Abstract
Background and Purpose
Midline shift determined on MRI or CT images is a well-validated marker of mass effect after large hemispheric infarction and associated with mortality. In this study, we targeted a population with moderately sized strokes. We compared midline shift to other imaging markers and determined their ability to predict long-term outcome.
Methods
MRI scans were studied from the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET) cohort. Midline shift, acute stroke lesion volume, lesional swelling volume, change in ipsilateral hemisphere volume, the ratio of ipsilateral to contralateral hemisphere volume and the reduction in lateral ventricle volume were measured. The relationships of these markers with poor outcome (modified Rankin scale score 3–6 at day 90) was assessed. Receiver operating characteristic (ROC) curves were generated to compare the performance of each metric.
Results
Of the 71 included patients, 59.2% had a poor outcome which was associated with significantly larger values for midline shift, lesional swelling volume, and ratio of hemisphere volumes. Lesional swelling volume, change in hemisphere volume, ratio of hemisphere volumes and lateral ventricle displacement were each correlated with midline shift (Spearman r=0.60, 0.49, 0.61 and −0.56, respectively; all p<0.0001). ROC curve analysis showed that lesional swelling volume (area under the curve (AUC)=0.791) predicted poor outcome better than midline shift (AUC=0.682). For predicting mortality, ROC curve analysis showed that these three markers were equivalent.
Conclusion
The ratio of ipsilateral to contralateral hemisphere volume, baseline lesion volume and lesional swelling volume best predicted poor outcome across a spectrum of stroke sizes.
Keywords: Ischemia, Stroke, Brain Edema, Magnetic Resonance Imaging, Mass Effect
Introduction
After acute ischemic stroke, development of edema 2–5 days after onset is a feared complication and often leads to secondary neurological injury.1–3
Several measures of mass effect have been evaluated in malignant edema, including change in hemisphere volume,4 cerebral spinal fluid (CSF) volumetric analysis,5 and midline shift.6 Midline shift is considered the gold standard,7 and is measured as horizontal septum pellucidum displacement on computed tomography (CT) or magnetic resonance imaging (MRI). Midline shift is closely correlated with poor outcome6 and also predicts early mortality.8
However, midline shift may be visible only if the stroke lesion volume is large with substantial edema formation. Alternative markers such as change in lateral ventricle volume or hemisphere volume may be more sensitive to smaller changes in swelling, but have only been evaluated in large hemispheric stroke patients.4
Alternatively, a region-of-interest-based analysis that compares baseline and follow-up MRI images from day 3 to 5 is able to distinguish infarct growth from lesional swelling volume.9, 10 The presence of lesional swelling as determined with this method independently predicted poor outcome and mortality.9 Lesional swelling is also associated with early neurological deterioration after stroke.11 It has also been shown that lower lesional swelling volume was associated with early reperfusion.10
To determine which imaging measures of mass effect are most suitable to predict outcome across a range of stroke volumes, we evaluated the relative performance of each marker in the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET) cohort. We compared lesional swelling volumes, hemispheric swelling, and hemisphere ratio as well as change in lateral ventricle volume and midline shift, and compared their ability to predict long-term outcome.
Methods
Patients
Patients enrolled in the Echoplanar Imaging Thrombolysis Evaluation Trial (EPITHET, NCT00238537) were retrospectively analyzed. The EPITHET study was a randomized double-blind controlled phase 2 trial of alteplase t-PA in acute stroke performed in 15 centers in Australia, New Zealand, Belgium, and the UK. Details of the study cohort have been described.12 In brief, acute hemispheric ischemic stroke patients with a National Institutes of Health stroke scale (NIHSS) score of more than 4 were included, received MRI imaging and were treated with intravenous alteplase or placebo 3–6 hr after stroke onset. Global functional outcome was assessed at 90 days using the modified Rankin Scale (mRS) score, and outcome was dichotomized into good (mRS 0–3) versus poor (mRS 4–6) outcome. Imaging was performed on 1.5 Tesla MRI scanners. Standardized diffusion-weighted imaging (DWI), perfusion imaging, and magnetic resonance angiography sequences were obtained before treatment, at day 3–5 as well as on day 90 to assess final infarct size when available. DWI consisted of 13–27 axial slices with a thickness of 5–7 mm. For the present analysis we included only patients that received baseline and follow-up MRI examination 3–5 days after onset with DWI.
