Summary
Cell competition employs comparisons of fitness to selectively eliminate cells sensed as less healthy. In Drosophila, apoptotic elimination of the weaker ‘loser’ cells from growing wing discs is induced by a signaling module consisting of the Toll ligand Spätzle (Spz), several Toll related receptors and NFkB factors. How this module is activated and restricted to competing disc cells is unknown. Here, we use Myc-induced cell competition to demonstrate that loser cell elimination requires local, wing disc synthesis of Spz. We identify Spz Processing Enzyme (SPE) and Modular Serine Protease (ModSP) as activators of Spz-regulated competitive signaling, and show that ‘winner’ cells trigger elimination of nearby WT cells by boosting SPE production. Moreover, Spz requires both Toll and Toll-8 to induce apoptosis of wing disc cells. Thus, during cell competition, Spz-mediated signaling is strictly confined to the imaginal disc, allowing errors in tissue fitness to be corrected without compromising organismal physiology.
eTOC blurb
Low levels of the Toll ligand Spätzle and its activating proteases are synthesized continuously by wing disc cells, but signaling is unproductive. Alpar et al. show that Myc super-competitor cells boost production of the proteases, thereby triggering Spätzle activation and inducing a killing signal that selectively eliminates nearby wild-type cells.

Introduction
Genetic or phenotypic heterogeneities in growing tissues can lead to competitive interactions between cells that eliminate the weaker population and stimulate expansion of the stronger (Johnston, 2009). These interactions, known as cell competition, are thought to function in a homeostatic role to preserve cooperative cell behavior and rid tissues of cells that are potentially dangerous to the tissue and animal. Two canonical paradigms of cell competition occur in tissues mosaic for the function of a ribosomal protein (Rp) or the growth-regulating transcription factor, Myc. Although inherently viable, when in mosaics the mutant cells are perceived as less fit and selectively instructed to die by apoptosis (Johnston et al., 1999; Morata and Ripoll, 1975; Moreno et al., 2002). A variant of cell competition, called “super-competition”, leads to death of wild-type (WT) cells when they grow near cells that express extra Myc (de la Cova et al., 2004; Moreno and Basler, 2004). Cell competition is thought to be a surveillance mechanism that senses suboptimal cells that could interfere with normal development. By exploiting the same principles, super-competition could be used by emergent cancer cells to stabilize as a population and establish territory within healthy tissues.
We previously identified a cohort of genes that is required to eliminate the less fit, ‘loser’ cells in both Rp-induced cell competition and Myc-induced super-competition (Meyer et al., 2014). All of these genes encode proteins used in the Drosophila immune response and include several Toll related receptors (TRRs), the neurotrophin family member and Toll ligand Spz, and three NFkB transcription factors. Genetic experiments indicated that during cell competition these genes function together in novel signaling modules (here called ‘competitive signaling’), triggered by apparent differences in cell fitness (Meyer et al., 2014). In Myc-induced super-competition, the competitive signaling module consists of Spz, the receptors Toll-2, Toll-3, Toll-8 and Toll-9 and the NF-kB factor Relish (Rel) – the downstream effector of the IMD immune pathway. Cell competition induced between RpL14−/+ and WT cells relies on a related module, again consisting of Spz, Toll-3 and Toll-9, but mediated by the activity of the canonical Toll pathway effectors, Dorsal (dl) and Dorsal-related immunity factor (Dif), rather than Rel. In both competitive contexts, the final outcome of signal activation is death of the weaker cells via expression of pro-apoptotic factors. In Myc-induced super-competition, Rel activity in WT loser cells induces the pro-apoptotic factor Head Involution Defective (Hid), while in Rp-induced cell competition, apoptosis of RpL14−/+ loser cells is mediated by Reaper (Rpr) (de la Cova et al., 2004; de la Cova et al., 2014; Meyer et al., 2014).
How competitive signaling is activated is unknown. Spz is well-known as the Toll ligand in innate immunity and in embryonic dorsal-ventral patterning and is also required for cell and Myc super-competition, thus we explored its role in the signaling between competing cell populations. Spz, a secreted protein, is synthesized as an inactive pro-protein that must be activated to function as a ligand for Toll. This event is controlled by endoproteolysis to release the active Spz C-terminal domain (C-106) from its N-terminal pro-domain (Arnot et al., 2010; Schneider et al., 1994). Activation of Spz is controlled extracellularly by secreted serine proteases (SPs) (Buchon et al., 2014; Stein and Stevens, 2014), which organize into cascades often consisting of a modular SP and two clip-domain SPs (Kellenberger et al., 2011; Veillard et al., 2016). Each SP in the cascade is produced and secreted as a zymogen that relies on an active upstream SP for its activation (Dissing et al., 2001). In addition, SPs can be activated by a local increase in their effective concentration (Buchon et al., 2009; Cho et al., 2012; Cho et al., 2010).
Control of SP cascade activity and subsequent cleavage of Spz is the decisive event that determines where and when Toll signaling is activated (Chasan et al., 1992; Dissing et al., 2001; El Chamy et al., 2008; Jang et al., 2006; Lemaitre et al., 1996; Morisato, 2001). In the embryo, precise spatial regulation of Spz activation within the perivitelline space (PVS) ensures that Toll signaling is restricted to a ventral domain of cells (Chasan et al., 1992; Cho et al., 2012; Cho et al., 2010; Roth et al., 1989; Rushlow et al., 1989; Steward, 1989). Conversely, in larval and adult stages, pro-Spz and its upstream SP zymogens circulate within the openly circulating hemolymph, where they are activated upon encounters with infecting pathogens (Buchon et al., 2009; Irving et al., 2005; Mulinari et al., 2006; Shia et al., 2009; Yamamoto-Hino et al., 2015). Activation of the SP cascade creates the active Spz ligand, which then activates Toll signaling in broadly distributed immune tissues (Shia et al., 2009).
Although several molecules used in the immune response for host defense (against pathogens) have roles in cell competition (against potentially threatening self-cells), the modules used in competitive signaling carry out non-immune-related functions and thus different outcomes. Whereas the immune response results in production of antimicrobial peptides (AMPs) to attack pathogens at a systemic level, cell competition is a distinctly local process that targets a specific group of non-immune, proliferating cells for apoptosis. As pro-Spz is constitutively present in the hemolymph, an attractive idea is that it functions as a circulating sensor of growth or cell fitness. However, widespread activation of Spz can damage the animal by triggering an inflammatory response (Parisi et al., 2014). Arguably, to benefit the organism, cell competition must be shielded from immune response activation, to eliminate suboptimal cells in specific tissues without disrupting the physiology of the whole animal.
We sought to determine the mechanism by which Spz activation occurs during competition, using Myc super-competition as a model. Contrary to the idea that circulating pro-Spz functions in cell competition, we demonstrate that its production by wing disc cells is specifically required. We identify two Spz-activating SPs that are required for cell competition. We show that expression of these SPs is elevated in Myc super-competitor cells, and that this increase is required to eliminate WT loser cells from the tissue. Finally, we demonstrate that Spz-mediated cell death in wing discs requires both Toll and Toll-8. Our results thus provide a mechanism for precise regulation of signal activation in cell competition that leads to Toll and Toll-8 dependent elimination of less fit cells.
Results
Spz and its receptor, Toll are required for cell competition in wing imaginal discs
Previous work showed that Toll-RNAi was unable to suppress elimination of loser cells, although subsequent experiments revealed that the knockdown was incomplete (Fig. S1A) (Meyer et al., 2014). We took advantage of a temperature sensitive, heteroallelic combination of Tl mutants (Tlr3/Tlr4) to circumvent their late embryonic lethality (Anderson et al., 1985) and readdress the role of Toll in cell competition. We used the tub>myc>Gal4 cell competition assay (de la Cova et al., 2004), wherein cell clones are generated via stochastic Flp/FRT-mediated excision of a >myc> cassette (where > denotes FRT) that expresses Myc at less than 2-fold over endogenous levels (Wu and Johnston, 2010). Cassette excision allows clonal expression of Gal4 and thus UAS-GFP to mark the clones (de la Cova et al., 2004). Since the GFP-positive clones are WT with respect to myc but surrounded by cells retaining the >myc> cassette (and thus GFP-negative), they are subject to cell competition and eliminated (de la Cova et al., 2004) (Fig. 1A–C). The acquisition of ‘loser’ status of cells in the clones is manifested by their Hid-dependent apoptosis, which reduces clone size relative to controls (de la Cova et al., 2004; de la Cova et al., 2014) (Fig. 1B–C, E–F). Cell competition is most pronounced in clones located within the wing pouch (WP) region of the disc (Fig. S1F–G).
Figure 1: Spz and Toll are required for cell competition in wing imaginal discs.
(A) Schematic of clonal assay for Myc-induced cell competition. Clones generated by ‘Flp-out’ of an FRT (>) flanked cassette in tub>CD2>Gal4 (left) or tub>myc>Gal4 (right), marked by Gal4 controlled green fluorescent protein (GFP) expression. Neutral, GFP+ clones generated in a WT background (left) are controls for clone growth in a non-competitive context. GFP+ clones of WT cells surrounded by tub>myc>Gal4 cells become ‘losers’. (B-D) Wing discs with non-competing control clones marked by nuclear (n) GFP (B), UAS-nGFP+ loser clones (C), and UAS-CD8-GFP+ loser clones in the null spzKG05402 background (D). (E-F) Results of competition assays in (E) Tl and (F) spz null larvae. Tukey plot of (median and quartile) size of control clones in WT and mutant larvae (white), loser clones in WT larvae (light grey,) and loser clones in Tl or spz null larvae (dark grey). Clone number scored/genotype are in box plots. *** P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction). (G) Results of qPCR showing spz expression in wing discs (WD), central nervous system (CNS), fat body (FB), salivary glands (SG), gut (G) and hemocytes (HC) of 3rd instar larvae. Error bars, SD. (See also Fig. S1).
In control clones, loss of Toll had no effect, indicating that the Tl null background does not affect normal growth or cell survival. However, loser cells were no longer eliminated in the Tl null background, indicating cell competition is prevented by loss of Tl (Fig. 1E). Similarly, cell competition was prevented in larvae homozygous for a null allele of spz (spzrm7 or spzKG05402), encoding the ligand for Toll (Meyer et al., 2014) (Fig. 1D, F). These data indicate that both Toll and Spz are required in cell competition to eliminate the loser cells, consistent with their well-established receptor-ligand relationship.
