Abstract
The risk for post-traumatic osteoarthritis is elevated after anterior cruciate ligament reconstruction (ACLR), and may be especially high among individuals with aberrant walking mechanics, such as medial tibiofemoral joint underloading six months post-operatively. Rehabilitation training programs have been proposed as one strategy to address aberrant gait mechanics. We developed the anterior cruciate ligament specialized post-operative return-to-sports (ACL-SPORTS) randomized control trial to test the effect of 10 post-operative training sessions consisting of strength, agility, plyometric, and secondary prevention exercises (SAPP) or SAPP plus perturbation (SAPP+PERT) training on gait mechanics after ACLR. Forty male athletes (age 23 ± 7 years) after primary ACLR were randomized to SAPP or SAPP+PERT training and tested at three distinct, post-operative time points: 1) after impairment resolution (Pre-training); 2) following 10 training sessions (Post-training); and 3) two years after ACLR. Knee kinematic and kinetic variables as well as muscle and joint contact forces were calculated via inverse dynamics and a validated electromyography-informed musculoskeletal model. There were no significant improvements from Pre-training to Post-training in either intervention group. Smaller peak knee flexion angles, extension moments, extensor muscle forces, medial compartment contact forces, and tibiofemoral contact forces were present across group and time, however the magnitude of interlimb differences were generally smaller and likely not meaningful two years post-operatively. Neither SAPP nor SAPP+PERT training appears effective at altering gait mechanics in men in the short-term, however meaningful gait asymmetries mostly resolved between Post-training and two years after ACLR regardless of intervention group.
Keywords: anterior cruciate ligament (ACL), rehabilitation, ACL-SPORTS Training, gait biomechanics, musculoskeletal modeling
Introduction
Posttraumatic osteoarthritis (OA) is a major concern for individuals after anterior cruciate ligament (ACL) reconstruction (ACLR). OA prevalence after ACLR may approach or exceed 50%.1,2 One factor that may contribute to the development and progression of knee OA after ACLR is walking gait mechanics. Wellsandt and colleagues found smaller involved limb medial compartment tibiofemoral joint loading during gait six months after ACLR is associated with radiographic OA five years post-operatively.3 Recent findings from Pietrosimone et al. suggest lower involved limb peak vertical ground reaction forces and peak knee adduction moments during walking six months after ACLR are associated with biochemical markers (greater plasma matrix metalloproteinase-3) indicative of early joint degeneration.4 Gait mechanics two and five years after ACLR are also related to poorer long-term patient reported outcomes and a higher prevalence of radiographic OA, respectively.5,6 Restoring walking gait mechanics, therefore, may be a critical component of OA prevention in individuals after ACLR.
Gait mechanics after ACLR may be influenced by a number of different factors. Individuals with quadriceps muscle weakness after ACLR walk more asymmetrically, including smaller knee flexion angles and moments in their involved limb, than both healthy controls and stronger ACL-reconstructed individuals.7 Quadriceps strength, however, does not fully explain knee gait asymmetries. Even individuals who restore quadriceps strength symmetry six months after ACLR still walk asymmetrically at that timepoint.8 Asymmetric hamstring strength is another factor that has been linked to more asymmetries during both walking and jogging two or more years after ACLR.9 In addition, performance on a battery of functional tests, including four single-legged hop tests, correlates to kinematic and kinetic symmetry variables during gait six months after ACLR.10 Taken together, these studies suggest that improving lower extremity strength, strength symmetry, and/or functional performance may improve knee symmetry during walking.
Developing interventions to address the aforementioned risk factors or to target directly the gait asymmetries themselves have been proposed. Rehabilitation programs incorporating strength and neuromuscular training programs have improved walking patterns and/or neuromuscular control strategies among individuals who are ACL-deficient (rather than ACL-reconstructed).11–15 Risberg and colleagues found improvements in knee extension moment during gait, but no changes in sagittal plane hip or knee excursions, among ACL-injured participants after 20 sessions of rehabilitation consisting of strength and neuromuscular training exercises.11 Hartigan and colleagues found that individuals who received pre-operative perturbation training (a specific type of neuromuscular training)13 plus strength training had more symmetrical knee excursions during gait compared to a group that received preoperative strength training alone.12 Chmielewski et al. found that perturbation training improved knee kinematics and reduced lower extremity muscle co-contraction among ACL-deficient participants.15 Perturbation training16,15,13,17,12 consists of a series of physical therapist applied perturbations while the patient stands on an unstable surface (i.e., rollerboard or rockerboard). Patients are taught to resist these multi-directional perturbations through selective muscle activation,13,17,12 rather than overpowering the movement or using a gross co-contraction strategy. Perturbation training aims to improve neuromuscular activation patterns and facilitate dynamic knee stability.18,15,13,12,14 Despite pre-operative perturbation training, gait impairments persist after ACLR19,8, thus post-operative interventions including perturbation training may be necessary to improve gait after reconstructive surgery. We developed the anterior cruciate ligament specialized post-operative return-to-sports (ACL-SPORTS) randomized control trial to test whether or not post-operative strength training with and without the addition of perturbation training had an effect on functional measures, clinical outcomes, and walking gait mechanics.16
Limited evidence exists on the effect of post-operative neuromuscular training after ACLR.20,21 Two recently published studies presented a portion of the primary outcomes in men of the ACL-SPORTS trial.20,21 We found no differences in these studies on clinical, functional or kinematic or kinetic gait variables among male athletes 1 and 2 years after primary ACLR who received 10 post-operative training sessions consisting of strength, agility, plyometric, and secondary prevention exercises (SAPP) or this SAPP training plus perturbation training (SAPP+PERT).20,21 These studies, however, did not examine the immediate before and after intervention effects of these two training paradigms on gait mechanics nor did they investigate at any time muscle forces or tibiofemoral joint loading, which is correlated to OA development.3 Given that perturbation training targets muscular activation patterns and, when delivered to ACL-injured individuals, reduces knee kinematic asymmetry and inappropriate muscle co-contraction during gait,15 it is plausible that post-operative perturbation training could facilitate knee kinematic, kinetic, and muscle and joint contact force symmetry during gait. Evaluating medial tibiofemoral joint loading is of particular interest given the relationship between medial tibiofemoral joint loading six months to two years after ACLR and bone and cartilage health.3,22 The purpose of the present study, therefore, was to investigate tibiofemoral loading, muscle forces, and the immediate before and after intervention knee kinematics and kinetics during walking after ACLR in men of the ACL-SPORTS randomized control trial. We hypothesized that knee gait asymmetries would be present before training and improve in both groups after training. We also hypothesized that the SAPP+PERT group would demonstrate greater improvement in knee gait symmetry compared to the SAPP only group.
