Abstract
Tumor immunoediting consisting of three phases of elimination, equilibrium or dormancy, and escape, has been supported by preclinical and clinical data. A comprehensive understanding of the molecular mechanisms by which anti-tumor immune responses regulate these three phases are important to developing highly tailored immunotherapeutics that can control cancer. To this end, IFN-γ produced by Th1 cells, cytotoxic T cells, NK cells and NKT cells is a pleiotropic cytokine that is involved in all three phases of tumor immunoediting, as well as during inflammation-mediated tumorigenesis processes. This essay presents a review of literature and suggests that overcoming tumor escape is feasible by driving tumor cells into a state of quiescent but not indolent dormancy in order for IFN-γ producing, tumor-specific T cells prevent tumor relapse.
Keywords: Interferon gamma, tumor immunoediting, tumor dormancy, immunotherapy
Introduction
Tumors display high levels of heterogeneity because of genetic instability, a characteristic of malignancy [1]. This results in a multitude of responses of tumor to the host immune responses or immunotherapeutics such that some tumor clones undergo apoptosis while other clones lay dormant and may later escape from the immune response and lead to distant metastasis. Anti-tumor immune responses utilize four major pathways to fight the tumor. Firstly, activated lymphocytes produce perforin to poke a hole in the extracellular membrane of target tumor cells as well as granzyme B to enter tumor cells and cleave caspases for the induction of apoptosis [2]. Secondly, they also express Fas-L to engage with Fas receptor on tumor cells and induce apoptosis [2]. Thirdly, they produce TNF-related apoptosis-inducing ligand (TRAIL) to engage with TRAIL receptors on tumor cells and in turn induce tumor cell apoptosis [3]. Finally, activated lymphocytes produce IFN-γ, which is a pleiotropic cytokine with a wide range of activity; IFN-γ simultaneously induces apoptosis, tumor dormancy, and immunoediting in tumor cells that could lead to tumor relapse and progression [4–8]. Paradoxically, chronic exposure of cells to IFN-γ facilitates the development of hepatocellular carcinoma [9], colorectal carcinoma [10], and papilloma [11]. Therefore, understanding the distinct mechanisms by which IFN-γ affects the tumor could lead to the development of highly tailored immunotherapeutics that could control the tumor without inducing tumor escape and relapse. IFN-γ is primarily produced by T cells, NK cells and NKT cells. The receptor for IFN-γ is comprised of two subunits which include IFN-γ receptor alpha (IFN-γ Rα) and IFN-γ receptor beta (IFN-γ Rβ). Binding of IFN-γ to its cell surface receptor IFN-γ Rα induces dimerization of IFN-γ Rα, thereby forming a site for the assembly with IFN-γ Rβ. Upon heterodimerization of IFN-γ Rα/IFN-γ Rβ, their intracellular janus family kinases, JAK1 and JAK2, respectively, dimerize and become phosphorylated. This phosphorylation creates binding sites for the signal transducer and activator of transcription (STAT) proteins, primarily STAT1 [12]. Phosphorylated STAT1 homodimers are then translocated into the nucleus to bind the interferon regulatory factor-1 (IRF-1) gene gamma-activated sequence (GAS) sites on the promoters of downstream target genes [13]. This, in turn, activates diverse pathways in different tumor clones.
IFN-γ induces apoptosis in tumor cells
IFN-γ exerts its tumor killing function directly by the induction of apoptosis or by facilitating non-apoptotic cell death, as well as indirectly by rendering tumor cells susceptible to apoptosis inducing function of the immune response or chemotherapies. For instance, IFN-γ induces IRF1, a tumor suppressor gene, which in turn reduces Bcl2 and increases Bak. These events facilitate the release of cytochrome c from mitochondria and activation of caspases, resulting in apoptosis [14]. Reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) at low concentrations are associated with cell proliferation. However, tumor cells that produce high amounts of RNI and ROS in response to IFN-γ tend to undergo apoptosis [15]. IFN-γ can also induce non-apoptotic cell death through the induction of autophagy in human hepatocellular carcinoma (HCC) [16]. IFN-γ-induced activation of STAT1 enhances the expression of the death receptor FAS and its ligand FAS-L in hepatoma and colon adenocarcinoma cells [17], and of TRAIL and its receptor death receptor 5 (DR5) in human tumor cell lines [18–20]. Accordingly, activated STAT1 sensitizes tumor cells to FAS or TRAIL mediated apoptosis. Also, activation of STAT1 by IFN-γ inhibits the expression of the p53 inhibitor murine double minute 2 (MDM2), thereby enhancing p53-induced apoptosis by doxorubicin and cisplatin [21].
