Abstract
Background
Gastric emptying is a complex physiological process regulating the division of a meal into smaller partitions for the small intestine. Disrupted gastric emptying contributes to digestive disease, yet current measures may not reflect different mechanisms by which the process can be altered.
Methods
We have developed high temporal resolution solid and liquid gastric emptying breath tests in mice using [13C]-octanoic acid and off axis- integrated cavity output spectroscopy (OA-ICOS). Stretched gamma variate and two-component stretched gamma variate models fit measured breath excretion data.
Key Results
These assays detect acceleration and delay using pharmacological (7.5 mg/kg atropine) or physiological (nutrients, cold exposure stress, diabetes) manipulations and remain stable over time. High temporal resolution resolved complex excretion curves with two components, which was more prevalent in mice with delayed gastric emptying following streptozotocin-induced diabetes. There were differences in the gastric emptying of Balb/c versus C57Bl6 mice, with slower gastric emptying and a greater occurrence of two-phase gastric emptying curves in the latter strain. Gastric emptying of C57Bl6 could be accelerated by halving the meal size, but with no effect on the occurrence of two-phase gastric emptying curves. A greater proportion of two-phase gastric emptying was induced in Balb/c mice with the administration of PYY (8–80 nmol) 60 minutes following meal ingestion.
Conclusions and Inferences
Collectively, these results demonstrate the utility of high temporal resolution gastric emptying assays. Two-phase gastric emptying is more prevalent than previously reported, likely involves intestinal feedback, but contributes little to the overall rate of gastric emptying.
Keywords: gastrointestinal physiology, murine, ileal brake, intestinal feedback
Abbreviated abstract
High temporal resolution gastric emptying breath tests can measure accelerated or delayed gastric emptying of liquids and solids and resolve one-phase versus two-phase gastric emptying. These analyses discovered that two-phase gastric emptying is more prevalent that previously described, is strain-dependent and may involve intestinal feedback, yet contribute little to the overall rate of gastric emptying.
Introduction
The [13C]-octanoic acid breath test has been an important technique for studying gastric emptying, especially in preclinical non-human longitudinal animal studies.1, 2 Earlier techniques required euthanasia of the experimental animal and retrieval of the postmortem meal,3, 4 radioactive beads,5 or marker dyes.6 Less invasive imaging techniques, such as scintigraphy,7 SPECT,8, 9 and magnetic resonance10 have been used; however, use of these for routine screening of the same animal during a longitudinal study remains impractical due to high expense and a slower throughput of data. In addition to providing a technique for studying normal physiological conditions or those of a disease state, the breath test also allows for longitudinal studies of changes in gastric emptying in the same mouse due to age or disease progression. [13C]-octanoic acid as a tracer to determine gastric emptying is effective because the rapid absorption in the duodenum, metabolism to 13CO2 in the liver and excretion through the lungs makes gastric emptying the rate limiting step in breath excretion.2, 7
Our research group has used this test to evaluate gastric emptying in mice in several disease models, most commonly diabetic gastroparesis.11–19 Utilizing off axis- integrated cavity output spectroscopy (OA-ICOS) with a multiple input unit to measure 13CO2, mitigates the need to concentrate air samples, thus increasing temporal resolution and throughput of data. Thus, the first aim of this study is to validate the responsiveness and reliability of OA-ICOS by pharmacologically accelerating and delaying gastric emptying.
Occasionally during a gastric emptying breath test, there is evidence for a second phase of gastric emptying, which appears as a second peak in the plotted data.2 Originally, we applied a single gamma variate model that was used for human solid gastric emptying but had been developed by Symonds et al. for gastric emptying in mice.2 This model allowed us to obtain a T1/2 (time to half emptying), but was still relatively weak in fitting later time points of a study and could not adapt to the presence of a second peak. We have developed two models20 to eliminate these two weaknesses -- a stretched gamma variate model (GVS) and a two-component stretched gamma variate model (GVS2) that could be used with two peak curves. Now that this second phase of gastric emptying can be properly fit, there is the ability to study the underlying physiological basis for its presence. Thus, the second aim of this study is to test the physiological or pathological factors that affect the development of the second peak.