Methods
Participants
This study is a prospective, randomized control trial (level of evidence: 1). The study was performed at the University of Delaware between November 2011 and August 2016. The study was registered at clinicaltrials.gov (NCT01773317) and was approved by the University of Delaware Institutional Review Board. Informed consent was obtained from all participants as well as a parent or guardian when the participant was a minor.
Participants were eligible for enrollment if they were between 3 and 10 months after primary, unilateral ACLR and previously participated in level I or II sports (i.e., sports involving jumping, cutting, and pivoting)23,24 for at least 50 hours per year. Participants were required to resolve all post-operative impairments, operationally defined as full and symmetric knee range of motion, minimal to no effusion,25 at least 80% quadriceps strength limb symmetry index, initiation of a running progression, and the ability to hop without pain on each leg. Individuals were excluded from participation if they had a previous ACLR or other severe lower extremity injury, concomitant grade III ligament (e.g., medial collateral ligament) injury, or an osteochondral defect of 1 cm2 or larger. After patients completed enrollment paperwork and signed informed consents, a research administrator (MC) randomized participants to receive either SAPP or SAPP+PERT training (Figure 1). Participants were randomized to treatment group (SAPP vs. SAPP+PERT) using a random number generator and stratified by sex so that 20 men received SAPP training and 20 men received SAPP+PERT training. The research administrator performed scheduling only, and all physical therapist researchers who performed clinical, functional, and/or motion analysis testing were blinded to group assignment (single-blinded study). An a priori power analysis indicated that 36 men were needed to detect differences between groups in sagittal plane knee kinematics (primary outcome) based on previously established minimal clinically important differences18 (β = 0.20; α = 0.05, medium effect size = 0.30); we enrolled 40 participants to allow for 10% attrition.16
Figure 1.
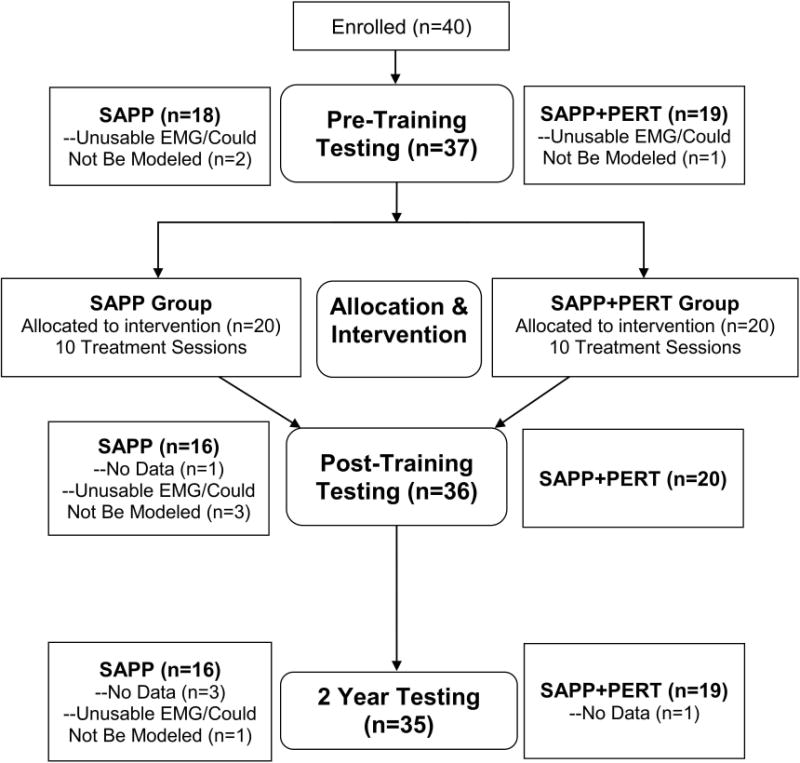
The flow chart provides reasons for missing data at each testing session.
Interventions
Details of the ACL-SPORTS randomized control trial have been described previously by White et al.16 and presented recently by Arundale et al.20 Briefly, all participants received 10 post-operative training sessions (approximately 2×/week) consisting of strengthening, agility, plyometric, and secondary prevention exercises (SAPP). The SAPP program included Nordic hamstrings, standing squats progressing to tuck jumps, drop jumps, and triple single leg hopping. Quadriceps strengthening was also performed in the clinic by participants who enrolled with a quadriceps strength index of between 80% and 90%; participants who enrolled with quadriceps strength index of 90% or greater were instructed to continue quadriceps strengthening on their own. The SAPP program also included agility drills (i.e., forward/backward running, side shuffles, cariocas, figure 8’s, circles, and 90° turns), which progressed by increasing the intensity gradually from 50% to 100% effort and incorporating sport-specific distractions, such as throwing or catching a ball. Participants in the SAPP training arm of the ACL-SPORTS randomized control trial received this training plus a sham intervention during which the athlete stood on one leg on a stable surface and performed hip flexion against a resistance band with the opposite limb. In contrast, participants in the SAPP+PERT intervention group received the same SAPP training plus 10 sessions of perturbation training13. The perturbation training component lasted approximately 30 minutes per session and was delivered by a licensed physical therapist. The general progression (e.g., increasing the speed, magnitude, and/or variability of the perturbations, or adding distractions) was standardized across participants; however, rate of progression was based on participant response and distractions (e.g., ball toss/catch, kick, etc.) were tailored to each participant’s primary sport. Readers may refer to White et al.16 for the ACL-SPORTS trial protocol and to Chmielewski et al.15 for a more thorough description of perturbation training progression.
