Key Points
IMRT resulted in improved survival compared with 3D-CRT in early-stage NKTCL.
A risk-adapted survival benefit profile offered by IMRT can be used to select patients and make treatment decisions.
Abstract
This study evaluated the survival benefit of intensity-modulated radiation therapy (IMRT) compared with 3-dimension conformal radiation therapy (3D-CRT) in a large national cohort of patients with early-stage extranodal nasal-type natural killer/T-cell lymphoma (NKTCL). This retrospective study reviewed patients with early-stage NKTCL treated with high-dose radiation therapy (RT; ≥45 Gy) at 16 Chinese institutions. Patients were stratified into 1 of 4 risk groups based on the number of risk factors: low risk (no factors), intermediate-low risk (1 factor), intermediate-high risk (2 factors), and high-risk (3-5 factors). Of the 1691 patients, 981 (58%) received IMRT, and 710 (42%) received 3D-CRT. Unadjusted 5-year overall survival (OS) and progression-free survival (PFS) were 75.9% and 67.6%, respectively, for IMRT compared with 68.9% (P = .004) and 58.2% (P < .001), respectively, for 3D-CRT. After propensity score match and multivariable analyses to account for confounding factors, IMRT remained significantly associated with improved OS and PFS. The OS and PFS benefits of IMRT persisted in patients treated with modern chemotherapy regimens. Compared with 3D-CRT, IMRT significantly improved OS and PFS for high-risk and intermediate-high–risk patients but provided limited benefits for low-risk or intermediate-low–risk patients. A risk-adapted survival benefit profile of IMRT can be used to select patients and make treatment decisions.
Visual Abstract
Introduction
Although rare globally, extranodal nasal-type natural killer/T-cell lymphoma (NKTCL) is prevalent in China, accounting for 10% to 20% of all non-Hodgkin lymphomas and 40% to 50% of peripheral T-cell lymphomas.1-5 This distinct entity is most common in young men, frequently originates from the upper aerodigestive tract (UADT), and is associated with Epstein-Barr virus; most patients have good performance status (PS) and early-stage disease.5-8 NKTCL exhibits diverse clinical presentations that mainly depend on the primary site.2,8-11 NKTCL is resistant to chemotherapy but sensitive to radiotherapy (RT); upfront RT and new chemotherapy regimens have improved outcomes.12-15
RT is essential for early-stage NKTCL.13-18 Risk-adapted therapy consisting of RT alone for low-risk patients and RT, followed by chemotherapy, for high-risk patients provides favorable outcomes, with 5-year overall survival (OS) rates of 70% to 90%.15 Moreover, optimal RT techniques play an important curative role in early-stage NKTCL. Appropriate high-dose RT with an extended involved-site field is associated with improved disease control and survival.13,15,18 In contrast, inadequate low-dose RT with a small field or omission of RT results in very poor outcomes, with 5-year OS < 50%.12,18-21 Furthermore, we demonstrated that RT exerts a dose-dependent effect on survival, with an association between improved locoregional control and prolonged survival.18 These findings highlight the importance of high-quality RT in early-stage NKTCL.
Advanced RT techniques, such as intensity-modulated radiation therapy (IMRT), enhance the therapeutic ratio by delivering a highly conformal dose distribution to the tumor and minimizing the dose to adjacent normal organs; IMRT has been widely adopted over the last decade.22-24 Dosimetric comparisons have shown that IMRT significantly improves tumor coverage and spares normal tissues compared with 3-dimension conformal radiation therapy (3D-CRT) in NKTCL.25-27 In our prior single-institution studies of early-stage NKTCL, high-dose and extended involved-site IMRT provided favorable outcomes with minimal toxicities.28,29 However, the clinical advantages of IMRT over 3D-CRT have not been specifically addressed, and the benefits of innovative RT techniques need to be validated in large multicenter studies. We hypothesized that advanced RT techniques provide a survival benefit over 3D-CRT in early-stage NKTCL. Using a multi-institutional database, we comprehensively analyzed the trends in RT techniques, compared the survival benefits of IMRT and 3D-CRT, and established a risk-adapted benefit profile for early-stage NKTCL.
Methods
Population and database
A cohort of 2640 patients with NKTCL treated at 16 Chinese institutions between 2000 and 2015 was recruited from the China Lymphoma Collaborative Group. All patients were diagnosed with the histopathological features of NKTCL, according to the World Health Organization classification. As described previously,5 routine staging procedures included physical and endoscopic examination, blood chemistry, computed tomography scan (CT) and magnetic resonance imaging of the head and neck, CT scan of the chest, abdomen and pelvis, and a bone marrow examination. Positron emission tomography (PET) has been recommended as a routine examination since 2012. The institutional review boards approved the study protocol and waived the need for informed consent because patient data were de-identified in the dataset.
