Summary
Twenty years ago, accidental bisphenol A (BPA) exposure caused a sudden increase in chromosomally abnormal eggs from our control mice [1]. Subsequent rodent studies demonstrated developmental effects of exposure with repercussions on adult health and fertility (e.g., [2–9], reviewed in [10–17]). Studies in monkeys, humans, fish, and worms suggest BPA effects extend across species (e.g., [18–30] reviewed in [31–33]). Widespread use has resulted in ubiquitous environmental contamination and human BPA exposure. Consumer concern resulted in “BPA free” products produced using structurally similar bisphenols that are now detectable environmental and human contaminants (e.g., [34–41]). We report here studies initiated by meiotic changes mirroring our previous BPA experience and implicating exposure to BPS (a common BPA replacement) from damaged polysulfone cages. Like BPA [1,2,5], our data show exposure to common replacement bisphenols induces germline effects in both sexes that may affect multiple generations. These findings add to growing evidence of the biological risks posed by this class of chemicals. Rapid production of structural variants of BPA and other EDCs circumvents efforts to eliminate dangerous chemicals, exacerbates the regulatory burden of safety assessment, and increases environmental contamination. Our experience suggests these environmental contaminants not only pose a risk to reproductive health but also to the integrity of the research environment. EDCs, like endogenous hormones, can affect diverse processes. The sensitivity of the germline allows us to detect effects that, although not immediately apparent in other systems, may induce variability that undermines experimental reproducibility and impedes scientific advancement.
Results and Discussion
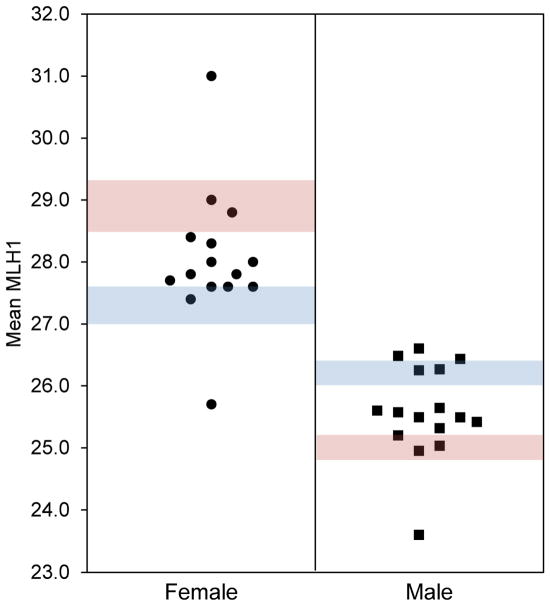
In the course of meiotic studies in male and female mice, we observed variation in meiotic recombination (measured by the number of MLH1 foci in pachytene stage meiocytes), with levels in some controls reaching values characteristic of BPA-exposed animals [2,5]. Although the change in pooled data was subtle, variation among litters was striking (Figure 1). Given our previous experience with BPA leaching from polycarbonate cages and water bottles [1], damaged materials were an obvious suspect. When white residue was evident on the surface of some polysulfone cages in our facility (Figure 2A), we suspected that exposure to chemicals leaching from the damaged polymer was eliciting meiotic effects.
Figure 1. Variation in control data suggests environmental contamination.
Data from 15 litters (1–2 fetuses each) of C57BL/6 females (circles) and 16 litters (2–4 adults each) of 129S1/SvimJ males (squares) showing variation in mean MLH1 counts in control animals analyzed during a 6-month period. Shaded bars denote historical laboratory means ± SEM for control (blue) and exposed (pink) animals, showing an exposure-induced increase in females and decrease in males as reported previously [2,5,53].
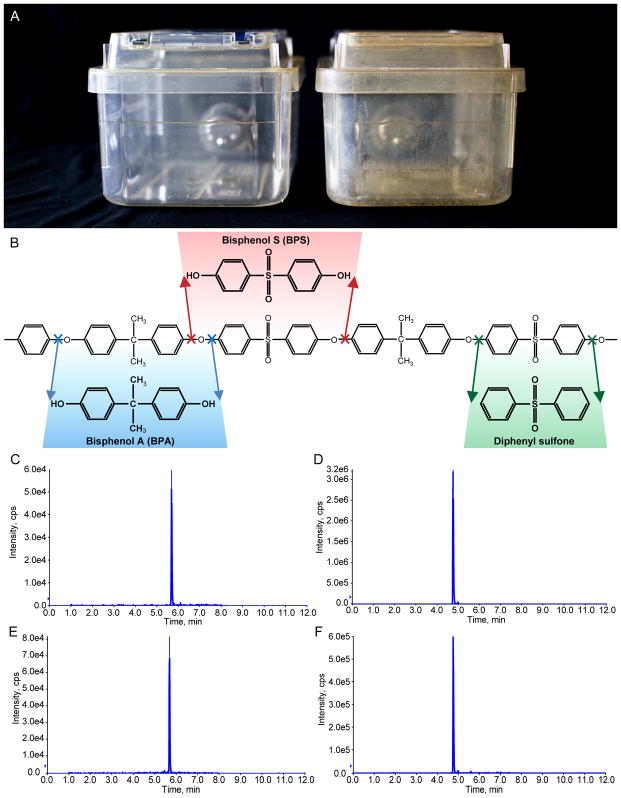
Figure 2. BPA and BPS are released from damaged polysulfone.
(A) Comparison of an undamaged polysulfone cage (left) and cage with white residue indicative of damage (right). (B) Structure of the BPA-diphenyl sulfone dimer that comprises polysulfone. Arrows denote cleavage sites that would result in the release of BPA (blue), BPS (red), and diphenyl sulfone (green). (C–F) Extracted ion chromatogram results showing BPA and BPS standards at 10 ng/mL (C and D, respectively) and results from the analysis of BPA and BPS in white residue scraped from a damaged cage (E an d F, respectively). The concentration of BPS detected in the damaged cage was greater than BPA (note differences in y-axis).