Imaging analysis
Midline shift
Using a DICOM viewer (RadiAnt, Version 2.2, Medixant), midline shift was measured at the point of maximal deviation from the line drawn between the anterior and posterior attachment of the falx cerebri on follow-up DWI only.13, 14
Lesional swelling volume
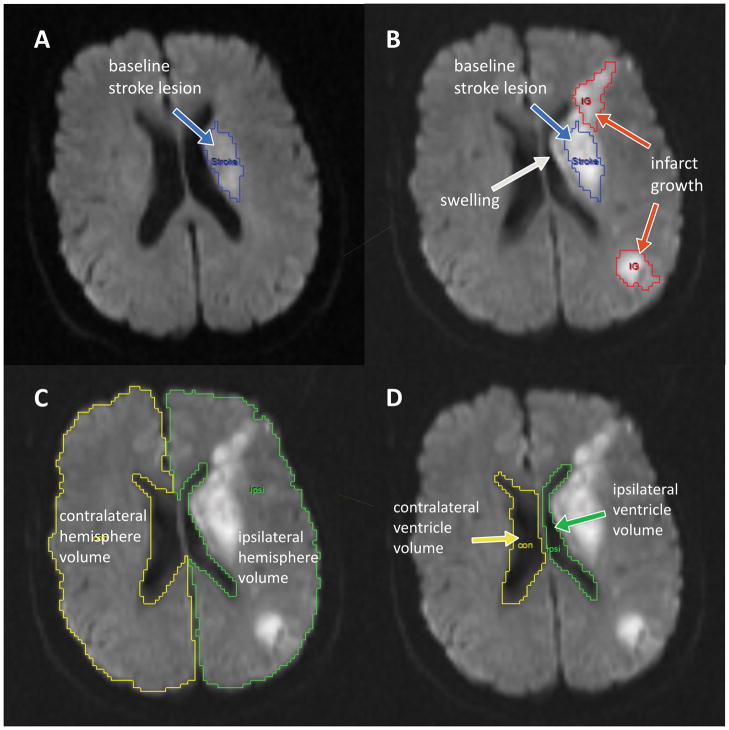
The rest of the imaging analysis was performed with Analyze 11.0 (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Analyze is an established image processing tool that has been used for the manual or semi-automated segmentation of biomedical images in numerous studies concerning varying neurological issues.9, 15–19 The option to use the semi-automatic edge-finding tool makes it easier to segment regions of interest, like acute stroke lesions and hemisphere volumes. For determination of lesional swelling volumes we used a method described in detail previously.9–11 Briefly, DWI lesion volumes were determined on the baseline (BL) and the follow-up (FU) images independently, and stroke lesion expansion (ΔDWI) was calculated. Subsequently, the FU image was coregistered onto the BL DWI and the mask for the BL lesion was superimposed onto the FU image (Figure 1B). Changes in stroke regions between BL and FU were designated infarct growth, swelling or hemorrhagic transformation by comparing BL and FU MRI scans side-by-side in axial, sagittal and transverse planes in the following way: Infarct growth was defined as involvement of new anatomic territory either adjacent to or distinct from the baseline lesion and delineated accordingly (Figure 1A and B). Hemorrhagic tissue was outlined as well. The final volumes were determined based on the relationship: Swelling volume = ΔDWI - infarct growth - hemorrhage volume.
Figure 1.
Illustration of the different imaging markers of mass effect.
A) shows the baseline (BL) diffusion-weighted image (DWI) with the stroke lesion outlined in blue. B) shows the coregistered follow-up (FU) DWI with the baseline lesion superimposed in blue. Newly infarcted tissue is outlined in red, and is excluded from the swelling volume measurement. The swelling volume corresponds to the region surrounding the blue baseline stroke lesion outline. C) Outline of the ipsilateral (green) and contralateral (yellow) hemisphere. The relative change in hemisphere volume is the FU hemisphere volume minus the BL hemisphere volume divided by the BL hemisphere volume. The hemisphere ratio is calculated as the FU ipsilateral hemisphere volume divided by the FU contralateral hemisphere volume. D) Outline of the ipsilateral (green) and contralateral (yellow) lateral ventricle volume. An analogous approach as in C) was used to calculate CSF displacement measurements, except that the lateral ventricle volumes were used.
