Abstract
Three-dimensional-porous scaffolds of bone graft substitutes play a critical role in both cell targeting and transplantation strategies. These scaffolds provide surfaces that facilitate the response of stem cells related to attachment, survival, migration, proliferation, and differentiation.
Objective
The aim of this study was to evaluate the in vitro behavior of human dental pulp mesenchymal stem cells cultured on scaffolds of polylactic/polyglycolic acid with and without hydroxyapatite.
Method
We performed an in vitro experimental study using dental pulp stem cells obtained from samples of premolars, molars. The cells were cultured on scaffolds with osteogenic differentiation medium. Cell proliferation, adhesion and cell differentiation to an osteoblastic linage in the biomaterial were evaluated at three different time points: 7, 15 and 30 days. Each experiment was performed in triplicate. Analysis of the data was performed with the Split Plot block and MANOVA model.
Results
The differentiation capability of hDPSCs towards the osteoblast lineage was better in the scaffold of PLGA/HA at 7, 15 and 30 days, as indicated by the high expression of osteogenic markers RUNX2, ALP, OPN and COL-I, compared with differentiation in the PLGA scaffold. No statistically significant differences were found in cell adhesion between the two types of scaffolds.
Conclusion
The PLGA/HA scaffold provided better physical and chemical signals, as judged by the ability of dental pulp stem cells to adhere, proliferate and differentiate toward the osteogenic lineage.
Keywords: Dentistry, Cell biology, Molecular biology
1. Introduction
Human dental pulp stem cells (hDPSCs) possess the capability of multi-lineage differentiation (Gronthos et al., 2000). These cells have great potential in tissue engineering because of their low morbidity after collection, easy surgical access, ability to be cryopreserved, ability to be recombined with many scaffolds and immuno-privilege and anti-inflammatory abilities (Bansal et al., 2015; Yan et al., 2011). Several studies have reported that hDPSCs can differentiate into osteoblasts and thus have a great potential for bone tissue engineering (Das and Zouani, 2014; Graziano et al., 2008).
Millions of patients worldwide have bone defects as a result of trauma, congenital abnormalities, cancer resection or deforming diseases (Rose and Oreffo, 2002). The frequency of these conditions is bound to the continuous growth in world population, particularly due to the increase in life expectancy (Michel et al., 2015). Conventional solutions to these issues include replacing the damaged bone tissue with either autologous or allogeneic bone grafts. Autologous grafts are the gold standard during bone restoration but present disadvantages for patients related to the increase of pain, morbidity and a limited supply of obtained bone. The disadvantages of allogeneic sources include immunogenic response and tissue rejection, which decrease the possibility of host integration (Mata et al., 2002). Bone tissue engineering has emerged as a highly promising approach to develop biologically active bone substitutes to restore, maintain and improve bone tissue (Boskey, 2001; Martino et al., 2012).
The focus of this research was the use of bone tissue engineering as a therapeutic strategy to handle bone critical size defects. It is well known that cells respond differently to surfaces of different materials. In this perspective, Caplan and Shah (2009) suggest that cell interpretation in response to a biomaterial reflects a central paradigm in tissue engineering studies. Following this research scope, we suggest that the understanding of the intracellular signaling mechanisms involved with cell adhesion, proliferation and differentiation on the implant surface is fundamental for the conception of new materials.
The purpose of the study was to analyze the in vitro behavior of dental pulp stem cells on Poly-(lactide-co-glycolide-acid) scaffolds with and without hydroxyapatite.
2. Materials & methods
This research was approved by the Ethics Committee in Dental Research, Faculty of Dentistry, of the Universidad Nacional de Colombia in its session of December 03, 2012, act number 0-20.
2.1. Isolation and culture of human dental pulp stem cells
Three wisdom teeth were obtained from one male (age 19 years) and two females (age 14 years and 22 years) donors at the Dental Clinic at El Bosque University with written informed consent approved by the Ethics Committee. After separation of the crown and root, pulp tissue was digested with 3 mg/mL collagenase type I (Sigma, USA) and 4 mg/mL dispase (Sigma, USA) for 2 h at 37 °C. The cells were then cultured in Dulbecco's modified Eagle's medium low glucose (DMEM) (HyClone) supplemented with 10% fetal bovine serum (FBS, HyClone), 100 U/mL penicillin G and 100 mg/mL streptomycin at 37 °C under 5% CO2.
Characterization of the hDPSCs primary culture, BMSCs cells (ATCC® PCS-500-012™), ADSCs (Lonza Catalog#: PT-5006) was conducted by flow cytometry using antibodies against CD34, CD73, CD90, CD105 and CD45 (MACS Miltenyi Biotec), according to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy to define human MSCs consensus (Dominici et al., 2006; Kimmelman et al., 2016). hDPSCs from passage 5 were employed for subsequent experiments. The doubling time (DT) was determined from the growth curves at 24, 48 and 72 hours, by using the Siegel's formula (Siegel et al., 2013) and Alamar blue spectrophotometric assay.