IFN-γ arrests cancer growth by driving tumor cells into a state of dormancy
Although the IFN-γ/STAT1 pathway induces tumor cell apoptosis, activation of STAT1 can also result in the inhibition of tumor cell growth and establishment of dormancy. In melanoma, activation of the IFN-γ/STAT1 pathway results in the downregulation of cyclin E and cyclin A with consequent tumor cell dormancy [22]. Activated STAT1 can also interact with cyclins D1, D2, D3 and CDK4 and result in cell cycle arrest in fibrosarcoma cells [23]. Tumor inhibitory function of IFN-γ-induced STAT1 activation is also mediated by the upregulation of the miRNA-29 family and a consequent downregulation of CDK6 in melanoma cells [24]. IFN-γ mediated tumor dormancy can also be induced independent from STAT1 signaling. Tumor clones that highly express indolamine 2,3-dioxygenase 1 (IDO1) and kynurenine (Kyn)-aryl hydrocarbon receptor (AhR) respond to IFN-γ by upregulating the cell cycle inhibitor p27, consequently preventing STAT1 signaling and inducing tumor dormancy [25]. In fact, p21 and p27 facilitate hypo-phosphorylation of the tumor suppressor Rb, thereby suppressing the activity of E2F transcription factor and inhibiting the activation of genes involved in cell proliferation. In a T antigen (Tag)-induced multistage carcinogenesis in pancreatic islets, IFN-γ producing CD4+ T cells inhibit tumor cell proliferation and establish tumor dormancy without destroying malignant cells [26]. It was also reported that CD8+ T cells maintain murine B cell lymphoma (BCL1) in the state of dormancy by producing IFN-γ [6]. Radiation-induced tumor dormancy is also mediated by the production of IFN-γ in Balb/c neu transgenic mice such that neutralization of IFN-γ reversed radiation-induced tumor dormancy and resulted in tumor relapse [27]. It has been demonstrated that levels of the expression of IFN-γ Rα on mammary tumor cells determine whether IFN-γ eliminates the tumor or establishes tumor dormancy. While low expression of IFN-γ Rα in tumor cells results in tumor dormancy, high levels of IFN-γ Rα expression result in tumor elimination in the presence of IFN-γ producing, neu-specific CD8+ T cell responses in FVB mice [7]. Given that STAT1 activation by IFN-γ results in the upregulation of major histocompatibility complex class I (MHC class I) molecules, which present antigens to T cells [28], dormant tumor cells could become more susceptible to the immune surveillance.
IFN-γ edits tumor cells and facilitates tumor escape and relapse
In addition to apoptosis inducing and tumor inhibitory functions, IFN-γ can also induce aberrant DNA methylation [29, 30] or genetic alteration in tumor cells [4], resulting in tumor progression and relapse. IFN-γ-induced tumor immunoediting is mediated through several mechanisms which include the induction of tumor antigen loss [30–34], upregulation of PD-L1 in tumor cells [35], recruitment of myeloid-derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs) to the tumor site [36, 37]. IFN-γ-induced HER2/neu loss has been reported in FVBN202 transgenic mouse model of breast cancer [30], and patients with HER2/neu positive ductal carcinoma in situ (DCIS) or breast cancer [32–34]. Activation of STAT1 by IFN-γ results in the induction of the immune checkpoint protein PD-L1 in tumor cells [38]. In addition, chronic IFN-γ signaling in tumor cells increases resistance to immune checkpoint blockade through STAT1-related epigenetic and transcriptomic alterations, rendering melanoma resistant to radiation therapy and immune checkpoint inhibitors [39]. It was suggested that the genomic instability induced by IFN-γ during tumor progression is due to adaptation of the tumor to an immunologically hostile microenvironment [4]. This phenomenon has been predicted by the adaptation model of immunity [40, 41]. Recent studies suggested that the state of tumor dormancy could determine whether IFN-γ may keep dormant cells in check or may edit dormant tumor cells and result in tumor relapse. Specifically, Ki67low indolent tumor cells are susceptible to immunoediting and escaping from immunotherapy whereas Ki67- quiescent dormant cells fail to undergo immunoediting and thus remain dormant by IFN-γ producing T cells [8]. Quiescent dormancy is due to lack of tumor cell proliferation and tumor cell arrest in G0 whereas indolent dormancy is due to a balance between tumor cell apoptosis and proliferation. Since genetic and epigenetic changes take place during cell division, indolent cells remain susceptible to immunoediting and escape from immunotherapy. We have reported that IFN-γ induces the expression of PD-L1 on Ki67low indolent, but not on Ki67- quiescent, dormant cells [8] . The detection of circulating tumor cells in breast cancer survivors even 22 years after mastectomy without clinical evidence of disease [42] suggests the existence and maintenance of tumor dormancy in cancer survivors.