Hemisphere volumes
Using Analyze 11.0, the cerebral hemisphere ipsi- and contralateral to the acute stroke on all relevant slices, but excluding cerebellum and brain stem, were delineated on BL and FU DWI (Figure 1C). Major sulci, cisterns, and ventricles were excluded. From these measurements, the following metrics were derived:
Change in ipsilateral hemisphere volume (in %): (ipsilateral hemisphere volume at FU – ipsilateral hemisphere volume at BL)/(ipsilateral hemisphere volume at BL)*100
Ratio hemisphere volume FU: (ipsilateral hemisphere volume at FU)/(contralateral hemisphere volume at FU)
Lateral ventricle volumes
In the same way, the volume of the ipsi- and contralateral lateral ventricle was measured on all relevant slices for BL and FU (Figure 1D). The following metrics were derived from these measurements:
Change in lateral ventricle volume (in %): (lateral ventricle volume at FU – lateral ventricle volume at BL)/(lateral ventricle volume at BL)*100
Ratio lateral ventricle volume: (ipsilateral lateral ventricle volume at FU)/ (contralateral lateral ventricle volume at FU)
Statistics
Baseline characteristics were presented as median and interquartile range or frequency and percentage. Patients were split into subgroups with good (mRS 0–2) and poor (mRS 3–6) outcome at day 90. The differences between the analyzed markers of mass effect between the two groups were tested with the Mann-Whitney U test. Correlation between different markers of mass effect was assessed using Spearman testing. Logistic regression models were constructed to evaluate the association between imaging measures, with poor outcome and mortality as the dependent variables. Performance of each model was assessed using receiver operating characteristic (ROC) curves, and the optimal threshold was identified by maximizing the sensitivity and specificity. These statistical analyses were performed using JMP Pro 11.0 (SAS Institute, Cary, NC) or MATLAB R2014b. ROC curves for the different imaging measures were compared using the StAR (Statistical Comparison of ROC Curves) online tool.20 This tool is based on a non-parametric approach for comparison of two or more paired ROC curves.21
Results
Of 101 subjects enrolled in the EPITHET study, 17 subjects were excluded due to lack of DWI with sufficient quality, and 13 lacked follow-up MRI. The clinical characteristics of the 71 patients included in the study are presented in Table 1. Of the 71 included patients, 29 patients (40.8%) had a good outcome (mRS 0–2), while 42 patients (59.2%) had a poor outcome (mRS 3–6) at 90 days. Median values for the different imaging marker of mass effect in these two subgroups of patients with good and poor outcome at 90 days can be seen in Table 2. Patients with poor outcome showed significantly greater midline shift, larger lesional swelling volumes, a larger change in ipsilateral hemisphere volume and a larger ratio of hemisphere volumes compared to patients with good outcome.
Table 1.
Clinical characteristics of the study population (n = 71).
| Age [years] | 75 (65–83) |
| Gender [male] | 33 (46%) |
| Baseline NIHSS | 13 (9–17) |
| Day 90 mRS | 3 (2–5) |
| Mortality rate at day 90 | 12 (16.9%) |
| Time from onset to baseline MRI [h] | 4.0 (3.4 – 4.7) |
| Time from onset to follow-up MRI [h] | 74.9 (50.5 – 99.6) |
| Intravenous thrombolysis treatment | 31 (43.7%) |
| DWI lesion volume at baseline [ml] | 21.3 (9.3 – 44.9) |
| Comorbidities: | |
| Hypertension | 48 (68%) |
| Diabetes mellitus | 18 (25%) |
| Hyperlipidemia | 29 (41%) |
| Atrial fibrillation | 30 (42%) |
| Current or past smoker | 25 (35%) |
Values are median (interquartile range) or frequency (percentage). n = sample size; NIHSS = national institutes of health stroke scale; mRS = modified Rankin Scale; DWI = diffusion-weighted imaging.
Table 2.
