Abstract
Objective
To evaluate imaging findings and complications from transcatheter interventional treatment of hepatocellular carcinoma via the inferior phrenic arteries.
Material & Methods
40 procedures in 25 patients (19 men; age range, 57–89 years) were retrospectively reviewed in this study. In all procedures, a micro-catheter was selectively inserted in the right inferior phrenic artery (n = 39) or left inferior phrenic artery (n = 1), and transcatheter arterial chemoembolization (n = 39) or transcatheter arterial embolization (n = 1) was performed. Imaging findings and patient charts were reviewed, and complications until time of discharge (median hospitalization period, 10.5 days; range, 3–21) were assessed.
Results
On angiography or computed tomography during angiography, collateral circulation from the right inferior phrenic artery to the pulmonary artery was seen in eight of 39 procedures (seven patients, 28%). In seven of these procedures, Lipiodol deposition was seen on the unenhanced computed tomography just after the procedure (post-procedure computed tomography) in the pulmonary arteries or pleura, and in six procedures, the deposited Lipiodol was noted to have spread into adjacent lung fields on the one week follow-up computed tomography. Branches of the right inferior phrenic artery were seen along the right margin of the heart in 18 procedures, and Lipiodol deposition was seen along the right margin of the heart on post-procedure computed tomography in four procedures. Complications occurred in 21 of 39 procedures of right inferior phrenic artery intervention (53%): shoulder pain in 18 (45%), pleural effusion in 14 (35%), basal atelectasis in 11 (28%), paroxysmal atrial fibrillation in two (5%) and hemoptysis in one (3%). In 14 procedures (35.9%), pleural effusion was seen on follow-up computed tomography examinations, and 11 (28.2%) of these procedures also showed basal atelectasis. However, only three procedures with pleural effusion showed Lipiodol deposition on the post-procedure computed tomography. In one patient who underwent transcatheter arterial chemoembolization twice via the right inferior phrenic artery, atrial fibrillation occurred after both procedures.
Conclusions
Transcatheter arterial chemoembolization or transcatheter arterial embolization via the inferior phrenic artery in patients with hepatocellular carcinoma was relatively safe. Shoulder pain was the most frequent complication, and required only conservative treatment. There was no clear connection between pleural effusion or basal atelectasis and collateral circulation from the right inferior phrenic artery to the pulmonary artery depicted on angiography, computed tomography during angiography or post-procedure computed tomography.
Abbreviations: IPA, inferior phrenic artery; HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; TAE, transcatheter arterial embolization; AG, angiography; CT-AG, CT during angiography; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events
Keywords: Hepatocellular carcinoma, Transcatheter arterial embolization, Therapeutic chemoembolization, Inferior phrenic artery, Complication
1. Introduction
Transcatheter treatment procedures, such as transcatheter arterial chemoembolization (TACE), transcatheter arterial embolization (TAE) and transcatheter arterial infusion (TAI) have been widely used to treat hepatocellular carcinoma (HCC) [1,2]. Since HCCs are occasionally multicentric and frequently relapse after treatment, many patients require repeated treatment to prolong survival time [1,2].
Tumors are generally thought to develop collateral arterial circulation as transcatheter treatment is repeated [3], and HCC protruding from the liver may be fed by extrahepatic arteries even without past treatment [[3], [4], [5], [6]]. In these cases, the interventional radiologist must sometimes treat tumors via various collateral arteries such as the inferior phrenic artery (IPA), internal mammary artery and intercostal artery [[3], [4], [5],7,8], in addition to the usual treatment via the hepatic arteries. The IPA is the artery most frequently involved in collateral circulation [[3], [4], [5]], and various complications related to transcatheter IPA treatment have been reported [3,5,6,[9], [10], [11], [12], [13], [14], [15]]. Collateral circulation between the IPA and extrahepatic arteries are common and considered a cause of extrahepatic complications [3,5,6,9,15].
The aim of this study was to evaluate the imaging findings--angiography (AG), computed tomography (CT) during angiography (CT-AG), unenhanced CT just after the procedure (post-procedure CT) and follow-up CT--and complications of transcatheter treatment via the IPA in patients with HCC.
2. Material & methods
The institutional ethical committee approved our research protocol. Written informed consent was obtained from all patients before treatment, but informed consent related to this study was waived because of its retrospective nature.