Study participants
Patients with early-stage NKTCL who received RT (with or without chemotherapy) were eligible for the analysis of treatment trends in RT techniques (2015/2640 patients). Patients with early-stage NKTCL treated with chemotherapy alone (n = 310) or with advanced-stage NKTCL (n = 315) were excluded.
The primary objective of this study was to determine the impact of RT technique on survival in patients with early-stage NKTCL receiving RT, with or without chemotherapy. Patients receiving ≥45 Gy were included, because 45-Gy RT achieved good outcomes, and guidelines recommend doses of 45 to 50 Gy.30,31 We excluded patients who received low-dose RT (<45 Gy, n = 172) because of its associated poor outcome12,18 and patients with unknown RT techniques (n = 152). Of 2015 patients, 1691 patients were eligible for comparison of survival outcomes between RT modalities.
Treatment
Patients treated with IMRT, volumetric-modulated arc therapy, or tomotherapy were included in the IMRT group (n = 981), and those treated with 3D-CRT and conventional 2-dimensional RT (2D-RT) were included in the 3D-CRT group (n = 710). Extended involved-site RT encompassed the primary tumor and involved regional lymph nodes.28,29 Generally, IMRT involved a 5-9–fixed field or arc plan, 3D-CRT involved a 3-5–field forward plan, and 2D-RT involved plans with a single anterior field or bilateral opposed fields. All treatments were administered as a 1.8 to 2–Gy once-daily fraction, 5 days per week. The median dose was 50 Gy (range, 45-74).
Of the 1691 patients, 1348 patients received additional chemotherapy, and 343 patients did not. Chemotherapy included regimens not containing doxorubicin (n = 730) and those containing doxorubicin (n = 618). The median number of chemotherapy cycles was 4 (range, 1-14).
Risk stratification
We established an NKTCL prognostic index (NKTCL-PI) to stratify early-stage patients and investigate the risk-adapted benefit profile of IMRT vs 3D-CRT. Based on our previous study,5 5 risk factors (age >60 years, Eastern Cooperative Oncology Group [ECOG] score ≥2, stage II, elevated lactate dehydrogenase [LDH], and primary tumor invasion [PTI]) were used to design the NKTCL-PI for early-stage disease. Patients were stratified into 1 of 4 risk groups based on the number of risk factors present: low risk (no factors), intermediate-low risk (1 factor), intermediate-high risk (2 factors), and high risk (≥3 factors).
Statistical analysis
Primary end points were OS (date of treatment until death from any cause) and progression-free survival (PFS; until disease progression, relapse, or death). Survival was assessed via the Kaplan-Meier product limit method and compared using log-rank tests stratified by prognostic factors and NKTCL-PI. A Cox regression model was used to identify risk factors for survival. Propensity score matching (PSM) was conducted to balance prognostic factors and generate comparable study arms.