Markers of mass effect in patients with good versus poor outcome at day 90.
| Patients with good outcome (mRS 0–2) | Patients with poor outcome (mRS 3–6) | p-value | |
|---|---|---|---|
| n | 29 | 42 | |
| Midline shift [mm] | 0.0 (0.0 – 2.0) | 2.1 (0.0 – 3.3) | 0.007* |
| Lesional swelling volume [ml] | 4.4 (1.1 – 10.2) | 28.3 (8.8 – 53.3) | <0.001* |
| Change in ipsilateral hemisphere volume [%] | 1.5 (−1.6 – 3.6) | 5.5 (1.2 – 9.1) | 0.004* |
| Ratio hemisphere volumes FU | 1.05 (1.04 – 1.09) | 1.13 (1.07 – 1.18 ) | <0.001* |
| Change in ipsilateral ventricle volume [%] | −9.0 (−26.3 – 3.5) | −21.2 (−41.4 – −1.9) | 0.245 |
| Ratio lateral ventricle volumes FU | 0.86 (0.66 – 1.01) | 0.7 (0.6 – 0.9) | 0.046 |
Values are median (interquartile range); n = sample size; mRS = modified Rankin Scale; FU = follow-up. p-values are calculated with the Mann Whitney U test.
p <0.01
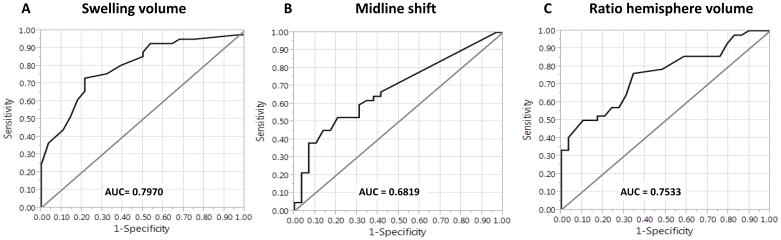
All of the examined markers of mass effect showed a high correlation with midline shift, as can be seen in Table 3. Table 3 also shows the results of the ROC analysis for predicting poor outcome or mortality. Lesional swelling volume best discriminated patients with good versus poor outcome, with an area under the curve (AUC) of 0.791. The ratio of hemisphere volumes had a similar AUC of 0.753. The difference between the AUC for swelling volumes and the AUC for ratio of hemisphere volumes was not significant (p = 0.51). The other measures, including midline shift, performed less well in comparison (see Table 3 and Figure 2). The AUC for midline shift was significantly lower than the AUC for lesional swelling volume (0.682 versus 0.791, p = 0.047). However, the AUC for baseline DWI volume was not significantly different from the AUC for swelling volume (0.716 versus 0.791, p = 0.17).
Table 3.
Association of markers of mass effect with outcome.
| Spearmans p | ROC (for mRS 3–6 at day 90) | ROC (for mortality at day 90) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Criterion value | Sensitivity | Specificity | AUC | Criterion value | Sensitivity | Specificity | ||
| DWI lesion volume BL [ml] | 0.455** | 0.716 | 27.3 | 79.3% | 57.1% | 0.482 | 90.6 | 88.1% | 33.3% |
| Swelling volume [ml] | 0.597** | 0.791 | 10.4 | 79.3% | 73.8% | 0.661 | 3.4 | 30.5% | 100.0% |
| Midline shift [mm] | -- | 0.682 | 2.00 | 79.3% | 52.4% | 0.662 | 3.2 | 88.1% | 50.0% |
| Change in ipsilateral hemisphere volume BL to FU [%] | 0.486** | 0.700 | 3.6 | 75.9% | 61.9% | 0.688 | 9.8 | 91.5% | 41.7% |
| Ratio hemisphere volumes FU | 0.612** | 0.753 | 1.06 | 65.5% | 76.2% | 0.645 | 1.14 | 81.4% | 58.3% |
| Change in ipsilateral ventricle volume BL to FU [%] | −0.561** | 0.583 | −18.4 | 54.8% | 69.0% | 0.541 | −61.8 | 100.0% | 16.7% |
| Ratio lateral ventricle volumes FU | −0.589** | 0.645 | 0.98 | 88.1% | 34.5% | 0.571 | 0.54 | 93.2% | 33.3% |
Spearmans p is shown for correlation with midline shift. AUC = area under the curve; ROC = receiver operating characteristics; mRS = modified Rankin Scale; BL = baseline; FU = follow-up.
p = <0.0001,
p <0.01
Figure 2.
Receiver operating characteristics curves for the discrimination between good and poor outcome.
Receiver operating characteristics (ROC) curve analysis showed that swelling volume (A) best discriminated patients with a modified Rankin Scale score of 3–6 at 90 days with an area under the curve (AUC) of 0.79. The AUC for midline shift (B) and the ratio of hemisphere volumes (C) were lower. The difference between the AUC for swelling volume and the AUC for midline shift was significant (p = 0.047).