How is Spz regulated during cell competition? Several larval tissues express spz mRNA, including the fat body (FB), salivary gland (SG) and hemocytes (HC), raising the possibility that the remote production of Spz by these tissues leads to its use in cell competition (Irving et al., 2005; Meyer et al., 2014; Shia et al., 2009) (Fig. 1G). However, spz mRNA is also expressed at low levels in the wing disc, where cell competition takes place, prompting us to look closer at expression of Spz in situ (Meyer et al., 2014) (Fig. 1G). We constructed a bacterial artificial chromosome (BAC) containing C-terminally tagged Spz in its endogenous locus (Spz-mCherry) and generated transgenic flies. SpzmCherry partially rescues the sterility phenotype of spz null females, indicating that it can functionally replace wild type Spz (Fig. S2A). Spz-mCherry is present in all of the larval tissues where we detected spz mRNA by qRT-PCR, at comparable relative levels (Fig. 2A and Fig. S2 C–E). In immature wing discs (72 hr after egg laying, AEL) SpzmCherry is present at low levels and can be detected intracellularly and near the apical cell surface (Fig. 2B). In mature wing discs (96 hr AEL), Spz-mCherry accumulates at higher levels in the disc lumen (Fig 2A) and also within cells, where it is enriched apically at wing disc cell membranes (Fig. 2A”). A similar expression pattern is seen with antibodies against Spz (Fig. S2F).
Figure 2: Local expression of spz is required to eliminate loser cells in wing discs.
(A-B) Anti-mCherry staining of Spz-mCherry expression in wing discs from (A) 96 hr or (B) 72hr larvae. A and A’ show apical and medial sections, respectively, from the same disc. F-actin labeling marks the apical cell surface. (A” and B’) Z-sections of the wing discs (positions shown by red dashed lines on xy-plane images). Images are sum projections of multiple sections. Scale bars, 50 m. (C) Schematic of tub>myc>lexA/lexO competition assay with tissue-specific spz-RNAi knock-down. (D-E) Tukey box plots show clone size distribution in controls with no knockdown, or in larvae with UAS-spz-RNAi expression induced with (D) Hml-Gal4, R4-Gal4, and (E) C10-Gal4 or C765-Gal4. Unshaded boxes, non-competing control clones (C); shaded boxes, loser (L) clones. *** P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction). The numbers in boxes are number of clones scored per genotype. (See also Figs. S2 and S3).
Expression of Spz by wing disc cells is essential for cell competition
The presence of spz mRNA and protein within wing disc cells intrigued us because Spz has no known function in this or other imaginal tissues. In principle, the lumenal presence of Spz in mature wing discs could be due to its uptake from the circulating hemolymph (Irving et al., 2005; Levashina et al., 1999; Shia et al., 2009) (Yamamoto-Hino et al., 2015). Or, loser cell elimination during cell competition could require local, wing disc synthesis of Spz. To distinguish between these mechanisms, we designed assays to determine whether the specific synthesis of Spz by the FB, HCs or wing disc (WD) cells was required to mediate competition. We constructed LexA-based versions of the tub>myc> and tub>CD2> gene cassettes that deploy the lexA-lexO expression system instead of Gal4/UAS to generate and mark cell clones (Fig. 2C). These cassettes allowed us to measure cell competition and at the same time, selectively disrupt spz in the FB, HC, or WD cells with UAS-spz-RNAi and tissue-specific Gal4 drivers. The tissue specificity of each driver was confirmed using the G-TRACE lineage tracing system (Evans et al., 2009) (Fig. S3). We found that knock down of spz in either HC or FB had no effect on elimination of loser cells in the WDs, indicating that these tissues contribute little Spz, if any, for cell competition (Fig. 2D). Strikingly, however, cell competition was robustly suppressed by the specific expression of spz-RNAi in WDs (Fig. 2E). Thus, the expression of Spz in WDs is sufficient to mediate competitive signaling, without input of systemic Spz made remotely by other tissues.
Activation of Spz within the wing disc induces local cell death
Cell competition requires fairly close proximity between the competing cell populations (de la Cova et al., 2004; Morata and Ripoll, 1975; Simpson and Morata, 1981). The finding that elimination of loser cells during competition requires that WD cells synthesize Spz suggests that the activated, ligand form of Spz functions as a local signal to induce cell killing. To determine if this was the case, we asked whether increasing expression of Spz in WD cells was sufficient to compromise their survival. We used the nubbin-Gal4 (nubGal4) driver to express pro-Spz (UAS-SpzFL), the unprocessed and inactive form of the protein, or the active form of Spz, C-106 (UASSpzAct) (Ligoxygakis et al., 2002b) specifically in the WP region of the disc and examined cell death by staining for the activated form of the Dcp1 caspase. Expression of UASSpzFL did not lead to accumulation of the cleaved, active C106 (Fig. 3A, lane 2), and had no effect on wing disc cell survival (Fig. 3B), indicating that expression of full-length Spz in WDs is not sufficient for its activation.
Figure 3: Activation of Spz in the wing disc induces Toll-mediated cell death (A-B).
Expression in WDs of pro-Spz leads to negligible Spz processing to the active C106 form (A) and does not induce cell death (n=17, 26 in order on graphs) (B). (A) Western blot of extracts from disc cells with nubGal4 alone (lane 1), or nubGal4 + UAS-SpzFL-HA (lane 2), + UAS-SPEAct and UAS-SpzFL-HA (lane 3), or + UAS-ModSPFL and UAS-SpzFLHA (lane 4), probed with anti-HA antibodies. SpzFL is processed to SpzC106 only when expressed with SPEAct or ModSPFL. (B) Tukey plots of cell death in WDs expressing nubGal4/UAS-SpzFL and in nubGal4/UAS-GFP controls. (C) Cell death in WDs of nubGal4/UAS-SpzAct, nubGal4/UAS-GFP, and nubGal4/UAS-SpzAct; Tlr3/Tlr26 larvae (n=14, 19, 20); n= number WD scored/genotype. ***P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction). (D-I) SpzAct (Spz C106) induces expression of the pro-apoptotic factors Rpr and Hid in WDs. (D-D’) Control WD expressing ptcGal4, UAS-GFP, rpr-lacZ. rpr-lacZ is expressed in a stereotypical posterior lateral pattern in all discs (Wells and Johnston, 2011) (D’) but is induced by UAS-SpzAct in some cells (E-E’). (F) Control nubGal4; UAS-GFP/ hid-lacZ.(G) SpzAct induces hid-lacZ expression in some WD cells. Images are sum projections of multiple sections. Scale bars, 50 m. (See also Fig. S4).
In striking contrast, expression of UAS-SpzAct led to a highly significant, 7-fold increase in apoptosis in WP cells over nub-Gal4 controls (Fig. 3C and Fig. S4B). This was accompanied by activation of transcriptional reporters for both hid and reaper (rpr) (Fig. 3D–G), the pro-apoptotic factors responsible for inducing apoptosis in loser cells in Myc- and Minute induced competition, respectively, consistent with the requirement for Spz in both genetic contexts of cell competition (de la Cova et al., 2004; de la Cova et al., 2014; Meyer et al., 2014). In contrast, immune response targets such as the Drosomycin anti-microbial peptide (Fehlbaum et al., 1994; Manfruelli et al., 1999) were not activated in WDs or in other tissues (Fig. S4 E–H), confirming our previous results that activation of NFkB signaling in wing discs does not induce immune targets, either cell autonomously or non- autonomously (Meyer et al., 2014). To determine whether the activity of SpzAct in WP cells was mediated by Toll signaling, we carried out epistasis experiments between SpzAct and Tl and confirmed that Toll is required for SpzAct to induce death of WP cells. In the Tlr3/Tlr26 background, cell death induced by nub-Gal4, SpzAct expression was substantially suppressed (Fig. 3C). Thus, WD-specific expression of SpzAct induces a Toll-mediated response - independent of their role in immune signaling - that activates Hid and Rpr, the known apoptotic effectors of cell competition, and leads to wing disc cell death. Altogether, these results provide compelling evidence that once activated, Spz provides a local killing signal that is mediated by Toll during cell competition.
The serine proteases ModSP and SPE are required for cell competition
Our experiments indicate that the expression and function of Spz in wing disc cells is necessary to kill loser cells during competition, and that Spz activity is sufficient to induce apoptosis in wing disc cells. However, only expression of the processed, active version of Spz, SpzAct was able to provoke this response, consistent with the accepted view that Toll binds only to the processed form of Spz, C106 (Gangloff et al., 2008; Morisato and Anderson, 1994; Weber et al., 2003). The results imply that the activity of the proteases that process pro-Spz into the C106 ligand form is normally limiting in wing discs, allowing Spz activation to be under tight control in this tissue. This is consistent with the fact that the lumen within the folded epithelium of wing imaginal disc is an enclosed space where the concentration of signaling molecules can be controlled locally for precise spatial regulation, similar to the PVS where Spz activation is restricted to ventral cells during embryonic DV patterning (Cho et al., 2010).