2.2. Preparation and characterization of porous PLGA and PLGA/HA scaffolds
Deproteinized bovine hydroxyapatite (HA) was prepared by sintering. The structure of the HA was confirmed by X-ray diffraction analysis and the results compared with International Centre for Diffraction Data (ICDD) PDF-2 database using the HighScore Plus software. The PLGA/HA composite material (1:3 proportion) was fabricated by a solvent casting/freeze drying/particulate leaching and pressure forming method. The PLGA/HA composite (PLA:PGA = 50:50; Mw 24.000 to 38.000) blocks were formed into criovials, cut into 1-mm thickness and 5-mm-diameter sections and sterilized by UV-ray irradiation for 2 hours. PLGA scaffolds were prepared by a leaching method without HA particles. The microstructure and pore morphology of the scaffolds were characterized by Scanning Electron Microscopy (SEM). An X-ray diffractometer (XRD) was used to determine the phase structure of the PLGA and PLGA/HA scaffolds.
2.3. Assessment of In vitro behavior of hDPSCs seeded on scaffolds
In this study, we evaluated cell proliferation, cell adhesion and osteoblastic differentiation of hDPSC in PLGA/HA and PLGA scaffolds. hDPSCs (2,5 × 105 cells per scaffold) were seeded on 3D scaffolds in 12-well plates (n = 3). The cell–scaffold constructs were cultured under basal or osteogenic conditions (STEMPRO®) for 7, 15 and 30 days. Monolayer cultures of hDPSC and the NHOst – human osteoblasts (Lonza Catalog #: CC-2538) seeded on scaffolds were used as control group. The cells could adhere for 24 hours on scaffolds and were cultured for 7, 15 or 30 days.
The cellular viability and proliferation of hDPSCs on scaffolds were assessed by Alamar Blue assay. In brief, at 7, 15 or 30 days, the cell-seeded scaffolds were rinsed three times with PBS and then incubated with fresh medium containing 10% (v/v) Alamar Blue indicator in the dark at 37 C under 5% CO2 for 4 h. Absorbance of the extracted dye, which is proportional to the number of cells in the scaffolds, was measured by spectrophotometer (TECAN) at wavelengths of 570 and 590 nm.
Osteogenic differentiation markers (RUNX2, COL-I, OPN and ALP) were evaluated by quantitative real time polymerase chain reaction (qRT-PCR) at 7, 15 or 30 days using the SYBR green kit (Zymo Research). Primer sequences are shown in Table 1. The relative quantification of the target gene was normalized to the transcript levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated using the 2−ΔΔCt method. hDPSC monolayer cultures were used as control group. The cell morphology on scaffolds were characterized by Scanning Electron Microscopy (SEM).
Table 1.
Primer sequences used in the study.
| Access number of gene bank | Gene symbol | Primer forward 5′-3′ | Primer reverse 5′-3′ | Size od PCR product |
|---|---|---|---|---|
| NM_001015051.3 | RUNX2 | CATCTAATGACACCACCAGGC | GCCTACAAAGGTGGGTTTGA | 168 |
| NM_001040058.1 | OPN | TGAAACGAGTCAGCTGGATGACCA | TGGCTGTGAAATTCATGGCTGTGG | 168 |
| NM_000478.4 | ALP | TCAGAAGCTCAACACCAACG | GTCAGGGACCTGGGCATT | 199 |
| NM_000088.3 | COL I | TGACCTCAAGATGTGCCACT | ACCAGACATGCCTCTTGTCC | 197 |
| NM_002046.3 | GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 226 |
2.4. Statistical analysis
Experimental results are shown as the mean ± standard deviation. The split plot statistical model was used for analyzing block data and multivariate analysis of variance (MANOVA) for semiparametric data. The statistical analysis was performed with R program. Differences were considered significant at p < 0.05.
3. Results
3.1. Isolation and characterization of hDPSCs
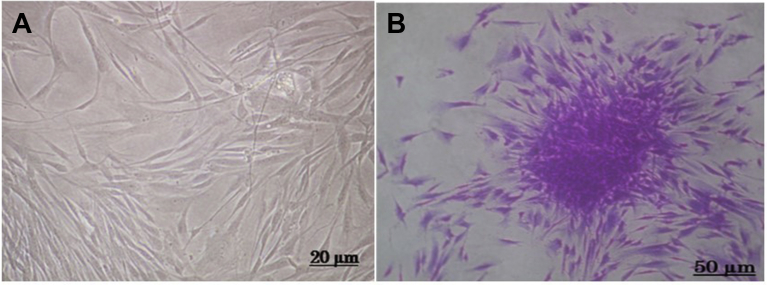
A heterogeneous cell population was obtained from extracted human dental pulp. Under an inverted microscope, the cells obtained after 5 days of incubation were fibroblast-like and non-refringent and had well-defined spherical nuclei (Fig. 1A). Fibroblast colony-forming units (CFU-F) typical of mesenchymal stem cells were also observed (Fig. 1B).
Fig. 1.
In vitro morphology of DPSCs. A. Human dental pulp stem cells with fibroblast-like morphology. B. DPSC colony forming unit (CFU-F) typical of mesenchymal stem cells.