Chronic exposure to IFN-γ facilitates tumorigenesis
Although IFN-γ is known for its ant-tumor function during anti-tumor immune responses, chronic exposure of normal cells to IFN-γ can also facilitate malignant transformation. In fact, IFN-γ appears to be pro-tumorigenic early during cell transformation whereas it manifests anti-tumor function against established tumors. For instance, IFN-γ has been reported to be involved in the initiation stage, but not in the promotion stage, of diethylnitrosamine-induced hepatocellular carcinoma due to its inflammatory function [9]. Suppressor of cytokine signaling-1 (SOCS1)-deficient mice are not able to inhibit IFN-γ inflammatory signaling. These mice develop spontaneous colorectal carcinoma because of the IFN-γ-induced hyperactivation of STAT1, which results in the induction of carcinogenesis-related enzymes, cyclooxygenase-2 and inducible nitric oxide synthase [10]. In the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced papilloma model, IFN-γ is involved in the development of papilloma by enhancing a Th17-associated inflammatory response [11]. IFN-γ producing macrophages were detected in 70% of human melanomas [43]. To this end, ultraviolet (UV)-induced cutaneous malignant melanoma can be abolished by systemic blockade of IFN-γ [43]. Non-alcoholic fatty liver disease (NAFLD) is also associated with the dominance of M1 macrophages which produce inflammatory cytokines, including IFN-γ [44, 45]. In fact, IFN-γ-induced protein 10 (IP-10) is elevated in patients with progressive NAFLD [46]. Dietary saturated fatty acids are major contributors to NAFLD through the activation of NF-kB, which is a key transcription factor for M1 macrophage activation [44, 47]. This, in turn, leads to inflammation-induced liver damage in nonalcoholic steatohepatitis (NASH) disease [45] and consequent progression to HCC [48, 49]. Even in the absence of NF-kB signaling, IFN-γ producing NKT cells actively participate in the pathogenesis of NASH disease [50]. Also, a higher frequency of IFN-γ producing Th1 cells is evident as NAFLD progresses to NASH disease [51].
Concluding remarks
IFN-γ is a pleiotropic cytokine which could manifest opposing effects on host cells ranging from cell transformation in the context of chronic inflammation, monocytes/macrophages, to anti-tumor effects, cytotoxic T cells (CTL), Th1, NK, NKT cells, during the immune response (Figure 1). The anti-tumor function of IFN-γ also varies depending on heterogeneity of the tumor cells and tumor microenvironment. IFN-γ can induce tumor cell apoptosis, directly or indirectly by upregulating the expression of FAS and DR5 on tumor cells. This cytokine can also induce cell cycle arrest and establish tumor cell dormancy. A dual function of IFN-γ appears to be due to low expression of IFN-γ Rα in tumor cells. Depending on the type of tumor dormancy, IFN-γ producing T cells can maintain tumor dormancy or result in tumor escape and relapse. In fact, IFN-γ could induce tumor immunoediting in indolent dormant cells (Ki67low) whereas it maintains quiescent dormant cells (Ki67-) in the state of dormancy without clinical evidence of disease. To this end, CD8+ T cells, Th1 cells, NK cells, NKT cells could be involved in the process of tumor immunoediting. Therefore, we suggest that establishment of quiescent tumor dormancy in residual disease by novel therapeutics may render dormant cells highly responsive to immunotherapy without risk of recurrence.
Figure 1. Multifaceted role of IFN-γ in cancer.