Motion Analysis Testing
Motion analysis testing during over ground walking occurred at three distinct, post-operative time points: 1) after impairment resolution (Pre-training); 2) following 10 training sessions (Post-training); and 3) two years post-operatively (2 years). Prior to motion capture, we first prepared for surface electromyography (EMG) electrode placement by shaving and abrading the skin with alcohol-soaked gauze to improve conductance. We then placed surface EMG electrodes (MA-300 EMG System, Motion Lab Systems, Baton Rouge, LA) on seven lower extremity muscles per limb crossing the knee joint, including the medial and lateral gastrocnemii, vasti, and hamstrings as well as the rectus femoris. We next recorded EMG during maximal volitional contractions, as described previously.26 We normalized EMG to maximal volitional isometric contractions or dynamic contractions, whichever was highest. EMG data during both normalization procedures and walking trials were collected at 1080 Hz. EMG data were high-pass filtered at 30 Hz using a 2nd order Butterworth filter, rectified, and low-pass filtered at 6 Hz to create a linear envelope. We then affixed 39 retroreflective markers to the bilateral lower extremities and pelvis in accordance with previous work.27
Participants walked at a self-selected gait speed maintained to within ± 5% both throughout and across all testing sessions. We collected kinematic data during gait at 120 Hz using an eight camera motion analysis system (VICON, Oxford, UK). We collected kinetic data at 1080 Hz using an embedded force platform (Bertec Corporation, Columbus, OH). We used commercial software (Visual3D, C-Motion, Germantown, MD) to calculate kinematic and kinetic variables via inverse dynamics. Kinematic and kinetic variables of interest included: peak knee flexion angle (pKFA), peak internal knee extension moment, peak knee adduction angle, and peak internal knee abduction moment. Moments were normalized by mass*height (kg*m) to allow comparisons across participants.28
EMG-Driven Musculoskeletal Model
Walking data were also analyzed using an EMG-driven musculoskeletal modeling approach.29–31 This previously validated31 model uses a Hill-type muscle fiber in series with an elastic tendon, and applies an iterative, simulated annealing process to best fit a forward dynamics knee flexion moment curve to the same moment curve derived through inverse dynamics, as described above. The forward dynamics knee flexion curve is varied by allowing several muscle parameters and coefficients to vary within ± 2 standard deviations of physiological norms. This simulated annealing process is designed to minimize the root mean square error between the forward and inverse dynamics knee flexion moment curves. The process was completed for 5 walking trials per limb for each participant, at each time point. Next, each trial was predicted using the derived muscle parameters and coefficients. Three trials per limb per participant were selected by maximizing the R2 values and minimizing the root mean square error of the predicted trials. Individual muscle forces were calculated for each of the three predicted trials. Medial and lateral tibiofemoral joint contact forces were estimated using the Winby frontal plane moment algorithm.32 The total tibiofemoral joint contact force was estimated as the sum of the individual compartment contact forces. Modeling derived variables of interest included combined knee extensor (i.e., quadriceps) muscle forces at pKFA, knee flexor (i.e., combined hamstrings and gastrocnemii) muscle forces at pKFA, first peak medial tibiofemoral compartment contact force, lateral tibiofemoral compartment contact force at pKFA, and first peak total tibiofemoral contact force. Tibiofemoral joint contact forces were constrained to the first half (0-50%) of stance due to previous work finding loading parameters during this portion of the gait cycle to be associated with knee joint degeneration and OA development after ACLR.4,3 Muscle and joint contact forces were normalized by body weight (BW) to allow comparison across participants.28
Statistical Analysis
A repeated measures linear mixed effects model was used to compare the effects of intervention (SAPP versus SAPP+PERT training), limb, and time for all biomechanical gait variables of interest. Three time points (Pre-training, Post-training, and 2 years) were analyzed for muscle and joint contact forces while only two time points (Pre-training and Post-training) were analyzed for kinematic and kinetic variables because we previously published one and two year outcomes for kinematic and kinetic variables (no meaningful differences between groups).21 Tests for significant main effects and interactions were conducted using an F-test with the Kenward-Roger approximation for the degrees of freedom to account for unbalanced groups over time due to subjects with partially observed data33 (Figure 1). Post-hoc comparisons were made for statistically significant effects (α = 0.05); Bonferroni corrections were made to account for multiple comparisons. (Post hoc p-values with Bonferroni correction were also compared to α = 0.05.) Chi-Square tests of proportions and t-tests were used to compare demographic characteristics between experimental groups (α = 0.05). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), Microsoft Excel (Redmond, WA), and SPSS version 24.0 (IBM Corporation, Armonk, New York, USA).
Results
Groups were similar for all relevant demographic and clinical variables and gait speed (Table 1).
Table 1.
Demographic characteristics were similar between the SAPP and SAPP+PERT groups.
| Demographic Variable | SAPP Group | SAPP+PERT Group | P-value |
|---|---|---|---|
| Age (years) | 23.5 ± 8.7 | 23.1 ± 5.8 | .859 |
| Height (m) | 1.79 ± 0.07 | 1.78 ± 0.06 | .505 |
| Mass (kg) | 85.3 ± 12.9 | 86.3 ± 10.0 | .791 |
| Body Mass Index (kg/m2) | 26.5 ± 1.8 | 27.3 ± 2.5 | .372 |
| Graft Type | 6 allograft, 5 BPTB, 9 hamstring | 7 allograft, 3 BPTB, 10 hamstring | .730 |
| Time from Surgery to Pre-training (months) | 5.2 ± 1.8 | 5.1 ± 1.6 | .848 |
| Gait Speed (m/s) | 1.54 ± 0.12 | 1.58 ± 0.11 | .328 |
Abbreviation: SAPP: strength, agility, plyometric, and secondary prevention; SAPP+PERT: strength, agility, plyometric, and secondary prevention plus perturbation training; allograft: soft-tissue allograft; hamstring: hamstring autograft; BPTB: bone patellar-tendon bone autograft
Kinematic & Kinetic Variables
There was a main effect of limb for peak knee flexion angle (F1,64.1 = 10.14, p = 0.0022; Figure 2). Participants walked using smaller peak knee flexion angles in the involved (19.5 ± 5.1°) compared to uninvolved (22.9 ± 4.9°) limb, regardless of group or time. The mean interlimb difference (3.5° less in the involved limb [discrepancy due to rounding]) exceeded both minimal detectable change (MDC)34 and minimal clinically important difference (MCID)18 values of 2.9° and 3°, respectively. There were no detectable main effects of group or time or any interaction effects for peak knee flexion angle.
Figure 2.