An unexpected contaminant
Polysulfone is comprised of BPA and diphenyl sulfone (Figure 2B), thus we suspected these were the contaminants of interest. LC-MS/MS analysis of a methanol extraction of damaged cages, however, demonstrated the presence of both BPA and BPS (Figure 2C–F). Because polymeric aromatic ethers, like their monomeric counterparts, cannot undergo nucleophilic substitution to generate an unsubstituted aromatic ring at the reaction site, degradation results in the formation of a phenolic group. Hence, damaged polysulfone is, in fact, more likely to generate BPS than diphenyl sulfone (Figure 2B). Unfortunately, high signal levels in both control and solvent blanks made it impossible to determine if diphenyl sulfone was a significant contaminant.
Replacement bisphenols have rapidly emerged in consumer products, and studies of them are limited. However, plastics containing them can leach estrogenic chemicals [42,43], and exposure has been reported to induce adverse effects similar to BPA (e.g., [44–51] reviewed in [52]). Our findings suggest that, although newer polymers like polysulfone are more resistant to chemical damage than polycarbonate, damage can occur in the course of normal use, and may result in the release of contaminants that are not constituent components of the polymer.
Bisphenol analogs elicit meiotic effects
To eliminate contamination, all caging materials in the facility were replaced, new breeding stocks purchased, and studies conducted to confirm that control values in both sexes had returned to expected levels. To verify that the contaminant bisphenols elicit meiotic effects, we designed experimental studies.
Our previous studies in mice suggest that a brief, appropriately timed exposure to BPA can impact the entire germ cell population in both sexes, although the timing and mechanisms differ. In females, all oocytes enter meiosis in the fetal ovary, and in utero exposure coinciding with meiotic onset increases levels of meiotic recombination [2,18]. The subtle changes induced are compatible with continued oocyte survival but increase the frequency of aneuploid eggs and embryos produced by the adult female [2]. In contrast, in males, BPA and other estrogenic exposures also can affect the entire germline, not by epigenetically modifying cells entering meiosis, but rather the germline stem cells. Neonatal exposure coinciding with the establishment of the spermatogonial stem cell (SSC) population of the testis causes a permanent reduction in recombination levels in all descendant spermatocytes [5,53].
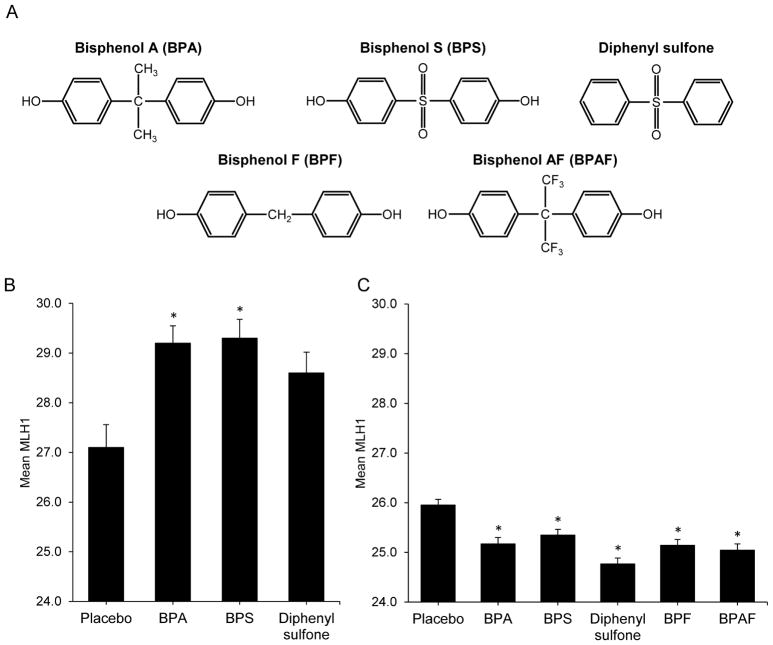
While rebuilding our colony and confirming that contamination had been eliminated, we initiated studies to assess the effects of the putative contaminants BPS and diphenyl sulfone using timed pregnant females purchased from The Jackson Laboratory. Oral doses of 20 ng/g BPA (positive control), BPS, diphenyl sulfone (Figure 3A), or placebo (vehicle only) control were administered on 14 and 15 days post coitum (dpc) to coincide with the time of meiotic entry in the fetal ovary. Twenty ng/g is below the US EPA tolerable daily intake level for BPA (50 ng/g/day), and thus is a low dose with human relevance. By comparison with unexposed female fetuses, BPA and BPS exposure induced a significant increase in mean MLH1 counts (27.1 ± 0.5, 29.2 ± 0.3, and 29.3 ± 0.4, respectively; post-hoc p < 0.01; Figure 3B). Diphenyl sulfone also elicited an increase (28.6 ± 0.4) but was not significant due to the limited sample size. Our previous studies in both mice and monkeys demonstrated similarly increased levels of meiotic recombination in developing oocytes as a result of maternal BPA exposure [2,18].
Figure 3. Bisphenol analog exposure elicits male and female meiotic effects.
(A) Chemical structures of BPA and four replacement bisphenols. (B) Mean MLH1 counts ± SEM for females treated 14–15 dpc with placebo, or 20 ng/g BPA, BPS, or diphenyl sulfone. Groups represent 37, 100, 88, and 56 cells for 3 placebo, 7 BPA, 6 BPS, and 6 diphenyl sulfone females, respectively). (C) Mean MLH1 counts ± SEM for males treated from 1–8 dpp with placebo or 20 ng/g BPA, BPS, diphenyl sulfone, BPF, or BPAF. Groups represent 420, 270, 330, 300, 385, and 270 for 14 placebo, 9 BPA, 11 BPS, 10 diphenyl sulfone, 13 BPF, and 9 BPAF males, respectively). Groups were compared by one-way ANOVA (F = 4.1, p < 0.01 for females; F = 11.4, p < 0.0001 for males). Significant differences were determined by Tukey-Kramer post-hoc test (asterisk denotes p < 0.01 post-hoc comparison with the placebo). See also Figure S1.