The expression of surface antigens was assayed on cells after in vitro expansion, at passage 5, and compared with commercial human primary cells adipose derived mesenchymal stem cells (ASC) and bone marrow mesenchymal stem cells (BMSC). The hDPSC and BMSC populations showed high expression of CD105 marker, a type I membrane glycoprotein located on cell surfaces (91,7% and 97,38% respectively), all cells were highly positive for the surface antigen expression of CD73 a lymphocyte differentiation marker, hDPSC (73, 04%) with BMSC (97,62%) and ASC (89,79%). The hDPSC and ASC showed low expression of CD45 (36, 98% and 32,11%) and were consistently negative for CD34 a marker for hematopoietic stem cells (Fig. 2).
Fig. 2.
Immunophenotyping of dental pulp stem cells (DPSC) compared with adipose-derived stem cells (ASC), and bone marrow mesenchymal stem cells for the markers CD105, CD90, CD73, CD45 and CD34.
To evaluate the growth kinetics of hDPSCs in monolayers culture, doubling time was analyzed using the Siegel formula at 24, 48 and 72 hours (Siegel et al., 2013). Based on the results, this mesenchymal cells tended to double their population in an average of 20.63 ± 5.05 hours.
3.2. Morphology of the PLGA/HA and PLGA scaffolds
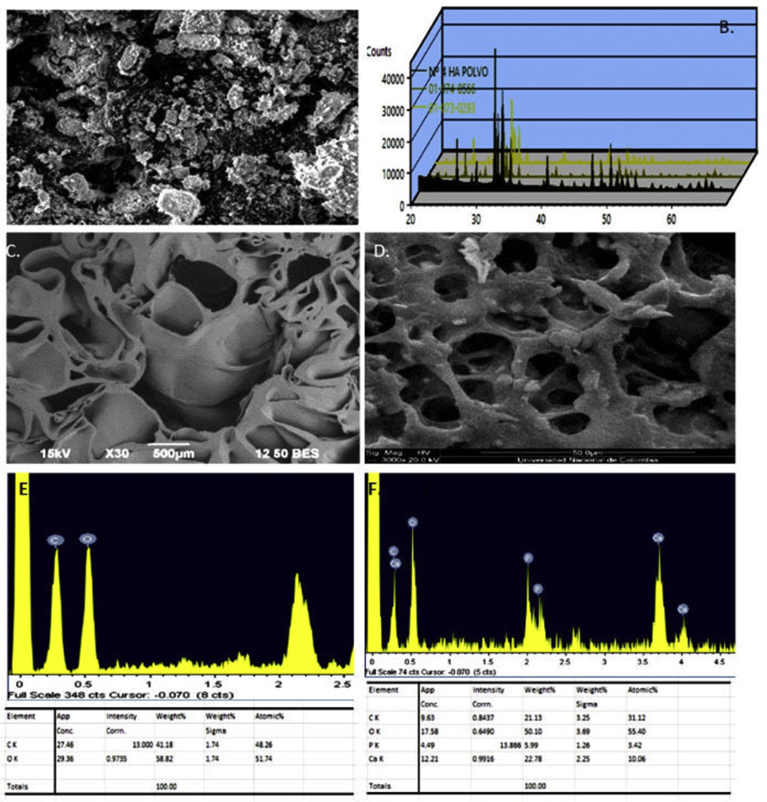
Chemical analysis of HA revealed a Ca/P molar ratio of 1.58, which is compatible with natural calcium hydroxyapatites. The X ray diffraction analysis confirmed that HA displayed the characteristic profile of pure hydroxyapatite. The HA crystals revealed characteristic peaks in the XRD pattern that were consistent with JCDS 2001 (Joint Committee on Powder Diffraction Standards, ICDD – International Center for Diffraction Data). Ca10 (PO4)6(OH)2 (01-074-0566) and Ca5 (PO4)3 (OH) (01-073-0293) were the dominant phases in samples (Figure 3A and B) (Wong-Ng et al., 2001).
Fig. 3.
Characterization of scaffolds. SEM micrographs of HA (A), diffractogram of the HA (B). SEM micrographs of PLGA/HA and (C) PLGA scaffolds (D). EDX scan spectra of PLGA/HA (E) and PLGA scaffolds (F).
The prepared PLGA (Fig. 3C) and PLGA/HA scaffold (Fig. 3D) were observed by SEM. The inner structure of the two scaffolds showed a spongy interconnected porosity. However, the porosity of the PLGA constructs was lower than that of the PLGA/HA constructs.
The pore in PLGA/HA scaffolds was uniform, and coherent, with diameters in the range of 10–311 μm. HA particles dispersed uniformly on the surface of the PLGA/HA scaffold. The sizes of HA particles range from 20 to 106 μm. In contrast, the PLGA scaffold was loose, irregular and had a larger average diameter. This difference in the inner structure of the scaffolds could be attributed to the presence of HA that promoted stabilization of its structure.
The EDX spectrum of scaffolds is illustrated in Fig. 3. The presence of HA in the PLGA/HA scaffolds was confirmed by the appearance of characteristic peaks for calcium, phosphorous, and oxygen, which are the main components of hydroxyapatite (Fig. 3E). Additional carbon peaks were also observed in the EDX spectra. These carbon peaks were attributed to the presence of PLGA (Fig. 3F). The EDX spectra of PLGA scaffolds showed the expected pattern of oxygen and carbon, which are the main components of this polymer (Fig. 3E).