Results
Trends in RT techniques
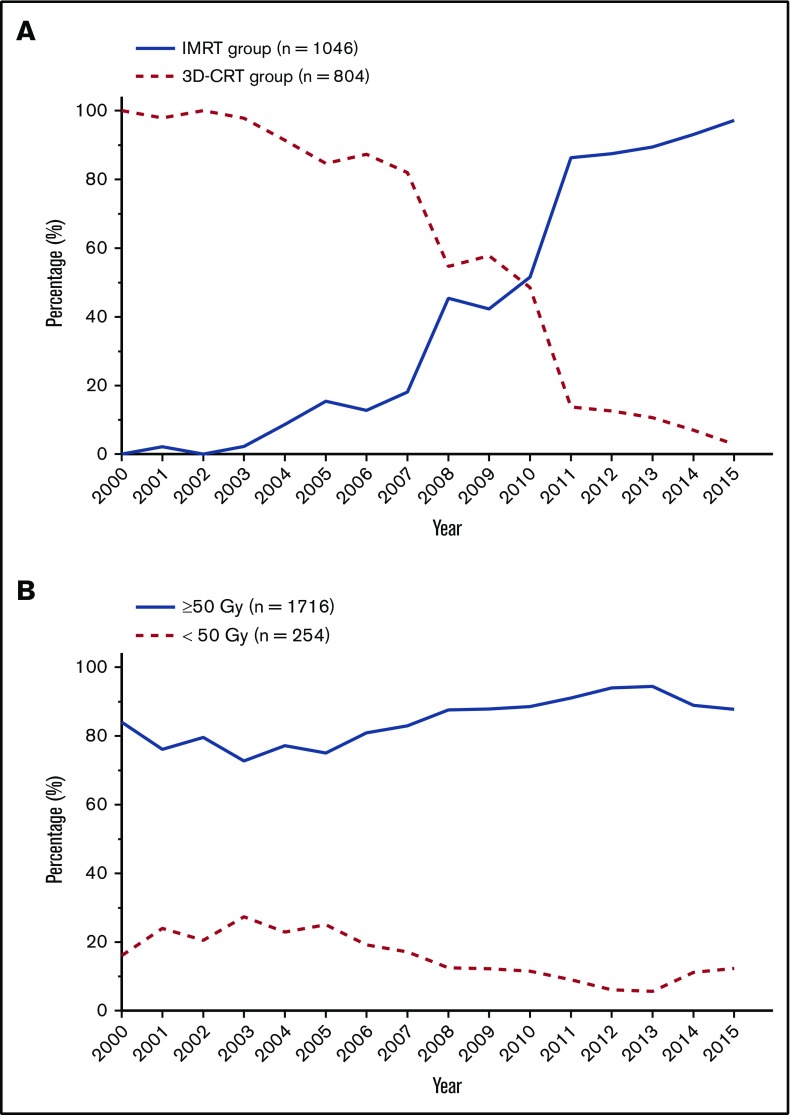
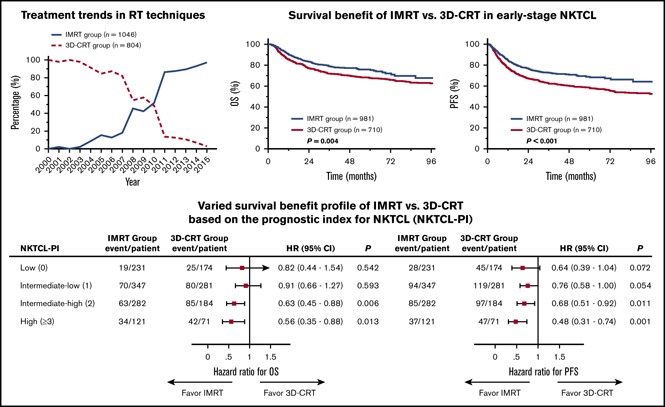
Of the 2015 patients with early-stage NKTCL receiving RT, 1850 patients were eligible for RT technique analysis: 1046 (56.5%) received IMRT, and 804 (43.5%) received 3D-CRT. IMRT was first used in 2001; its use gradually increased until 2007 and then increased from 45.4% in 2008% to 97.1% in 2015 (Figure 1A). Median dose was 50 Gy (range, 10-74); 1716 patients (85.2%) received ≥50 Gy, 82 (4.1%) received 45-49 Gy, 172 (8.5%) received <45 Gy, and 45 patients (2.2%) were not eligible for dose analysis. The proportion of patients receiving <50 Gy remained at 20% to 28% between 2001 and 2005 and then decreased slightly from 25% in 2005 to 12.3% in 2015 (Figure 1B). Therefore, most patients received the optimal RT dose over time, and IMRT was widely applied in recent years.
Figure 1.
Treatment trends in RT techniques and doses for evaluable patients with early-stage NKTCL. Proportion of patients receiving IMRT vs 3D-CRT (A) and RT doses ≥ 50 Gy vs <50 Gy (B) between 2000 and 2015.
Clinical features
The clinical features of the 1691 patients with early-stage NKTCL receiving definitive RT are presented in Table 1. Median age was 43 years (range, 3-85), and male/female ratio was 2.4:1. Most patients had primary disease in the nasal cavity (n = 1344), followed by extranasal UADT (n = 331) and disease at extra-UADT sites (n = 16). B symptoms and elevated LDH were observed in 37.4% and 25.9% of patients, respectively. Most patients (69.0%) had stage I disease, 54.2% presented with PTI, and 95.9% had good PS (ECOG score 0 or 1).
Table 1.
Clinical characteristics of patients with early-stage NKTCL before and after PSM stratification by RT modality
| IMRT group | 3D-CRT group | P | |
|---|---|---|---|
| Before PSM | |||
| Total | 981 | 710 | |
| Male | 689 (70.2) | 498 (70.1) | .967 |
| Age >60 y | 120 (12.2) | 95 (13.4) | .484 |
| B symptoms | 378 (38.5) | 255 (35.9) | .273 |
| Elevated LDH | 228 (23.2) | 210 (29.6) | .003 |
| ECOG ≥2 | 31 (3.2) | 38 (5.4) | .025 |
| Stage II | 355 (36.2) | 170 (23.9) | <.001 |
| PTI | 554 (56.5) | 363 (51.1) | .029 |
| After PSM | |||
| Total | 649 | 649 | |
| Male | 453 (69.8) | 453 (69.8) | 1.000 |
| Age >60 y | 79 (12.2) | 79 (12.2) | 1.000 |
| B symptoms | 226 (34.8) | 226 (34.8) | 1.000 |
| Elevated LDH | 162 (25.0) | 162 (25.0) | 1.000 |
| ECOG ≥2 | 15 (2.3) | 15 (2.3) | 1.000 |
| Stage II | 162 (25.0) | 162 (25.0) | 1.000 |
| PTI | 330 (50.8) | 330 (50.8) | 1.000 |
Data are n (%).
Patients receiving IMRT were more likely to have locally extensive disease, including PTI (56.5% vs 51.1%, P = .029) and stage II disease (36.2% vs 23.9%, P < .001). In contrast, more patients receiving 3D-CRT than IMRT had elevated LDH (29.6% vs 23.2%, P = .003) and a ECOG score ≥2 (5.4% vs 3.2%, P = .025).