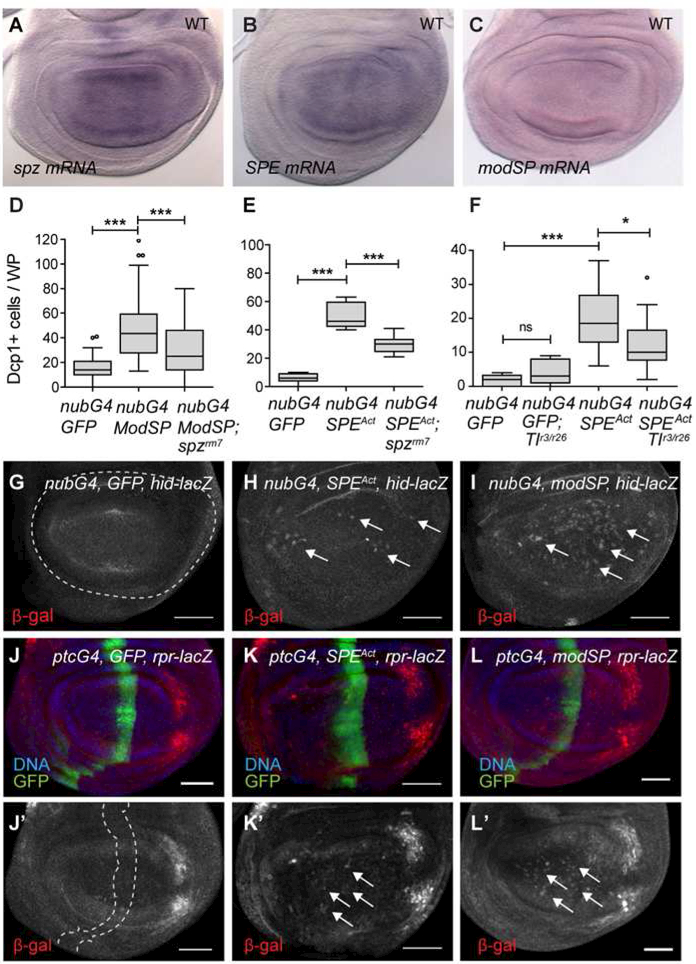
How is Spz cleaved and activated for signaling in cell competition? In embryonic patterning and the immune response, processing of Spz is controlled by the activity of two distinct SP cascades (Veillard et al., 2016) (Fig. 4A). We carried out quantitative RTPCR assays on RNA isolated from WDs and detected mRNA expression for five of these SPs, easter (ea), SPE, grass, spirit and modSP (Fig. 4B). We screened all SPs with known Spz-regulatory roles for a requirement in the elimination of loser cells (with the exception of spirit, for which no mutant alleles are available) to determine their function during cell competition. Cells in loser clones were successfully eliminated in the background of mutations in ea (ea1/ea4), snk (snk1/snk4), gd (gd7), or ndl (ndlrm5), indicating that none of the embryonic SPs are required during cell competition (Fig. 4CF). In addition, none of the mutants altered clone or larval growth in non-competitive controls (Fig. S1E). Likewise, loser cells were still eliminated from WDs in larvae carrying either psh1or grassHer null alleles (Fig. 4G, H). However, consistent with their WD expression (Fig. 4B), cell competition was suppressed by loss of either modSP (modSP1), the apical immune SP, or SPE (SPESK6), the SP with direct Spz-cleaving activity (Fig. 4H, I). Although significant, the effect of modSP loss was milder than loss of SPE (Fig. 4H). As Psh and ModSP can independently activate SPE we considered there might be redundancy between them (Ligoxygakis et al., 2002b), but this was not the case, because psh1; modSP1 double mutants behaved like modSP1 alone (Fig. 4H). Our results thus demonstrate that activation of Spz during Myc-induced cell competition is mediated by the activities of ModSP and SPE.
Figure 4: The serine proteases SPE and ModSP are required for cell competition.
(A) Schematic diagram of SP cascades that control Spz activity in the immune response and embryonic patterning. Above, pathogen recognition by innate immune cells activates ModSP, triggers cascade activation that culminates in Spz cleavage by SPE. Below, in the embryonic SP cascade, GD is the initial SP and Easter the terminal SP in Spz processing. (B) qRT-PCR results for SP expression in WDs. Data from 3 independent experiments. Error bars, SEM. (C-I) Loser clone size is not increased in mutants of (C) ndl (ndlrm5) (n=51, 28, 12), (D) gd (gd7) (n=97, 14, 9), (E) snk (snk1/4) (n=42, 21, 15), (F) ea (ea1/4) (n=116, 44, 18), (G) grass (grassHer) (n=140, 58, 24) or (H) psh (psh1) (n=36, 35, 73, 20, 87, 35). In contrast, null modSP1 (H) and SPESK6 (I) null mutants increased loser clone size in tub>myc assays (n=54, 83, 23, 25). Tukey plots of clone size distributions from ≥ three independent experiments. Non-competing controls (white), losers (shaded). *** P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction). (See also Fig. S1)
Expression of SPE or ModSP is sufficient to activate Spz in the wing disc
Since Spz must be in its active form to induce death of WD cells, we wondered if Spz activation is controlled locally within the WD during cell competition. Both SPE and modSP are expressed in WD cells. Interestingly, mRNA expression of SPE, and also of spz, is highest within the WP region of the disc (Fig. 5A, B and Fig. S5), where cell competition is most robust (Fig. S1F–G). modSP expression is weaker and more diffuse in the disc (Fig. 5C and Fig. S5). These SPs are produced as zymogens and require endoproteolytic activation (Fig. 4A) but can be also activated by increasing their effective concentration (Buchon et al., 2009; Cho et al., 2012; Cho et al., 2010). We stimulated the activity of these SPs in the WD in two ways: by expressing full-length, unprocessed ModSP, or expressing the processed and activated form of SPE (UASSPEAct) (Jang et al., 2006). Expression of UAS-ModSPFL with nub-Gal4 induced cell death in the WP nearly 3-fold over controls, Fig. 5D), and expression of UAS-SPEAct resulted in a greater than 8-fold increase in cell death over controls (Fig. 5E, F). The cell death induced by either SP was accompanied by expression of rpr-lacZ and hid-lacZ in many cells (Fig. 5G-L). To verify that this cell death was due to Spz activation, we monitored the cleavage of HA-tagged UAS-SpzFL in discs that also expressed UASModSPFL or UAS-SPEAct in the WP (Fig. 3A). Co-expression of SpzFL and either SPEAct or ModSPFL resulted in production of the cleaved form of Spz, C106 (Fig. 3A). Moreover, WD cell death induced by UAS-ModSPFL or UAS-SPEAct was significantly suppressed in spz null mutant larvae, indicating that the cells’ response to SP expression required Spz (Fig. 5D, E). Likewise, in a Tl null mutant background, cell death induced by SPEAct expression was reduced by 50% (Fig. 5F). Together, these results demonstrate that expression of ModSP or activated SPE in the WD is sufficient to cleave and activate Spz and induce Toll-dependent signaling that kills WD cells.
Figure 5: Spz is activated by expression of SPE or ModSP in the wing disc.
(A) RNA in situ hybridization (ISH) shows that spz, (B) SPE and (C) modSP mRNA are expressed in WT discs. (D-F) Spz-dependent cell death is induced by ModSPFL or SPEAct. Tukey plots of WP cell death in WD with (D) nubGal4/UAS-ModSP vs. nubGal4/UAS-ModSPFL; spzrm7 (n=33, 56, 40), (E) nubGal4/UAS-SPEAct vs. nubGal4/UAS-SPEAct; spzrm7 (n=10, 5, 11) or (F) nubGal4/UAS-SPEAct vs. nubGal4/ UAS-SPEAct; Tlr3/Tlr26 (n=6, 5, 24, 22). ***P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction). (G-L) hid and rpr are induced by SP activity in the WP. (G-I) hid-lacZ reporter activity in (G) control, (H) nubGal4/UAS-SPEAct or (I) nubGal4/UASModSPFL WDs. Arrows point to cells with hid-lacZ induction. (J-L) rpr-lacZ is induced (arrows) by SPEAct (K) or ModSPFL (L) expression. Images are sum projections of multiple sections. Dashed lines mark nubGal4 or ptcGal4 expression domains. Scale bars, 50 m. (See also Figs. S4 and S5).
Expression of Spz, SPE and ModSP is enhanced in Myc expressing cells
The ability of WP-specific expression of ModSP to induce cleavage of Spz and lead to Spz/Toll-dependent apoptosis of those cells confirmed that its activation in WDs can be achieved by increasing its effective concentration. Indeed, expression of UASModSPFL in the WP was sufficient to trigger its processing into its cleaved active form (Fig. S6A) (Buchon et al, 2009). These findings raised the possibility that a local increase in SP expression during cell competition could be a mechanism for triggering Spz activation. We tested this idea by examining the expression of these SPs in wing discs in which cell competition was induced. We generated Flp-out clones of cells expressing Myc in WDs, which leads to competition-induced death of nearby WT cells (de la Cova et al., 2004). Strikingly, expression of both modSP and SPE mRNA was significantly elevated within the Myc-expressing clones, compared to surrounding cells (Fig. 6A, B and Fig. S5 H). To track the endogenous SPE protein expressed from its locus, we constructed and introduced a BAC carrying C-terminally tagged SPE (SPEYFP) into the genome. When expressed in SPE null mutant animals, the SPE-YFP BAC restored Drs induction in response to bacterial infection (Fig. S7A). SPE-YFP is expressed throughout the WD, and in tissue cross-sections appeared apically enriched in the cells (Fig. 6D, D’). Like SPE mRNA, SPE-YFP was strongly and cell autonomously elevated in Myc-expressing WD cells (Fig. 6E). Moreover, spz mRNA (Fig. 6C and Fig. S5G) and Spz protein (Fig. 6F) are also elevated in Myc-expressing cells. To examine the basis for the enhanced expression we carried out a series of controls. Dp110, the catalytic subunit of the Drosophila PI3K, does not induce cell competition (de la Cova et al., 2004; Senoo-Matsuda and Johnston, 2007), but like Myc, increases cellular biosynthesis and thus cell size. However, Dp110 expression did not induce spz, SPE, or modSP mRNA (Fig. S5I–K). To test whether competitive interactions were required for Myc to enhance expression of these genes, we expressed Myc homogeneously with the ubiquitous tub-Gal4 driver, blocking its super-competitor function (de la Cova et al., 2004; Simpson, 1979). Expression of spz, SPE and modSP was still elevated in WDs with homogenous Myc overexpression, implying that competitive interactions are not required for their regulation (Fig. S6C). Intriguingly, while expression of Myc in wing discs elevated SPE-YFP levels, in FB cells Myc expression reduced SPE-YFP, suggesting SPE is under tissue-specific regulation (Fig. S7B–C). Thus, the enhanced expression of Spz and two of its regulatory SPs appears to be regulated by a Myc-regulated transcriptional program that is intrinsic to wing discs.
Figure 6: Elevated SPE in Myc winner cells promotes the elimination of loser cells.
(A-C) ISH to (A) SPE, (B) modSP and (C) spz mRNA in WDs with Myc-expressing Flp out clones. (D) SPE-YFP protein expression in WT and (E) ptcGal4/UAS-Myc WDs. (F) anti-Spz staining of WDs with Myc-expressing Flp-out clones. Dashed lines outline clones. Images are sum projections of multiple sections. Scale bars, 50mm. (G) Schema of MARCM cell competition assay. FRT-mediated mitotic recombination in hsFlp, tubGal4, UAS-GFP; UAS-Myc; FRT82B tubGal80 hsCD2/FRT82B larvae yields sibling clones with no Gal80 allowing UAS-Myc and UAS-GFP expression (green), or with Gal80 and CD2 (blue) (middle panel). Clones expressing UAS-Myc become winners (W), while their siblings are losers (L). FRT82B spzrm7 or FRT82B SPESK6 chromosomes were used to remove spz or SPE function only in W clones (bottom). Non-competing (NC) clones (top) are induced and marked similarly but lack UAS-Myc. (H-I) Clone size distribution scatter plots of pairs of sibling clones as indicated. Mean and SD are shown. P-values: paired t-test for comparison of sibling clone pairs; unpaired t-test with Welch’s correction for all others. *** P<0.0005, ** P<0.005, * P<0.05. (See also Figs. S5–S7).