3.3. Behavior of dental pulp stem cells seeded in scaffolds
The in vitro cell response to the PLGA/HA and PLGA scaffolds was assessed in terms of cell adhesion, proliferation and osteogenic differentiation. Fig. 4 describes the SEM images of the hDPSCs that adhered to the PLGA scaffold (Fig. 4A, C and E) and PLGA/HA (Fig. 4B, D and F) at 7, 15 and 30 days in culture, respectively. The SEM images reveal the bioactive properties of HA with a preferential formation of vesicles from cells on the PLGA/HA scaffolds (Fig. 4B and D) in contrast to the PLGA-only scaffolds (Fig. 4A and C). At 7 days, the hDPSCs on the PLGA scaffolds developed short filopodia at their apical pole, spreading from lamellipodia, which is usually observed in fibroblast-like cells (Fig. 4A).
Fig. 4.
Representative SEM images of hDPSC in PLGA (A, C, E) and PLGA/HA scaffolds (B, D, F) at 7, 15 and 30 days in culture, respectively (white arrows). On day 7, hDPSCs attached to the PLGA/HA scaffolds form vesicles (4b, black arrows). On day 15 and 30, abundant vesicles were secreted in the PLGA/HA group and calcified nodules deposited on the cell surface (F).
As shown in Fig. 4, we found that the formation of vesicles on the PLGA/HA scaffolds was better compared to formation on the PLGA only scaffolds, suggesting that HA is effective in accelerating the interaction and cell differentiation. The increase in vesicles formation of hDPSCs on the scaffolds was proportional to the incubation time; at longer periods of time, there were more vesicles on the PLGA/HA scaffolds (Fig. 4B, D and F) compared to that on PLGA only scaffolds (Fig. 4A, C and E).
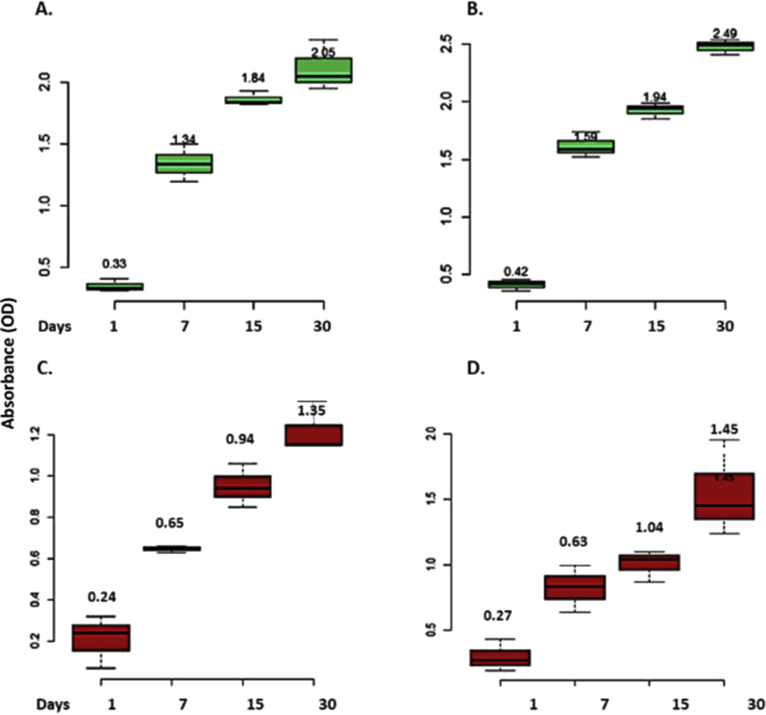
The difference in proliferation behavior of cells on scaffolds was determined by Alamar Blue assay. The cells cultured on the PLGA and PLGA/HA scaffolds for 7, 15 or 30 days revealed that cells can proliferate on the scaffolds. However, an increase in cell proliferation was observed on the PLGA/HA in basal medium compared to PLGA scaffolds, suggesting that HA is important for the osteogenic differentiation process (Fig. 5).
Fig. 5.
Proliferation of DPSC on scaffolds. Graph represents increase in absorbance at 570–600 nm from the Alamar Blue assay indicating an increase of hDPSC cell number over time in in vitro culture, in osteogenic (A, C), and basal medium (B, D). An increase in cell proliferation was observed on the PLGA/HA compared to PLGA scaffolds p = 0,05.
The differentiation of mesenchymal stem cells on scaffolds is one of the key factors regarding bone regeneration. The mRNA expression of the genes RUNX2, ALP, OPN and COLI is considered an important tool for determining the differentiation of mesenchymal stem cells (osteogenesis). Fig. 6 shows the dendrogram and unsupervised hierarchical clustering heat map of expression of osteogenic markers in hDPSCs on the PLGA/HA and PLGA scaffolds compared with osteoblasts. The vertical distances on each branch of the dendrogram represent the degree of similarity between scaffolds types' gene expression profiles of these genes in hDPSCs cultured on the PLGA/HA and PLGA scaffolds for 30 days compared with osteoblasts. It is evident that the cells initiate the differentiation process at 7 days on PLGA/HA scaffolds by the high expression of RUNX2 in contrast with expression on PLGA only scaffolds. The gene expression of ALP was significantly up-regulated (p < 0.001) in PLGA/HA scaffold groups compared to that in PLGA-only cultures at day 15, whereas the expression of OPN, ALP and COLI was significantly up-regulated (p < 0.001) in PLGA/HA scaffolds compared to that in PLGA cultures at day 30. The gene expression of the osteoblast-specific transcription factor RUNX2 at days 15 and 30 was down-regulated in the cells seeded in the PLGA/HA scaffolds but remained higher in the cells seeded in the PLGA scaffolds.