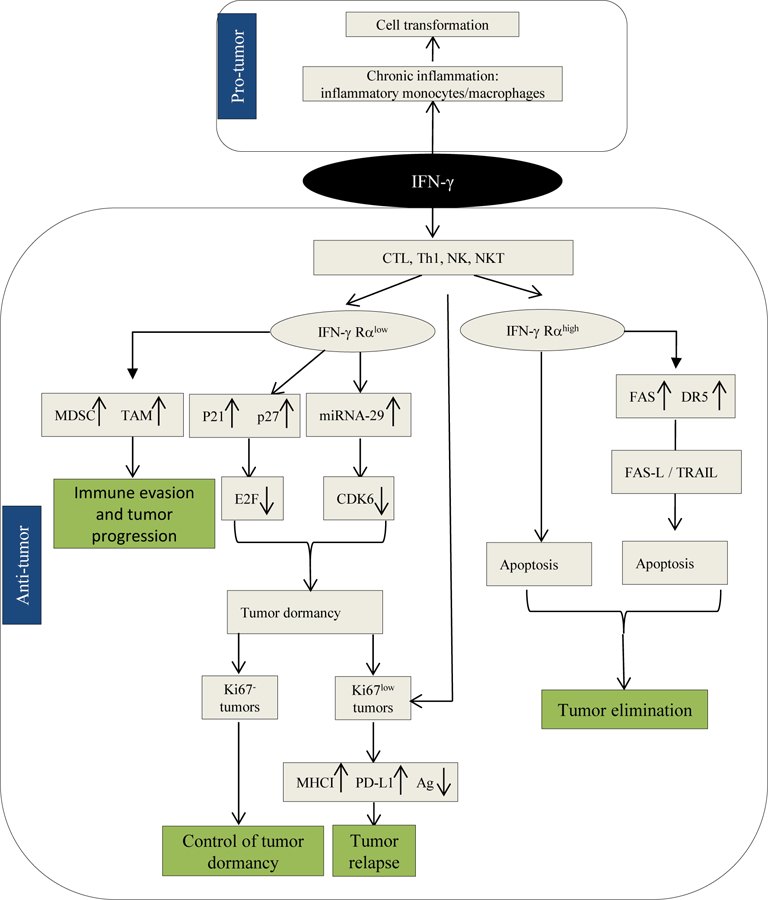
Pro-tumor function of IFN-γ is mediated by chronic inflammation involving inflammatory monocytes and macrophages. Anti-tumor function of IFN-γ is mediated by cells of the adaptive immune system (CTL and Th1), NK cells and NKT cells. The outcome of anti-tumor immune responses is determined by the status of the expression of IFN-γ Rα on target cells such that high levels of IFN-γ Rα render the tumor susceptible to apoptosis while low levels of IFN-γ Rα could result in tumor immunoediting and relapse or maintenance of immunogenic tumor dormancy depending on the type of tumor dormancy being Ki67- quiescent or Ki67low indolent, respectively.
Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program under Award No. W81XWH-14-1-0087, and a pilot funding from the VCU Massey Cancer Center supported, in part, with funding from NIH/NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Disclosures: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Department of Defense. The authors declare no conflicts of interest.
References
- 1.McGranahan N, Swanton C (2015) Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer.Cell 27, 15–26. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Figueira SK, Vrazo AC, Binkowski BF, Butler BL, Tabata Y, Filipovich A, Jordan MB, Risma KA (2014) Real-time detection of CTL function reveals distinct patterns of caspase activation mediated by Fas versus granzyme B. J.Immunol 193, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wennerberg E, Sarhan D, Carlsten M, Kaminskyy VO, D’Arcy P, Zhivotovsky B, Childs R, Lundqvist A (2013) Doxorubicin sensitizes human tumor cells to NK cell- and T-cell-mediated killing by augmented TRAIL receptor signaling. Int.J.Cancer 133, 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda K, Nakayama M, Hayakawa Y, Kojima Y, Ikeda H, Imai N, Ogasawara K, Okumura K, Thomas DM, Smyth MJ (2017) IFN-gamma is required for cytotoxic T cell-dependent cancer genome immunoediting. Nat.Commun 8, 14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda H, Old LJ, Schreiber RD (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13, 95–109. [DOI] [PubMed] [Google Scholar]
- 6.Farrar JD, Katz KH, Windsor J, Thrush G, Scheuermann RH, Uhr JW, Street NE (1999) Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-gamma in establishing and maintaining the tumor-dormant state. J.Immunol 162, 2842–2849. [PubMed] [Google Scholar]
- 7.Kmieciak M, Payne KK, Wang XY, Manjili MH (2013) IFN-gamma Ralpha is a key determinant of CD8+ T cell-mediated tumor elimination or tumor escape and relapse in FVB mouse. PLoS One 8, e82544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne KK, Keim RC, Graham L, Idowu MO, Wan W, Wang XY, Toor AA, Bear HD, Manjili MH (2016) Tumor-reactive immune cells protect against metastatic tumor and induce immunoediting of indolent but not quiescent tumor cells. J.Leukoc.Biol [DOI] [PMC free article] [PubMed]
- 9.Matsuda M, Nakamoto Y, Suzuki S, Kurata T, Kaneko S (2005) Interferon-gamma-mediated hepatocarcinogenesis in mice treated with diethylnitrosamine. Lab.Invest 85, 655–663. [DOI] [PubMed] [Google Scholar]
- 10.Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, Kato S, Yoshimura A (2006) IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J.Exp.Med 203, 1391–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z (2009) IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res 69, 2010–2017. [DOI] [PubMed] [Google Scholar]
- 12.Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat.Rev.Immunol 5, 375–386. [DOI] [PubMed] [Google Scholar]
- 13.Boehm U, Klamp T, Groot M, Howard JC (1997) Cellular responses to interferon-gamma. Annu.Rev.Immunol 15, 749–795. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Weyman CM, Liu H, Almasan A, Zhou A (2008) IFN-gamma induces apoptosis in HL-60 cells through decreased Bcl-2 and increased Bak expression. J.Interferon Cytokine Res 28, 65–72. [DOI] [PubMed] [Google Scholar]