Materials and Methods
Animals
Virgin female NOD/ShiLtJ, Balb/cJ, B6;C3Fe a/a-Csf1<+>, and C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) purchased at 6 to 8 weeks of age were used in this study. The estrous cycles of mice were not accounted for in this study. Mice were housed in a room maintained at 20–23°C with a 10 to 14 hour light-dark cycle. Cages were cleaned weekly for non-diabetic mice and daily for diabetic mice; diabetic mice were housed individually or in pairs of two. Gastric emptying was determined weekly, starting at 6–8 weeks of age. Some mice were exposed to a cold stress test, which has been demonstrated to significantly accelerate gastric emptying.21, 22 Cages containing these mice were placed in a 4°C walk-in refrigerator for a period of 15 minutes ending 30 minutes prior to testing. Drugs or vehicle controls were delivered to mice via intraperitoneal (i.p.) injection 30 minutes prior to testing with the exception of PYY (Tocris, Avonmouth, Bristol, United Kingdom) and its vehicle, which were administered 60 minutes into a solid gastric emptying test. The volume of i.p. injections were adjusted to 10ml/kg according to the weight of each animal. All protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Gastric Emptying
Mice were fasted overnight (no more than 16 hours) on a metal mesh bottom with free access to water. At the time of testing, mice were placed in a non-restraining, clear chamber they had previously been habituated to over a two week period. The chamber was connected to a house hydrocarbon free air source (approximately 500 ppm CO2) at a flow rate controlled so that CO2 concentrations exiting the chamber were maintained between 1000–1500 ppm. Two to four periods of collection (every 5 min) were used to establish baseline readings before receiving the 13C-containing meal. For solid gastric emptying, mice received 0.1 or 0.2 g of cooked egg yolk containing 2.5 µmol [13C]-octanoic acid16 and were allowed to freely ingest the meal. The same dose of octanoic acid was delivered regardless of meal size. Mice were allowed 5 min to ingest the egg meal. For liquid gastric emptying, after baseline measurements, the mice received a gavage of 0.2 mL of water or a liquid nutrient meal (Ensure) containing 2.5 µmol [13C]-acetic acid, or a liquid nutrient meal (Ensure) containing 2.5 µmol [13C]-octanoic acid and returned to the breath collection chamber. Air containing the exhaled breath from each chamber was automatically sampled every five minutes and analyzed by Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) using the Los Gatos Research Multiple Input Unit and Carbon Dioxide Carbon Isotope Analyzer (Mountain View, CA, USA).
Diabetes
Previous studies from our group have demonstrated delayed or accelerated gastric emptying in various models of diabetes.14, 15, 17 All data from these studies were stored in our custom Electronic Animal Research Record (EARR) relational database. This affords us to retrospectively analyze these data using our newly developed LINUX-based software.20 We randomly retrieved data from two different mouse models of diabetes, NOD/ShiLtJ and B6;C3Fe a/a-Csf1<+>, from the database using search criteria of diabetic (>250 mg/dl), non-diabetic (<150 mg/dl), and normal versus delayed gastric emptying using criteria set by the published study.12, 14 To increase the power of the contingency tests, gastric emptying tests from B6;C3Fe a/a-Csf1<+> and NOD/ShiLtJ mice from more recent unpublished studies were also included in our analysis.
Corticosterone Measurements
Either immediately following a four hour gastric emptying test, or at the same time of day (1 pm) from animals within their home cages, 100 µL of blood was obtained by laceration of the submandibular veins. Serum samples were analyzed by ELISA (Assaypro LLC, St. Charles, MO) according to the manufacturer’s instructions.
RITC-Dextrose Transit
Three milligrams of rhodamine isothiocyanate (RITC)-conjugated dextran (10kDa; Sigma, St. Louis, MO) and 10 µL [13C]-octanoic acid was thoroughly mixed with 5 g of egg yolk prior to cooking. A 0.2 g egg meal containing 250 µg octanoic acid and 120 µg RITC-dextran was delivered to animals as described above. 240 minutes after initial egg time, mice were euthanized by CO2 exposure and the GI tract was removed and divided into five pieces: stomach, proximal small intestine, distal small intestine, cecum, and colon. These tissues and luminal contents were homogenized and diluted into water for a final volume (including tissue) of 4 mL. Six 0.2 g of the egg meal were also homogenized and diluted into water for a final volume (including egg) of 4 mL. 100 µl of each 4 ml sample was loaded onto a 96 well plate and fluorescence (570nm excitation; 595±9nm emission) was measured (SynergyMx; Biotek; Winooski, VT).