A local increase in SPE in Myc winner cells is critical for loser cell elimination
If the enhanced expression of Spz and its activating SPs in Myc winner cells is important for triggering activation of local Spz-mediated signaling in cell competition, then removal of Spz or either SP specifically from the Myc winner cell population would be predicted to prevent loser cell elimination. To test this idea, we used a MARCM-based cell competition assay that allows us to mark and follow winner and loser cell clones in tandem. In this assay, FRT-mediated mitotic recombination generates two daughter cells whose progeny form sibling clones. One clone expresses Myc under Gal4 control and its cells become winners, while its sibling clone carries the Gal4 inhibitor Gal80 and remains WT for Myc expression, thus its cells behave as losers (Fig. 6G). Neutral (non-competing) control sibling clones grow at the same rate and thus are similar in size after a defined growth period. Expression of Myc induces cell-autonomous growth and thus results in larger clone sizes compared to WT control clones. Importantly, the WT siblings of Myc-expressing clones are reduced in size relative to WT controls due to cell loss induced by cell competition (compare neutral and competing clone sizes in Fig. 6H, I) (de la Cova et al, 2004).
To determine if elevated expression of Spz or SPE in Myc clones was important for activation of competitive signaling, we generated FRT spzrm7 and FRT SPESK6 chromosomes to independently eliminate each gene’s function specifically from the GFP-expressing clones and measured the impact of this loss on sibling clone size (Fig. 6H, I). The size of neutral control clones was not altered by loss of spz or SPE. Likewise, the growth capacity of Myc-expressing cell clones that lacked spz or SPE was not impaired and they remained larger than controls. Loss of spz specifically from Myc winner clones did not significantly change the size of their WT sibling loser clones, possibly because of the abundance of Spz-expressing cells in their vicinity (Fig. 6H). However, the size difference between pairs of spz mutant Myc winner clones and sibling WT loser clones was less pronounced than control winner-loser sibling pairs, implying that Spz up-regulation in Myc winner clones may contribute to signaling activity.
However, loss of SPE specifically from Myc winner clones completely suppressed competitive signaling, so that their sibling WT loser clones were the same size as non-competing controls (Fig. 6I). This is in striking contrast to RNAi-mediated knockdown of either SPE or spz solely in the loser cells, which did not prevent their competitive elimination (Fig. S5L, M). Hence, despite widespread expression of SPE throughout the WP, its specific expression in Myc winner cells is required to kill loser cells. The up-regulation of SPs in the Myc expressing population and the specific requirement for SPE in those cells for competitive signaling implies that the increased level of SPs in those cells contributes to spatially restricted activation of Spz during cell competition.
The local response to Spz signaling in wing discs requires Toll and Toll-8
Altogether, five TRRs - Toll, Toll-2, Toll-3, Toll-8 and Toll-9 - are required, in non-redundant fashion, to eliminate WT loser cells subject to Myc super-competition (Meyer et al., 2014) (Fig. 1C). Our experiments reveal that Toll itself is required for much of Spz activity in WDs, but it remained possible that Spz, alone or with Toll, also interacts with the other TRRs in cell competition. To determine whether these TRRs play a role in controlling the response to active Spz, we carried out a series of epistasis tests.
We used validated RNAi transgenes (Fig. S1D–E) against Toll-2, Toll-3, Toll-8 and Toll-9 to individually inhibit their function in nub-Gal4, SPEAct-expressing WP cells, and tested whether the SPE-induced cell death was suppressed. Expression of RNAi targeting Toll-2, Toll-3 or Toll-9 did not alter the extent of death induced by SPEAct (Fig. 7A, B, D). However, Toll-8-RNAi significantly decreased death induced by SPEAct (Fig. 7C). A similar suppression of cell death occurred upon co-expression of Toll-8-RNAi with SpzAct (Fig. 7E). Hence, cell death induced by expression of activated Spz, or by Spz activation via SPE activity, is at least partially Toll-8-dependent, suggesting that Toll-8 can respond to the active, ligand form of Spz. Since loss of Tl also prevents death due to both SpzAct and SPEAct (Fig. 3C, Fig. 5F), our results place both Toll and Toll-8 downstream of the Spz ligand, raising the intriguing possibility that these two receptors interact to mediate Spz-dependent signaling during cell competition.
Figure 7: Spz-mediated competitive signaling in wing discs requires Toll-8.
(A-D) Quantification of cell death (Tukey plots) in nubGal4/UAS-SPEAct discs with (A) Toll2/18w-RNAi (n=35, 31, 64, 31), (B) Toll3/MstProx-RNAi (n=43, 35, 54, 21), (C) Toll8/Tollo-RNAi (n=44, 43, 16, 26), or (D) Toll-9-RNAi (n=45, 46, 19, 20). (E) Cell death in nubGal4/UAS-SpzAct discs co-expressing UAS-Toll8/Tollo-RNAi (n=14, 12, 5). (F) Model of local Spz-mediated competitive signaling in WDs. See text for details. ***P<0.0005, ** P<0.005, * P<0.05 (unpaired t-test with Welch’s correction).
Discussion
Our results provide a mechanistic explanation for how Spz-mediated signaling between Myc super-competitor and WT cells is initiated in wing discs. We show that Spz must be produced locally in wing disc cells for signaling to occur, and identify two proteases, SPE and ModSP, as upstream mediators of cell competition. Both SPs and also Spz itself are expressed at low levels in WT wing discs, but are specifically upregulated in Myc winner cells. The local SPE increase in winner cells is necessary to kill WT cells in Myc-induced cell competition, implying that signal activation is driven by a Myc-regulated boost of protease expression. Finally, our results show that the death-inducing Spz signal in loser cells requires both Toll and Toll-8. Based on our findings, we propose a model of signal initiation in Myc super-competition. Principles of this model could apply to competitive signaling induced by other genetic contexts.
Local control of signal activation
Wing discs are semi-enclosed sacs of epithelial cells that reside in the larva, bathed by constantly recirculating hemolymph in which Spz and its activating SPs are present. We were thus surprised that for Myc-induced cell competition to occur, this systemic Spz is not required or sufficient; rather, Spz must be synthesized locally within the wing disc. Interestingly, spz, modSP and SPE are all endogenously expressed in WDs, but no signaling activity is detected under normal conditions. However, the expression of each is enhanced in Myc-expressing cells, and the specific induction of SPE in the winners is critical for the competitive outcome: loss of SPE from winners completely blocked elimination of loser cells. Mechanistically, the elevated SP expression likely increases their effective concentration and triggers activity by nucleating auto- and/or extra-catalytic interactions (Buchon et al.; Cho et al., 2012; Cho et al., 2010; Dissing et al.; El Chamy et al., 2008; Han et al., 2000; Ligoxygakis et al., 2002a). Our results thus suggest that locally elevated SPE is a key trigger of signal activation during Myc super-competition. Supporting this, SPE processing is detected upon expression of a stabilized form of SPEFL in the WP (SPEFL-SA, Fig. S6B) (Jang et al., 2006). However, as overexpressed SPEFL remains predominantly in its inactive form, upregulation of SPE alone may not be sufficient. Since both SPE and ModSP are elevated in Myc-expressing cells, the collective increase could trigger local Spz activation and lead to competitive signaling. Other factors may also participate, for instance unidentified SPs - made by either winner cells or loser cells, or byproducts of metabolic changes that occur during cell competition, which could influence signaling between winner and loser cell populations (de la Cova et al., 2014).
Spz is also required for cell competition between WT and Minute/+ cells (Meyer et al., 2014), thus an important area for future study is whether the same SPs activate Spz in Minute and other contexts of cell competition, and if competitive signaling in such contexts is also regulated by differential expression of these SPs. This possibility is hinted at by the observations that SPE expression is reduced in RpL14+/− WD cells (Fig. S6D) and in RpS3+/− and mahj−/− WD cells (Kucinski et al., 2017) relative to WT disc cells.
Myc super-competitor cells are insensitive to Spz killing activity
We find that Myc winner cells drive Spz activation by providing sufficiently high levels of SPs, yet these cells survive to populate the tissue, implying they are unresponsive to the death-inducing signal. Furthermore, Myc winner cells have a critical role in expressing the activating factors that allow competitive signaling and cell death of WT loser cells. Conversely, the genes controlling the response to competitive signaling – encoding the TRRs and transducing factors Relish and DREDD - are specifically required in the loser cells (Meyer et al., 2014). This implies that Myc winner cells produce a killing signal that specifically targets loser cells. How is signaling restricted to the losers? One answer may be that expression of several TRRs required for cell competition is significantly less in Myc-expressing cells than in WT cells (Meyer et al., 2014). Similarly, expression from a Toll-8 transcriptional reporter in wing discs is reduced in Myc expressing clones (Fig. S6E). Moreover, RNAi-mediated reduction of Toll-8 protects cells from SpzAct – induced death (Fig. 7E), suggesting that the immediate response to active Spz can be controlled by differential expression and/or regulation of TRRs. Spz and Toll-8 are not known binding partners, but since both Toll and Toll-8 are required downstream of Spz in cell competition, perhaps Toll mediates interactions between Spz and Toll-8. Pairings between different TRRs could also define alternative responses. Overall, our results imply that Myc cells gain a competitive advantage by inducing activation of a killing signal while downregulating the receptors required to respond to it (Fig. 7F). As disc cell induction of hid and rpr reporter activity and apoptosis in response to activated Spz, SPE or ModSP appears stochastic, a related implication is that each cell’s response is individually regulated. What determines the response of cells to Spz signaling is unclear, but it could reflect inherent differences in cellular fitness.
How is cell fitness sensed? Our results demonstrate that disc cells make low levels of Spz and the SPs needed for its activation. We speculate that the continued presence of components of this potentially dangerous signal (Spz, SPE, ModSP) is instructive, allowing each cell to survey its neighbors’ fitness. Healthy disc cells will tolerate the signal and continue to proliferate, but cells that are “less fit” will not, allowing signaler cells to outcompete them (Fig. 7F). Implicit in this model is that no cell can ‘cheat’ by over-producing the signal unless they are fit enough to tolerate it themselves (e.g., Myc super-competitors). Although based on Myc super-competition, this model might also apply to the Minute competitive context, where Rp/+ cells, making less of signaling components than WT cells, would be more sensitive to the signal and thus outcompeted.