Of the 71 included patients, 12 patients (16.9%) did not survive the first 90 days after stroke.
For the discrimination of mortality, ROC curve analysis showed that lesional swelling volume, midline shift and ratio of hemisphere volumes were equivalent (0.661, 0.662 and 0.645, respectively, see Table 3).
Discussion
In this comparative analysis study, we demonstrated that several markers of mass effect, including midline shift, lesional swelling volume, CSF displacement, and hemispheric swelling, were intercorrelated, as expected. However, in the setting of moderately sized stroke, we demonstrated that the traditional gold standard midline shift may not perform as well as lesional swelling volume, hemisphere ratio, or baseline DWI stroke lesion volume in predicting outcome.
Midline shift discriminates patients with risk of early death in very large stroke lesions.8 Our study demonstrates a less robust performance in predicting poor outcome, likely due to the smaller size in baseline stroke volumes. To shift the midline, swelling must be substantial, this makes midline shift less sensitive to smaller changes in swelling. Ventricle volume is also less sensitive, which may be related to the relatively low image quality on DWI and apparent diffusion coefficient (ADC) scans. Although validated in malignant edema,5 CSF displacement may not be as sensitive for smaller lesions due to location-specific effects. In contrast, lesional swelling volume and hemisphere ratio, as well as baseline DWI stroke lesion volume were each strongly associated with outcome. It has been previously shown that in stroke patients with smaller stroke volumes, the amount of swelling predicts outcome.9 Therefore the precise measurement of swelling is an essential part in understanding and predicting clinical outcome progression after stroke, regardless of the stroke severity.
Several secondary neurological injury processes are associated with subsequent brain injury after the initial stroke.22 Prediction of the course of the disease and potentially decisions about management and therapeutic options are dependent upon accurate assessment of the types of secondary neurological injury, including edema. A multitude of parameters, including the pre-stroke disability, NIHSS, comorbidities, age, family support and quality of rehabilitation greatly impacts the outcome of stroke patients at 90 days. This might be even more so in patients with mild to moderate stroke sizes. Our results suggest that the precise measurement of swelling volumes might be a useful parameter to consider in this process. Using measure like lesional swelling volumes or ratio or hemispheric volumes would improve the prediction of worse outcome in comparison to using midline shift or other markers.
Our study has several limitations. All the compared methods rely on spatial resolution of the images and movement artifacts and poor quality images might affect them differently. Although we showed that lesional swelling volume is a better predictor of poor outcome than some other methods in our cohort, measuring it requires serial MRI and post-acquisition analysis using specialized software. DWI baseline volume, midline shift and ratio of hemisphere volumes have the advantage that the measurements can be made on a single scan, and are compatible with either CT or MR imaging acquisition. These measures might be an alternative to midline shift for more mildly affected stroke patients. Although some of the methods evaluated may not be practical for routine clinical care, they may nevertheless be of value as intermediate endpoints in studies that seek to understand the role of edema or in interventional studies that are designed to reduce edema formation. Another limitation is that the current techniques measure mass effect, a downstream consequence of edema accumulation, but they do not measure water content directly. Whereas mass effect may have particular clinical relevance, the non-invasive measurement of water content, such as quantitative MRI23 or CT hypoattenuation,24 may provide additional information about brain edema and may further refine the predictive ability for outcome in patients. Further development of each of these approaches will facilitate the study of brain edema and its link to neurological outcome.