Data Analysis
Output tables generated by the CO2 analyzer containing delta13C values, the sampled chamber and time, were analyzed using a custom Linux based system described previously.20 Only the empirical models GVS and GVS2 were used for the current study. The GVS model produced output values of T1/2, the time (min) to half emptying, Tlag, the time (min) to peak gastric emptying, and Max R, or maximal rate of 13CO2 production. In addition to these three variables, GVS2 produced Tpeak2, the time (min) to the second peak. The preferred model for each gastric emptying curve was determined by which had the lowest corrected Akaike Information Criterion (AICc). As described previously, overfitting by GVS2 despite a lower AICc was revealed by either peaks that had a half width of less than 24 min or by an output value of 0.00 min for Tpeak2. In these cases, GVS was selected as the preferred model.
Statistical Analyses
Contingency tables of GVS versus GVS2 were analyzed using a Fisher’s exact test for two groups and chi-square with three or more groups. Comparisons in continuous value data sets were made using a two-tailed, unpaired t-test for two groups and ANOVA with Neuman-Keuls Multiple Comparisons test for three or more groups. Fractional 13C dose/hr curves and fluorescent meal transit data were analyzed with a 2-way ANOVA with Bonferroni Multiple Comparisons test. For all tests, significance was determined by P<0.05.
Results
High Temporal Resolution and Throughput Gastric Emptying
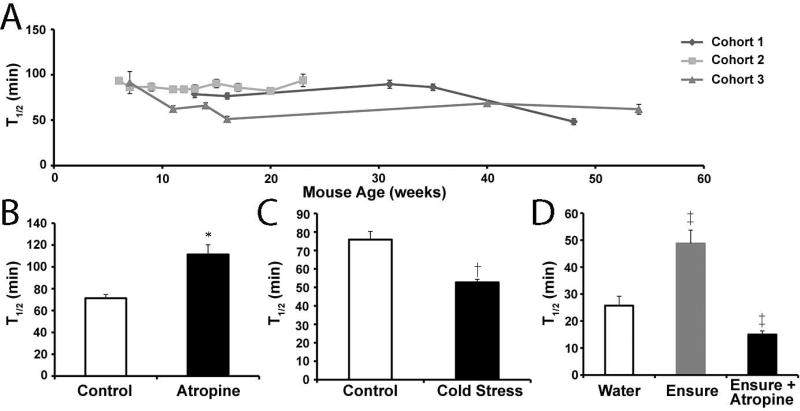
Non-invasive measures of gastric emptying repeatedly in the same animal allows for longitudinal studies of diseases and treatments. Measuring 13C enrichment in exhaled breath samples provides a robust and reproducible measure of gastric emptying that has been extensively validated. Our research group has used manual breath collection and 13C analysis by isotope gas chromatography,14 automated sampling and 13C analysis by infrared spectroscopy,11, 13, 14, 18, 19 and most recently by OA-ICOS.12, 15–17 As described previously,16 the latter system allows for simultaneous measurements in 12 animals with approximately 15 distinct measurements after stabilization every 5 minutes for high temporal resolution of a 4–6 hour gastric emptying test. We measured gastric emptying on a weekly basis in 3 cohorts of 10, 12, and 16 Balb/cJ mice, respectively, between 6 and 54 weeks of age. On some weeks these mice received interventions as described below. T1/2 values obtained for solid gastric emptying on weeks where there were no interventions were evaluated and found to be very consistent as the mice aged (Figure 1A). The coefficient of variation over time was 0.21, 0.05 and 0.20 for cohorts 1, 2 and 3, respectively.
Figure 1.