Fig. 6.
Dendrogram and unsupervised hierarchical clustering heat map of osteogenic markers expression in hDPSCs on the PLGA/HA and PLGA scaffolds compared with osteoblasts (3 replicates each), using uncentered Pearson correlation and centroid linkage. The vertical distances on each branch of the dendrogram represent the degree of similarity between two scaffolds types gene expression profiles (expression level is color coded: red for over-expressed, black for unchanged expression, and green for under-expressed genes). hDPSCs differentiation at 7 days on PLGA/HA scaffold shown by the high expression of RUNX2. ALP was significantly up-regulated (p < 0.001) in PLGA/HA at day 15, whereas OPN, ALP and COL-I expression was significantly up-regulated (p < 0.001) at day 30.
4. Discussion
Current therapeutic approaches for lost bone tissue have included the use of grafts (autologous, homologous, and heterologous) and biomaterials but have not yet provided completely satisfactory results. Therefore, bone tissue engineering is a promising strategy that involves the use of osteogenic cells seeded on scaffolds that mimic the extracellular bone matrix and a progressive investigation in the design of these supports for bone tissue regeneration is needed (Cancedda et al., 2007; Gemini-Piperni et al., 2014).
The interaction between cells and biomaterial scaffolds is a complex process that is affected by numerous aspects such as cell behavior, material surface properties and environmental factors. To date, several groups have demonstrated that surface topography and chemical composition could drive cell adhesion, proliferation, migration and differentiation (D'Anto et al., 2016).
The adhesion and proliferation of cells on scaffolds determine the initial success of the cell-scaffold interaction and influence the subsequent behavior of the target cells. Therefore, the objective of this study was to evaluate the in vitro behavior of hDPSCs on PLGA scaffolds with and without hydroxyapatite (HA).
hDPSCs are a kind of mesenchymal stem cell with the potential for cell-mediated therapy and tissue engineering applications. Previous work has reported that hDPSCs have significant osteogenic differentiation ability for bone regeneration (Asutay et al., 2015; Khanna-Jain et al., 2012).
PLGA is an FDA-approved, synthetic polymer that has been widely used for in vitro and in vivo bone regeneration studies because of its desirable mechanical properties, biocompatibility and biodegradability (Kim et al., 2013; Park et al., 2012). Although its by-products, lactic and glycolic acid, can cause moderate local inflammation, its compatibility and biosafety in bone healing have been shown in both experimental and clinical studies (Baksh et al., 2007). However, despite being biocompatible, clinical application of pure PLGA for bone regeneration is hampered by poor osteoconductivity and suboptimal mechanical properties. Therefore, PLGA is often used in combination with ceramics in order to make PLGA more biomimetic and able to enhance bone regeneration for tissue engineering and regenerative medicine applications (Pan and Ding, 2012). HA is among the family of calcium phosphate-based bioceramics (Arahira and Todo, 2014; Miao et al., 2008), due to its non-toxicity, bioactivity and osteoconductivity and the similarity of its chemical structure to that of nature bone minerals. The incorporation of HA into a polymer matrix is assumed to mimic the natural bone structure, enhance the cell growth response and improve the mechanical properties of scaffolds (Ao et al., 2017; Asutay et al., 2015). In such strategies, the interaction between stem cells and the material applied as scaffold plays a critical role in the generation of a cell-friendly microenvironment, which must be conducive to the regeneration of dental structures (Conde et al., 2016).
Flow cytometric analysis showed the typical immunophenotypic characterization of mesenchymal cells. Thus, our findings excluded any presence of primitive hematopoietic progenitors and are consistent with previously reported data (Atari et al., 2012).
Morphology and structure of the pure PLGA and PLGA/HA scaffolds were observed under SEM, as shown in Fig. 3D. Both PLGA and PLGA/HA scaffolds showed an interconnected and highly porous structure. The diameter of these pores in the PLGA/HA scaffolds ranged from 10 to 311 μm in diameter, which is important for the bioresorbability of the material and plays an important role in the osteoconductivity of the scaffold (Penk et al., 2013). The minimum recommended pore size for a bone substitute is 100 μm, but subsequent studies have shown better osteogenesis for substitutes with pores >300 μm (Dogan et al., 2014; Penk et al., 2013).