Geographical and functional separation of Spz-mediated signaling
A striking outcome of this study is that Spz activity in wing discs is independent of, and isolated from, the systemic immune response. Under conditions where Spz activity is induced in discs, we found no evidence of immune activation in discs or in any larval tissues (Fig. S4E–L). Moreover, in the absence of spz expression in discs, the high levels of Spz produced by the FB and HCs are insufficient to regulate cell competition. Interestingly, SPE expression is regulated by Myc differently in the immunocompetent FB than in discs (Fig S7B–C). These data indicate that the two Spz-controlled signaling contexts in larvae are mechanistically distinct and geographically isolated. A benefit of tight compartmentalization of signaling mediated by Spz during cell competition is that it will not trigger a costly system-wide inflammatory response, which can suppress animal growth (Baker et al., 2011; DiAngelo et al., 2009; Steel and Whitehead, 1994). Signal partitioning also appears to protect healthy imaginal cells from aberrant signal activation during an infection (Meyer et al 2014).
However, neoplastic transformation of imaginal disc cells (e.g., scribbled mutant) can trigger a Spz/Toll-dependent systemic immune response, which then contributes to restraining tumor growth (Parisi et al., 2014). In this context, Spz is contributed by the FB and/or HCs, the latter being attracted to disruptions in the basement membrane (BM) of the disc (Pastor-Pareja et al., 2008). In addition, AMP genes, which are direct targets of the immune response, are upregulated in neoplastic discs (Bunker et al., 2015). In contrast, wing discs containing Myc super-competitors show no increase in recruitment of circulating HCs, maintain an intact BM and overall disc architecture, and do not induce AMP gene expression (Fig. S4M–O). Collectively, our results argue that Myc super-competitor cells are ‘cheaters’, exploiting mechanisms of cell competition to gain tissue territory, while benefiting from resistance to competitive signaling within discs and isolation from systemic tumor-suppressing immune surveillance. As such, the mechanisms of cell and super-competition provide important models for understanding how pre-malignant cancers can covertly overtake tissues.
STAR methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Laura Johnston (lj180@columbia.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila stocks and care
Males and females from the following fly strains were used in this study (followed by source): spzrm7and psh1 (gifts of C. Hashimoto); SPESK6 (gift of S. Goto); ea4 (E. LeMosy), ea1, ea2, Tlr26 (gifts of D. Stein); spzKG05402, snk1, snk4, gd7, ndlrm5, modSP1, grassHer, Tlr3, Tlr4, R4-Gal4, ptc-Gal4, UAS-spz-RNAi, UAS-Toll2-RNAi, UAS-Toll3-RNAi, UAS-Toll8-RNAi, UAS-Toll9-RNAi, hid-lacZ (hidW−05014), and Tollo-lacZ (tollo (Bloomington Drosophila Stock Center (BDSC)); C10-Gal4 (gift of G. Struhl); C765-Gal4; Hml-Gal4 (gift of E. Bach); nub-Gal4 (gift of R. Mann); tub>CD2>Gal4 (gift of E. Moreno); UAS-Myc (Johnston et al, 1999); UAS-SpzAct (gift of J. Royet); UAS-SPEAct and UAS-modSP-GFP (gifts of B. Lemaitre); rpr-lacZ 150 (gift of M. Brodsky); D4-lacZ (gift of U. Banerjee); UAS-RedStinger UAS-Flp Ubi>STOP>eGFP (gift of M. Hardwick); UAS SPE-RNAi (gift of R. Ueda); dipt-lacZ drs-GFP (gift of J. M. Reichhart). (See also Table S1).
FRT82B spzrm7 and FRT82B SPESK6 were constructed by standard recombination methods. The recombinant spzrm7 flies were screened for the embryonic patterning phenotype (Anderson and Nusslein-Volhard, 1984; Morisato and Anderson, 1994b). The recombinant SPESK6 flies were screened for the presence of the mutation by sequencing with the following primers: SPE-CHK-263F: CTAAGCACTTGACCTTGTTGATTGT;SPE CHK+273R: CCGCAGACGTCATTTCCAG (Goto et al, 2015). The original spzrm7 and SPESK6 strains carried developmental delay or larval lethality phenotypes, respectively. Both phenotypes were absent in the FRT recombinant lines generated, thus the recombinant FRT82B spzrm7 or FRT82B SPESK6 strains were used in all experiments requiring spzrm7 or SPESK6 alleles.
Flies were raised in uncrowded conditions at 25°C on yeasted cornmeal-molasses food supplemented with penicillin and streptomycin. Eggs from appropriate crosses were collected on grape-agar plates for 2–3 hours at 25°C. First instar larvae were transferred onto standard molasses food supplemented with fresh yeast at 24hrs after egg-laying (AEL) and raised at 25°C (≤ 50 larvae per food vial). Where specific developmental times are not stated, larvae used in the experiments were dissected as 3rd instar wandering larvae (generally approximately 115 hr AEL).
For experiments in the temperature sensitive heteroallelic Tlr3/Tlr26 background, larvae were raised at 18°C for 72 hours, and then switched to the restrictive temperature, 29°C, for 72 hours prior to dissection. For all RNAi lines used in the study, the efficiency of transcription inhibition was checked by RT-PCR, with RNA extracted from tub-Gal4, UAS-RNAi larvae. UAS-Toll-RNAi lines did not completely knock down Tl mRNA, providing an explanation for why Tl RNAi knock down did not prevent cell competition (Meyer et al, 2014). All other RNAi lines used in experiments showed strong to complete inhibition of transcription (Fig. S1A, B).
METHODS DETAILS
Cell competition assays
Eggs from appropriate crosses were collected on grape-agar plates for 2–3 hours at 25°C to obtain ywhsFlp1.22; tub>myc>Gal4, UAS-GFP (for competition) or yw hsFlp1.22; tub>CD2>Gal4 / UAS-GFP (for control) larvae. First instar larvae were transferred onto standard molasses food supplemented with fresh yeast at 24hrs after egg-laying (AEL) and raised at 25°C. To induce FLP recombinase the larvae were heat-shocked at 37ˆC for 10 minutes (for the tub>myc cassette) or 8 minutes (for the tub>CD2 cassette) at 45 hrs AEL for clone induction, and dissected at 96 hrs AEL. Male and female larvae were pooled, except when X chromosome mutants were used; hemizygous males were then selected for analysis (also in controls). Clone areas were measured using ImageJ, for clones in the wing pouch only, where cell competition is strongest (Fig. S1). For clonal experiments in the temperature sensitive heteroallelic Tlr3/Tlr4 background, eggs from appropriate crosses were collected on grape plates for 2–3 hours at 18°C. The larvae were raised at 18°C for 96 hours and then heat shocked at 37°C as described above. Following heat shock, the larvae were switched to the restrictive temperature, 29°C, for 48 hours prior to dissection.
For tissue-specific spz knock-down, transgenic flies carrying ywhsFlp 1.22, lexOGFP and tub>myc>lexA or tub>CD2>lexA cassettes were used to induce loser or control clones. Clones were induced by heat-shocking for 20 minutes at 37°C. The rest of the experimental procedure was the same as for the comparable Gal4 cassettes, described above.
MARCM competition assays were performed as described (de la Cova et al, 2004) using FRT82B spzrm7 or FRT82B SPESK6 recombinant flies (this work) to obtain spz mutant winner, or SPE mutant winner clones, respectively. The recombinant FRT mutant flies were crossed to ywhsFlp1.22 tub-Gal4, UAS-GFP;; FRT82B hsCD2 tub-Gal80 or ywhsFlp1.22 tub-Gal4, UAS-GFP; UAS-Myc; FRT82B hsCD2 tub-Gal80 flies. Eggs from appropriate crosses were collected on grape plates for 3–4 hours at 25°C. First instar larvae were transferred onto standard molasses food supplemented with fresh yeast at 24hrs AEL and raised at 25°C. The larvae were heat-shocked at 37°C for 20 minutes at 30 hrs AEL for clone induction, and dissected at 100 hrs AEL. To induce CD2 expression, larvae were heat shocked at 37°C for 50 minutes, and allowed to recover for 30 minutes at RT immediately prior to dissection. CD2 was detected by immunostaining. Clone areas were measured using ImageJ, for clone pairs in the wing pouch only. Myc-overexpressing ‘Flp-out’ clones were induced by heat-shock of ywywhsFlp; act>CD2>Gal4, UAS-GFP; UAS-Myc larvae for 6 minutes at 37°C at 48hrs AEL.
Construction of spz-mCherry and SPE-YFP transgenic flies
The BACs CH322–164M23 (for spz) and CH322–35F14 (for SPE) – from the attBP[acman]-CAM library (H. Bellen Lab) – were used to insert the coding sequences of mCherry or sfYFP (gift of B. Glick) fluorescent tags to the C-terminal ends of spz or SPE sequences respectively. Tagging was carried out at the BAC-Recombineering Core Facility at the University of Chicago. The modified Spz-mCherry and SPE-YFP BACs were injected into y1 M{vas-int.Dm}ZH-2A w*; M{3×P3-RFP.attP’}ZH-51C flies (BDSC #24482) for insertion into the attP site at 51C1 on chromosome 2 by FC31-mediated transgenesis (BestGene).
The Spz-mCherry and SPE-YFP constructs were tested for function by assessing rescue of mutant phenotypes. spz-mCherry; spzrm7 female flies did not show the sterility phenotype of spzrm7 females (Fig. S2A). To test for immune response phenotypes, ywhsFlp 1.22; +; +, ywhsFlp 1.22;; SPESK6 or ywhsFlp 1.22; SPE-YFP; SPESK6 larvae were pricked with a sterile tungsten needle dipped (or not dipped for controls) in a concentrated pellet of Micrococcus luteus culture; larvae were checked for Drs expression by qRT-PCR 12 hours after the immune challenge (Romeo and Lemaitre, 2008). While SPESK6 mutant larvae were deficient in Drs induction as described before (Yamamoto-Hino and Goto, 2016), SPE-YFP; SPESK6 larvae responded to the immune challenge by increased Drs expression at levels comparable to WT (Fig. S7A).