- 15.Rakshit S, Chandrasekar BS, Saha B, Victor ES, Majumdar S, Nandi D (2014) Interferon-gamma induced cell death: Regulation and contributions of nitric oxide, cJun N-terminal kinase, reactive oxygen species and peroxynitrite. Biochim.Biophys.Acta 1843, 2645–2661. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Du Q, Cao Z, Guo Z, Evankovich J, Yan W, Chang Y, Shao L, Stolz DB, Tsung A, Geller DA (2012) Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1). Cancer Lett 314, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Fu XY, Plate J, Chong AS (1998) IFN-gamma induces cell growth inhibition by Fas-mediated apoptosis: requirement of STAT1 protein for up-regulation of Fas and FasL expression. Cancer Res 58, 2832–2837. [PubMed] [Google Scholar]
- 18.Shin EC, Ahn JM, Kim CH, Choi Y, Ahn YS, Kim H, Kim SJ, Park JH (2001) IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. Int.J.Cancer 93, 262–268. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong MJ, Stang MT, Liu Y, Yan J, Pizzoferrato E, Yim JH (2015) IRF-1 inhibits NF-kappaB activity, suppresses TRAF2 and cIAP1 and induces breast cancer cell specific growth inhibition. Cancer.Biol.Ther 16, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KB, Choi YH, Kim IK, Chung CW, Kim BJ, Park YM, Jung YK (2002) Potentiation of Fas- and TRAIL-mediated apoptosis by IFN-gamma in A549 lung epithelial cells: enhancement of caspase-8 expression through IFN-response element. Cytokine 20, 283–288. [DOI] [PubMed] [Google Scholar]
- 21.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A (2004) STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J.Biol.Chem 279, 5811–5820. [DOI] [PubMed] [Google Scholar]
- 22.Kortylewski M, Komyod W, Kauffmann ME, Bosserhoff A, Heinrich PC, Behrmann I (2004) Interferon-gamma-mediated growth regulation of melanoma cells: involvement of STAT1-dependent and STAT1-independent signals. J.Invest.Dermatol 122, 414–422. [DOI] [PubMed] [Google Scholar]
- 23.Dimco G, Knight RA, Latchman DS, Stephanou A (2010) STAT1 interacts directly with cyclin D1/Cdk4 and mediates cell cycle arrest. Cell.Cycle 9, 4638–4649. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt MJ, Philippidou D, Reinsbach SE, Margue C, Wienecke-Baldacchino A, Nashan D, Behrmann I, Kreis S (2012) Interferon-gamma-induced activation of Signal Transducer and Activator of Transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell.Commun.Signal 10, 41-811X-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Liang X, Yin X, Lv J, Tang K, Ma J, Ji T, Zhang H, Dong W, Jin X, Chen D, Li Y, Zhang S, Xie HQ, Zhao B, Zhao T, Lu J, Hu ZW, Cao X, Qin FX, Huang B (2017) Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-gamma-induced immunologic dormancy of tumor-repopulating cells. Nat.Commun 8, 15207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Forster I, Huss R, Weber WA, Kneilling M, Rocken M (2008) TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer.Cell 13, 507–518. [DOI] [PubMed] [Google Scholar]
- 27.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, Fu YX (2013) Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J.Immunol 190, 5874–5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F (2009) Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int.Rev.Immunol 28, 239–260. [DOI] [PubMed] [Google Scholar]
- 29.Katsurano M, Niwa T, Yasui Y, Shigematsu Y, Yamashita S, Takeshima H, Lee MS, Kim YJ, Tanaka T, Ushijima T (2012) Early-stage formation of an epigenetic field defect in a mouse colitis model, and non-essential roles of T- and B-cells in DNA methylation induction. Oncogene 31, 342–351. [DOI] [PubMed] [Google Scholar]
- 30.Kmieciak M, Knutson KL, Dumur CI, Manjili MH (2007) HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur.J.Immunol 37, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, Knutson KL (2009) Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 69, 2887–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namjoshi P, Showalter L, Czerniecki BJ, Koski GK (2016) T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget [DOI] [PMC free article] [PubMed]