In males, we assessed the effects of neonatal exposure to the putative contaminants, BPS and diphenyl sulfone, and two other common replacement bisphenols, BPF and BPAF (Figure 3A). Males were given daily oral doses of 20 ng/g BPA, BPS, diphenyl sulfone, BPF, BPAF, or placebo from 1–8 days post-partum (dpp) and meiotic analyses conducted on 6-week-old adults. As shown in Figure 3C, all bisphenols induced significant meiotic effects. By comparison with controls (26.0 ± 0.1), mean MLH1 counts in exposed males were significantly reduced, with diphenyl sulfone eliciting the strongest effect: 25.2 ± 0.1, 25.3 ± 0.1, 24.8 ± 0.1, 25.1 ± 0.1, and 25.0 ± 0.1 for BPA, BPS, diphenyl sulfone, BPF, and BPAF, respectively (Figure 3C, post-hoc p < 0.01).
Low recombination rates are deleterious because spermatocytes with homologs that fail to undergo recombination face certain death due to the actions of a robust spindle assembly checkpoint mechanism that causes arrest and demise of cells with unpartnered chromosomes at metaphase I [5,54,55]. As predicted on the basis of previous studies [5,53], reduced recombination levels in bisphenol exposed males resulted in an increase in the frequency of spermatocytes with at least one synaptonemal complex lacking an MLH1 focus (i.e., MLH1 null SCs; Figure S1).
Although “BPA free” is a valuable marketing tool and most consumers interpret this label as an indication of a safer product, our findings add to growing evidence from studies in C. elegans [56], zebrafish [45,48,51,57–59], mice [46,49,50,60–62], rats [63–65], and human in vitro studies [25,44,47,66] that replacement bisphenols have the potential to induce adverse effects similar to those reported for BPA. Meiosis is both a sensitive indicator of environmental contamination and, because recombination directly affects the amount of genetic diversity in a population, an evolutionary driver. Thus, exposures that influence recombination are cause for concern. Importantly, meiotic effects of bisphenol exposure are clearly not limited to mice. Remarkably similar effects of BPA and replacement bisphenols have been reported in C. elegans, although subtle mechanistic differences among bisphenols are evident [56]. While understanding the mechanism of action of individual chemicals is important, our data suggest that bisphenols as a class should be considered germline toxicants.
Exposure effects persist in males for several generations
Meiotic recombination is quantitative, making it a powerful means of tracing exposure effects across generations. Our previous studies suggest meiotic effects induced by neonatal estrogenic exposure in male mice are transmitted to offspring and exposure effects intensify with successive generations of exposure [53]. Thus, inadvertent exposure of our animals provided an opportunity to determine if and for how long exposure effects persisted after the elimination of environmental bisphenol contamination. Three 129S1/SvimJ males from the exposed colony served as founders (F0) for an analysis of four successive generations of unexposed male descendants. F0s were born and weaned in contaminated cages but transferred as adults to new cages with sibling females to produce F1 males. On average, ten males from at least three litters were produced each generation for each family. Male progeny from new 129S1/SvimJ breeding stock served as unexposed controls. Our analysis of over 120 male progeny provided evidence both of the variability of the exposure effect on our animals and that exposure effects spanned several generations.
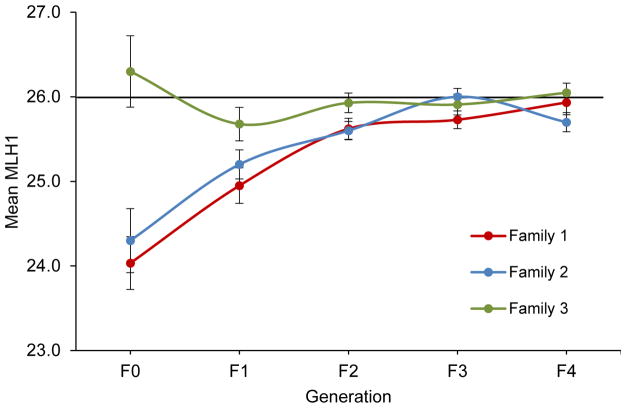
As shown in Figure 4, families 1 and 2 exhibited similar trends, with a significant reduction in recombination levels by comparison with controls for the first three generations (F0–F2; assessed by one-way ANOVA; Figure 4). In contrast, the MLH1 mean of the founder for family 3 (26.3 ± 0.4) was in the control range and subsequent generations of offspring did not deviate significantly. The variation among founder males is consistent with the inter-litter variation that characterized the exposure effect (Figure 1), but effectively reduced our generational study to the analysis of two families.
Figure 4. Effects of inadvertent exposure on male meiotic recombination rate persist for several generations.
Black line denotes MLH1 mean for new colony 129S1/SvimJ males (26 ± 0.0 foci/cell, n = 1848 cells from 63 males from 22 litters). Colored lines show MLH1 mean ± SEM for three different founder males (F0) from the exposed colony and four subsequent generations of unexposed male offspring (F1 – F4). Twenty-five to 30 pachytene cells were analyzed per male, and F1 groups consisted of 4–6 males/family, F2 of 11–14 males/family, F3 of 12–16 males/family, and F4 of 8–12 males/family. Means for each generation were compared to the new colony mean using one-way ANOVA (F = 13.4, p < 0.0001 for family 1; F = 12.0, p < 0.0001 for family 2; and F = 1.6, p = 0.2 for family 3), and significant differences between groups were assessed using a Tukey-Kramer post-hoc. For both family 1 and 2, the F0, F1, and F2 generations had significantly lower mean MLH1 values by comparison with the new colony (post-hoc p < 0.05); the F2, F3, and F4 generations had significantly higher mean MLH1 values by comparison with the F0 (post-hoc p < 0.05).
