumi T, Escudero Contreras A, Khamashta MA, Hughes GR. The value of IgA antiphospholipid testing for diagnosis of antiphospholipid (Hughes) syndrome in systemic lupus erythematosus. J Rheum 2001; 28:2637–43. [PubMed] [Google Scholar]
- 21. Molina JF, Gutierrez‐Urena S, Molina J et al. Variability of anticardiolipin antibody isotype distribution in 3 geographic populations of patients with systemic lupus erythematosus. J Rheumatol 1997; 24:291–6. [PubMed] [Google Scholar]
- 22. Danowski A, Kickler TS, Petri M. Anti‐beta2‐glycoprotein I: prevalence, clinical correlations, and importance of persistent positivity in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Rheumatol 2006; 33:1775–9. [PubMed] [Google Scholar]
- 23. Samarkos M, Davies KA, Gordon C, Loizou S. Clinical significance of IgA anticardiolipin and anti‐beta2‐GP1 antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Clin Rheumatol 2006; 25:199–204. [DOI] [PubMed] [Google Scholar]
- 24. Petri M, Orbai AM, Alarcon GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreoli L, Fredi M, Nalli C et al. Clinical significance of IgA anti‐cardiolipin and IgA anti‐beta2glycoprotein I antibodies. Curr Rheumatol Reports 2013; 15:343. [DOI] [PubMed] [Google Scholar]
- 26. Marchetti T, Ribi C, Perneger T et al. Prevalence, persistence and clinical correlations of classic and novel antiphospholipid antibodies in systemic lupus erythematosus. Rheumatology (Oxford, UK). 2018. doi: 10.1093/rheumatology/key095 Epub ahead of print. [DOI] [PubMed]
- 27. Gladman D, Ginzler E, Goldsmith C et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–9. [DOI] [PubMed] [Google Scholar]
- 28. Frodlund M, Dahlstrom O, Kastbom A, Skogh T, Sjowall C. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: analysis of a regional Swedish register. Br Med J Open 2013; 3:e003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustafsson JT, Gunnarsson I, Kallberg H et al. Cigarette smoking, antiphospholipid antibodies and vascular events in systemic lupus erythematosus. Ann Rheum Dis 2015; 74:1537–43. [DOI] [PubMed] [Google Scholar]
- 30. Vitali C, Bombardieri S, Jonsson R et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002; 61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–24. [DOI] [PubMed] [Google Scholar]
- 32. Martinsson K, Johansson A, Kastbom A, Skogh T. Immunoglobulin (Ig)G1 and IgG4 anti‐cyclic citrullinated peptide (CCP) associate with shared epitope, whereas IgG2 anti‐CCP associates with smoking in patients with recent‐onset rheumatoid arthritis (the Swedish TIRA project). Clin Exp Immunol 2017; 188:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruce IN, Clark‐Soloninka CA, Spitzer KA, Gladman DD, Urowitz MB, Laskin CA. Prevalence of antibodies to beta2‐glycoprotein I in systemic lupus erythematosus and their association with antiphospholipid antibody syndrome criteria: a single center study and literature review. J Rheumatol 2000; 27:2833–7. [PubMed] [Google Scholar]
- 34. Meijide H, Sciascia S, Sanna G, Khamashta MA, Bertolaccini ML. The clinical relevance of IgA anticardiolipin and IgA anti‐beta2 glycoprotein I antiphospholipid antibodies: a systematic review. Autoimmun Rev 2013; 12:421–5. [DOI] [PubMed] [Google Scholar]
- 35. Devreese KM. Antiphospholipid antibody testing and standardization. Int J Lab Hematol 2014; 36:352–63. [DOI] [PubMed] [Google Scholar]
- 36. Tebo AE, Willis R, Jaskowski TD et al. Clinical significance and correlations between anti‐beta2 glycoprotein I IgA assays in antiphospholipid syndrome and/or systemic lupus erythematosus. Clin Chim Acta 2016; 460:107–13. [DOI] [PubMed] [Google Scholar]
- 37. Martinez‐Flores JA, Serrano M, Alfaro J et al. Heterogeneity between diagnostic tests for IgA anti‐beta2 glycoprotein I: explaining the controversy in studies of association with vascular pathology. Anal Chem 2013; 85:12093–8. [DOI] [PubMed] [Google Scholar]
- 38. Pericleous C, Ferreira I, Borghi O et al. Measuring IgA anti‐beta2‐glycoprotein I and IgG/IgA anti‐domain I antibodies adds value to current serological assays for the antiphospholipid syndrome. PLOS ONE 2016; 11:e0156407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murthy V, Willis R, Romay‐Penabad Z et al. Value of isolated IgA anti‐beta2 ‐glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum 2013; 65:3186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Despierres L, Beziane A, Kaplanski G et al. Contribution of anti‐beta2glycoprotein I IgA antibodies to the diagnosis of anti‐phospholipid syndrome: potential interest of target domains to discriminate thrombotic and non‐thrombotic patients. Rheumatology (Oxford, UK) 2014; 53:1215–8. [DOI] [PubMed] [Google Scholar]
- 41. Mattia E, Ruffatti A, Tonello M et al. IgA anticardiolipin and IgA anti‐beta2 glycoprotein I antibody positivity determined by fluorescence enzyme immunoassay in primary antiphospholipid syndrome. Clin Chem Lab Med 2014; 52:1329–33. [DOI] [PubMed] [Google Scholar]
- 42. Pengo V, Ruffatti A, Legnani C et al. Incidence of a first thromboembolic event in asymptomatic carriers of high‐risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–8. [DOI] [PubMed] [Google Scholar]
- 43. Cardoso CR, Signorelli FV, Papi JA, Salles GF. Initial and accrued damage as predictors of mortality in Brazilian patients with systemic lupus erythematosus: a cohort study. Lupus 2008; 17:1042–8. [DOI] [PubMed] [Google Scholar]
- 44. Bruce IN, O'Keeffe AG, Farewell V et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015; 74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gustafsson JT, Simard JF, Gunnarsson I et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther 2012; 14:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bertolaccini ML, Amengual O, Andreoli L et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev 2014; 13:917–30. [DOI] [PubMed] [Google Scholar]
- 47. Pasoto SG, Chakkour HP, Natalino RR et al. Lupus anticoagulant: a marker for stroke and venous thrombosis in primary Sjogren’s syndrome. Clin Rheumatol 2012; 31:1331–8. [DOI] [PubMed] [Google Scholar]
- 48. Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep 2006; 8:100–8. [DOI] [PubMed] [Google Scholar]
- 49. Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 2003; 30:36–40. [PubMed] [Google Scholar]
- 50. Castaneda S, Nurmohamed MT, Gonzalez‐Gay MA. Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol 2016; 30:851–69. [DOI] [PubMed] [Google Scholar]
- 51. Agca R, Heslinga SC, Rollefstad S et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017; 76:17–28. [DOI] [PubMed] [Google Scholar]
- 52. Arkema EV, Svenungsson E, Von Euler M, Sjowall C, Simard JF. Stroke in systemic lupus erythematosus: a Swedish population‐based cohort study. Ann Rheum Dis 2017; 76:1544–9. [DOI] [PubMed] [Google Scholar]
- 53. Goncalves MJ, Sousa S, Ines LS et al. Characterization of damage in Portuguese lupus patients: analysis of a national lupus registry. Lupus 2015; 24:256–62. [DOI] [PubMed] [Google Scholar]
- 54. Erkan D, Aguiar CL, Andrade D et al. 14th International Congress on Antiphospholipid antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014; 13:685–96. [DOI] [PubMed] [Google Scholar]