Construction of lexA ‘flp-out’ cassettes
The fused sequence for α-tubulin promoter, FRT, myc cDNA and α-tubulin trailer was amplified from a tub>myc>Gal4 transformation vector (pDA470, gift of P. Gallant) and inserted into the mENTRY vector (Harvard Plasmid Database, Ni et al, 2009). A second FRT site was constructed by annealing the single-stranded oligos AAATCTAGAgaagttcc tattccgaagttcctattctctagtaagtataggaaCAATTGGGGCCCAAAandTTTGGGCCCCAATTGttcctatacttactagagaataggaacttcggaataggaacttcTCTAGATTT (lower case, FRT sequence, upper case, added restriction sites), and inserted Xba I/Mfe I downstream of the α-tubulin trailer into the tub>myc-mENTRY vector. The tub>CD2>-mENTRY vector was made by replacing the myc cDNA-α-tubulin trailer on the tub>myc>-mENTRY vector with the fused sequence of CD2 cDNA and α-tubulin trailer, amplified from the pDA481 vector. tub>myc> and tub>CD2> sequences were then inserted into the pBnlsLexA: GADflUw destination vector (Pfeiffer et al, 2010) by Gateway cloning. The resulting tub>myc>lexA and tub>CD2>lexA destination vectors were then injected into y1 M{vasint.Dm}ZH-2A w*; M{3×P3-RFP.attP’}ZH-51C flies (BDSC #24482) for insertion into the attP site at 51C1 on chromosome 2 or into y1w67c23; P{CaryP}attP2 flies (BDSC #8622) for insertion into the attP site at 68A4 on chromosome 3 (BestGene). The resulting fly strains were confirmed to generate GFP-positive clones in a heat shock dependent manner.
Immunohistochemistry
Wing imaginal discs and other tissues were fixed in 4% paraformaldehyde:PBS for 20 minutes at room temperature and washed with PBS 0.01% Tween-20. Hoechst 33258 or 4′,6-diamidino-2-phenylindole (DAPI) was used to stain DNA. The following primary antibodies were used at the given concentrations: rabbit anti-mCherry (1:500 – Abcam Cat# ab167453, RRID:AB_2571870), rat anti-Spz (1:400 – gift of S. Goto), AlexaFluor 647-phalloidin (1:40 – Thermo Fisher Scientific Cat# A22287, RRID:AB_2620155), mouse anti-Digoxygenin (1:1000 – Roche Cat# 11093274910, RRID:AB_514497), rabbit anti-Dcp1 (1:100 – Cell Signaling Technology Cat# 9578, RRID:AB_2721060), rabbit anti-βGal (1:1000 – Cappel), mouse anti-V5 (1:200 - Thermo Fisher Scientific Cat# MA1–81617, RRID:AB_935874), mouse anti-CD2 (1:400 – BD Biosciences Cat# 554826, RRID:AB_395538). Secondary antibodies were preadsorbed with fixed embryos for an hour at room temperature and used at the following concentrations: Alexa Fluor 488 Goat anti-Rat IgG (1:1000, Molecular Probes Cat# A-11006, RRID:AB141373); Alexa Fluor 488 Goat anti-Mouse IgG (1:1000, Thermo Fisher Scientific Cat# A-11001, RRID:AB_2534069); Alexa Fluor 555 Goat anti-Rabbit IgG (1:1000, Molecular Probes Cat# A-21429, RRID:AB_141761). (See also Table S2).
As a control for anti-Spz staining in wing discs, staining was done in which the primary antibody incubation step was omitted from the standard staining protocol. No fluorescence signal was detected in these wing discs (Fig. S2B).
RNA in situ hybridizations were carried out using digoxygenin-labeled RNA probes transcribed from spz, SPE, or modSP cDNA (DGRC clones FI05217, GH28857, and LD43740), as described (Johnston and Sanders, 2003).
Western blotting
The anterior 1/3 of 20–30 3rd instar larvae were dissected in PEM buffer (0.1 M PIPES, 2mM EGTA, 1mM MgSO4), and all tissues except imaginal discs were removed from the carcass. The larval tissues were then homogenized and centrifuged to remove debris. Protein concentration in the homogenates were determined using the Bio-Rad Protein Assay reagent (Bio-Rad Laboratories) and for each sample, a volume corresponding to 30–50ug total protein was separated on denaturing polyacrylamide gels (8% for ModSP and SPE, and 15% for SpzFL-HA blots. Stacking gel concentration was 4%). Following transfer to nitrocellulose membranes, the primary antibodies rat anti-HA (1:5000 – Roche Cat# 11867423001, RRID:AB_390918), mouse anti-V5 (1:2000, Thermo Fisher Scientific Cat# MA1–81617, RRID:AB_935874), rabbit anti-GFP (1:5000, Thermo Fisher Scientific Cat# A-6455, RRID:AB_221570) and mouse anti-tubulin (1:1000, Sigma-Aldrich Cat# T9026, RRID:AB_477593), and horseradish peroxidase-conjugated secondary antibodies (anti-Mouse IgG (1:10000, Jackson ImmunoResearch Labs Cat# 115-035-003, RRID:AB_10015289), anti-Rabbit IgG (1:20000, Jackson ImmunoResearch Labs Cat# 111-035-144, RRID:AB_2307391) and anti-Rat IgG (1:5000)) were used to detect the respective proteins with the Immobilon Western Chemiluminescent HRP Substrate Kit (Millipore, WBKLS0500). (See also Table S2).
Imaging and image analysis
For clonal assays and in situ hybridizations, wing discs were imaged with a Zeiss Axiophot 2. All other images were taken with a Leica SP5 inverted conventional confocal microscope. ImageJ was used for clone area measurements and cell death counts.
qRT-PCR and analysis
Wing imaginal discs from 30–40 larvae (ywhsFlp 1.22; +; +), or larval tissues from 10 larvae (ywhsFlp 1.22; +; +), were dissected in PBS. For hemolymph collection, wandering 3rd instar larvae (ywhsFlp 1.22; +; +) were cleaned by rinsing first in ethanol than in PBS, and bled on ice, and hemolymph from 30 larvae were pooled. The discs with homogenous Myc expression were dissected from ywhsFlp; tubGal80ts; tubGal4/UAS-Myc larvae, raised at 18°C for 6 days, then switched to 29°Cfor 24–30 hrs and then dissected. Total RNA from all tissue samples was isolated by Trizol (Invitrogen), and treated with RNAse-free DNAse I. cDNA was synthesized from 2–4ug total RNA, using oligo-dT and Superscript RT-III (Invitrogen). qRT-PCR was then performed using Applied Biosystems Power SYBR Green or Roche FastStart Universal SYBR Green Master Mix, in an Applied Biosystems 7900HT Real-Time PCR Systems or Roche LightCycler 480 qRT-PR machine. The following primer pairs were used:
act5c, F: TGTGACGAAGAAGTTGCTGCT, R: AGGTCTCGAACATGATCTGG;
tubα1, F: GCCAGATGCCGTCTGACAA, R: AGTCTCGCTGAAGAAGGTGTTGA;
spz, F: CTCTCGCTGTCGTGTGTTCT, R: TTCCTTTGCACGTTTGCGAG;
SPE, F: ACCAATACGACCCTCTGGGA, R: GCAGTCAGGATCGGTACGAG;
grass, F: ACATCCTGACTGCTGCTCAC, R: GAACTCGACCATTTTCGGCG;
spirit, F: GAGTTCTTCGTGTCGGTGGT, R: AAGCTGGTCTGCCCATAACC;
modSP, F: GCCGGAGAATTCGATGGCTA, R: CGGCGGTTATGACTAGGTCC;
psh, F: CTGCAAGAAGATTCGCGAGC, R: CCAGATAGGACGAGACACGC;
ea, F: TTTACCTGAGTCGCAGCCAG, R: CCAAACGAACACCGGACAAC;
snk, F: CCGAAGTACAGATCCTCGGC, R: GCTTGCAGGTCATTTGTGGG;
gd, F: GGTGAACCAAAGAGCTCCGA, R: AGGCAAGCGGGTCGAATAAA;
ndl, F: CGCCTGCCAATTTCCGTATG, R: CGTTTGGCAAAGTCCTGTGG;
Drs,F:TTGTTCGCCCTCTTCGCTGTCCT,R:GCATCCTTCGCACCAGCACTTCA.
act5C or tubα1 mRNA was used for normalization. (See also Table S2).
Detailed Genotypes
Figure 1:
(B) ywhsFlp; tub>CD2>Gal4/UAS-GFP.
(C) ywhsFlp; tub>myc>Gal4, UAS-GFP.
(D) ywhsFlp; tub>myc>Gal4/UAS-GFP; spzKG05402.
(E) ywhsFlp; tub>CD2>Gal4 /UAS-GFP; ywhsFlp; tub>CD2>Gal4/ UAS-GFP; Tlr3/Tlr4; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; Tlr3/Tlr4.
(F) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>CD2>Gal4/UAS-GFP; spzKG05402; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; spzKG05402; ywhsFlp; tub>myc>Gal4, UAS-GFP; FRT82B spzrm7.
(G) ywhsFlp; +; +.
Figure 2:
A) ywhsFlp; spz-mCherry; FRT82B spzrm7.
B) ywhsFlp; spz-mCherry; FRT82B spzrm7.
D) ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; Hml-Gal4/UAS-spz-RNAi; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; Hml-Gal4/UAS-spz-RNAi; ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; R4-Gal4/UAS-spz-RNAi; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; R4-Gal4/UAS-spz-RNAi.
E) ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; C10-Gal4/UAS-spz-RNAi; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; C10-Gal4/UAS-spz-RNAi; ywhsFlp; tub>CD2>lexA (attP40), lexO-mCherry; C765-Gal4/UAS-spz-RNAi; ywhsFlp; tub>myc>lexA (attP40), lexO-mCherry; C765-Gal4/UAS-spz-RNAi.
Figure 3:
(A) nubGal4/UAS-GFP; nubGal4; UAS-SpzFL-HA.
(B) nubGal4; nubGal4;UAS-SpzFL-HA; nubGal4/ UAS-SPEAct; UAS-SpzFL-HA; nubGal4/UAS-modSPFL; UAS-SpzFL-HA.
(C) nubGal4/UAS-GFP; nubGal4/UAS-SpzAct; nubGal4/ UAS-SpzAct; Tlr3/Tlr26.
(D) ptcGal4,UAS-GFP, rpr-150-lacZ.