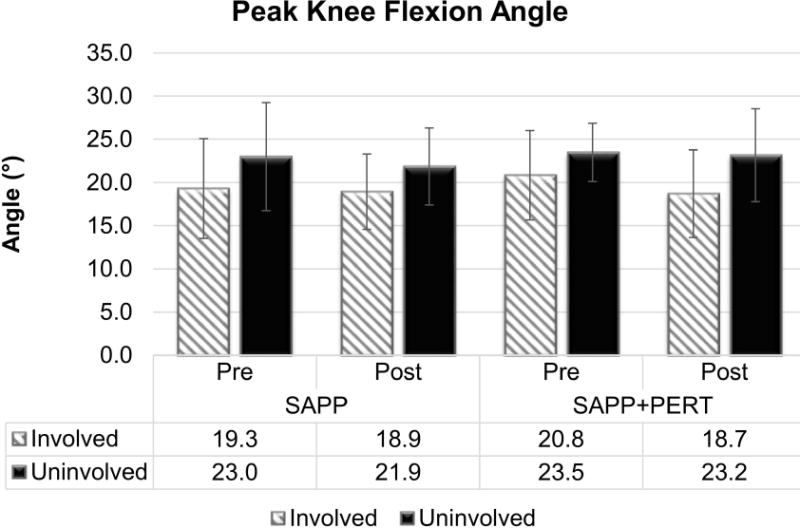
Participants walked with smaller peak knee flexion angles in the involved versus uninvolved limb regardless of group or time. There were no significant effects of group or time or any interaction effects.
There was also a main effect of limb for peak knee extension moment (F1,71.1 = 14.22, p = 0.0003; Figure 3). Participants walked using smaller peak knee extension moments in the involved (0.40 ± 0.13 N*m/kg*m) compared to uninvolved (0.50 ± 0.13 N*m/kg*m) limb, regardless of group or time. The mean interlimb difference (0.10 N*m/kg*m less in the involved limb) exceeded both MDC34 and MCID18 values. There were no detectable main effects of group or time or any interaction effects for peak knee extension moment.
Figure 3.
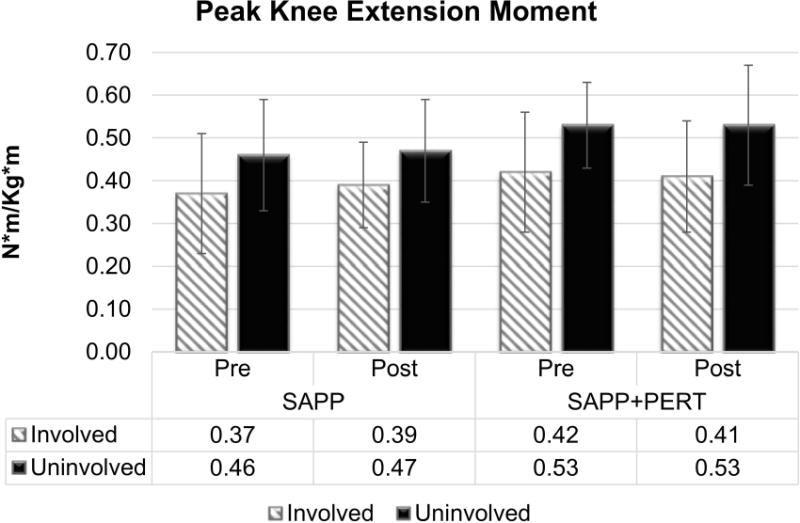
Participants walked using smaller peak knee extension moments in the involved compared to uninvolved limb. There were no detectable main effects of group or time or any interaction effects for peak knee extension moment.
There were no statistically significant effects for peak knee adduction angle or peak knee abduction moment (Figures S-1 and S-2). Moreover, none of the interlimb difference values for either peak knee adduction angle or abduction moment met or exceeded MDC values34, suggesting that any observed differences were smaller than could be reasonably detected.
Muscle Forces
A significant main effect of limb (F1,71.7 = 10.50, p = 0.0018) and a significant 3-way (limb by group by time) interaction effect (F4,44.8 = 3.35, p = 0.0175) were present for knee extensor muscle forces at pKFA (Figure 4). Collapsing across group and time, participants walked with smaller knee extensor muscle forces in the involved (2.17 ± 0.55 BW) compared to the uninvolved limb (2.53 ± 0.58 BW). When analyzing each group more closely, however, changes occurred differently over time. At Pre-training, participants in the SAPP group walked with relatively symmetric knee extensor muscle forces (p = 0.2977, Bonferroni adjusted p = 1.0), whereas participants in the SAPP+PERT group tended to walk with smaller involved versus uninvolved limb knee extensor muscle forces at Pre-training (p = 0.0147, Bonferroni adjusted p > 0.05). At Post-training, participants in both groups walked with asymmetrically smaller involved versus uninvolved limb knee extensor muscle forces, although only the interlimb difference for the SAPP+PERT group was statistically significant after adjusting for multiple comparisons (SAPP: p = 0.0118, Bonferroni adjusted p > 0.05; SAPP+PERT: p = 0.0003, Bonferroni adjusted p = 0.0231). By 2 years, both groups walked with knee extensor muscles forces at pFKA that were not different across limbs or between groups. There were no statistically significant main effects of group or time for knee extensor muscle forces at pKFA, nor were there any two-way interaction effects detected for knee extensor muscle forces at pKFA.
Figure 4.
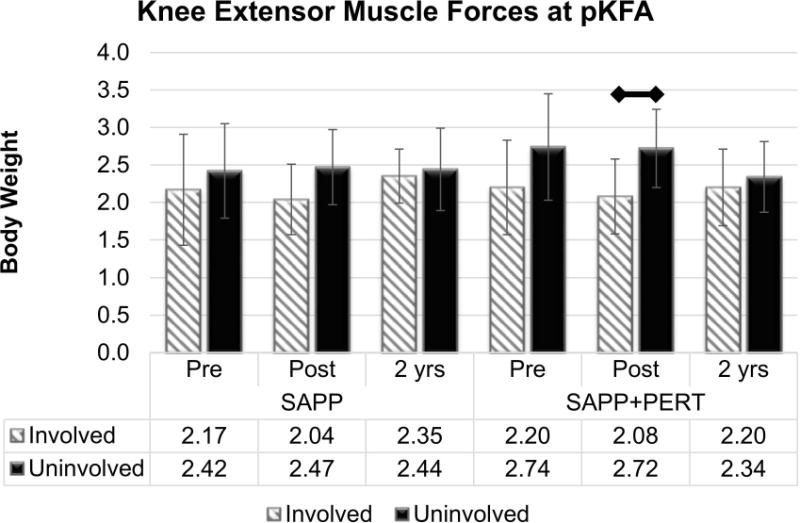
Knee extensor muscle forces at peak knee flexion angle (pKFA) were smaller in the involved versus uninvolved limb at Post-training, but became relatively symmetric in both the SAPP and SAPP+PERT groups two years post-operatively. (After adjusting for multiple comparisons, only the difference between involved and uninvolved limb in the SAPP+PERT group at Post-training was statistically significant.)