The diagnosis of HCC was made by a combination of imaging findings and evaluation of specific tumor markers, or by needle biopsy. The radiological imaging included contrast-enhanced CT, contrast-enhanced magnetic resonance imaging (MRI) using gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA; EOB·Primovist®, Bayer Yakuhin Co. Ltd., Tokyo, Japan), and ultrasonography with or without contrast media (Perflubutane, Sonazoid®, Daiichi-Sankyo Co. Ltd., Tokyo, Japan). The treatment strategies of HCC were selected through discussion by hepatologists, surgeons, pathologists, diagnostic radiologists and interventional radiologists. Therapeutic chemoembolization was selected based on performance status, hepatic functional reserve, the size or number of tumors and patients’ wishes.
A 4- or 5-Fr sheath was inserted in the femoral artery. The celiac artery and superior mesenteric artery was selected with 4- or 5-Fr shepherd hook catheter or cobra catheter and enhanced. We performed CTs during hepatic arteriography (CTHA) and arterial portography (CTAP) in all procedures. Involvement of an extrahepatic artery as an HCC feeding artery was suspected if the HCC was not enhanced on CTHA and CTAP. We evaluated the origin of the IPA before procedures on enhanced CT. The IPA was carefully selected with a 1.9–2.1-Fr micro-catheter (Progreat Σ; Terumo, Tokyo, Japan in most cases) and selective AG was performed. CT-AG via the IPA was performed at the physician’s discretion. If the tumor enhanced on AG or CT-AG of the IPA, the IPA was considered to be the feeding artery of the HCC. When performing TACE or TAE, we spared IPA branches that did not feed the targeted tumors as much as possible. TACE was performed by infusing an emulsion of Lipiodol (Fuji Pharma Co., Ltd., Tokyo, Japan) and chemotherapeutic agents (epirubicin (Epirubicin; Nippon Kayaku, Tokyo, Japan), miriplatin (Miripla; Dainippon Sumitomo Pharma, Osaka, Japan), and cisplatin (IA-call; Nippon Kayaku, Tokyo, Japan). Gelatin sponge particles (1mm-Gelpart; Nippon Kayaku, Tokyo, Japan) were frequently used as an adjuvant embolizing agent. When the tumors received additional vascular supply from branches of the hepatic arteries, TACE or TAE via these arteries was also performed in the same session. We did not perform coil embolization of the IPA.
All patients underwent unenhanced CT at the end of the procedure (post-procedure CT) and were carefully monitored in the ward. Follow-up CT of the upper abdomen was usually performed about a week after the procedure to evaluate deposition of Lipiodol in HCC following TACE. Additional CT or plain chest radiographs were obtained when patients developed symptoms such as prolonged fever or decreased 02 saturation following procedures.
We searched the interventional radiology reports of transcatheter procedures via IPAs for the treatment of HCC performed in our department from January 2010 to January 2015. We reviewed the imaging findings and patient charts. Complications until discharge were assessed, recorded and classified according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 [16]. In this study, only descriptive statistical analyses were performed.
3. Results
We found 39 TACE procedures and one TAE procedure via the right IPA (n = 39) or left IPA (n = 1) for the treatment of HCC in 25 patients (19 men and 6 women; mean age, 69.9 years; range, 57–89). 35 (87.5%) were classified as Child-Pugh class A, and five (12.5%) as class B at the time of treatment. Five (20%) patients had viral hepatitis B, 12 (48%) had viral hepatitis C, and three (12%) had alcoholic liver cirrhosis. The etiology of liver disease was not proved in 5 (20%) patients.
None of the patients showed any evidence of pleural effusion, pleural thickening or calcification suggestive of old pleuritis, or any evidence of previous thoracic surgery nor trauma on enhanced CT prior to the procedure. Eight (32%) patients had previous surgical resection of the liver. In one case, there was no history of previous TAE or TACE, but all other patients had undergone at least one previous TACE or TAE for HCCs (mean ± SD, 5.0 ± 3.2 times; range, 0–13). The targeted tumors were in segment 7 in 21 (52.5%) procedures, 8 in 10 (25%) procedures, 5 in three (7.5%) procedures, 4 in four (10%) procedures, 3 in one (2.5%) procedure and 1 in one (2.5%) procedure. The size of the tumor ranged 10–151 mm in maximum diameter (34 ± 23.8 mm).