Gastric emptying studies in Balb/c mice illustrating stability of tests over time and detection of both accelerated and delayed gastric emptying for both solid and liquid test meals. A) Mean T1/2 values (± SEM) obtained for solid gastric emptying on weeks where there were no interventions plotted versus the age of the mice for three distinct cohorts of mice. B) Mean T1/2 values (± SEM) obtained for solid gastric emptying of sixteen mice on contiguous weeks where they received an intraperitoneal injection of saline (open bar) or 7.5 mg/kg atropine (closed bar) 30 minutes prior to testing. *P<0.05 T-test compared to saline. C) Mean T1/2 values (± SEM) obtained for solid gastric emptying of sixteen mice on contiguous weeks where they were exposed to cold (cold stress; closed bar) or room temperature (control; open bar) 30 minutes prior to testing. †P<0.05 T-test compared to control. D) Mean T1/2 values (± SEM) obtained for liquid gastric emptying of six mice on contiguous weeks where the gavaged meal was water (open bar) or Ensure (shaded and closed bars). ‡ P<0.05 ANOVA with Neuman-Keuls multiple comparisons test compared to water control.
To test whether OA-ICOS analysis can measure accelerated or delayed gastric emptying of a solid egg meal, we used several interventions delivered 30 minutes prior to testing. Atropine (7.5 mg/kg, i.p, Sigma, St. Louis, MO) significantly delayed gastric emptying (T1/2 = 111 ± 9 min) compared to vehicle control (saline, i.p.) (T1/2 = 71 ± 3 min, N=16, P<0.05 Paired T-test; Figure 1B), and exposure to cold stress significantly accelerated gastric emptying (T1/2 = 53 ± 2 min) compared to naïve control (T1/2 = 67 ± 3 min, N=16, P<0.05 Paired T-test; Figure 1C). Bethanechol (20 mg/kg), has been demonstrated to cause accelerated gastric emptying of solids in mice.14, 23 For the current study, this dose of bethanechol caused watery eyes and agitation so that the mice did not eat the meal within the five minute feeding period. A lower dose of bethanechol (10 mg/kg) still caused watery eyes in the mice but reduced the agitation so that the animals consumed the test meal. This dose, however, did not alter the rate of gastric emptying (T1/2 = 62 ± 3 min, N=13) compared to vehicle control (saline, i.p.) (T1/2 = 64 ± 3 min, N=13, P>0.05 Paired T-test).
For liquid gastric emptying using [13C]-acetic acid, the presence of nutrients in the gavage solution delayed gastric emptying (T1/2 = 40 ± 8 min) compared to water alone (T1/2 = 25 ± 5 min; N=). Furthermore, atropine (7.5 mg/kg) significantly accelerated liquid gastric emptying of the nutrient meal (T1/2 = 15 ± 1 min, N=6, P<0.05 ANOVA Neuman-Keuls Multiple Comparisons Test; Figure 1D). Sumatriptan delays liquid gastric emptying in humans24, 25 but causes contractions of the fundus in mice.26, 27 Sumatriptan (0.1mg/kg) had no effect on gastric emptying of the liquid nutrient meal (T1/2 = 42 ± 4 min, N=6, P>0.05 ANOVA Neuman-Keuls Multiple Comparisons Test). The effect of atropine on liquid gastric emptying was surprising given previous