The cell adhesion of hDPSCs seeded on PLGA and PLGA/HA scaffolds was evaluated by SEM. We observed that after 7 days, the cells had spread well, with an intimate contact with the surface of the PLGA/HA and PLGA scaffolds, and had migrated through the pores inside the cavities and were able to adhere to the wall of the pores. In addition, we observed differences in the formation of matrix vesicles among the scaffolds. In particular, while we observed matrix vesicles (MVs) after 7 days in hDPSCs cultured on PLGA/HA, these matrix vesicles were not observed until 15 days in hDPSCs seeded on PLGA only scaffolds. This result suggests that hDPSCs seeded on PLGA/HA scaffolds differentiate into osteoblasts faster than do hDPSCs seeded on PLGA only. These findings are consistent with those found in the study by Arahira and Todo, who evaluated the proliferation and differentiation of mesenchymal stem cell (hMSCs) on three-dimensional collagen/tricalcium phosphate β scaffolds. Using SEM, they observed the appearance of spherical structures on cells compatible with matrix vesicles and mineralization nodules produced by osteoblasts differentiated from hMSCs at 14 days of culture with osteogenic culture medium. At 21 days of culture, they observed structures of the extracellular matrix consistent with collagen fibers. Spherical structures were associated with vesicles of the matrix because they had a diameter of 40 to 200 nm and because these have been considered to play a fundamental role in the process of bone calcification (Arahira and Todo, 2014; Tatsuhiro et al., 2018). Besides, the presence of HA crystals in the scaffold offers suitable mechanical proprieties, and roughness which can increase cell adhesion (Geckil et al., 2010; Lou et al., 2012).
PLGA/HA scaffolds were well tolerated by the hDPSCs and did not affect the proliferation rate of cells which promotes the biocompatibility of scaffolds. The amount in the absorbance values in Alamar Blue assay demonstrated a statistically significant increase in hDPSCs and osteoblasts over the culture period. However, proliferation was greater in hDPSCs cultured in basal medium than it was in hDPSCs cultured in medium with osteogenic supplements. Cell proliferation was higher in the PLGA/HA scaffolds compared to that in the PLGA only scaffolds. This result agrees with Karadzic et al. (2015), who evaluated cell proliferation in different scaffolds (HA, PLGA/HA, Alginate/HA, vinyl ethylene acetate/HA and Bio-oss) and found that proliferation was greater after 30 days in scaffolds composed of PLGA/HA (Karadzic et al., 2015). On the other hand, it has been described that precursor cells continue division before acquiring a fully differentiated state, while terminal differentiation usually coincides with proliferation arrest and permanent exit from the division cycle (Ruijtenberg and van den Heuvel, 2016).
We noted interesting results regarding the time-dependent expression of bone-related genes inside the hDPSCs constructs after initiating the 3D culture. The data obtained showed the expected expression profiles of the osteoblast phenotype. Results showed an up-regulation of all osteogenic markers after day 14 and 30, compared to control hDPSCs. The gene expression of RUNX2, COL-I, OPN and ALP was higher in hDPSCs cultured with osteogenic medium on PLGA/HA scaffolds than hDPSCs cultured with osteogenic medium on PLGA only scaffolds. Compared with osteoblasts, the hDPSC in the PLGA/HA constructs exhibited significantly up-regulation of RUNX2 expression on day 7 after culture initiation. Moreover, the expression of RUNX2 decreases after 15 and 30 days of culture suggesting that this gene is downregulated in mature osteoblasts (Komori, 2010). Besides we found increase in expression of ALP, OPN, and COL-I marker genes in the middle-to-late stages of the osteoblast differentiation process until day 30 of the culture. These data are in agreement with the temporal gene expression demonstrated during osteogenesis (Kulterer et al., 2007).
This result suggests that the process of osteoblastic differentiation of these cells occurs better on PLGA/HA scaffolds (p = 0.038). This is probably due to hydroxyapatite, which has been reported to induce the expression of genes such as ALP, COL-I, OPN and RUNX2 (Aonuma et al., 2012; Balaji Raghavendran et al., 2014; He et al., 2016; Lin et al., 2009). RUNX2 is a transcription factor involved in osteoblastic differentiation and skeletal morphogenesis. It has been shown to affect the expression of type I collagen and OPN by binding to the promoters of these genes. RUNX2 and COL-I are known to be early markers of osteoblastic differentiation while OPN is involved in initiating mineralization and promoting mineral crystal formation during bone formation, but the highest expression is observed in mature osteoblasts at sites of bone remodeling. ALP appears to be intimately related to pre-osseous cellular metabolism and to the elaboration of a calcifying bone matrix (Ris et al., 1975).
In addition, the characteristics of surface polymer phase and of the biocomposite, such as the level of hydration, surface tension, surface charge and topography, have a great influence on cell adhesion and proliferation (Arahira and Todo, 2014; Das and Zouani, 2014; Wang et al., 2012). Our results suggest that the scaffolds used in this study were adequately designed with regard to their surface morphology, which allowed cell adhesion, proliferation and differentiation. However, the appearance of matrix vesicle-like structures in the hDPSCs seeded on PLGA/HA scaffolds with the complete culture medium may suggest that this biocomposite is bioactive and has an osteoconductivity and osteoinductive effect due to the presence of hydroxyapatite.
5. Conclusions
In conclusion, the in vitro data demonstrated that the PLGA/HA scaffold has suitable proprieties in supporting hDPSCs adhesion, proliferation and osteogenic differentiation. This biomaterial could be a promising candidate for bone tissue engineering and regenerative medicine applications. However, further studies are needed to more thoroughly evaluate the behavior of hDPSCs in vivo using this type of biocomposite.