There were no statistically significant differences in knee flexor muscle forces at pKFA (Figure S-3).
Joint Contact Forces
There was a group by time interaction effect for peak medial compartment contact force (pMCCF: F2,62 = 7.76, p = 0.0010; Figure 5). Irrespective of limb, the SAPP group walked with lower pMCCF at Post-training (2.71 ± 0.50 BW) versus 2 years (3.03 ± 0.53 BW, Bonferroni adjusted p-value = 0.0066), while there was no detectable difference for pMCCF across time in the SAPP+PERT group. There was also a main effect of limb (F1,70.8 = 5.88, p = 0.0179) with lower pMCCF in the involved (2.66 ± 0.50 BW) versus uninvolved (2.89 ± 0.49 BW) limb across group and time. This interlimb difference (0.23 BW less in the involved limb), however, was smaller than the MDC of 0.30 BW34. Only the interlimb differences at Pre-training and Post-training in the SAPP+PERT group exceeded the MDC of 0.30 BW34. However, the involved and uninvolved limb values at Pre-training and Post-training were similar within each intervention group (i.e., the changes between Pre-training and Post-training were similar for SAPP and SAPP+PERT), suggesting that neither SAPP nor SAPP+PERT training altered medial compartment contact force in the short term. There were no other significant main effects or interaction effects for pMCCF.
Figure 5.
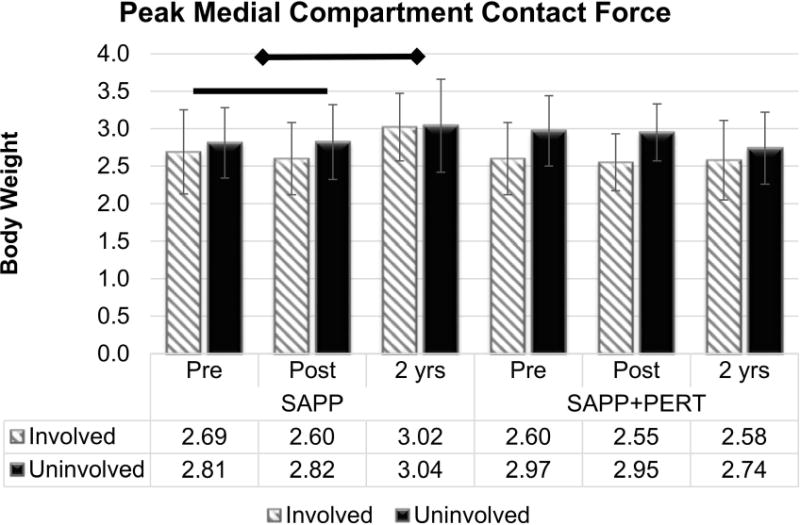
Peak medial compartment contact force increased from Pre-training and Post-training to two years post-operatively in the SAPP group only. A main effect of limb was also present, with smaller values in the involved versus uninvolved limb. Only the interlimb differences at Pre-training and Post-training in the SAPP+PERT group exceeded the MDC of 0.30 BW34. However, the involved and uninvolved limb values at Pre-training and Post-training were similar within each intervention group, indicating that neither SAPP nor SAPP+PERT training altered medial compartment contact force.
There was a main effect of limb (F1,72.8 = 8.56, p = 0.0046; Figure 6) for peak tibiofemoral joint contact force with smaller values in the involved (3.98 ± 0.69 BW) compared to the uninvolved (4.36 ± 0.69 BW) limb. This interlimb difference (0.38 BW less in the involved limb), however, was smaller than the MDC of 0.66 BW34. There were no other statistically significant main effects or interaction effects for peak tibiofemoral joint contact force.
Figure 6.
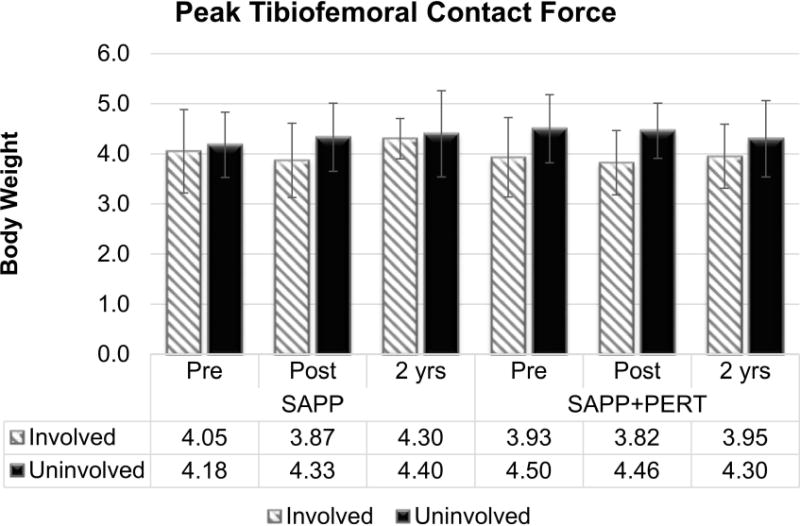
Peak tibiofemoral joint contact force was smaller in the involved versus uninvolved limb, regardless of group of time; however, the differences did not exceed the MDC of 0.66 BW34.
There were also no statistically significant effects for lateral compartment contact force at pKFA (Figure S-4).
Discussion
The purpose of this study was to investigate the effect of strength, agility, plyometric, and secondary prevention (SAPP) training versus SAPP plus perturbation (SAPP+PERT) training on knee kinematic, kinetic, muscle force, and joint contact loading variables during gait in the men of the ACL-SPORTS randomized control trial. Specifically, we aimed to test the hypotheses that knee symmetry during gait would improve over time in both groups and that the SAPP+PERT intervention group would demonstrate greater improvement compared to the SAPP only group. Neither hypothesis was supported, with the exception of improved knee extensor muscle force symmetry at 2 years, but not at Post-training. The lack of change from Pre-training to Post-training across gait variables and treatment groups suggests neither SAPP nor SAPP+PERT training alters gait mechanics in the short term.