(E) UAS-SpzAct; ptcGal4,UAS-GFP, rpr-150-lacZ.
(F) nubGal4; UAS-GFP/ hid-lacZ.
(G) nubGal4/ UAS-SpzAct; hid-lacZ.
Figure 4:
(B) ywhsFlp; +; +.
(C) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ndlrm5.
(D) tub>CD2>Gal4, UAS-GFP/hsFlp; tub>myc>Gal4, UAS-GFP/hsFlp; gd7; tub>myc>Gal4, UASGFP/hsFlp.
(E) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; snk1/4.
(F) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ea1/4.
(G) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; grassHer.
(H) ywhsFlp; tub>CD2>Gal4/UAS-GFP; psh1; tub>CD2>Gal4, UAS-GFP/hsFlp; modSP1; ywhsFlp; tub>myc>Gal4, UAS-GFP; psh1; tub>myc>Gal4, UAS-GFP/hsFlp; ywhsFlp; tub>myc>Gal4, UAS-GFP; modSP1; psh1; tub>myc>Gal4, UAS-GFP/hsFlp; modSP1.
(I) ywhsFlp; tub>CD2>Gal4/UAS-GFP; ywhsFlp; tub>CD2>Gal4/UAS-GFP; FRT82B SPESK6/Df(3R)Bsc491; ywhsFlp; tub>myc>Gal4, UAS-GFP; ywhsFlp; tub>myc>Gal4, UAS-GFP; FRT82B SPESK6/Df(3R)Bsc491.
Figure 5:
(A) ywhsFlp; +; +.
(B) ywhsFlp; +; +.
(C) ywhsFlp; +; +.
(D) nubGal4/UAS-GFP; nubGal4/UAS-modSPFL-GFP; nubGal4/ UAS-modSPFL-GFP; FRT82B spzrm7.
(E) nubGal4/UAS-GFP; nubGal4/UAS-SPEAct; nubGal4/ UAS-SPEAct; FRT82B spzrm7.
(F) nubGal4/UAS-GFP; nubGal4/UAS-GFP; Tlr3/r26; nubGal4/UAS-SPEAct; nubGal4/ UAS-SPEAct; Tlr3/r26.
(G) nubGal4/UAS-GFP; hid-lacZ;
(H) nubGal4 /UAS-SPEAct; hid-lacZ.
(I) nubGal4 /UAS-modSPFL-GFP; hid-lacZ.
(J) UAS-GFP; ptcGal4, UAS-GFP, rpr-150-lacZ.
(K) UAS-SPEAct; ptcGal4, UAS-GFP, rpr-150-lacZ.
(L) UAS-modSPFL-GFP; ptcGal4, UAS-GFP, rpr-150-lacZ.
Figure 6:
(A) ywhsFlp; act>CD2>Gal4; UAS-Myc.
(B) ywhsFlp; act>CD2>Gal4; UAS-Myc.
(C) ywhsFlp; act>CD2>Gal4; UAS-Myc.
(D) SPE-YFP.
(E) SPE-YFP; ptcGal4/UAS-Myc.
(F) ywhsFlp; act>CD2>Gal4; UAS-Myc.
(H) ywhsFlp, tubGal4, UAS-GFP;;FRT82B tubGal80 hsCD2/FRT82B; ywhsFlp, tubGal4, UASGFP;; FRT82B tubGal80 hsCD2/FRT82B spzrm7; ywhsFlp, tubGal4, UAS-GFP; UAS-Myc; FRT82B tubGal80 hsCD2/FRT82B; ywhsFlp, tubGal4, UAS-GFP; UAS-Myc; FRT82B tubGal80 hsCD2/FRT82B spzrm7.
(I) ywhsFlp, tubGal4, UAS-GFP;; FRT82B tubGal80 hsCD2/FRT82B; ywhsFlp, tubGal4, UASGFP;; FRT82B tubGal80 hsCD2/FRT82B SPESK6; ywhsFlp, tubGal4, UAS-GFP; UAS-Myc; FRT82B tubGal80 hsCD2/FRT82B; ywhsFlp, tubGal4, UAS-GFP; UAS-Myc; FRT82B tubGal80 hsCD2/FRT82B SPESK6.
Figure 7:
(A) nubGal4/UAS-GF; nubGal4/UAS-GFP; UAS-Toll2/18w-RNAi; nubGal4 / UAS-SPEAct; UAS GFP; nubGal4 / UAS-SPEAct; UAS-Toll2/18w-RNAi.
(B) nubGal4 /UAS-GFP; nubGal4/UAS-GFP; UAS-Toll3/MstProx-RNAi; nubGal4 / UAS-SPEAct; UAS-GFP; nubGal4 / UAS-SPEAct; UAS-Toll3/MstProx-RNAi.
(C) nubGal4/UAS-GFP; nubGal4/UAS-GFP; UAS-Toll8/Tollo-RNAi; nubGal4 / UAS-SPEAct; UAS GFP; nubGal4 / UAS-SPEAct; UAS-Toll8/Tollo-RNAi.
(D) nubGal4 /UAS-GFP; nubGal4/UAS-GFP; UAS-Toll9-RNAi; nubGal4 / UAS-SPEAct; UAS-GFP; nubGal4/UAS-SPEAct; UAS-Toll9-RNAi.
(E) nubGal4/UAS-GFPnubGal4 / UAS-SpzAct; UAS-GFP; nubGal4 / UAS-SpzAct; UAS-Toll8/Tollo-RNAi.
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed using Prism v5.0. Statistical details of experiments can be found in figures and figure legends.
Supplementary Material
Highlights.
In Myc super-competition, Spz signals via Toll and Toll-8 to kill loser cells
Spz is activated for competitive signaling by the proteases SPE and ModSP
Regulation of protease production and receptor expression by Myc allows ‘cheating’
Wing disc-restricted Spz keeps competitive signaling isolated from immune tissues
Acknowledgments
We thank members of the Johnston lab for advice, S. Goto for the gift of anti-Spz antibodies, B. Glick for sfYFP, J. Moran and BRCF at U Chicago for tagged BAC construction, and numerous colleagues and the BDSC for fly stocks. We gratefully acknowledge the valuable resources provided by the DGRC, FlyBase, and DHSB. Funded by the NIH (RO1GM0786464, to LAJ), the NCI (R01CA192838, to LAJ) and the Leukemia & Lymphoma Society (to CB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Anderson KV, Bokla L, and Nusslein-Volhard C (1985). Establishment of dorsal ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791–798. [DOI] [PubMed] [Google Scholar]
- Anderson KV, and Nusslein-Volhard C (1984). Information for the dorsal--ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature 311, 223–227. [DOI] [PubMed] [Google Scholar]
- Arnot CJ, Gay NJ, and Gangloff M (2010). Molecular mechanism that induces activation of Spatzle, the ligand for the Drosophila Toll receptor. J Biol Chem 285,19502–19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, and Ghosh S (2011). NF-kappaB, inflammation, and metabolic disease. Cell Metab 13, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, and Abrams JM (2000). Drosophila p53 binds a damage response element at the reaper locus. Cell 101, 103–113. [DOI] [PubMed] [Google Scholar]
- Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, and Lemaitre B (2009). A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106, 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Silverman N, and Cherry S (2014). Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nat Rev Immunol 14, 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capilla A, Karachentsev D, Patterson RA, Hermann A, Juarez MT, and McGinnis W (2017). Toll pathway is required for wound-induced expression of barrier repair genes in the Drosophila epidermis. Proc Natl Acad Sci U S A 114, E2682–E2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan R, and Anderson KV (1989). The role of easter, an apparent serine protease, in organizing the dorsal-ventral pattern of the Drosophila embryo. Cell 56, 391–400. [DOI] [PubMed] [Google Scholar]
- Chasan R, Jin Y, and Anderson KV (1992). Activation of the easter zymogen is regulated by five other genes to define dorsal-ventral polarity in the Drosophila embryo. Development 115, 607–616. [DOI] [PubMed] [Google Scholar]
- Cho YS, Stevens LM, Sieverman KJ, Nguyen J, and Stein D (2012). A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity. Curr Biol 22, 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Stevens LM, and Stein D (2010). Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol 20, 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, and Johnston LA (2004). Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Senoo-Matsuda N, Ziosi M, Wu DC, Bellosta P, Quinzii CM, and Johnston LA (2014). Supercompetitor status of Drosophila Myc cells requires p53 as a fitness sensor to reprogram metabolism and promote viability. Cell Metab 19, 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing M, Giordano H, and DeLotto R (2001). Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J 20, 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Chamy L, Leclerc V, Caldelari I, and Reichhart JM (2008). Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat Immunol 9, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do MT, et al. (2009). G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6, 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, and Hoffmann JA (1994). Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem 269, 33159–33163. [PubMed] [Google Scholar]
- Foldi I, Anthoney N, Harrison N, Gangloff M, Verstak B, Nallasivan MP, AlAhmed S, Zhu B, Phizacklea M, Losada-Perez M, et al. (2017). Three-tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J Cell Biol 216, 1421–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff M, Murali A, Xiong J, Arnot CJ, Weber AN, Sandercock AM, Robinson CV, Sarisky R, Holzenburg A, Kao C, et al. (2008). Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spaetzle. J Biol Chem 283, 14629–14635. [DOI] [PubMed] [Google Scholar]
- Han JH, Lee SH, Tan YQ, LeMosy EK, and Hashimoto C (2000). Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo. Proc Natl Acad Sci U S A 97, 9093–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, Hoffmann JA, Hetru C, and Meister M (2005). New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7, 335–350. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, Kambris Z, Brun S, Hashimoto C, Ashida M, et al. (2006). A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell 10, 45–55. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, and Gallant P (1999). Drosophila myc regulates cellular growth during development. Cell 98, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, and Sanders AL (2003). Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol 5, 827–833. [DOI] [PubMed] [Google Scholar]
- Kellenberger C, Leone P, Coquet L, Jouenne T, Reichhart JM, and Roussel A (2011). Structure-function analysis of grass clip serine protease involved in Drosophila Toll pathway activation. J Biol Chem 286, 12300–12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski I, Dinan M, Kolahgar G, and Piddini E (2017). Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat Commun 8, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, and Hoffmann JA (1996). The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, and Reichhart JM (1999). Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science 285, 1917–1919. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Bulet P, and Reichhart JM (2002a). Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defense of Drosophila. EMBO Rep 3, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, and Reichhart JM (2002b). Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297, 114–116. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, and Lemaitre B (1999). A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18, 3380–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SN, Amoyel M, Bergantinos C, de la Cova C, Schertel C, Basler K, and Johnston LA (2014). An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler JD (1977). Developmental genetics of the Drosophila egg. I. Identification of 59 sex-linked cistrons with maternal effects on embryonic development. Genetics 85, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, and Ripoll P (1975). Minutes: mutants of Drosophila autonomously affecting cell division rate. Developmental Biology 42, 211–221. [DOI] [PubMed] [Google Scholar]
- Moreno E, and Basler K (2004). dMyc transforms cells into super-competitors. Cell 117, 117–129. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, and Morata G (2002). Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759. [DOI] [PubMed] [Google Scholar]
- Morisato D (2001). Spatzle regulates the shape of the Dorsal gradient in the Drosophila embryo. Development 128, 2309–2319. [DOI] [PubMed] [Google Scholar]
- Morisato D, and Anderson KV (1994a). The Spaetzle Gene Encodes a Component of the Extracellular Signaling Pathway Establishing the Dorsal-Ventral Pattern of the Drosophila Embryo Cell 76, 677–688. [DOI] [PubMed] [Google Scholar]
- Morisato D, and Anderson KV (1994b). The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell 76, 677–688. [DOI] [PubMed] [Google Scholar]
- Mulinari S, Hacker U, and Castillejo-Lopez C (2006). Expression and regulation of Spatzle-processing enzyme in Drosophila. FEBS Lett 580, 5406–5410. [DOI] [PubMed] [Google Scholar]
- Pan D, and Courey AJ (1992a). The same dorsal binding site mediates both activation and repression in a context-dependent manner. EMBO J 11, 1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DJ, and Courey AJ (1992b). The same dorsal binding site mediates both activation and repression in a context-dependent manner. EMBO J 11, 1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi F, Stefanatos RK, Strathdee K, Yu Y, and Vidal M (2014). Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep 6, 855–867. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, and Xu T (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech 1, 144–154; discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RA, Juarez MT, Hermann A, Sasik R, Hardiman G, and McGinnis W (2013). Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila. PLoS One 8, e61773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo Y, and Lemaitre B (2008). Drosophila immunity: methods for monitoring the activity of Toll and Imd signaling pathways. Methods Mol Biol 415, 379–394. [DOI] [PubMed] [Google Scholar]