- 33.Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K, Zhang P, Koski G, Czerniecki BJ (2012) HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 118, 4354–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR, Weinstein S, Orel SG, Vonderheide R, Coukos G, DeMichele A, Araujo L, Spitz FR, Rosen M, Levine BL, June C, Zhang PJ (2007) Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res 67, 1842–1852. [DOI] [PubMed] [Google Scholar]
- 35.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M (2015) IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br.J.Cancer 112, 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hix LM, Karavitis J, Khan MW, Shi YH, Khazaie K, Zhang M (2013) Tumor STAT1 transcription factor activity enhances breast tumor growth and immune suppression mediated by myeloid-derived suppressor cells. J.Biol.Chem 288, 11676–11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tymoszuk P, Charoentong P, Hackl H, Spilka R, Muller-Holzner E, Trajanoski Z, Obrist P, Revillion F, Peyrat JP, Fiegl H, Doppler W (2014) High STAT1 mRNA levels but not its tyrosine phosphorylation are associated with macrophage infiltration and bad prognosis in breast cancer. BMC Cancer 14, 257-2407-14-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J (2015) Interferon-gamma-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L 1 expression. Oncoimmunology 4, e1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ (2016) Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 167, 1540–1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manjili MH (2014) The adaptation model of immunity. Immunotherapy 6, 59–70. [DOI] [PubMed] [Google Scholar]
- 41.Manjili MH (2017) Tumor Dormancy and Relapse: From a Natural Byproduct of Evolution to a Disease State. Cancer Res 77, 2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, Haley B, Morrison L, Fleming TP, Herlyn D, Terstappen LW, Fehm T, Tucker TF, Lane N, Wang J, Uhr JW (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin.Cancer Res 10, 8152–8162. [DOI] [PubMed] [Google Scholar]
- 43.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA, Hornyak TJ, Arnheiter H, Trinchieri G, Meltzer PS, De Fabo EC, Merlino G (2011) Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature 469, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo W, Xu Q, Wang Q, Wu H, Hua J (2017) Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci.Rep 7, 44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E (2017) The Role of Tissue Macrophage-Mediated Inflammation on NAFLD Pathogenesis and Its Clinical Implications. Mediators Inflamm 2017, 8162421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CC, Wu CL, Su WW, Shih KL, Tarng DC, Chou CT, Chen TY, Kor CT, Wu HM (2015) Interferon gamma-induced protein 10 is associated with insulin resistance and incident diabetes in patients with nonalcoholic fatty liver disease. Sci.Rep 5, 10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cusi K (2012) Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 142, 711–725.e6. [DOI] [PubMed] [Google Scholar]
- 48.Wong RJ, Cheung R, Ahmed A (2014) Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 59, 2188–2195. [DOI] [PubMed] [Google Scholar]
- 49.Sanyal A, Poklepovic A, Moyneur E, Barghout V (2010) Population-based risk factors and resource utilization for HCC: US perspective. Curr.Med.Res.Opin 26, 2183–2191. [DOI] [PubMed] [Google Scholar]
- 50.Locatelli I, Sutti S, Vacchiano M, Bozzola C, Albano E (2013) NF-kappaB1 deficiency stimulates the progression of non-alcoholic steatohepatitis (NASH) in mice by promoting NKT-cell-mediated responses. Clin.Sci.(Lond) 124, 279–287. [DOI] [PubMed] [Google Scholar]
- 51.Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N, Geier A (2016) Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J.Immunol 196, 97–105. [DOI] [PubMed] [Google Scholar]