Recombination levels in founders from both family 1 and 2 were low by comparison with controls (24.0 ± 0.3, 24.3 ± 0.4, and 26.0 ± 0.0, respectively; post-hoc p < 0.01; Figure 4). In both families, F1 males showed an increase in mean MLH1 levels (24.9 ± 0.2 and 25.2 ± 0.2, respectively) by comparison with their fathers, although the difference did not reach significance. By the F2 generation, mean MLH1 values reached an intermediate level (25.6 ± 0.1 in both families) that was significantly different (post-hoc p < 0.05) from both the F0 and the new colony mean, providing evidence of a transgenerational effect. However, in the F3 generation, mean values for both families (25.7 ± 0.1 and 26.0 ± 0.1, respectively) were not significantly different from the new colony, and this return to expected control values was evident in the F4 generation (Figure 4).
Although we cannot pinpoint the onset of the accidental exposure in our 129S1/SvimJ colony, evidence of exposure effects in some families but not others suggests that the duration of exposure was limited to a single generation. Indeed, one founder male exhibited no evidence of exposure. Thus, while our data suggest eradication of male germline effects after several generations, they do not allow us to draw conclusions about a scenario with greater human relevance, i.e., the resolution of effects following multiple generations of exposure. This is an important consideration in view of our recent finding that the testis phenotype is exacerbated by successive generations of exposure [53].
Brave new world
DuPont’s 20th century slogan “better living through chemistry” has been borne out. Remarkable technical advances allow us to synthesize molecules and create subtle variations in them. Innovation, however, has outpaced our ability to understand the implications of the release of rapidly generated families of structurally similar chemicals into our environment. Our data add to and extend the growing concern about the harmful reproductive effects of one such family, the bisphenols. Although most data derive from rodent studies, given the developmental and reproductive similarities, concerns almost certainly extend to humans. Importantly, bisphenols are not the only chemical family with an ever-increasing array of diverse members; other prominent environmental contaminant families include the parabens, perfluorinated compounds (PFCs), phthalates, flame retardants, and quaternary ammonium compounds.
The ability to rapidly enhance the properties of a chemical has tremendous potential for treating cancer, enhancing medical and structural materials, and controlling dangerous infectious agents. Importantly, this technology has paved the way for “green chemistry”, a healthier future achieved by engineering chemicals to ensure against hazardous effects (e.g., [67]). Currently, however, regulatory agencies charged with assessing chemical safety cannot keep pace with the introduction of new chemicals. Further, as replacement bisphenols illustrate, it is easier and more cost effective under current chemical regulations to replace a chemical of concern with structural analogs rather than determine the attributes that make it hazardous.
The environmental exposure underlying this study is the third such inadvertent environmental contamination encountered in the course of studies in our laboratory [1,68]. The sensitivity of the germ cell endpoints we study has made it possible to rapidly detect the effects of these environmental contaminants, but identifying and eliminating them has impeded our research. Because we study environmental effects, we are vigilant about controlling the animal environment and testing contact materials. Thus, repeated inadvertent contamination in the course of our studies is an indicator of the sheer number of contaminants and their ubiquitous presence in daily life. This not only represents a hazard to human health, but also to the ability of scientists to conduct sound and meaningful studies. For example, initial data suggest that inadvertent contamination may have compromised the CLARITY-BPA project sponsored by the U.S. Food and Drug Administration (FDA) and the National Toxicology Program (NTP) [69,70]. CLARITY-BPA is a multi-investigator initiative conducted under federal oversight and designed to comprehensively test the effects of BPA exposure. Thus, because findings from this initiative will inform regulatory decisions regarding BPA in the U.S., evidence of possible contamination of control animals in the CLARITY-BPA project is disturbing. As our data demonstrate, common EDCs that are prevalent environmental contaminants have the potential to introduce significant variability in research studies. The NIH considers rigor and reproducibility “the cornerstones of science advancement.” Thus, compromised studies in both our laboratory and the CLARITY-BPA project suggest that, by interfering with reproducibility of results, environmental contamination can undermine scientific interpretation.
STAR★Methods
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Patricia Hunt (pathunt@wsu.edu).