Declarations
Author contribution statement
Nury Tatiana Jiménez: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Juan Carlos Munévar, Jose Manuel González, Clementina Infante: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Sandra Janneth Perdomo Lara: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Universidad Nacional de Colombia (202010020528) and Universidad El Bosque Grant.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ao C., Niu Y., Zhang X., He X., Zhang W., Lu C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017;97:568–573. doi: 10.1016/j.ijbiomac.2016.12.091. [DOI] [PubMed] [Google Scholar]
- Aonuma H., Ogura N., Takahashi K., Fujimoto Y., Iwai S., Hashimoto H., Ito K., Kamino Y., Kondoh T. Characteristics and osteogenic differentiation of stem/progenitor cells in the human dental follicle analyzed by gene expression profiling. Cell Tissue Res. 2012;350(2):317–331. doi: 10.1007/s00441-012-1477-6. [DOI] [PubMed] [Google Scholar]
- Arahira T., Todo M. Effects of proliferation and differentiation of mesenchymal stem cells on compressive mechanical behavior of collagen/beta-TCP composite scaffold. J. Mech. Behav. Biomed. Mater. 2014;39:218–230. doi: 10.1016/j.jmbbm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Asutay F., Polat S., Gul M., Subasi C., Kahraman S.A., Karaoz E. The effects of dental pulp stem cells on bone regeneration in rat calvarial defect model: micro-computed tomography and histomorphometric analysis. Arch. Oral Biol. 2015;60(12):1729–1735. doi: 10.1016/j.archoralbio.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Atari M., Caballé-Serrano J., Gil-Recio C., Giner-Delgado C., Martínez-Sarrà E., García-Fernández D.A., Barajas M., Hernández-Alfaro F., Ferrés-Padró E., Giner-Tarrida L. The enhancement of osteogenesis through the use of dental pulp pluripotent stem cells in 3D. Bone. 2012;50(4):930–941. doi: 10.1016/j.bone.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Baksh D., Yao R., Tuan R. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cell. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Balaji Raghavendran H.R., Puvaneswary S., Talebian S., Murali M.R., Naveen S.V., Krishnamurithy G., McKean R., Kamarul T. A comparative study on in vitro osteogenic priming potential of electron spun scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for tissue engineering application. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R., Jain A., Mittal S. Current overview on challenges in regenerative endodontics. J. Conserv. Dent. 2015;18(1):1–6. doi: 10.4103/0972-0707.148861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A.L. Musculoskeletal disorders and orthopedic conditions. J. Am. Med. Assoc. 2001;285(5):619–623. doi: 10.1001/jama.285.5.619. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Giannoni P., Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28(29):4240–4250. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Caplan M.R., Shah M.M. Translating biomaterial properties to intracellular signaling. Cell Biochem. Biophys. 2009;54(1-3):1–10. doi: 10.1007/s12013-009-9048-5. [DOI] [PubMed] [Google Scholar]
- Conde M.C., Chisini L.A., Demarco F.F., Nor J.E., Casagrande L., Tarquinio S.B. Stem cell-based pulp tissue engineering: variables enrolled in translation from the bench to the bedside, a systematic review of literature. Int. Endod. J. 2016;49(6):543–550. doi: 10.1111/iej.12489. [DOI] [PubMed] [Google Scholar]
- D'Anto V., Raucci M.G., Guarino V., Martina S., Valletta R., Ambrosio L. Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. J. Tissue Eng. Regen. Med. 2016;10(2):E147–E154. doi: 10.1002/term.1768. [DOI] [PubMed] [Google Scholar]
- Das R.K., Zouani O.F. A review of the effects of the cell environment physicochemical nanoarchitecture on stem cell commitment. Biomaterials. 2014;35(20):5278–5293. doi: 10.1016/j.biomaterials.2014.03.044. [DOI] [PubMed] [Google Scholar]
- Doğan A., Demirci S., Bayir Y., Halici Z., Karakus E., Aydin A., Cadirci E., Albayrak A., Demirci E., Karaman A., Ayan A.K., Gundogdu C., Sahin F. Boron containing poly-(lactide-co-glycolide) (PLGA) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;44:246–253. doi: 10.1016/j.msec.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Geckil H., Xu F., Zhang X., Moon S., Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond) 2010;5(3):469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemini-Piperni S., Takamori E.R., Sartoretto S.C., Paiva K.B., Granjeiro J.M., de Oliveira R.C., Zambuzzi W.F. Cellular behavior as a dynamic field for exploring bone bioengineering: a closer look at cell-biomaterial interface. Arch. Biochem. Biophys. 2014;561:88–98. doi: 10.1016/j.abb.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Graziano A., d'Aquino R., Laino G., Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4(1):21–26. doi: 10.1007/s12015-008-9013-5. [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Lin K.F., Sun Z., Song Y., Zhao Y.N., Wang Z., Bi L., Liu J. Effects of nano-hydroxyapatite/poly(DL-lactic-co-glycolic acid) microsphere-based composite scaffolds on repair of bone defects: evaluating the role of nano-hydroxyapatite content. Artif. Organs. 2016;40(7):E128–E135. doi: 10.1111/aor.12741. [DOI] [PubMed] [Google Scholar]