Chemotherapeutic agents used were epirubicin in 22 (55%) procedures (mean ± SD, 28.3 ± 10.2 mg; range, 10–50), miriplatin in 16 (40%) procedures (mean ± SD, 92.8 ± 26.3 mg; range, 30–120), and cisplatin in one (2.5%) procedure (80 mg). The dose of Lipiodol (mean ± SD) was 4.2 ± 1.9 ml (range, 0–10). These volumes were the total dose used for TACE or TAE, and usually less than 20% was used for IPA administration. In 37 (92.5%) procedures, branches of the hepatic arteries were embolized simultaneously with gelatin sponge particles. In 10 (25%) procedures, an additional extrahepatic collateral artery was embolized, such as the intercostal artery (four procedures, 10%), right renal capsular artery (three procedures, 7.5%), right adrenal artery (one case, 2.5%) and right gastroepiploic artery (one case, 2.5%).
The hospitalization period (mean ± SD) was 11.5 ± 4.2 days (median, 10.5 days; range, 3–21). Patients underwent unenhanced (n = 15) or enhanced (n = 25) follow-up CT after the procedures (median, 7 days; range, 4–46).
3.1. IPA AG, CT-AG and post-procedure CT findings
Collateral circulation from the right IPA to the pulmonary artery was depicted on AG and/or CT-AG in eight (20.5%) procedures and in 7 (28%) patients. Three (12%) patients had undergone hepatectomy before interventional therapy to the IPA. In seven of these eight procedures in which collateral circulation was identified, Lipiodol deposition in the pulmonary arteries or pleura was depicted on the post-procedure CT. In six of these procedures, the deposition was seen to have spread into the adjacent lung fields on follow-up CTs (Fig. 1, Fig. 2, Fig. 3). In one procedure, collateral circulation from the right IPA was not depicted on AG or AG-CT, but the post-procedure CT showed Lipiodol deposition in the pleura and the inferior branch of the pulmonary artery.
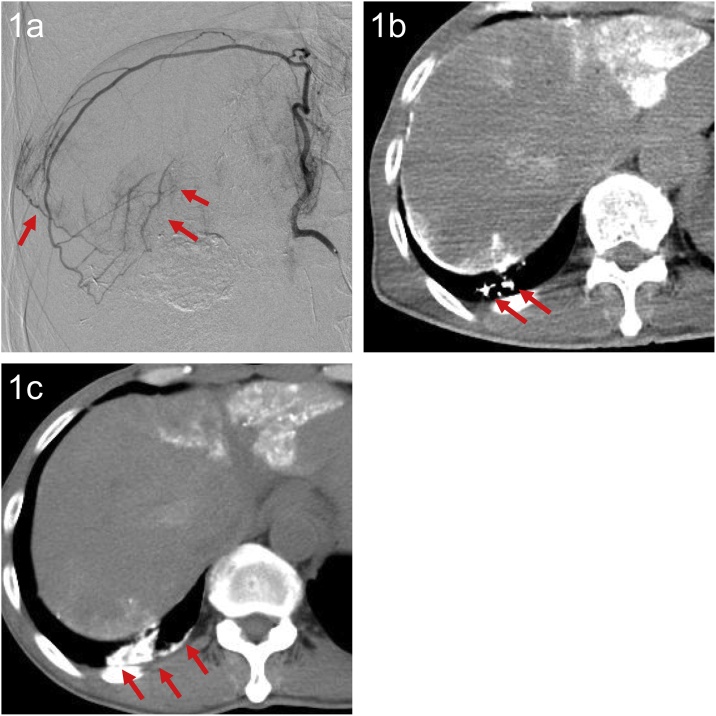
Fig. 1.
A 61-year-old man with HCC.
(a) AG of the right IPA showed communication with an inferior branch of the right pulmonary artery (arrow). TACE was performed via this artery, using an emulsion of epirubicin, Lipiodol and 1-mm Gelpart.
(b) Post-procedure CT showed Lipiodol deposition in the inferior branch of the right pulmonary artery (arrow).
(c) Unenhanced CT seven days after TACE showed Lipiodol deposition in the collapsed right lower lobe (arrow). Lipiodol had spread into the adjacent lung fields.