Interlimb asymmetries were present in several variables, including peak knee flexion angle, peak knee extension moment, knee extensor muscle forces at pKFA, peak medial compartment contact force, and peak tibiofemoral joint contact force. Only some of these interlimb differences, however, were likely clinically meaningful. Collapsing across group and time, interlimb differences in peak knee flexion angle and extension moments exceeded previously established MDC34 and MCID18 values, indicating smaller involved limb peak knee flexion angles and extension moments at both Pre-training and Post-training. This finding was not surprising given that sagittal plane kinematic and kinetic asymmetries persisted in this cohort at one year and, to a lesser degree, even two years after ACLR21. Knee extensor muscle forces were more variable over time: smaller involved limb knee extensor muscle forces were present in both groups at Post-training, but became approximately symmetric in both groups by 2 years. To the authors’ knowledge, no MCID values exist for knee extensor muscle forces. Pooling across group and time, interlimb differences for peak tibiofemoral joint contact force and medial compartment contact force were smaller than MDC values thus may not be meaningful clinically, but merit further discussion.
Knee loading variables were relatively symmetric in both intervention groups by two years post-operatively, which suggests favorable long-term outcomes6. Interestingly, only the SAPP+PERT group walked with peak medial compartment contact force underloading at earlier time points. Underloading during this time-frame (5-7 months after ACLR) may place individuals at higher risk of radiographic knee OA 5 years post-operatively3. It is unlikely that the SAPP+PERT training influenced or altered this risk, however, because underloading in the SAPP+PERT group occurred at both Pre-training and Post-training and was consistent across these two early time points (Figure 5). The SAPP group demonstrated more symmetrical loading at Pre-training, but also did not change significantly from Pre-training to Post-training, suggesting that neither SAPP nor SAPP+PERT training altered medial compartment tibiofemoral loading symmetry in the short-term. It is unclear, however, why the SAPP group increased medial compartment loading from Pre-training and Post-training to 2 years. Ongoing, long-term radiographic follow-up is needed to evaluate the implications of these findings as well as the effect of SAPP and SAPP+PERT training on knee joint health. Future studies may also investigate the effect of increasing joint loading on knee health, as the directionality of the relationship between joint unloading and knee degeneration after ACLR3,22 is not well established.
Frontal plane gait asymmetries were not detected in the present study, and did not change following intervention. Previous studies have reported conflicting evidence regarding the presence and even direction of interlimb asymmetry in frontal plane gait mechanics after ACLR35–41, although this may be due to not controlling for concomitant meniscal treatment.42 Therefore, it is presently unclear if, or which, participants after ACLR need interventions to address frontal plane mechanics. Nevertheless, neither SAPP nor SAPP+PERT training appears to alter gait kinematics or kinetics in the frontal plane, at least among male athletes who, as a group, walked similarly between limbs in these variables prior to training.
Our null findings are not overly surprising. A recent systematic review and meta-analysis by Kaur et al. found that it may take an average of six years after ACLR to restore joint kinematics during gait43. The failure of the ACL-SPORTS training program to restore symmetry in every knee gait biomechanical variable assessed following training, at only seven months after primary ACLR, may be unremarkable in light of the findings from Kaur and colleagues. Other post-operative rehabilitation interventions also have been unsuccessful at improving gait biomechanics in various populations. For example, Eitzen et al. found a 12-week supervised exercises therapy program did not change frontal or sagittal plane hip, knee, or ankle kinematics or kinetics in patients with hip osteoarthritis44. Previous studies investigating strength and perturbation training on gait mechanics in ACL-deficient participants have found a greater response in women compared to men, who walked with similar patterns before and after training14,27. Women may respond differently to the ACL-SPORTS training program and further investigation is needed.
One potential explanation for why the ACL-SPORTS training was largely ineffective at improving gait mechanics in male athletes is that participants were required to meet stringent inclusion criteria, including 80% quadriceps strength index, prior to enrollment. If the study included athletes earlier after ACLR or allowed participation among athletes with significant quadriceps strength deficits (i.e., < 80% quadriceps strength index), larger interlimb gait asymmetries would likely have been present at enrollment (Pre-training)7. If larger asymmetries had been present initially, there would have been greater room for improvement and perhaps there would have been response to intervention. More likely, however, changing something we do continuously throughout the day like walking in late stages of rehabilitation will require more direct intervention or continuous rather than intermittent interventions.
Bioinspired technologies present exciting possibilities for future gait retraining interventions.45 Shull and colleagues used haptic biofeedback to train individuals with medial compartment knee OA to walk with a toe-in gait, reducing the first peak knee adduction moment.46,47 These reductions corresponded to improved symptoms and function and were maintained at one-month follow-up.47 Pizzolato and colleagues recently provided real-time visual biofeedback of medial tibiofemoral loading to five healthy participants; interestingly, all five participants were able to increase medial tibiofemoral loading both with and without suggestions but only three were able to decrease medial tibiofemoral loading and required suggestions in order to do so.48 Individualized suggestions were more effective gait retraining strategies among these participants48 and in 20 healthy individuals receiving vibrotactile biofeedback.49 Future work should investigate direct, individualized training paradigms and continuous surveillance and/or biofeedback using wearable sensors and other remote technology in individuals after ACLR.
There are several limitations to consider when interpreting the results of this study. First, we analyzed men only, thus the effect of SAPP versus SAPP+PERT training on the gait mechanics of women is unknown. Second, we did not control for surgical factors such as surgeon or graft type, which may make our findings more generalizable, although individualized training paradigms could be considered in future studies.48,49 Third, joint contact and muscle forces were estimated rather than measured directly. The musculoskeletal modeling approach used in this study, however, has been previously validated29,31 and was applied consistently across all participants. Finally, the implications of SAPP and SAPP+PERT training on long-term knee joint health are unknown. Further investigation, including ongoing long-term radiographic follow-up and the effect of SAPP and SAPP+PERT training on gait mechanics in women, is warranted.
In conclusion, neither SAPP nor SAPP+PERT training appear effective at improving gait mechanics in male athletes in the short term. The addition of post-operative perturbation training also does not appear to alter gait mechanics, including model estimations of muscle forces and joint loading, in men after ACLR. Further analysis of the effect of ACL-SPORTS trial and other rehabilitation and gait retraining programs on gait biomechanics and long-term knee health is warranted.
Supplementary Material
Figure S-1. There were no statistically significant effects for peak knee adduction angle. None of the interlimb difference within a group met the MDC of 1.7°34.
Figure S-2. There were no statistically significant nor clinically meaningful effects for peak knee abduction moment (MDC = 0.06 N*m/kg*m)34.
Figure S-3. Knee flexor muscle forces at peak knee flexion angle (pKFA) were relatively similar across limbs, group, and time.