Survival benefit of IMRT vs 3D-CRT
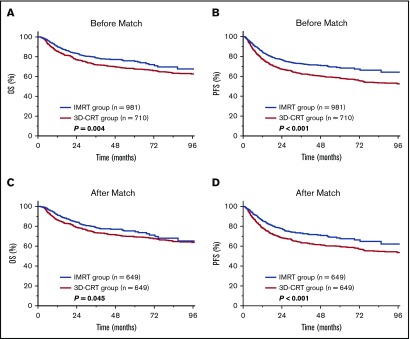
We compared the efficacy of IMRT with 3D-CRT in early-stage NKTCL. Median follow-up for surviving patients was 70 months for the 3D-CRT group and 37 months for the IMRT group. Five-year OS, PFS, and locoregional control (LRC) were 71.9%, 63.9%, and 84.8%, respectively, for the entire cohort. IMRT provided superior survival and LRC compared with 3D-CRT. Unadjusted 5-year OS and PFS were 75.9% and 67.6% for IMRT compared with 68.9% (P = .004; Figure 2A) and 58.2% (P < .001; Figure 2B) for 3D-CRT. Five-year LRC was 85.9% for IMRT compared with 82.9% for 3D-CRT (P = .038).
Figure 2.
Survival benefit of IMRT over 3D-CRT in early-stage NKTCL. OS and PFS after IMRT or 3D-CRT for the entire cohort (A-B) and for the PSM cohort (C-D).
Analysis of the well-balanced propensity score–matched cohort (649 patients in each RT group; Table 1) demonstrated a similar survival improvement for IMRT over 3D-CRT. Adjusted 5-year OS and PFS were 75.3% and 68.3% for IMRT vs 69.4% (P = .045; Figure 2C) and 59.9% (P < .001; Figure 2D) for 3D-CRT. In multivariable analysis, age (as continuous variable), ECOG PS, stage, and PTI were independently significant for OS and PFS (Table 2). In addition, RT technique (3D-CRT vs IMRT) remained an independent prognostic factor for OS (hazard ratio [HR], 1.38; 95% confidence interval [CI], 1.13-1.69; P = .002) and PFS (HR, 1.51; 95% CI, 1.26-1.80; P < .001), favoring IMRT.
Table 2.
Multivariable analysis of associations between clinical variables and radiation technique with OS and PFS for all patients with early-stage NKTCL
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex (female vs male) | 0.88 | 0.71-1.09 | .257 | 0.96 | 0.80-1.16 | .684 |
| B symptoms (yes vs no) | 0.89 | 0.72-1.09 | .261 | 0.97 | 0.81-1.16 | .715 |
| Ann Arbor stage (II vs I) | 1.62 | 1.31-1.99 | <.001 | 1.38 | 1.15-1.65 | <.001 |
| PTI (yes vs no) | 1.82 | 1.47-2.25 | <.001 | 1.72 | 1.44-2.07 | <.001 |
| Age as continuous variable | 1.14 | 1.07-1.22 | <.001 | 1.08 | 1.02-1.14 | .010 |
| Elevated LDH level (yes vs no) | 1.27 | 1.03-1.56 | .024 | 1.17 | 0.97-1.40 | .098 |
| ECOG PS (≥2 vs 0-1) | 2.10 | 1.47-3.00 | <.001 | 2.16 | 1.57-3.00 | <.001 |
| RT technique (3D-CRT vs IMRT) | 1.38 | 1.13-1.69 | .002 | 1.51 | 1.26-1.80 | <.001 |
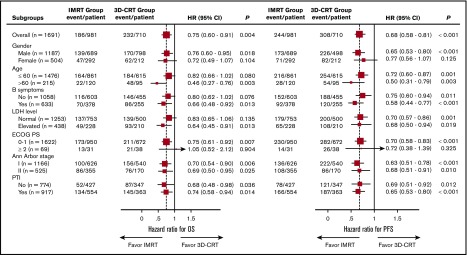
Survival benefit of IMRT in subgroup analyses
The advantage of IMRT over 3D-CRT was evaluated using subgroup analyses. The OS and PFS benefits of IMRT were maintained across most subgroups (Figure 3). Because the extent of locoregional disease affects tumor target delineation and selection of RT technique, we evaluated early-stage patients with a high primary tumor burden or lymph node involvement; IMRT retained a survival benefit over 3D-CRT. For patients with PTI, OS and PFS were 68.6% and 63.1%, respectively, for IMRT vs 58.0% (P = .014) and 47.5% (P < .001), respectively, for 3D-CRT. Similarly, the 5-year OS and PFS rates for patients without PTI were 85.0% and 76.4%, respectively, for IMRT vs 77.8% (P = .036) and 69.6% (P = .012), respectively, for 3D-CRT. In stage II NKTCL, the corresponding OS and PFS rates were 68.7% and 62.8%, respectively, for IMRT vs 55.6% (P = .025) and 51.9% (P = .010), respectively, for 3D-CRT; the corresponding rates for stage I were 79.9% and 72.7%, respectively, for IMRT vs 71.4% (P = .006) and 60.7% (P < .001), respectively, for 3D-CRT.