- Karadzic I., Vucic V., Jokanovic V., Debeljak-Martacic J., Markovic D., Petrovic S., Glibetic M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. 2015;103(1):350–357. doi: 10.1002/jbm.a.35180. [DOI] [PubMed] [Google Scholar]
- Khanna-Jain R., Mannerstrom B., Vuorinen A., Sandor G.K., Suuronen R., Miettinen S. Osteogenic differentiation of human dental pulp stem cells on beta-tricalcium phosphate/poly (l-lactic acid/caprolactone) three-dimensional scaffolds. J. Tissue Eng. 2012;3(1) doi: 10.1177/2041731412467998. 2041731412467998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Jeong S.Y., Ju Y.M., Yoo J.J., Smith T.L., Khang G., Lee S.J., Atala A. In vitro osteogenic differentiation of human amniotic fluid-derived stem cells on a poly(lactide-co-glycolide) (PLGA)-bladder submucosa matrix (BSM) composite scaffold for bone tissue engineering. Biomed. Mater. 2013;8(1) doi: 10.1088/1748-6041/8/1/014107. [DOI] [PubMed] [Google Scholar]
- Kimmelman J., Heslop H.E., Sugarman J., Studer L., Benvenisty N., Caulfield T., Hyun I., Murry C.E., Sipp D., Daley G.Q. New ISSCR guidelines: clinical translation of stem cell research. Lancet. 2016;387(10032):1979–1981. doi: 10.1016/S0140-6736(16)30390-7. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of osteoblast differentiation by Runx2. Adv. Exp. Med. Biol. 2010;658:43–49. doi: 10.1007/978-1-4419-1050-9_5. [DOI] [PubMed] [Google Scholar]
- Kulterer B., Friedl G., Jandrositz A., Sanchez-Cabo F., Prokesch A., Paar C., Scheideler M., Windhager R., Preisegger K.H., Trajanoski Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Chow K.L., Leng Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. 2009;89(2):326–335. doi: 10.1002/jbm.a.31994. [DOI] [PubMed] [Google Scholar]
- Lou W., Zhang H., Ma J., Zhang D., Liu C., Wang S., Deng Z., Xu H., Liu J. In vivo evaluation of in situ polysaccharide based hydrogel for prevention of postoperative adhesion. Carbohydr. Polym. 2012;90(2):1024–1031. doi: 10.1016/j.carbpol.2012.06.037. [DOI] [PubMed] [Google Scholar]
- Martino S., D'Angelo F., Armentano I., Kenny J.M., Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012;30(1):338–351. doi: 10.1016/j.biotechadv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Mata A., Boehm C., Fleischman A.J., Muschler G., Roy S. Growth of connective tissue progenitor cells on microtextured polydimethylsiloxane surfaces. J. Biomed. Mater. Res. 2002;62(4):499–506. doi: 10.1002/jbm.10353. [DOI] [PubMed] [Google Scholar]
- Miao X., Tan D.M., Li J., Xiao Y., Crawford R. Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly(lactic-co-glycolic acid) Acta Biomater. 2008;4(3):638–645. doi: 10.1016/j.actbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Michel J., Penna M., Kochen J., Cheung H. Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int. 2015;2015:305217. doi: 10.1155/2015/305217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2(3):366–377. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Park D.S., Shin J.W., Kang Y.G., Kim H.K., Yoon T.R., Shin J.W. Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J. Mater. Sci. Mater. Med. 2012;23(11):2671–2678. doi: 10.1007/s10856-012-4738-8. [DOI] [PubMed] [Google Scholar]
- Penk A., Förster Y., Scheidt H.A., Nimptsch A., Hacker M.C., Schulz-Siegmund M., Ahnert P., Schiller J., Rammelt S., Huster D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn. Reson. Med. 2013;70(4):925–935. doi: 10.1002/mrm.24541. [DOI] [PubMed] [Google Scholar]
- Ris M.M., Deitrich R.A., Von Wartburg J.P. Inhibition of aldehyde reductase isoenzymes in human and rat brain. Biochem. Pharmacol. 1975;24(20):1865–1869. doi: 10.1016/0006-2952(75)90405-0. [DOI] [PubMed] [Google Scholar]
- Rose F.R., Oreffo R.O. Bone tissue engineering: hope vs hype. Biochem. Biophys. Res. Commun. 2002;292(1):1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- Ruijtenberg S., van den Heuvel S. Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15(2):196–212. doi: 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G., Kluba T., Hermanutz-Klein U., Bieback K., Northoff H., Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuhiro F., Seiko T., Yusuke T., Reiko T.T., Kazuhito S. Dental pulp stem cell-derived, scaffold-free constructs for bone regeneration. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ma N., Kratz K., Xu X., Li Z., Roch T., Bieback K., Jung F., Lendlein A. The influence of polymer scaffolds on cellular behaviour of bone marrow derived human mesenchymal stem cells. Clin. Hemorheol. Microcirc. 2012;52(2-4):357–373. doi: 10.3233/CH-2012-1611. [DOI] [PubMed] [Google Scholar]
- Wong-Ng W., McMurdie H.F., Hubbard C.R., Mighell A.D. JCPDS-ICDD research associateship (cooperative program with NBS/NIST) J. Res. Natl. Inst. Stand. Technol. 2001;106(6):1013–1028. doi: 10.6028/jres.106.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Yu Y., Zhang G., Tang C., Yu J. A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev. 2011;7(1):161–171. doi: 10.1007/s12015-010-9155-0. [DOI] [PubMed] [Google Scholar]