Experimental model and subject details
Breeding stocks of adult inbred 129S1/SvimJ and C57BL/6J mice (The Jackson Laboratory) were mated in brother/sister breeding pairs with mating beginning at 6-weeks of age (sexual maturity). Pups resulting from these matings were weaned at 20 days post-partum (dpp) and housed in polysulfone cages (Allentown Inc.) separated by sex, with no more than 5 mice per cage. Cages were kept on ventilated racks (Allentown Inc., Jag 75 micro isolator model) on a 14-hr light/10-hr dark cycle, in a climate controlled, specific pathogen-free facility, monitored quarterly by sentinel mice. Cages contained Sanichip 7090A bedding (Harlan Laboratories) and a nestlet (Ancare) for enrichment. Drinking water in polysulfone bottles and irradiated food (Envigo Teklad 2920) were autoclaved and provided ad libitum. Littermates of the same sex were randomly assigned to experimental groups. All adult mice were killed using inhaled CO2 until cessation of breathing was observed, followed by secondary internal cervical dislocation. Fetal mice were euthanized using decapitation as specified by the Washington State University Institutional Care and Use Committee. All protocols were approved and followed the National Institute of Health standards established by the Guide for Care and Use of Laboratory Animals (The National Academies Press). Washington State University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Method details
Detection of contaminants in damaged cages
Cage extractions were obtained by sequentially rinsing five mouse cages with 100 mL of absolute methanol. The resultant methanol extraction was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an Agilent LC1260 (Agilent, Santa Cruz, CA)-AB Sciex 5500 Triple Quadrupole MS (AB Sciex, Foster City, CA) at the University of California San Francisco. Samples from cage filters and scrapings were extracted with methanol, evaporation, and reconstitution in 10% methanol for injection into the LC-MS/MS. One mL aliquots of methanol were included as a negative control. Extracts were injected into an Agilent Extend-C18 (2.1 × 100 mm, 1.8 μm) column, maintained at 50°C. Chromatographic separation of the analytes was achieved by gradient elution using water with 0.05% ammonium acetate (pH 7.8) as mobile phase A and methanol with 0.05% ammonium acetate (pH 7.8) as mobile phase B. The elution gradient employed was- 0–0.5 min = 30%B; 1 min = 75%B; 4 min = 100%B; 4–6 min = 100%B; and 6.01–12 min = 30% B. The limit of detection (LOD) was 0.2 ng/mL for BPA and 0.01 ng/mL for BPS. Data analysis was done using AB Sciex Analyst 1.6 software package. Identification and confirmation of each analyte in the sample was based on its retention time and the peak area ratio between its two transitions. A signal/noise (S/N) ration of > 3 was used to define qualitative signals.
Breeding paradigm for recovery analysis
Three males born and weaned in contaminated cages served as F0 founders of three families. At 6-weeks of age, founder males were placed in new, undamaged cages and paired with two female siblings to produce second-generation (F1) offspring. Each founder produced 2–4 litters, and each litter was weaned into new, undamaged cages. At 6-weeks of age, one male from one litter of each family was paired with two sister females to produce the third-generation (F2). Each male produced 3–4 litters. This pattern was repeated to produce fourth-generation (F3) and fifth-generation (F4) offspring for each family. In total, there were 3 F0, 15 F1, 39 F2, 41 F3, and 31 F4 129S1/SvimJ males from 2, 9, 10, 12, and 12 litters, respectively. Adult males of all generations were killed between 6–12-weeks of age as specified above and their testes surgically collected in phosphate-buffered saline (PBS: 136.9 mM NaCl, 53.7 mM KCl, 29.4mM KH2PO4, 129.6 mM Na2HPO4, pH 7.4).
Treatment solutions
For intentional exposure studies, new breeding stocks of 129S1/SvimJ and C57BL/6 mice were obtained from The Jackson Laboratory to generate offspring for analysis. To verify the elimination of contamination, we analyzed meiotic recombination levels in replacement animals born in our facility. Levels in 129S1/SvimJ males appeared normal, but slightly high mean recombination levels were observed in C57BL/6 females. When levels remained high after several months, we resorted to the use of timed pregnant females purchased from The Jackson Laboratory to experimentally assess the effects of exposure to the putative bisphenol contaminants. Females arrived on 13 days post coitum (dpc) and were treated 14–15 dpc with 20 ng/g BPA, BPS or diphenyl sulfone, or placebo (equal volume ethanol/corn oil vehicle). All chemicals were dissolved in 100% ethanol and diluted in tocopherol-stripped corn oil to a 2.5% (v/v) ethanol solution and administered orally by pipette as previously stated for the male mice. Female dams were given a 20 ng/g body weight dose as determined via electronic scale daily. Dams were killed on 17.5 dpc, and fetuses were collected in phosphate-buffered saline (PBS). Female sample size consisted of 3 placebo, 7 BPA, 6 BPS, and 6 diphenyl sulfone C57BL/6 females from 2, 3, 2, and 3 litters, respectively.
Male 129S1/SvimJ mice were treated from 1–8 dpp with 20 ng/g of either diphenyl sulfone, bisphenol A (BPA), bisphenol S (BPS), bisphenol F (BPF), bisphenol AF (BPAF), or placebo (an equal volume ethanol/corn oil vehicle). Treatment stock solutions were made by dissolving powder solid chemicals in 100% ethanol and reduced to treatment dosages by serial dilutions. All treatment solutions were made containing 1% (v/v) ethanol in tocopherol-stripped corn oil (MP Biomedicals), such that solution concentrations were 20 ng/μl of solution. Mice were dosed with one μL treatment solution per gram of body weight, with pup weights estimated as average weight for age and strain. This produced a final daily dose of 20 ng/g which was chosen for several reasons: First, it is below the US EPA tolerable intake level for BPA (50 ng/g/day), and thus represents a low dose with human relevance; second, a 20 ng/g dose of BPA elicits meiotic effects in male mice [5]; and third, using the same dose for all chemicals makes it possible to compare their relative potency. Adult males were killed at 6-weeks of age as specified above and their testes were surgically collected in PBS. Male samples consisted of 14 placebo, 9 BPA, 11 BPS, 10 diphenyl sulfone, 13 BPF, and 9 BPAF 129S1/SvimJ males from 5, 3, 3, 3, 3, and 4 litters, respectively.