reports of atropine causing delayed emptying of a liquid meal containing phenol red in mice28 or [13C]-acetic acid in rats.29 Therefore we retested this experiment in mice that received the nutrient meal with [13C]-octanoic acid. Atropine significantly accelerated liquid gastric emptying of the [13C]-octanoic acid nutrient test meal (T1/2 = 26 ± 2 min) compared to vehicle control (saline, i.p.) (T1/2 = 37 ± 7 min, N=6, P<0.05 Paired T-test). There was no difference in the rate of liquid emptying of [13C]-octanoic acid compared to [13C]-acetic acid, which is different than has been previously reported in rats.30
Two Peak Analysis of Gastric Emptying Curves from Diabetic Animals
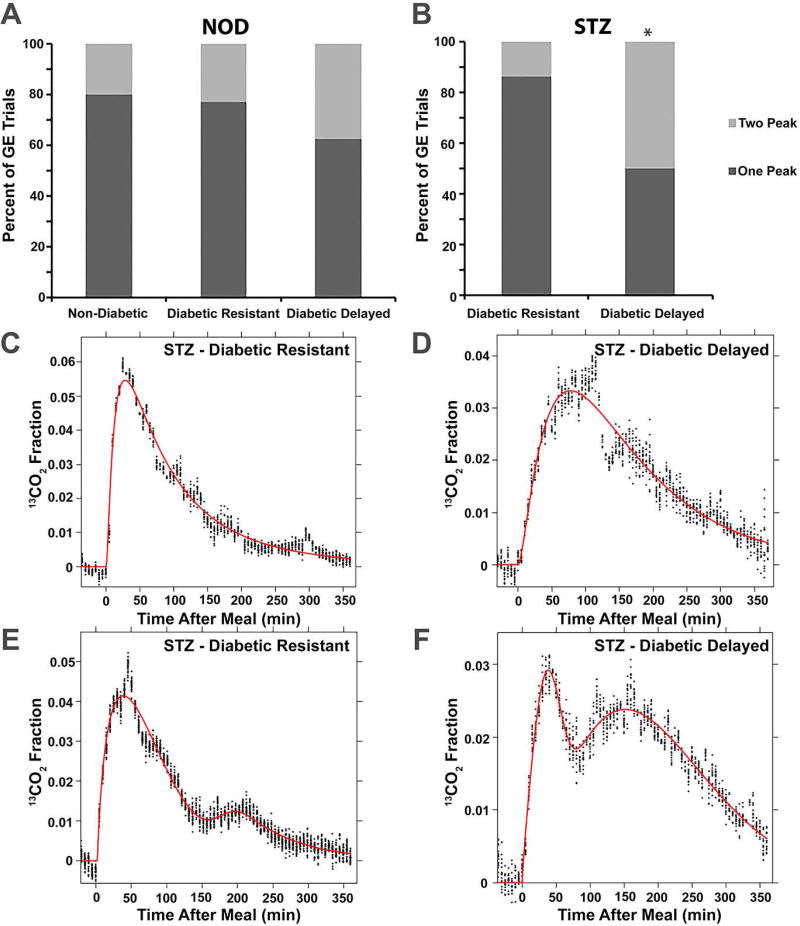
The recently developed tool for analysis of gastric emptying data20 is able to detect two-peak gastric emptying curves that may be an indication of saltatory rather than steady-state emptying. We tested the hypothesis that delayed gastric emptying in diabetic mice is associated with an increased occurrence of two peak gastric emptying curves. A single gastric emptying curve of 54 female NOD/ShiLtJ mice was analyzed at a time point of 5–10 weeks after the development of diabetes or in age-matched normoglycemic NOD/ShiLtJ mice. The relative frequencies of two-peak curves was not different between the three groups of NOD/ShiLtJ mice as six curves were better fit by the GVS2 model for each of the Non-Diabetic (n=25) and Diabetic Delayed (n=16) groups and three curves better fit by GVS2 for the Diabetic Resistant (Non-Delayed) (n=13; P>0.05, Chi-square; Figure 2A). Mice in the Diabetic Delayed group had the slowest T1/2 of the three groups and correspond with values reported previously.14 Likewise, Tlag and R values of the Diabetic Delayed group were significantly slower than Non-Diabetic and Diabetic Resistant groups. The Tpeak2 values, however, were not different between the three groups, suggesting diabetic induced GE in NOD/ShiLtJ mice does not affect the second peak (Table 1).
Figure 2.
Two-phase gastric emptying does not contribute to diabetic gastroparesis. A) The proportion of gastric emptying tests better fit by the GVS2 model (light gray; 2 peak) than the GVS model (dark gray; 1 peak) were not different in NOD/ShiLtJ mice that remained non-diabetic, were diabetic and had normal gastric emptying (resistant), or were diabetic and exhibited delayed gastric emptying (P>0.05, Chi-Square). B) In B6;C3Fe a/a-Csf1<+> treated with streptozotocin (STZ), there was a greater proportion of gastric emptying tests better fit by the GVS2 model (light gray; 2 peak) than the GVS model (dark gray; 1 peak) in delayed versus resistant mice (*P<0.05, Fishers Exact Test). Representative data from individual gastric emptying experiments that were better fit with the GVS model (C and D) or GVS2 model (E and F) for STZ-treated B6;C3Fe a/a-Csf1<+> mice with normal (C and E) or delayed (D and F) gastric emptying illustrates the similarities between 1-peak and 2-peak curves for delayed versus resistant mice.
Table 1.