- Roth S, Stein D, and Nusslein-Volhard C (1989). A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rushlow CA, Han K, Manley JL, and Levine M (1989). The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Hudson KL, Lin TY, and Anderson KV (1991). Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev 5, 797–807. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Jin Y, Morisato D, and Anderson KV (1994). A processed form of the Spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development 120, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, and Johnston LA (2007). Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci U S A 104, 18543–18548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, and Ligoxygakis P (2009). Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J Cell Sci 122, 4505–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P (1979). Parameters of cell competition in the compartments of the wing disc of Drosophila. Developmental Biology 69, 182–193. [DOI] [PubMed] [Google Scholar]
- Simpson P, and Morata G (1981). Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Developmental Biology 85, 299–308. [DOI] [PubMed] [Google Scholar]
- Steel DM, and Whitehead AS (1994). The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology today 15, 81–88. [DOI] [PubMed] [Google Scholar]
- Stein D, Roth S, Vogelsang E, and Nusslein-Volhard C (1991). The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell 65, 725–735. [DOI] [PubMed] [Google Scholar]
- Stein DS, and Stevens LM (2014). Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip Rev Dev Biol 3, 301–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R (1989). Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Veillard F, Troxler L, and Reichhart JM (2016). Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 122, 255–269. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, et al. (2009). Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods 6, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ANR, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler J-L, and Gay NJ (2003). Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nature Immunology 4, 794–800. [DOI] [PubMed] [Google Scholar]
- Wu DC, and Johnston LA (2010). Control of wing size and proportions by Drosophila myc. Genetics 184, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, and Nusslein-Volhard C (1985). Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791–798. [DOI] [PubMed] [Google Scholar]
- Arnot CJ, Gay NJ, and Gangloff M (2010). Molecular mechanism that induces activation of Spatzle, the ligand for the Drosophila Toll receptor. J Biol Chem 285, 19502–19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RG, Hayden MS, and Ghosh S (2011). NF-kappaB, inflammation, and metabolic disease. Cell Metab 13, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, and Lemaitre B (2009). A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106, 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Silverman N, and Cherry S (2014). Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nature reviews Immunology 14, 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker BD, Nellimoottil TT, Boileau RM, Classen AK, and Bilder D (2015). The transcriptional response to tumorigenic polarity loss in Drosophila. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasan R, Jin Y, and Anderson KV (1992). Activation of the easter zymogen is regulated by five other genes to define dorsal-ventral polarity in the Drosophila embryo. Development 115, 607–616. [DOI] [PubMed] [Google Scholar]
- Cho YS, Stevens LM, Sieverman KJ, Nguyen J, and Stein D (2012). A ventrally localized protease in the Drosophila egg controls embryo dorsoventral polarity. Curr Biol 22, 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Stevens LM, and Stein D (2010). Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol 20, 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, and Johnston LA (2004). Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116. [DOI] [PubMed] [Google Scholar]
- de la Cova C, Senoo-Matsuda N, Ziosi M, Wu DC, Bellosta P, Quinzii CM, and Johnston LA (2014). Supercompetitor status of Drosophila Myc cells requires p53 as a fitness sensor to reprogram metabolism and promote viability. Cell Metab 19, 470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, and Birnbaum MJ (2009). The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A 106, 20853–20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing M, Giordano H, and DeLotto R (2001). Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J 20, 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Chamy L, Leclerc V, Caldelari I, and Reichhart JM (2008). Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat Immunol 9, 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do MT, et al. (2009). G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6, 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, and Hoffmann JA (1994). Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem 269, 33159–33163. [PubMed] [Google Scholar]
- Gangloff M, Murali A, Xiong J, Arnot CJ, Weber AN, Sandercock AM, Robinson CV, Sarisky R, Holzenburg A, Kao C, et al. (2008). Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spaetzle. J Biol Chem 283, 14629–14635. [DOI] [PubMed] [Google Scholar]
- Han JH, Lee SH, Tan YQ, LeMosy EK, and Hashimoto C (2000). Gastrulation defective is a serine protease involved in activating the receptor toll to polarize the Drosophila embryo. Proc Natl Acad Sci U S A 97, 9093–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, Hoffmann JA, Hetru C, and Meister M (2005). New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7, 335–350. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, Kambris Z, Brun S, Hashimoto C, Ashida M, et al. (2006). A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell 10, 45–55. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, and Gallant P (1999). Drosophila myc regulates cellular growth during development. Cell 98, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger C, Leone P, Coquet L, Jouenne T, Reichhart JM, and Roussel A (2011). Structure-function analysis of grass clip serine protease involved in Drosophila Toll pathway activation. J Biol Chem 286, 12300–12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucinski I, Dinan M, Kolahgar G, and Piddini E (2017). Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat Commun 8, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, and Hoffmann JA (1996). The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, and Reichhart JM (1999). Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science 285, 1917–1919. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Bulet P, and Reichhart JM (2002a). Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defense of Drosophila. EMBO Rep 3, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, and Reichhart JM (2002b). Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297, 114–116. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, and Lemaitre B (1999). A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18, 3380–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SN, Amoyel M, Bergantinos C, de la Cova C, Schertel C, Basler K, and Johnston LA (2014). An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morata G, and Ripoll P (1975). Minutes: mutants of Drosophila autonomously affecting cell division rate. Developmental Biology 42, 211–221. [DOI] [PubMed] [Google Scholar]
- Moreno E, and Basler K (2004). dMyc transforms cells into super-competitors. Cell 117, 117–129. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K, and Morata G (2002). Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759. [DOI] [PubMed] [Google Scholar]
- Morisato D (2001). Spatzle regulates the shape of the Dorsal gradient in the Drosophila embryo. Development 128, 2309–2319. [DOI] [PubMed] [Google Scholar]
- Morisato D, and Anderson KV (1994). The Spaetzle Gene Encodes a Component of the Extracellular Signaling Pathway Establishing the Dorsal-Ventral Pattern of the Drosophila Embryo Cell 76, 677–688. [DOI] [PubMed] [Google Scholar]
- Mulinari S, Hacker U, and Castillejo-Lopez C (2006). Expression and regulation of Spatzle-processing enzyme in Drosophila. FEBS Lett 580, 5406–5410. [DOI] [PubMed] [Google Scholar]
- Parisi F, Stefanatos RK, Strathdee K, Yu Y, and Vidal M (2014). Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell reports 6, 855–867. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Wu M, and Xu T (2008). An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech 1, 144–154; discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Stein D, and Nusslein-Volhard C (1989). A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rushlow CA, Han K, Manley JL, and Levine M (1989). The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell 59, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Jin Y, Morisato D, and Anderson KV (1994). A processed form of the Spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development 120, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Senoo-Matsuda N, and Johnston LA (2007). Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc Natl Acad Sci U S A 104, 18543–18548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, and Ligoxygakis P (2009). Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J Cell Sci 122, 4505–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P (1979). Parameters of cell competition in the compartments of the wing disc of Drosophila. Developmental Biology 69, 182–193. [DOI] [PubMed] [Google Scholar]
- Simpson P, and Morata G (1981). Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Developmental Biology 85, 299–308. [DOI] [PubMed] [Google Scholar]
- Steel DM, and Whitehead AS (1994). The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunology today 15, 81–88. [DOI] [PubMed] [Google Scholar]
- Stein DS, and Stevens LM (2014). Maternal control of the Drosophila dorsal-ventral body axis. Wiley Interdiscip Rev Dev Biol 3, 301–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R (1989). Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Veillard F, Troxler L, and Reichhart JM (2016). Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie 122, 255–269. [DOI] [PubMed] [Google Scholar]
- Weber ANR, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler J-L, and Gay NJ (2003). Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nature Immunology 4, 794–800. [DOI] [PubMed] [Google Scholar]
- Wells BS, and Johnston LA (2011). Maintenance of imaginal disc plasticity and regenerative potential in Drosophila by p53. Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, and Johnston LA (2010). Control of wing size and proportions by Drosophila myc. Genetics 184, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M, Muraoka M, Kondo S, Ueda R, Okano H, and Goto S (2015). Dynamic regulation of innate immune responses in Drosophila by Senju-mediated glycosylation. Proc Natl Acad Sci U S A 112, 5809–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.