Meiocyte preparations and immunostaining
Meiocyte preparations were made according to the method developed by Peters and colleagues [71]. Testes were cleaned in PBS and incubated in hypotonic solution (30mM tris, pH 8.2–8.4; 50 mM sucrose; 17 mM sodium citrate; and 5 mM EDTA, pH 8.2) for 20 min. Several seminiferous tubules from each testis were separated and macerated in 500 mM sucrose. Cell suspensions were spread on slides coated in paraformaldehyde (PFA: 1% paraformaldehyde, 50 μL Triton X-100, pH 9.2). Ovaries were cleaned in PBS and incubated in hypotonic solution for 12 min. Both ovaries from each fetus were macerated together in 500 mM sucrose and the cell suspension was spread on slides coated in PFA. Slides were incubated in a humid chamber for 2 hrs. and washed with 0.4% Photo-flo 200 solution (Kodak Professional). Immunofluorescence staining of slides was performed as described previously [5,53]. Slides were stained with MLH1 primary antibody (BD Pharmingen, 550838, at 1:60) overnight followed by SYCP3 primary antibody (Novus, NB300-232, at 1:200) for 2 hrs., and counterstained with Alexa Fluor 488-conjugated AffiniPure Donkey Anti-Mouse (AFDAM) secondary antibody (Jackson Immunoresearch Laboratories, Inc., 715-545-150, at 1:75) and either 2 hrs. in Cy3-conjugated AffiniPure Donkey Anti-Rabbit (CDAR) secondary antibody for spermatocytes (Jackson Immunoresearch Laboratories, Inc., 711-165-152, at 1:1500) or 1 hr. in Rhodamine-conjugated AffiniPure Donkey Anti-rabbit (RDAR) secondary antibody for oocytes (Jackson Immunoresearch Laboratories, Inc., 711-025-152, at 1:200). All staining was done in 1X ADB (10X stock consisted of 10 mL normal donkey serum, 3.0 g BSA, 50 μL Triton-X 100, and 90 mL PBS that was then sterile filtered with a 45 μm filter), and slides were incubated at 37°C.
MLH1 analysis
Spermatocytes were imaged using the GenASI Scan & Analysis platform with an Olympus BX61 microscope. Oocytes were imaged using a Zeiss Axio Imager epifluorescence microscope. MLH1-FITC, SYCP3-TRITC, and DAPI were imaged sequentially, and brightness adjusted using either GenASI MetScan or Axiovision software. The number of MLH1 foci in composite MLH1/SYCP3 images was counted by two independent observers who were blinded with regard to exposure group.
Quantification and statistical analysis
Twenty-five to thirty pachytene spermatocytes were scored per animal, and minor counting discrepancies were resolved. No minimum or maximum number of oocytes was analyzed per animal. Cells with major scoring discrepancies, poor staining, or synaptic defects were excluded from analysis. In addition, cells with greater than two MLH1 null SCs were excluded to eliminate potential bias due to poor immunostaining.
Average MLH1 counts were determined for each animal and pooled averages were determined for each treatment group (n = number of cells). Male sample sizes comprised of: 3 F0 males (90 cells), 15 F1s (441 cells), 39 F2s (1167 cells), 41 F3s (1218 cells), 31 F4s (928 cells), and 91 new colony (2675 cells) for persistence of inadvertent exposure in the “old colony”; 14 placebo (420 cells), 9 BPA (270 cells), 11 BPS (330 cells), 10 diphenyl sulfone (300 cells), 13 BPF (385 cells), and 9 BPAF (270 cells). Female sample sizes consisted of 3 placebo (37 cells), 7 BPA (100 cells), 6 BPS (88 cells), and 6 diphenyl sulfone (56 cells). Differences in mean MLH1 foci counts and MLH1 null frequencies among exposure groups or generations were analyzed by one-way ANOVA. For statistically significant differences (p < 0.05), a Tukey-Kramer post-hoc test was performed to infer which groups differed. All statistical analysis was performed using Microsoft Excel.
Data and software availability
Data are available on request. Please contact the lead author.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Hannah Kiser, Alyssa Marre, Nitha Muntu, and Galen Gorence for their assistance with data collection and Terry Hassold for comments on the manuscript. Support for these studies was provided from NIH grant R01 HD083177to PH and R56 ES13527 to PH and RG.
Footnotes
Declaration of interests
The authors declare no competing interests.
Author contributions
Conceptualization, Methodology: PAH TSH. Investigation: TSH, HP, CL, RG, SM, MCG, CVS. Formal analysis: TSH, HP, CL, SM, RG, PAH. Visualization: TSH, CL, SM. Resources: PAH, RG. Funding Acquisition: PAH. Writing – original draft, review, and editing: TSH, PAH, HP, RG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 2.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Zhang W, Liu J, Wang W, Li H, Zhu J, Weng S, Xiao S, Wu T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod Toxicol. 2014;44:33–40. doi: 10.1016/j.reprotox.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015;11:e1004949. doi: 10.1371/journal.pgen.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284:354–362. doi: 10.1016/j.taap.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arambula SE, Fuchs J, Cao J, Patisaul HB. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: A CLARITY-BPA consortium study. Neurotoxicology. 2017;63:33–42. doi: 10.1016/j.neuro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, Harris EP, Zhou C, Flaws JA, Adli M, et al. Transgenerational effects of bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinology. 2018;159:132–144. doi: 10.1210/en.2017-00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao G, Zhao Y, Li H, Li W. Bisphenol A-elicited miR-146a-5p impairs murine testicular steroidogenesis through negative regulation of Mta3 signaling. Biochem Biophys Res Commun. 2018;50:478–485. doi: 10.1016/j.bbrc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suvorov A, Waxman DJ. Early programing of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies. Reprod Toxicol. 2015;57:59–72. doi: 10.1016/j.reprotox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartain CV, Hunt PA. An old culprit but a new story: Bisphenol A and “NextGen” bisphenols. Fertil Steril. 2016;106:820–826. doi: 10.1016/j.fertnstert.2016.07.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vrooman LA, Xin F, Bartolomei MS. Morphologic and molecular changes in the placenta: What we can learn from environmental exposures. Fertil Steril. 