Figure 3.
Forest plots representing OS and PFS after IMRT or 3D-CRT for different subgroups. HRs are derived from multivariate Cox proportional hazards models, with 95% CIs and P values for OS and PFS. The area of the squares is proportional to sample size. The vertical dashed line is the HR for comparison in the entire cohort.
Survival benefit of IMRT after new-regimen chemotherapy
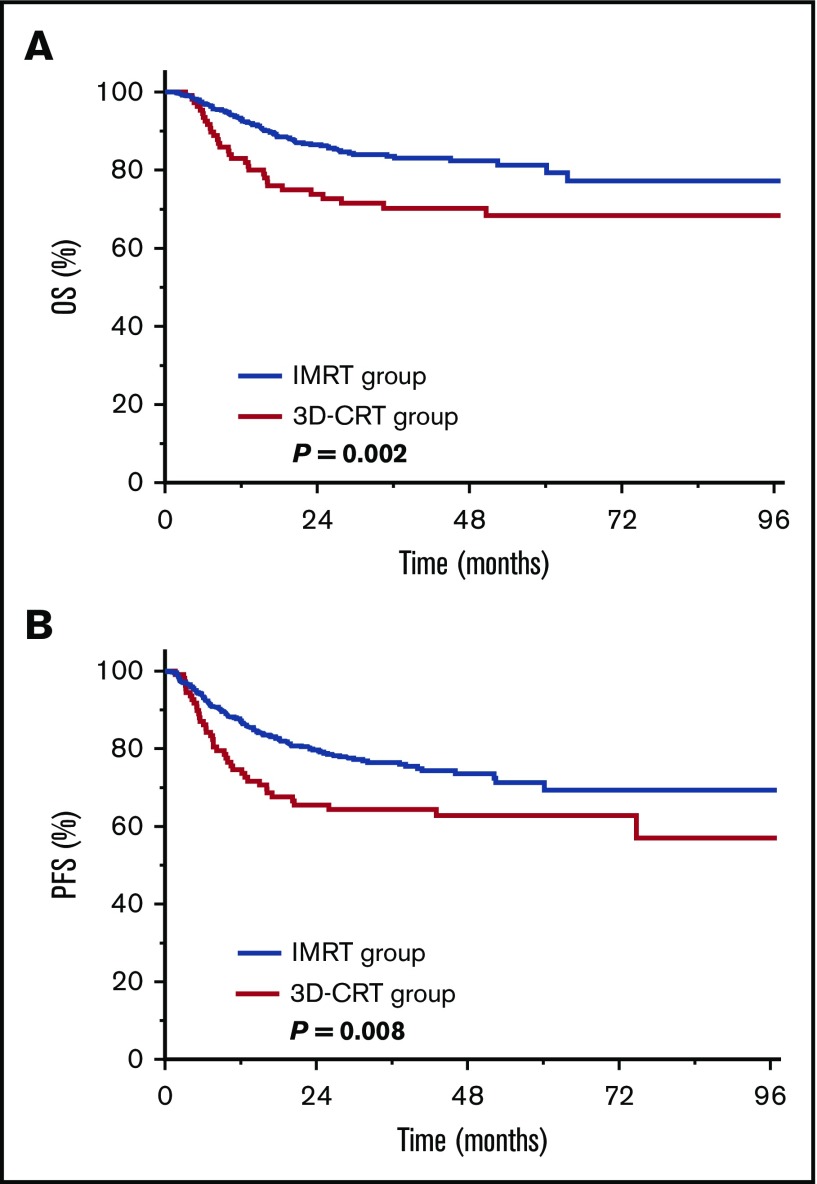
We investigated whether the survival benefit of IMRT persists after new-regimen chemotherapy. Of the 715 patients receiving definitive RT and asparaginase- or gemcitabine-based chemotherapy, 604 (84.5%) received IMRT, and 111 (15.5%) received 3D-CRT. The 5-year OS and PFS rates were 81.3% and 71.3%, respectively, for IMRT vs 68.4% (P = .002; Figure 4A) and 62.7% (P = .008; Figure 4B), respectively, for 3D-CRT. This finding indicates that patients still benefit from IMRT in the modern chemotherapy era.
Figure 4.
Improved survival for IMRT vs 3D-CRT after new-regimen chemotherapy. OS (A) and PFS (B) for IMRT or 3D-CRT in patients with early-stage NKTCL receiving asparaginase- or gemcitabine-based chemotherapy.