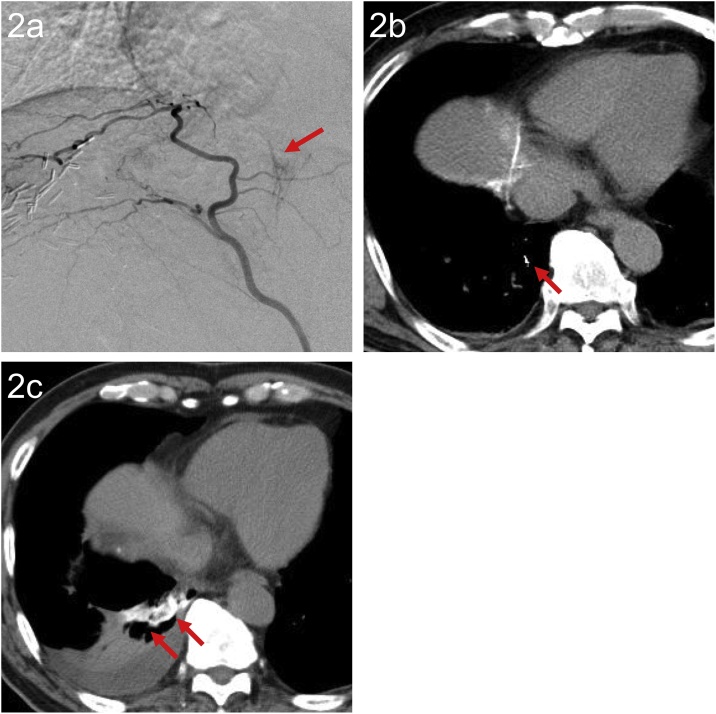
Fig. 2.
A 75-year-old man with HCC who developed hemoptysis four days after TACE.
(a) AG of the right IPA showed collaterals between the right IPA and the inferior branch of the right pulmonary artery (arrow). TACE using an emulsion of epirubicin and Lipiodol, and 1-mm Gelpart via the right IPA including collaterals was performed.
(b) Post-procedure CT revealed subtle Lipiodol accumulation along the inferior branch of the right pulmonary artery (arrow).
(c) Unenhanced CT at seven days after TACE showed deposition of Lipiodol in the collapsed right lower lobe (arrow). The deposited Lipiodol had spread into the lung parenchyma.
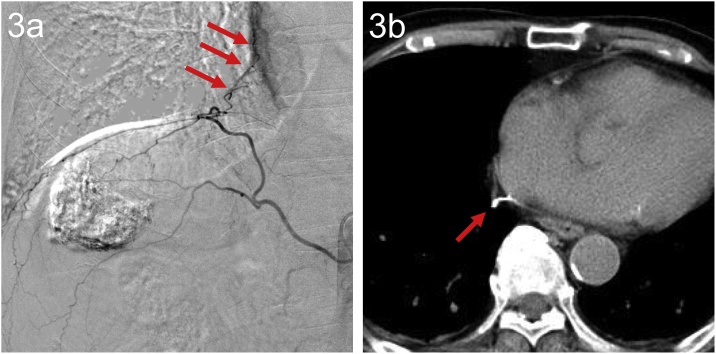
Fig. 3.
An 80-year-old man with HCC, showing Lipiodol deposition near the pericardium.
(a) AG of the right IPA showed enhancement along the right margin of the heart (arrow).
(b) Post-procedure CT showed Lipiodol deposition along the right margin of the heart (arrow). On the next day and 3 days after TACE, paroxysmal atrial fibrillation occurred (heart rate & 130), but improved after treatment with an antiarrhythmic drug.
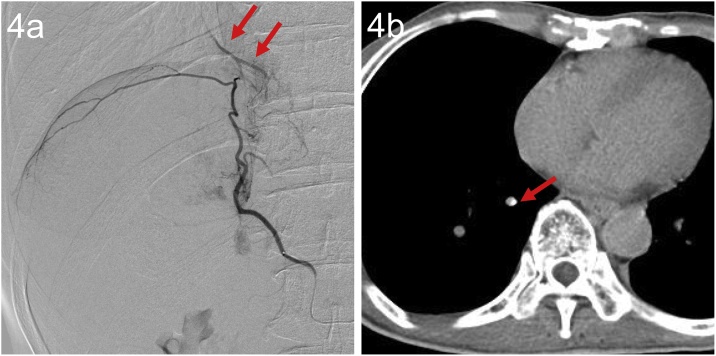
There was one procedure in which collateral circulation from the right IPA to the pulmonary vein was recognized on AG and AG-CT, but no Lipiodol deposition was seen on the post-procedure CT (Fig. 4).
Fig. 4.