2016;106:930–940. doi: 10.1016/j.fertnstert.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patisaul HB. Endocrine disruption of vasopressin systems and related behaviors. Front Endocrinol (Lausanne) 2017;8:134. doi: 10.3389/fendo.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassenaar PNH, Trasande L, Legler J. Systematic Review and Meta-Analysis of Early-Life Exposure to Bisphenol A and obesity-related outcomes in rodents. Environ Health Perspect. 2017;125:106001. doi: 10.1289/EHP1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata M, Kang JH. Bisphenol A (BPA) and cell signaling pathways. Biotechnol Adv. 2018;36:311–327. doi: 10.1016/j.biotechadv.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Nesan D, Sewell LC, Kurrasch DM. Opening the black box of endocrine disruption of brain development: Lessons from the characterization of Bisphenol A. Horm Behav. 2018 doi: 10.1016/j.yhbeh.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsworth JD, Jentsch JD, VandeVoort CA, Roth RH, Redmond DE, Jr, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calhoun KC, Padilla-Banks E, Jefferson WN, Liu L, Gerrish KE, Young SL, Wood CE, Hunt PA, VandeVoort CA, Williams CJ. Bisphenol A exposure alters developmental gene expression in the fetal rhesus macaque uterus. PLoS One. 2014;9:e85894. doi: 10.1371/journal.pone.0085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L, Wang S, Wang Y, He M, Liu D. Bisphenol A exposure accelerated the aging process in the nematode Caenorhabditis elegans. Toxicol Lett. 2015;235:75–83. doi: 10.1016/j.toxlet.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Brieño-Enriquez MA, Reig-Viader R, Cabero L, Toran N, Martinez F, Roig I, Garcia Caldes M. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod. 2012;18:171–183. doi: 10.1093/molehr/gar074. [DOI] [PubMed] [Google Scholar]
- 23.Vitku J, Heracek J, Sosvorova L, Hampl R, Chlupacova T, Hill M, Sobotka V, Bicikova M, Starka L. Associations of bisphenol A and polychlorinated biphenyls with spermatogenesis and steroidogenesis in two biological fluids from men attending an infertility clinic. Environ Int. 2016;89–90:166–173. doi: 10.1016/j.envint.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Olson MR, Su R, Flaws JA, Fazleabas AT. Bisphenol A impairs decidualization of human uterine stromal fibroblasts. Reprod Toxicol. 2017;73:339–344. doi: 10.1016/j.reprotox.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eladak S, Moison D, Guerquin MJ, Matilionyte G, Kilcoyne K, N’Tumba-Byn T, Messiaen S, Deceuninck Y, Pozzi-Gaudin S, Benachi A, et al. Effects of environmental bisphenol A exposures on germ cell development and Leydig cell function in the human fetal testis. PLoS One. 2018;13:e0191934. doi: 10.1371/journal.pone.0191934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Lau SW, Fan Y, Wu RSS, Ge W. Juvenile exposure to bisphenol A promotes ovarian differentiation but suppresses its growth – Potential involvement of pituitary follicle-stimulating hormone. Aquat Toxicol. 2017;193:111–121. doi: 10.1016/j.aquatox.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Santangeli S, Maradonna F, Gioacchini G, Cobellis G, Piccinetti CC, Dalla Valle L, Carnevali O. BPA-induced deregulation of epigenetic patterns: Effects on female zebrafish reproduction. Sci Rep. 2016;6:21982. doi: 10.1038/srep21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allard P, Colaiácovo MP. Mechanistic insights into the action of bisphenol A on the germline using C. elegans. Cell Cycle. 2011;10:183–184. doi: 10.4161/cc.10.2.14478. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Guo JY, Li X, Zhou HJ, Zhang SH, Liu XD, Chen DY, Fang YC, Feng XZ. Behavioural effect of low-dose BPA on male zebrafish: Tuning of male mating competition and female mating preference during courtship process. Chemosphere. 2017;169:40–52. doi: 10.1016/j.chemosphere.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Zhou D, Yang J, Li H, Lu Q, Liu Y, Lin K. Ecotoxicity of bisphenol A to Caenorhabditis elegans by multigenerational exposure and variations of stress response in vivo across generations. Environ Pollut. 2016;208:767–773. doi: 10.1016/j.envpol.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: A review (2007–2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ejaredar M, Lee Y, Roberts DJ, Sauve R, Dewey D. Bisphenol A exposure and children’s behavior: A systematic review. J Expo Sci Environ Epidemiol. 2017;27:175–183. doi: 10.1038/jes.2016.8. [DOI] [PubMed] [Google Scholar]
- 34.Česen M, Lenarčič K, Mislej V, Levstek M, Kovačič A, Cimrmančič B, Uranjek N, Kosjek T, Heath D, Dolenc MS, et al. The occurrence and source identification of bisphenol compounds in wastewaters. Sci Total Environ. 2018;616–617:744–752. doi: 10.1016/j.scitotenv.2017.10.252. [DOI] [PubMed] [Google Scholar]
- 35.Xue J, Liu W, Kannan K. Bisphenols, benzophenones, and bisphenol A diglycidyl ethers in textiles and infant clothing. Environ Sci Technol. 2017;51:5279–5286. doi: 10.1021/acs.est.7b00701. [DOI] [PubMed] [Google Scholar]
- 36.Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ Sci Technol. 2012;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Abualnaja KO, Asimakopoulos AG, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Minh TB, Moon HB, et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ Int. 2015;83:183–191. doi: 10.1016/j.envint.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- 39.Regueiro J, Wenzl T. Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A. 2015;1422:230–238. doi: 10.1016/j.chroma.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 40.Regueiro J, Wenzl T. Development and validation of a stable-isotope dilution liquid chromatography–tandem mass spectrometry method for the determination of bisphenols in ready-made meals. J Chromatogr A. 2015;1414:110–121. doi: 10.1016/j.chroma.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol. 2015;49:11834–11839. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. Most plastic products release estrogenic chemicals: A potential health problem that can be solved. Environ Health Perspect. 