Varied survival benefit profile of IMRT according to NKTCL-PI
To better understand which patients are most likely to benefit from IMRT, patients were stratified into 4 risk groups using NKTCL-PI. Significant survival differences were observed for the risk-stratified groups (Figure 5A-B): 5-year OS and PFS rates were 89.3% and 80.7%, respectively, for low-risk patients (n = 405), 72.0% and 63.0%, respectively, for intermediate-low–risk patients (n = 628), 62.6% and 55.3%, respectively, for intermediate-high–risk patients (n = 466), and 53.8% and 50.3%, respectively, for high-risk patients (n = 192; P < .001).
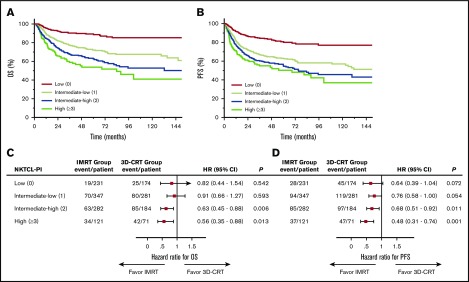
Figure 5.
Varied survival benefit profile of IMRT vs 3D-CRT based on NKTCL-PI. Patients with early-stage NKTCL were stratified into 4 risk groups with different OS (A) and PFS (B). HRs for OS (C) and PFS (D) for IMRT vs 3D-CRT within different risk subgroups defined by NKTCL-PI. Forest plots showing improved OS (C) and PFS (D) for IMRT compared with 3D-CRT in patients at intermediate-high or high risk but limited benefit of IMRT in patients at low or intermediate-low risk.
When examining the benefit profile of IMRT relative to 3D-CRT, patients with 2 to 5 risk factors had significantly better survival after IMRT than after 3D-CRT, whereas patients with no risk factors or 1 risk factor did not (Figure 5C-D). The magnitude of the improvement in survival among patients treated with IMRT correlated significantly with NKTCL-PI. Five-year OS was 91.2% for IMRT vs 87.6% for 3D-CRT (absolute difference, 3.6%; HR, 0.82; 95% CI, 0.44-1.54; P = .542) in low-risk patients, 73.2% vs 70.6% (absolute difference, 2.6%; HR, 0.91; 95% CI, 0.66-1.27; P = .593) in intermediate-low–risk patients, 71.0% vs 55.3% (absolute difference, 15.7%; HR, 0.63; 95% CI, 0.45-0.88; P = .006) in intermediate-high–risk patients, and 64.4% vs 42.4% (absolute difference, 22.0%; HR, 0.56; 95% CI, 0.35-0.88; P = .013) in high-risk patients. Similarly, 5-year PFS was 83.6% for IMRT vs 77.0% for 3D-CRT (absolute difference, 6.6%; HR, 0.64; 95% CI, 0.39-1.04; P = .072) in low-risk patients, 65.9% vs 59.1% (absolute difference, 6.8%; HR, 0.76; 95% CI, 0.58-1.00; P = .054) in intermediate-low-risk patients, 62.4% vs 48.8% (absolute difference, 13.6%; HR, 0.68; 95% CI, 0.51-0.92; P = .011) in intermediate-high-risk patients, and 65.7% vs 35.2% (absolute difference, 30.5%; HR, 0.48; 95% CI, 0.31-0.74; P = .001) in high-risk patients. This finding indicates a risk-adapted survival benefit profile for IMRT in early-stage NKTCL.
Discussion
Despite the absence of high-level data demonstrating the favorable impact of advanced RT techniques on survival in early-stage NKTCL, the use of IMRT has rapidly increased across China in recent years, with most patients receiving adequate RT doses. This is the first large-scale real-world multicenter comparison of IMRT with 3D-CRT in early-stage NKTCL. IMRT provided a significant survival benefit over 3D-CRT after adjusting for confounding variables via PSM and multivariable analyses. The clinical advantage of IMRT was consistent across most subgroups examined, regardless of primary tumor burden or modern chemotherapy. After stratifying patients into 4 risk groups using NKTCL-PI, the survival benefits of IMRT were more apparent for patients with higher numbers of risk factors, indicating a risk-adapted benefit profile for IMRT.
Treatment of early-stage NKTCL has evolved over the last decade, with the introduction of upfront RT and new chemotherapy regimens improving survival outcomes.4,8,15-17 Moreover, RT techniques have changed from 2D-RT or 3D-CRT to IMRT. Because RT is an essential component of first-line therapy for early-stage NKTCL,12-15 optimization of RT techniques may play a key role in the management of early-stage NKTCL.18-21,32 There are no published data describing the survival benefit of IMRT in a large multicenter comparative study. Current recommendations for RT techniques in NKTCL are largely based on dosimetric studies,25-27 small cohorts,28,29,33 or experience with other cancers,22-24,34 resulting in consensus-based, rather than evidence-based, recommendations.30,31,35 We provide the first confirmation that, compared with 3D-CRT/2D-RT, IMRT provides an incremental survival benefit in early-stage NKTCL. This favorable effect of IMRT was also observed in subgroups of patients with higher tumor burdens, including PTI and stage II disease.