A 62-year-old man.
(a) AG of the right IPA showed collaterals to the inferior branch of the right pulmonary vein (arrow).
(b) CT-AG showed enhancement along the inferior branch of the right pulmonary vein (arrow). Post-procedure CT and follow-up CT seven days after TACE showed no deposition of Lipiodol in the lung.
The pericardiacophrenic artery (a branch of the internal mammary artery) was depicted along the right margin of the heart in 19 (47.5%) procedures (13 patients, 52%) on AG and/or CT-AG, and four of these procedures showed Lipiodol deposition along the right margin of the heart on post-procedure CT (Fig. 3).
3.2. Complications
Complications were observed in 21 of 39 (53.8%; Table 1) procedures in which TACE or TAE was performed via the right IPA. No fatalities related with the procedure were observed.During or immediately after 18 of 39 (46.2%) procedures treated via the right IPA, patients complained of right shoulder pain. This symptom gradually improved within a few days with conservative analgesic treatment.
Table 1.
Complications observed after IPA TACE or TAE.
| Complications | Right IPA (n = 39) | Left IPA (n = 1) |
|---|---|---|
| shoulder pain | 18 (46.2%) | 0 |
| pleural effusion | 14 (35.9%) | 0 |
| basal atelectasis | 11 (28.2%) | 0 |
| hemoptysis | 1 (2.6%) | 0 |
| paroxysmal atrial fibrillation | 2 (5.1%) | 0 |
In 14 (35.9%) procedures, pleural effusion was seen on follow-up CT, and 11 of these procedures also showed basal atelectasis. However, only three procedures with pleural effusion showed Lipiodol deposition and collaterals from IPAs. In five of the eight procedures in which collateral circulation from the right IPA to the pulmonary artery was identified, pleural effusion was not observed on the follow-up CT. No subjective symptoms, such as dyspnea or desaturation, were observed, and no patients required supplemental oxygen.
One patient developed mild hemoptysis four days after TACE via the right IPA. No dyspnea or desaturation occurred. In this case, collateral circulation from the right IPA to the right pulmonary artery was seen on AG and AG-CT (Fig. 2). Post-procedure CT revealed Lipiodol deposition along the right IPA, and the unenhanced follow-up CT a week later showed increased deposition in the collapsed right lower lobe. Hemoptysis improved within a few weeks after treatment with a hemostatic agent.
One patient developed atrial fibrillation after each of two TACE procedures (Fig. 3). In the first TACE, AG of the IPA revealed collateral circulation toward the heart, and the post-procedure CT showed Lipiodol deposition in the mediastinum along the cardiac surface. This patient had no past history of arrhythmia, but asymptomatic atrial fibrillation was incidentally recognized a day after the procedure. Continuous oral administration of an antiarrhythmic drug was initiated. Atrial fibrillation occurred again two days after the second TACE via the right IPA, which was performed six months after the first one.
All of these complications were classified as CTCAE Grade 1 or 2.
4. Discussion
Shoulder pain was the most common complication, and required only conservative treatment. Pleural effusion and lung atelectasis were commonly seen on the follow-up CT, as described in previous studies [3,5,[9], [10], [11], [12]].
Severe pulmonary complications due to introduction of chemoembolic drugs into the pulmonary circulation have been reported previously [6,9,15], and arteriovenous shunts in the tumor and high volumes of Lipiodol administration have been suggested as risk factors of pulmonary embolization [15,17]. It is not surprising that chemoembolic drugs occasionally flow into the lungs through collateral circulation between the IPA and branches of the pulmonary artery, even without arteriovenous shunts in the tumor. In fact, in our study, collateral circulation from the right IPA to the pulmonary artery was identified on AG and/or CT-AG in eight procedures, and Lipiodol deposition in the pulmonary arteries or pleura was seen on post-procedure CT of eight procedures. It was noteworthy that Lipiodol identified in the pulmonary arteries on post-procedure CT spread into the pulmonary parenchyma on follow-up CT. However, only three of 14 procedures with pleural effusion showed collateral circulation during the procedures, and in five of eight procedures with collateral circulation from the right IPA to the pulmonary artery, there was no pleural effusion on follow-up CT. These findings suggest that there are invisible collaterals from the IPA to the pulmonary circulation, and that the detection of collaterals on CT or CT-AG does not always predict the development of pleural or pulmonary complications.