Gastric emptying parameters for NOD/ShiLtJ mice.
| Group | T1/2 (min) | Tlag (min) | Tpeak2 (min) | R (×10−3) (min−1) |
|---|---|---|---|---|
| Non-Diabetic (N=25) | 103 ± 4 | 55 ± 4 | 114 ± 23 (N=6) | 6.1 ± 0.3 |
| Diabetic Resistant (N=13) | 104 ± 7 | 52 ± 6 | 152 ± 19 (N = 3) | 6.7 ± 0.5 |
| Diabetic Delayed (N=16) | 212 ± 14a | 78 ± 6a | 127 ± 18 (N=6) | 3.0 ± 0.2a |
P<0.001 compared to Non-Diabetic and Diabetic Resistant groups, ANOVA with Neuman-Keuls Multiple Comparisons Test.
Normal T1/2 ranges for B6;C3Fe a/a-Csf1<+> have previously been reported.15 Five to six weeks following streptozotocin-induced diabetes, 30 -35% of diabetic animals developed delayed gastric emptying.15 A T1/2 of 119 min was used to separate diabetic B6;C3Fe a/a-Csf1<+> mice (n=38) into two groups, Resistant (n=22; 58%; T1/2 ≤119 min) or Delayed (n=16; 42%; T1/2 >119). A single gastric emptying curve was analyzed for each mouse. There were significantly more gastric emptying curves better fit by GVS2 in the Delayed group (n=8; 50%) compared to the Resistant Group (n=3; 14%; P<0.05, Fisher’s Exact Test; Figure 2B). While the T1/2, Tlag and R values were all slower in the Delayed group compared to the Resistant group, there was no difference in the Tpeak2 value between these two groups of mice (Table 2). Representative one-peak (Figures 2C and 2D) and two-peak (Figures 2E and 2F) gastric emptying curves for Resistant (Figures 2C and 2E) and Delayed (Figures 2D and 2F) mice demonstrate that delayed mice tend to have a greater proportion of total emptying that occurs during a second phase that happens at the same time.
Table 2.
Gastric emptying parameters for Diabetic B6;C3Fe a/a-Csf1<+> mice
| Group | T1/2 (min) | Tlag (min) | Tpeak2 (min) | R (×10−3) (min−1) |
|---|---|---|---|---|
| Diabetic Resistant (N=22) | 80 ± 5 | 47 ± 3 | 218 ± 69 (N = 3) | 9.8 ± 0.9 |
| Diabetic Delayed (N=16) | 166 ± 18a | 71 ± 9a | 157 ± 20 (N = 8) | 4.4 ± 0.3a |
P<0.0001 and
P=0.005 compared to Diabetic Resistant group, T-test.
Strain Differences
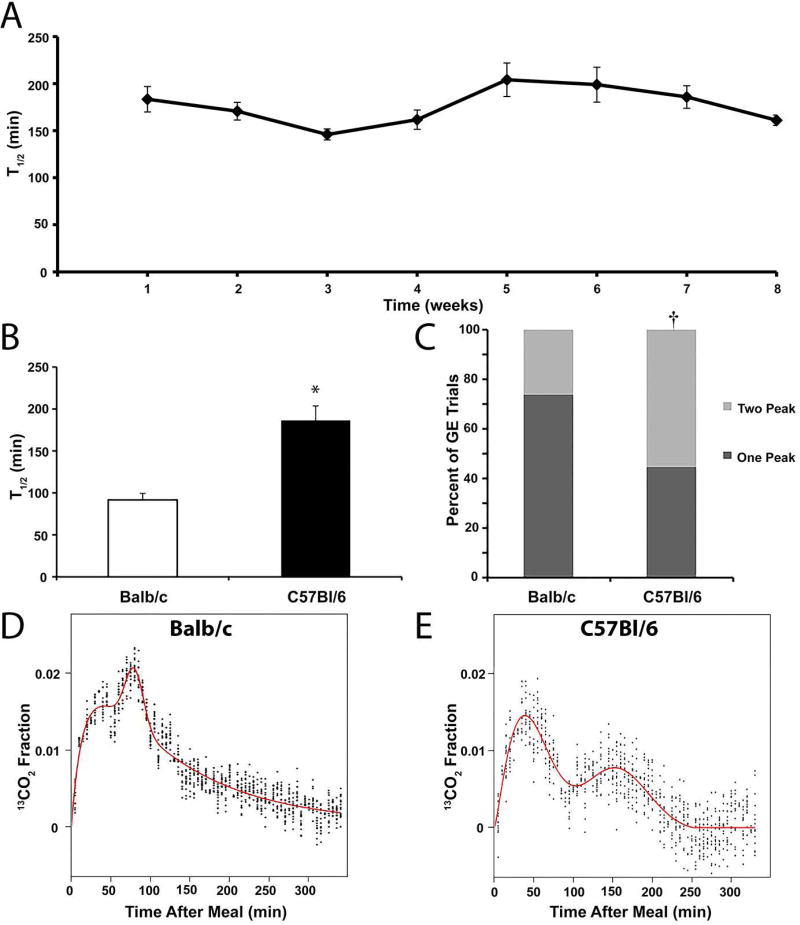