Figure S-4. Lateral compartment contact force at peak knee flexion angle (pKFA) was relatively similar across limbs, group, and time. No group or limb differences approached the minimal detectable change of 0.61 BW34.
Statement of Clinical Significance.
Neither SAPP nor SAPP+PERT training appears effective at altering gait mechanics in men in the short-term, however meaningful gait asymmetries mostly resolved between Post-training and two years after ACLR regardless of intervention group.
Acknowledgments
Funding was provided by the National Institutes of Health, including the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of General Medical Sciences: R01-AR048212, R37-HD037985, R01-HD087459, P30-GM103333, U54-GM104941, and T32-HD00749. JJC received funding from the University of Delaware: University Doctoral Fellowship Award (GRAD 112114). JJC’s work was supported in part by a Promotion of Doctoral Studies (PODS) – Level I Scholarship from the Foundation for Physical Therapy. Thank you to the National Institutes of Health (NIH); Martha Callahan and the Delaware Rehabilitation Institute Research Core; Angela H. Smith and the University of Delaware Physical Therapy Clinic; and Kathleen Cummer, P. Michael Eckrich, Georgia Gagianas, Celeste Dix, and Naoaki Ito for their assistance with data collection and processing.
Footnotes
Author Contributions: JJC contributed to research design, data acquisition, analysis, and interpretation, drafting the manuscript, and incorporating revisions. AK contributed to data acquisition, analysis, interpretation, and critical review. RZ and AJHA contributed to data acquisition, interpretation, and critical review. MLZ contributed to statistical analysis, interpretation, and critical review. KM contributed to data analysis, interpretation, and critical review. TSB and LSM contributed to research design, data interpretation, and critical review. All authors read and approved the final version prior to submission.
Disclosure Statement: No competing financial interests exist for any of the authors.
References
- 1.Lohmander LS, Englund PM, Dahl LL, Roos EM. The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. Available from: http://journal.ajsm.org/cgi/doi/10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander LS, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. Available from: http://onlinelibrary.wiley.com/doi/10.1002/art.20589/abstract. [DOI] [PubMed] [Google Scholar]
- 3.Wellsandt E, Gardinier ES, Manal K, et al. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44(1):143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017 doi: 10.1002/jor.23534. Available from: http://doi.wiley.com/10.1002/jor.23534. [DOI] [PMC free article] [PubMed]
- 5.Khandha A, Manal K, Wellsandt E, et al. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(3):625–633. doi: 10.1002/jor.23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Gait mechanics 2 years after anterior cruciate ligament reconstruction are associated with longer-term changes in patient-reported outcomes. J Orthop Res. 2017;35(3):634–640. doi: 10.1002/jor.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 8.Roewer BD, Di Stasi SL, Snyder-Mackler L. Quadriceps strength and weight acceptance strategies continue to improve two years after anterior cruciate ligament reconstruction. J Biomech. 2011;44(10):1948–1953. doi: 10.1016/j.jbiomech.2011.04.037. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring Strength Asymmetry at 3 Years After Anterior Cruciate Ligament Reconstruction Alters Knee Mechanics During Gait and Jogging. Am J Sports Med. 2017;45(1):97–105. doi: 10.1177/0363546516664705. Available from: http://ajs.sagepub.com/lookup/doi/10.1177/0363546516664705. [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am J Sports Med. 2013;41:1310–1318. doi: 10.1177/0363546513482718. Available from: http://journal.ajsm.org/cgi/doi/10.1177/0363546513482718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risberg M, Moksnes H, Storevold A, et al. Rehabilitation after anterior cruciate ligament injury influences joint loading during walking but not hopping. Br J Sports Med. 2009;43(6):423–428. doi: 10.1136/bjsm.2008.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartigan E, Axe MJ, Snyder-Mackler L. Perturbation training prior to ACL reconstruction improves gait asymmetries in non-copers. J Orthop Res. 2009;27(6):724–729. doi: 10.1002/jor.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald GK, Axe MJ, Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physical active individuals. Phys Ther. 2000;80(2):128–140. [PubMed] [Google Scholar]
- 14.Hurd WJ, Chmielewski TL, Snyder-Mackler L. Perturbation-enhanced neuromuscular training alters muscle activity in female athletes. Knee Surgery, Sport Traumatol Arthrosc. 2006;14(1):60–69. doi: 10.1007/s00167-005-0624-y. [DOI] [PubMed] [Google Scholar]
- 15.Chmielewski TL, Hurd WJ, Rudolph KS, et al. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther. 2005;85(8):740–754. [PubMed] [Google Scholar]
- 16.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament-specialized post-operative return-to-sports (ACL-SPORTS) training: a randomized control trial. BMC Musculoskelet Disord Mar. 2013;23(14):108. doi: 10.1186/1471-2474-14-108. Available from: http://www.biomedcentral.com/1471-2474/14/108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald GK, Axe M, Snyder-mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sport Phys Ther. 2000;30(4):194–203. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- 18.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech. 2012;27(4):360–365. doi: 10.1016/j.clinbiomech.2011.10.008. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardinier ES, Di Stasi S, Manal K, et al. Knee contact force asymmetries in patients who failed return-to-sport readiness criteria 6 months after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(12):2917–2925. doi: 10.1177/0363546514552184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arundale AJH, Cummer K, Capin JJ, et al. Report of the Clinical and Functional Primary Outcomes in Men of the ACL-SPORTS Trial: Similar Outcomes in Men Receiving Secondary Prevention With and Without Perturbation Training 1 and 2 Years After ACL Reconstruction. Clin Orthop Relat Res. 2017;475(10):2523–2534. doi: 10.1007/s11999-017-5280-2. Available from: http://link.springer.com/10.1007/s11999-017-5280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capin JJ, Zarzycki R, Arundale A, et al. Report of the Primary Outcomes for Gait Mechanics in Men of the ACL-SPORTS Trial: Secondary Prevention With and Without Perturbation Training Does Not Restore Gait Symmetry in Men 1 or 2 Years After ACL Reconstruction. Clin Orthop Relat Res. 2017;475(10):2513–2522. doi: 10.1007/s11999-017-5279-8. Available from: http://link.springer.com/10.1007/s11999-017-5279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxby DJ, Wang X, Bryant AL, et al. Tibiofemoral contact forces protect against articular tissue damage in the anterior cruciate ligament reconstructed knee, but not if there is concurent meniscal injury. Osteoarthr Cartil. 2016;24(2016):S94. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1063458416002144. [Google Scholar]