In the procedures we reviewed, the most severe complication that we could attribute with reasonably certainty to collaterals between the pulmonary arteries or pleura and the IPA was mild hemoptysis. Introducing chemoembolic drugs to the systemic circulation puts a patient at risk for severe complications such as cerebral Lipiodol embolization or ARDS [[9], [10], [11],13,14], but none of our patients experienced severe pulmonary or systemic complications. We suspect that the limited amount of Lipiodol administered into the IPAs is one possible reason.
The pericardiacophrenic artery, which is a branch of the right internal mammary artery, occasionally communicates with the right IPA [4,18]. In our study, the accumulation of Lipiodol along the pericardiacophrenic artery was depicted on post-procedure CT in four procedures. One patient, who had no history of arrhythmia, developed atrial fibrillation after TACE, which has not been previously reported as a complication of IPA intervention. However, the patient developed arrhythmia twice following TACE, suggesting the symptom may have been preexisting. Furthermore, since various factors are considered to cause arrhythmia, we cannot confirm any relationship between arrhythmia and TACE via the right IPA.
There were some limitations in our study. First, since we did not have a control group without TACE or TAE via the IPAs, we could not conclude that the complications observed in our study were directly related to TACE or TAE via the IPAs. Our study was retrospective, so there may be various unrecognized factors affecting our results. Second, the doses and types of infused drugs varied, and the accurate amount of Lipiodol and other drugs administered into the IPAs cannot be confirmed. We estimate the amount of Lipiodol was less than 2 ml for each procedure. Thirdly, the position of the tip of the micro-catheters during TACE or TAE in the IPA varied, and this may have affected the onset of complications (or lack thereof).
5. Conclusion
Collaterals between the IPA and the pulmonary circulation may be seen during interventional procedures to IPAs, and were occasionally associated with Lipiodol deposition in the lungs and pleura. However, there was no clear connection between these imaging findings and the occurrence of pleural effusion and atelectasis. Shoulder pain was the most frequent complication, and required only conservative treatment. TACE or TAE via the IPA in patients with HCC was found to be relatively safe if the amount of Lipiodol is limited.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The institutional ethics committee of our hospital approved our research protocol, and informed consent was obtained from all individual participants included in the study.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ikeda Kenji, Kumada Hiromitsu, Saitoh Satoshi, Arase Yasuji, Chayama Kazuaki. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150–2154. doi: 10.1002/1097-0142(19911115)68:10<2150::aid-cncr2820681011>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Camma Calogero, Schepis Filippo, Orlando Ambrogio, Albanese Maddalena, Shahied Lillian, Trevisani Franco, Andreone Pietro, Craxi Antonio, Cottone Mario. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 3.Miyayama Shiro, Matsui Osamu, Taki Keiichi, Minami Tetsuya, Ryu Yasuji, Ito Chiharu, Nakamura Koichi, Inoue Dai, Notsumata Kazuo, Toya Daisyu, Tanaka Nobuyoshi, Mitsui Takeshi. Extrahepatic blood supply to hepatocellular carcinoma: angiographic demonstration and transcatheter arterial chemoembolization. Cardiovasc. Interv. Radiol. 2006;29:39–48. doi: 10.1007/s00270-004-0287-y. [DOI] [PubMed] [Google Scholar]
- 4.Kim Hyo-Cheol, Wook Chung Jin, Lee Whal, Fae Hwan Fun, Park Fae Hyung. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. RadioGraphics. 2005;25:S25–S39. doi: 10.1148/rg.25si055508. [DOI] [PubMed] [Google Scholar]