Because IMRT is more complex to plan and deliver than 3D-CRT or 2D-RT, treatment planning for NKTCL is challenging as a result of the close proximity of the primary tumor to surrounding organs at risk in most patients with NKTCL.28,29 Variations or errors in target delineation and dosing can affect treatment outcomes.12,18-20 This large multi-institution study of patients treated with high-dose RT (≥45 Gy) in a relatively uniform manner minimizes the confounding effect of RT dose variations, allowing accurate analysis of the true benefit of IMRT. The superior OS and PFS achieved with IMRT suggest that tumor target definition is adequate and accurate in early-stage NKTCL across China in the modern RT era.
As IMRT becomes more widely used, the optimal treatment strategy becomes an increasingly important question. We identified 4 risk groups for early-stage NKTCL based on the NKTCL-PI model, which is based on age > 60 years, ECOG PS ≥ 2, stage II, elevated LDH, and PTI.5 Estimated 5-year OS rates varied substantially from 53.8% to 89.3% between low-risk and high-risk patients. Interestingly, the use of IMRT seems to be beneficial in patients with any risk, but the absolute magnitude of the benefit is smaller in low- or intermediate-low–risk patients. Because IMRT offers improved tumor target coverage and reduces normal tissue doses, the survival benefit observed in higher-risk patients is mostly attributed to the superiority of IMRT for older patients (>60 years) or those with poor PS (ECOG ≥ 2) who are less likely to tolerate 3D-CRT or 2D-RT or patients with larger or more infiltrative tumors (PTI, stage II disease, elevated LDH) whose treatment may be easier to plan with IMRT with complex geometric targets. In contrast, the limited benefits of IMRT observed in patients at lower risk probably relate to the good outcome of smaller or superficial tumors (stage I disease without PTI or localized cutaneous lesions) whose treatment may be easier to plan with 3D-CRT or 2D-RT. This is the first investigation of the survival benefit of IMRT based on individual risk factors. Using NKTCL-PI, we could identify a subset of early-stage patients who may benefit most from IMRT, which may help to guide individualized RT and counseling of patients. More work is required to determine which patients should be treated with IMRT or 3D-CRT combined with optimal chemotherapy.
This retrospective study has several limitations. First, the 2 RT groups were not randomly assigned, and follow-up was shorter for the IMRT group. The nonrandomized selection of RT technique confers a risk for confounding by prognostic factors across institutions and physicians. To address this, the protocol specified that patients eligible for final analysis received high-dose RT (≥45 Gy) reflecting uniform definitive RT, and the PSM and multivariable regression model incorporated all variables and RT technique. Furthermore, we believe that the use of individual patient-level data from multiple institutions, review of each institutions’ datasets by the primary investigator, and central data review ultimately provided a more reliable dataset than a simple retrospective review or population-based dataset, which have significantly variable contributions. In the absence of randomized trials, real-world consecutive series provide the strongest level of evidence. Second, PET-CT was not routinely performed at initial staging and response assessment. Because the majority of patients received IMRT in recent years, the survival benefit of IMRT may also be due to more accurate initial staging with advanced imaging technology, such as PET-CT and magnetic resonance imaging. Third, although there was not a significant difference in OS and PFS between IMRT and 3D-CRT in low-risk patients, data on the quality-of-life benefit and lower toxicity of IMRT were not available. Longer follow-up is needed to verify the survival advantages and reduced toxicity of IMRT.
In summary, advanced RT techniques, such as IMRT, significantly improve treatment outcomes in early-stage NKTCL. Along with previous data demonstrating the survival benefit of optimal RT field and dose,12-15,18,20 our findings emphasize the critical importance of high-quality RT in the first-line treatment of early-stage NKTCL. The risk-adapted survival benefit profile may help to inform patients and physicians when selecting RT techniques and making treatment decisions.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Key Projects of Research and Development of China (2016YFC0904600) and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1-001).
The sponsors had no role in the design and execution of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Authorship
Contribution: Y.-X.L. designed the research; T.W. and Y.Y. collected and analyzed data; Y.-X.L., T.W., and Y.Y. wrote the manuscript; C.H. performed statistical analyses; and all authors provided study materials or patient data and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the China Lymphoma Collaborative Group appears in the online appendix.
Correspondence: Ye-Xiong Li, State Key Laboratory of Molecular Oncology and Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; e-mail: yexiong12@163.com.