Recent studies in our group have begun using transgenic animals on a B6 background. In our initial testing of some of these mice, we noted that gastric emptying appeared both delayed and more variable in C57Bl/6J mice compared to Balb/cJ. Figure 3A demonstrates the mean T1/2 values for C57Bl/6J mice tested over time. The coefficient of variation over time for these mice was 0.11. To test if there were differences in gastric emptying between these two strains of mice, we analyzed 34 gastric emptying tests from 12 Balb/cJ mice and 27 tests from 9 C57Bl/6J mice using our Linux-based analysis tool. Figure 3B demonstrates the overall T1/2 for C57Bl/6J strain was 186 ± 17.3 min and 91 ± 8.2 min for Balb/cJ (P<0.0001, unpaired T test). Significant differences were also observed in the Tlag, Tpeak2 and R values between the two strains of mice (Table 3). A greater proportion of the gastric emptying curves were better fit by the GVS2 model in C57BL/6J mice (15/27; 56%) compared to Balb/cJ mice (9/34; 26%; P<0.05, Fishers Exact Test; Figure 3C). Representative curves fit with the GVS2 model for each strain demonstrate the differences between strains of the second peak that is reflected in the Tpeak2 value (Figures 3D and 3E).
Figure 3.
Strain differences in solid gastric emptying tests between Balb/cJ and C57Bl/6J mice. A) Mean T1/2 values (± SEM) obtained for solid gastric emptying for nine C57Bl6 mice over a contiguous eight-week period shows relative stability of gastric emptying over time. B) The mean T1/2 values (± SEM) for C57Bl/6J mice (N=27 tests; closed bars) are significantly delayed compared to Balb/cJ mice (N=34 tests; open bars). *P<0.0001 T-test compared to saline. C) A greater proportion of the gastric emptying curves were better fit by the GVS2 model (light gray; 2 peak) than the GVS model (dark gray; 1 peak) in C57BL/6J mice compared to Balb/cJ mice. †P<0.05, Fishers Exact Test. Representative data from individual gastric emptying experiments that were better fit with the GVS2 model for a Balb/cJ mouse (D) and a C57BL/6J mouse (E) illustrates the qualitative differences between 2-peak curves for the two strains of mice.
Table 3.
Gastric emptying parameters for Balb/cJ and C57BL/6J strain mice.
| Strain | T1/2 (min) | Tlag (min) | Tpeak2 (min) | R (×10−3) (min−1) |
|---|---|---|---|---|
| Balb/c | 91 ± 8 | 61 ± 4 | 87 ± 7 | 9.0 ± 0.5 |
| C57BL/6 | 186 ± 17a | 89 ± 6a | 159 ± 8a | 3.8 ± 0.2a |
P<0.001, compared to Balb/cJ group, T-test.