Acknowledgments
The original funding for the EPITHET trial was from the National Health and Medical Research Council, Australia. The analysis performed for this study was funded in part by the NIH/NINDS K23NS076597, R01 NS099209 and AHA 14GRNT19060044.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Kimberly WT, Sheth KN. Approach to Severe Hemispheric Stroke. Neurology. 2011;76:50–6. doi: 10.1212/WNL.0b013e31820c35f4. [DOI] [PubMed] [Google Scholar]
- 2.Bevers M, Kimberly W. Critical Care Management of Acute Ischemic Stroke. Curr Treat Options Cardiovasc Med. 2017;19:41. doi: 10.1007/s11936-017-0542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacke W, Schwab S, Horn M, Spranger M, Georgia M, van Kummer R. ‘Malignant’ Middle Cerebral Artery Territory Infarction. Clinical Course and Prognostic Signs. Arch Neurol. 1996;53:309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 4.Yoo AJ, Sheth KN, Kimberly WT, et al. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:742–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhar R, Yuan K, Kulik T, et al. CSF Volumetric Analysis for Quantification of Cerebral Edema After Hemispheric Infarction. Neurocrit Care. 2016;24:420–7. doi: 10.1007/s12028-015-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross DA, Olsen WL, Ross AM, Andrews BT, Pitts LH. Brain shift, level of consciousness, and restoration of consciousness in patients with acute intracranial hematoma. J Neurosurg. 1989;71:498–502. doi: 10.3171/jns.1989.71.4.0498. [DOI] [PubMed] [Google Scholar]
- 7.Ropper A. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–8. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 8.Pullicino P, Alexandrov A, Shelton J, Alexandrova N, Smurawska L, Norris J. Mass effect and death from severe acute stroke. Neurology. 1997;49:1090–5. doi: 10.1212/wnl.49.4.1090. [DOI] [PubMed] [Google Scholar]
- 9.Battey TW, Karki M, Singhal AB, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. 2014;45:3643–8. doi: 10.1161/STROKEAHA.114.006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine HJ, Ostwaldt AC, Bevers MB, et al. Reperfusion After Ischemic Stroke is Associated with Reduced Brain Edema. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17720559. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irvine HJ, Battey TW, Ostwaldt AC, et al. Early neurological stability predicts adverse outcome after acute ischemic stroke. Int J Stroke. 2016;11:882–9. doi: 10.1177/1747493016654484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 13.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15:1160–9. doi: 10.1016/S1474-4422(16)30196-X. [DOI] [PubMed] [Google Scholar]
- 14.Kimberly WT, Dutra BG, Boers AMM, et al. Association of Reperfusion With Brain Edema in Patients With Acute Ischemic StrokeA Secondary Analysis of the MR CLEAN Trial. JAMA Neurol. 2018 doi: 10.1001/jamaneurol.2017.5162. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bevers MB, Battey TWK, Ostwaldt AC, et al. Apparent Diffusion Coefficient Signal Intensity Ratio Predicts the Effect of Revascularization on Ischemic Cerebral Edema. Cerebrovasc Dis. 2018;45:93–100. doi: 10.1159/000487406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, Blumhardt LD, Constantinescu CS. The relationship of brain and cervical cord volume to disability in clinical subtypes of multiple sclerosis: a three-dimensional MRI study. Acta Neurol Scand. 2003;108:401–6. doi: 10.1034/j.1600-0404.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 17.Asdaghi N, Hameed B, Saini M, Jeerakathil T, Emery D, Butcher K. Acute perfusion and diffusion abnormalities predict early new MRI lesions 1 week after minor stroke and transient ischemic attack. Stroke. 2011;42:2191–5. doi: 10.1161/STROKEAHA.110.611376. [DOI] [PubMed] [Google Scholar]
- 18.Keihaninejad S, Heckemann RA, Fagiolo G, et al. A robust method to estimate the intracranial volume across MRI field strengths (1. 5T and 3T) Neuroimage. 2010;50:1427–37. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royle NA, Booth T, Valdés Hernández MC, et al. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol Aging. 2013;34:2726–33. doi: 10.1016/j.neurobiolaging.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergara IA, Norambuena T, Ferrada E, Slater AW, Melo F. StAR: a simple tool for the statistical comparison of ROC curves. BMC Bioinformatics. 2008;9:265. doi: 10.1186/1471-2105-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 22.Heiss WD, Kidwell CS. Imaging for prediction of functional outcome and assessment of recovery in ischemic stroke. Stroke. 2014;45:1195–201. doi: 10.1161/STROKEAHA.113.003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, Paczynski R, Venkatesan R, et al. Quantitative regional brain water measurement with magnetic resonance imaging in a focal ischemia model. Magn Reson Med. 1997;38:303–10. doi: 10.1002/mrm.1910380221. [DOI] [PubMed] [Google Scholar]
- 24.Dzialowski I, Weber J, Doerfler A, Forsting M, Von Kummer R. Brain Tissue Water Uptake after Middle Cerebral Artery Occlusion Assessed with CT. J Neuroimaging. 2004;14:42–8. [PubMed] [Google Scholar]