References
- 1.Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429-434. [DOI] [PubMed] [Google Scholar]
- 2.Au WY, Weisenburger DD, Intragumtornchai T, et al. ; International Peripheral T-Cell Lymphoma Project. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931-3937. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389-400. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi M, Suzuki R, Oguchi M, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. 2017;35(1):32-39. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Zhang YJ, Zhu Y, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015;29(7):1571-1577. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612-618. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZY, Liu QF, Wang H, et al. Clinical implications of plasma Epstein-Barr virus DNA in early-stage extranodal nasal-type NK/T-cell lymphoma patients receiving primary radiotherapy. Blood. 2012;120(10):2003-2010. [DOI] [PubMed] [Google Scholar]
- 8.Kim TM, Lee SY, Jeon YK, et al. ; Lymphoma Subcommittee of the Korean Cancer Study Group. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19(8):1477-1484. [DOI] [PubMed] [Google Scholar]
- 9.Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 2008;112(8):3057-3064. [DOI] [PubMed] [Google Scholar]
- 10.Li YX, Liu QF, Fang H, et al. Variable clinical presentations of nasal and Waldeyer ring natural killer/T-cell lymphoma. Clin Cancer Res. 2009;15(8):2905-2912. [DOI] [PubMed] [Google Scholar]
- 11.Liu QF, Wang WH, Wang SL, et al. Immunophenotypic and clinical differences between the nasal and extranasal subtypes of upper aerodigestive tract natural killer/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2014;88(4):806-813. [DOI] [PubMed] [Google Scholar]
- 12.Vargo JA, Patel A, Glaser SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States. Cancer. 2017;123(16):3176-3185. [DOI] [PubMed] [Google Scholar]
- 13.Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006;24(1):181-189. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Li YX, Wang WH, et al. Primary radiotherapy showed favorable outcome in treating extranodal nasal-type NK/T-cell lymphoma in children and adolescents. Blood. 2009;114(23):4771-4776. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood. 2015;126(12):1424-1432, quiz 1517. [DOI] [PubMed] [Google Scholar]
- 16.Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys. 2008;70(1):166-174. [DOI] [PubMed] [Google Scholar]
- 17.Li YX, Wang H, Jin J, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82(5):1809-1815. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Cao JZ, Lan SM, et al. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type NK/T-cell lymphoma. JAMA Oncol. 2017;3(1):83-91. [DOI] [PubMed] [Google Scholar]
- 19.Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol. 2000;18(1):54-63. [DOI] [PubMed] [Google Scholar]
- 20.Isobe K, Uno T, Tamaru J, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. 2006;106(3):609-615. [DOI] [PubMed] [Google Scholar]
- 21.Koom WS, Chung EJ, Yang WI, et al. Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys. 2004;59(4):1127-1137. [DOI] [PubMed] [Google Scholar]
- 22.Boero IJ, Paravati AJ, Xu B, et al. Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol. 2016;34(7):684-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kam MKM, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873-4879. [DOI] [PubMed] [Google Scholar]
- 24.Nutting CM, Morden JP, Harrington KJ, et al. ; PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomita N, Kodaira T, Tachibana H, et al. A comparison of radiation treatment plans using IMRT with helical tomotherapy and 3D conformal radiotherapy for nasal natural killer/T-cell lymphoma. Br J Radiol. 2009;82(981):756-763. [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Ma X, Hu W, Chen L, Huang J, Guo Y. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for stage I-II natural killer/T-cell lymphoma nasal type: dosimetric and clinical results. Radiat Oncol. 2013;8(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Huang E, Wang Y, et al. Dosimetric comparison of helical tomotherapy, VMAT, fixed-field IMRT and 3D-conformal radiotherapy for stage I-II nasal natural killer T-cell lymphoma. Radiat Oncol. 2017;12(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Li YX, Wang WH, et al. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys. 2012;82(3):1115-1121. [DOI] [PubMed] [Google Scholar]
- 29.Bi XW, Li YX, Fang H, et al. High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer’s ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys. 2013;87(5):1086-1093. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network Guidelines. Version 2.2017, valid from 21 February 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#t-cell. Accessed 1 September 2017.
- 31.d’Amore F, Gaulard P, Trümper L, et al. ; ESMO Guidelines Committee. Peripheral T-cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v108-v115. [DOI] [PubMed] [Google Scholar]
- 32.Wu RY, Liu K, Wang WH, et al. Patterns of primary tumor invasion and regional lymph node spread based on MRI in early-stage nasal NK/T-cell lymphoma: implications for clinical target volume definition and prognostic significance. Int J Radiat Oncol Biol Phys. 2017;97(1):50-59. [DOI] [PubMed] [Google Scholar]
- 33.Li YY, Lin HQ, Zhang LL, et al. Intensity-modulated radiotherapy has superior outcomes to three-dimensional conformal radiotherapy in patients with stage IE-IIE extranodal nasal-type natural killer/T-cell lymphoma. Oncotarget. 2017;8(36):60504-60513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh RR, Grossbard ML, Harrison LB, Yahalom J. Association of intensity-modulated radiation therapy on overall survival for patients with Hodgkin lymphoma. Radiother Oncol. 2016;118(1):52-59. [DOI] [PubMed] [Google Scholar]
- 35.Yahalom J, Illidge T, Specht L, et al. ; International Lymphoma Radiation Oncology Group. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92(1):11-31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.