- 23.Daniel DM, Lou Stone M, Dobson BE, et al. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 24.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form Knee Surgery. Sport Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 25.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sport Phys Ther. 2009;39(12):845–849. doi: 10.2519/jospt.2009.3143. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20032559. [DOI] [PubMed] [Google Scholar]
- 26.Capin JJ, Khandha A, Zarzycki R, et al. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J Orthop Res. 2017;35(9):1894–1901. doi: 10.1002/jor.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Stasi S, Hartigan EH, Snyder-Mackler L. Unilateral Stance Strategies of Athletes With ACL Deficiency. J Appl Biomech. 2012;28(4):374–386. doi: 10.1123/jab.28.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: A comparison of two techniques. J Biomech. 2003;36(4):599–603. doi: 10.1016/s0021-9290(02)00433-5. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20(4):367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardinier E, Manal K, Thomas B, Snyder-Mackler L. Gait and neuromuscular asymmetries after acute ACL rupture. Med Sci Sport Exerc. 2012;44(8):1490–1496. doi: 10.1249/MSS.0b013e31824d2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manal K, Buchanan TS. An electromyogram-driven musculoskeletal model of the knee to predict in vivo joint contact forces during normal and novel gait patterns. J Biomed Eng. 2013;135(2):21014. doi: 10.1115/1.4023457. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3705826&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42(14):2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. Available from: [DOI] [PubMed] [Google Scholar]
- 33.Littell RC, Milliken GA, Stroup WW, et al. SAS for Mixed Models, Second. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 34.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Minimum detectable change for knee joint contact force estimates using an EMG-driven model. Gait Posture. 2013;38(4):1051–1053. doi: 10.1016/j.gaitpost.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varma RK, Duffell LD, Nathwani D, McGregor AH. Knee moments of anterior cruciate ligament reconstructed and control participants during normal and inclined walking. BMJ Open. 2014;4(6):e004753. doi: 10.1136/bmjopen-2013-004753. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84901915809&partnerID=tZOtx3y1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster KE, Feller JA. The knee adduction moment in hamstring and patellar tendon anterior cruciate ligament reconstructed knees. Knee Surgery, Sport Traumatol Arthrosc. 2012;20(11):2214–2219. doi: 10.1007/s00167-011-1835-z. [DOI] [PubMed] [Google Scholar]
- 37.Webster KE, McClelland Ja, Palazzolo SE, et al. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2012;46(5):355–9. doi: 10.1136/bjsm.2010.080770. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21508075. [DOI] [PubMed] [Google Scholar]
- 38.Patterson MR, Delahunt E, Caulfield B. Peak knee adduction moment during gait in anterior cruciate ligament reconstructed females. Clin Biomech. 2014;29(2):138–142. doi: 10.1016/j.clinbiomech.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Webster KE, Feller JA, Wittwer JE. Longitudinal changes in knee joint biomechanics during level walking following anterior cruciate ligament reconstruction surgery. Gait Posture. 2012;36(2):167–171. doi: 10.1016/j.gaitpost.2012.02.004. Available from: [DOI] [PubMed] [Google Scholar]
- 40.Zabala ME, Favre J, Scanlan SF, et al. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013;46(3):515–520. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43(5):366–370. doi: 10.1136/bjsm.2008.052522. Available from: http://bjsm.bmj.com/cgi/doi/10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 42.Capin JJ, Khandha A, Zarzycki R, et al. Gait Mechanics Differ after ACL Reconstruction Based on Medial Meniscal Treatment. J Bone Jt Surg. 2018 doi: 10.2106/JBJS.17.01014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur M, Ribeiro DC, Theis JC, et al. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport Med. 2016;46(12):1869–1895. doi: 10.1007/s40279-016-0510-4. [DOI] [PubMed] [Google Scholar]
- 44.Eitzen I, Fernandes L, Nordsletten L, Risberg MA. No effects of a 12-week supervised exercise therapy program on gait in patients with mild to moderate osteoarthritis: a secondary analysis of a randomized trial. J Negat Results Biomed. 2015;14(5):1–11. doi: 10.1186/s12952-015-0023-y. Available from: http://www.jnrbm.com/content/14/1/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pizzolato C, Lloyd DG, Barrett RS, et al. Bioinspired Technologies to Connect Musculoskeletal Mechanobiology to the Person for Training and Rehabilitation. Front Comput Neurosci. 2017 Oct;11:1–16. doi: 10.3389/fncom.2017.00096. Available from: http://journal.frontiersin.org/article/10.3389/fncom.2017.00096/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shull PB, Shultz R, Silder A, et al. Toe-in gait reduces the first peak knee adduction moment in patients with medial compartment knee osteoarthritis. J Biomech. 2013;46(1):122–128. doi: 10.1016/j.jbiomech.2012.10.019. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31(7):1020–1025. doi: 10.1002/jor.22340. [DOI] [PubMed] [Google Scholar]
- 48.Pizzolato C, Reggiani M, Saxby DJ, et al. Biofeedback for Gait Retraining Based on Real-Time Estimation of Tibiofemoral Joint Contact Forces. IEEE Trans Neural Syst Rehabil Eng A Publ IEEE Eng Med Biol Soc. 2017;25(9):1612–1621. doi: 10.1109/TNSRE.2017.2683488. Available from: http://search.ebscohost.com/login.aspx?direct=true&AuthType=cookie,ip,shib,uid&db=cmedm&AN=28436878&site=ehost-live&scope=site&authtype=shib&custid=s8000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uhlrich SD, Silder A, Beaupre GS, et al. Subject-specific toe-in or toe-out gait modifications reduce the larger knee adduction moment peak more than a non-personalized approach. J Biomech. 2018;66:103–110. doi: 10.1016/j.jbiomech.2017.11.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S-1. There were no statistically significant effects for peak knee adduction angle. None of the interlimb difference within a group met the MDC of 1.7°34.
Figure S-2. There were no statistically significant nor clinically meaningful effects for peak knee abduction moment (MDC = 0.06 N*m/kg*m)34.
Figure S-3. Knee flexor muscle forces at peak knee flexion angle (pKFA) were relatively similar across limbs, group, and time.
Figure S-4. Lateral compartment contact force at peak knee flexion angle (pKFA) was relatively similar across limbs, group, and time. No group or limb differences approached the minimal detectable change of 0.61 BW34.