- 5.Chung Jin Wook, Park Jae Hyung, Han Joon Koo, Choi Byung Ihn, Kim Tae Kyung, Han Man Chung. Transcatheter oily chemoembolization of the inferior phrenic artery in hepatocellular carcinoma: the safety and potential therapeutic role. J. Vasc. Interv. Radiol. 1998;9:495–500. doi: 10.1016/s1051-0443(98)70306-9. [DOI] [PubMed] [Google Scholar]
- 6.Il Gwon Dong, Ko Gi-Young, Yoon Hyun-Ki, Sung Kyu-Bo, Moung Lee Fae, Fong Ryu Seok, Hee Seo Myong, Shim Fae-Chan, Fai Lee Ghi, Kim Ho Kyun. Inferior phrenic artery: anatomy, variations, pathologic conditions, and interventional management. RadioGraphics. 2007;27:687–705. doi: 10.1148/rg.273065036. [DOI] [PubMed] [Google Scholar]
- 7.Nakai Motoki, Sato Morio, Kawai Nobuyuki, Minamiguchi Hiroki, Masuda Mitsunori, Tanihata Hirohiko, Takeuchi Taizo, Terada Masaki, Kishi Kazushi. Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology. 2001;219:147–152. doi: 10.1148/radiology.219.1.r01mr28147. [DOI] [PubMed] [Google Scholar]
- 8.Park Sung Il, Lee Do Yun, Won Jong Yoon, Lee Jong Tae. Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J. Vasc. Interv. Radiol. 2003;14:461–468. doi: 10.1097/01.rvi.0000064856.87207.1e. [DOI] [PubMed] [Google Scholar]
- 9.Tajima Tsuyoshi, Honda Hiroshi, Kuroiwa Toshirou, Yabuuchi Hidetake, Okafuji Takashi, Yoshimitsu Kengo, Irie Hiroyuki, Aibe Hitoshi, Masuda Kouji. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J. Vasc. Interv. Radiol. 2002;13:893–900. doi: 10.1016/s1051-0443(07)61772-2. [DOI] [PubMed] [Google Scholar]
- 10.Shin Sung Wook, Do Young Soo, Choo Sung Wook, Lieu Wei Chiang, Cho Sung Ki, Park Kwang Bo, Yoo Byung Chul, Kang Eun Hae, Choo In-Wook. Diaphragmatic weakness after transcatheter arterial chemoembolization of inferior phrenic artery for treatment of hepatocellular carcinoma. Radiology. 2006;241:581–588. doi: 10.1148/radiol.2412051209. [DOI] [PubMed] [Google Scholar]
- 11.Kim Hyo-Cheol, Chung Jin Wook, Kim Won Hwa, An Sangbu, Seong Nak Jong, Jae Hwan Jun, Park Jae Hyung. Chemoembolization of the left inferior phrenic artery in patients with hepatocellular carcinoma: 9-year single-center experience. Am. J. Roentgenol. 2010;194:1124–1130. doi: 10.2214/AJR.09.3030. [DOI] [PubMed] [Google Scholar]
- 12.Suh Sang Hyun, Won Jong Yun, Do Yun Lee, Lee Jong Tae, Lee Kwang-Hun. Chemoembolization of the left inferior phrenic artery in patients with hepatocellular carcinoma: radiographic findings and clinical outcome. J. Vasc. Interv. Radiol. 2005;16(12):1741–1745. doi: 10.1097/01.RVI.0000182172.00168.1B. [DOI] [PubMed] [Google Scholar]
- 13.Zhi Li, Ni Rui-fang, Reddy Busireddy Kiran Kumar, Yong-hai Jin, Xin Zhao, Li Ming-ming, Chao Yang. Cerebral lipiodol embolism following transcatheter arterial chemoembolization for hepatocellular carcinoma: a report of two cases and literature review. Chin. Med. J. 2011;124(24):4355–4358. [PubMed] [Google Scholar]
- 14.Jia Zhong-Zhi, Tian Feng, Jiang Guo-Min. Cerebral lipiodol embolism after transarterial chemoembolization for hepatic carcinoma: a case report. World J. Gastroenterol. 2012;18(30):4069–4070. doi: 10.3748/wjg.v18.i30.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto Ichiro, Aso Nobuya, Nagaoki Kenji, Matsuoka Yojiro, Uetani Masataka, Ashizawa Kazuto, Iwanaga Soji, Mori Masakazu, Morikawa Minoru, Fukuda Toshio, Hayashi Kuniaki, Matsunaga Naofumi. Complications associated with transcatheter arterial embolization for hepatic tumors. RadioGraphics. 1998;18:605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute . Bethesda; 2009. Common Terminology Criteria for Adverse Events v.4.0.https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (Accessed 21 Feburary 2018) [Google Scholar]
- 17.Chung J.W., Park J.H., Im J.G., Han J.K., Han M.C. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187(3):689–693. doi: 10.1148/radiology.187.3.8388567. [DOI] [PubMed] [Google Scholar]
- 18.Loukas Marios, Hullett Joel, Wagner Teresa. Clinical anatomy of the inferior phrenic artery. Clin. Anat. 2005;18:357–365. doi: 10.1002/ca.20112. [DOI] [PubMed] [Google Scholar]