2011;119:989–996. doi: 10.1289/ehp.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bittner GD, Yang CZ, Stoner MA. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ Health. 2014;13:41, 1–14. doi: 10.1186/1476-069X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103:11–21. doi: 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Kinch CD, Ibhazehiebo K, Jeong JH, Habibi HR, Kurrasch DM. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A. 2015;112:1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roelofs MJ, van den Berg M, Bovee TF, Piersma AH, van Duursen MB. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology. 2015;329:10–20. doi: 10.1016/j.tox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Desdoits-Lethimonier C, Lesné L, Gaudriault P, Zalko D, Antignac JP, Deceuninck Y, Platel C, Dejucq-Rainsford N, Mazaud-Guittot S, Jégou B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum Reprod. 2017;32:1465–1473. doi: 10.1093/humrep/dex093. [DOI] [PubMed] [Google Scholar]
- 48.Le Fol V, Aït-Aïssa S, Sonavane M, Porcher JM, Balaguer P, Cravedi JP, Zalko D, Brion F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf. 2017;142:150–156. doi: 10.1016/j.ecoenv.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Liang S, Yin L, Shengyang Yu K, Hofmann MC, Yu X. High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol A and selected analogues in mouse spermatogonial cells. Toxicol Sci. 2017;155:43–60. doi: 10.1093/toxsci/kfw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi M, Sekulovski N, MacLean JA, Hayashi K. Effects of bisphenol A analogues on reproductive functions in mice. Reprod Toxicol. 2017;73:280–291. doi: 10.1016/j.reprotox.2017.06.134. [DOI] [PubMed] [Google Scholar]
- 51.Qiu W, Shao H, Lei P, Zheng C, Qiu C, Yang M, Zheng Y. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere. 2018;194:1–8. doi: 10.1016/j.chemosphere.2017.11.125. [DOI] [PubMed] [Google Scholar]
- 52.Rochester JR, Bolden AL. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horan TS, Marre A, Hassold T, Lawson C, Hunt PA. Germline and reproductive tract effects intensify in male mice with successive generations of estrogenic exposure. PLoS Genet. 2017;13:e1006885. doi: 10.1371/journal.pgen.1006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eaker S, Cobb J, Pyle A, Handel MA. Meiotic prophase abnormalities and metaphase cell death in MLH1-deficient mouse spermatocytes: Insights into regulation of spermatogenic progress. Dev Biol. 2002;249:85–95. doi: 10.1006/dbio.2002.0708. [DOI] [PubMed] [Google Scholar]
- 55.Vernet N, Mahadevaiah SK, Ojarikre OA, Longepied G, Prosser HM, Bradley A, Mitchell MJ, Burgoyne PS. The Y-encoded gene Zfy2 acts to remove cells with unpaired chromosomes at the first meiotic metaphase in male mice. Curr Biol. 2011;21:787–793. doi: 10.1016/j.cub.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Shu L, Qiu Z, Lee DY, Settle SJ, Que Hee S, Telesca D, Yang X, Allard P. Exposure to the BPA-Substitute Bisphenol S causes unique alterations of germline function. PLoS Genet. 2016;12:e1006223. doi: 10.1371/journal.pgen.1006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol. 2013;47:8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- 58.Naderi M, Wong MYL, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Shi J, Jiao Z, Zheng S, Li M, Zhang J, Feng Y, Yin J, Shao B. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere. 2015;128:252–257. doi: 10.1016/j.chemosphere.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 60.Gong M, Huai Z, Song H, Cui L, Guo Q, Shao J, Gao Y, Shi H. Effects of maternal exposure to bisphenol AF on emotional behaviors in adolescent mice offspring. Chemosphere. 2017;187:140–146. doi: 10.1016/j.chemosphere.2017.08.119. [DOI] [PubMed] [Google Scholar]
- 61.LaPlante CD, Catanese MC, Bansal R, Vandenberg LN. Bisphenol S alters the lactating mammary gland and nursing behaviors in mice exposed during pregnancy and lactation. Endocrinology. 2017;158:3448–3461. doi: 10.1210/en.2017-00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding ZM, Jiao XF, Wu D, Zhang JY, Chen F, Wang YS, Huang CJ, Zhang SX, Li X, Huo LJ. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem Biol Interact. 2017;278:222–229. doi: 10.1016/j.cbi.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Yin J, Jiao Z, Shi J, Li M, Shao B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol Lett. 2012;211:201–209. doi: 10.1016/j.toxlet.2012.03.802. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Sheng N, Cui R, Feng Y, Shao B, Guo X, Zhang H, Dai J. Gestational and lactational exposure to bisphenol AF in maternal rats increases testosterone levels in 23-day-old male offspring. Chemosphere. 2016;163:552–561. doi: 10.1016/j.chemosphere.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 65.Ullah H, Jahan S, Ain QU, Shaheen G, Ahsan N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere. 2016;152:383–391. doi: 10.1016/j.chemosphere.2016.02.125. [DOI] [PubMed] [Google Scholar]
- 66.Verbanck M, Canouil M, Leloire A, Dhennin V, Coumoul X, Yengo L, Froguel P, Poulain-Godefroy O. Low-dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS One. 2017;12:e0179583. doi: 10.1371/journal.pone.0179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albert O, Nardelli TC, Hales BF, Robaire B. Identifying greener and safer plasticizers: A 4-step approach. Toxicol Sci. 2018;161:266–275. doi: 10.1093/toxsci/kfx156. [DOI] [PubMed] [Google Scholar]
- 68.Melin VE, Potineni H, Hunt P, Griswold J, Siems B, Werre SR, Hrubec TC. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol. 2014;50:163–170. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, Gamboa da Costa G, Woodling KA, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–97. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, Delclos KB, Fisher JW, Doerge DR. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol Sci. 2014;139:4–20. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters AH, Plug AW, van Vugt MJ, de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–68. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.