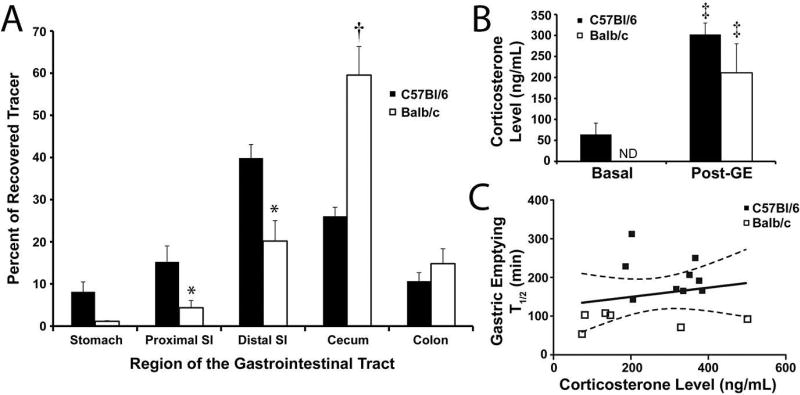
An alternative method of gastrointestinal transit was used to validate these strain-related differences revealed by the breath test: the distribution of fluorescent 10kDa dextran mixed in with the normal egg yolk meal. Four hours after ingestion, the GI tract was removed from C57Bl/6J (n=9) and Balb/cJ (n=12) mice and the percent of total tracer was calculated for each of the five gastrointestinal regions (Figure 4A). There was a significant difference in the distribution of RITC-dextran between strains, with the dextran traveling farther in Balb/cJ mice (P<0.05, 2-way ANOVA). The region with the largest difference in percent of total tracer observed between the two strains was the cecum (B6: 26.1 ± 2.1%; B/c: 59.5 ± 6.8%, mean ± SE, P<0.0001, 2-way ANOVA, Bonferroni’s Multiple Comparisons Test). At the four hour time point, there was 8.1 ± 2.3 % of total tracer (mean ± SEM) left in the stomach of the C57Bl/6J strain compared to the 1.1 ± 0.2 % left in the stomach of Balb/cJ mice (P>0.05, 2-way ANOVA, Bonferroni’s Multiple Comparisons Test).
Figure 4.
Gastrointestinal transit but not corticosterone levels differed between Balb/cJ and C57Bl/6J mice. A) The mean proportion (± SEM) of 10kDa RITC-dextran recovered from the lumen of five different segments of the gastrointestinal tract four hours after eating the egg yolk meal is plotted for Balb/cJ (N=12; closed bars) and C57Bl/6J mice (N=6; open bars). *P<0.05, 2-way ANOVA, Bonferroni’s Multiple Comparisons Test compared to C57Bl6 mice. †P<0.0001, 2-way ANOVA, Bonferroni’s Multiple Comparisons Test compared to C57Bl6 mice. B) Mean blood corticosterone levels (± SEM) in resting C57Bl6 mice (N=5) and C57Bl6 (N=9) and Balb/c (N=6) mice that had just completed a solid gastric emptying breath test. ‡P<0.05, ANOVA, Neuman-Keuls Multiple Comparisons test compared to resting C57Bl6 mice. C) Plot of T1/2 values from solid gastric emptying breath tests versus post-test corticosterone levels for Balb/cJ (open symbols) and C57Bl/6J (closed symbols) mice. There was no correlation between these measures (P>0.05, Pearson Correlation).
There are well known differences in immune and physiological processes between C57Bl/6J and Balb/cJ mice, including stress31–33 that is well known to modulate gastric emptying.34 Blood corticosterone levels were tested as a quantitative measure of stress from three groups of mice: baseline C57Bl/6J mice that did not undergo gastric emptying, and C57Bl/6J and Balb/cJ mice that underwent gastric emptying. We did not test baseline Balb/cJ corticosterone levels but based on published studies35 we anticipate these values to be near or below the baseline C57Bl/6J mice. The average basal measurement for C57Bl/6J mice (n=5) was 64 ± 10.0 ng/mL (mean ± SE). A post-GE measurement for both C57Bl/6J (n=9; 302 ± 27.1 ng/mL, mean ± SE) and Balb/cJ (n=6; 210.97 ± 69.2 ng/mL, mean ± SE) strains showed an increase in corticosterone levels from basal for the C57Bl/6J mice (P<0.05, ANOVA, Neuman-Keuls Multiple Comparisons test), but no difference between the two strains (P>0.05, ANOVA, Neuman-Keuls Multiple Comparisons test, Figure 4B). Additionally, there was no correlation between post-GE levels of corticosterone and the T1/2 obtained (P>0.05, Pearson Correlation, Figure 4C).
Effect of Meal Size on Gastric Emptying in C57BL/6J Mice
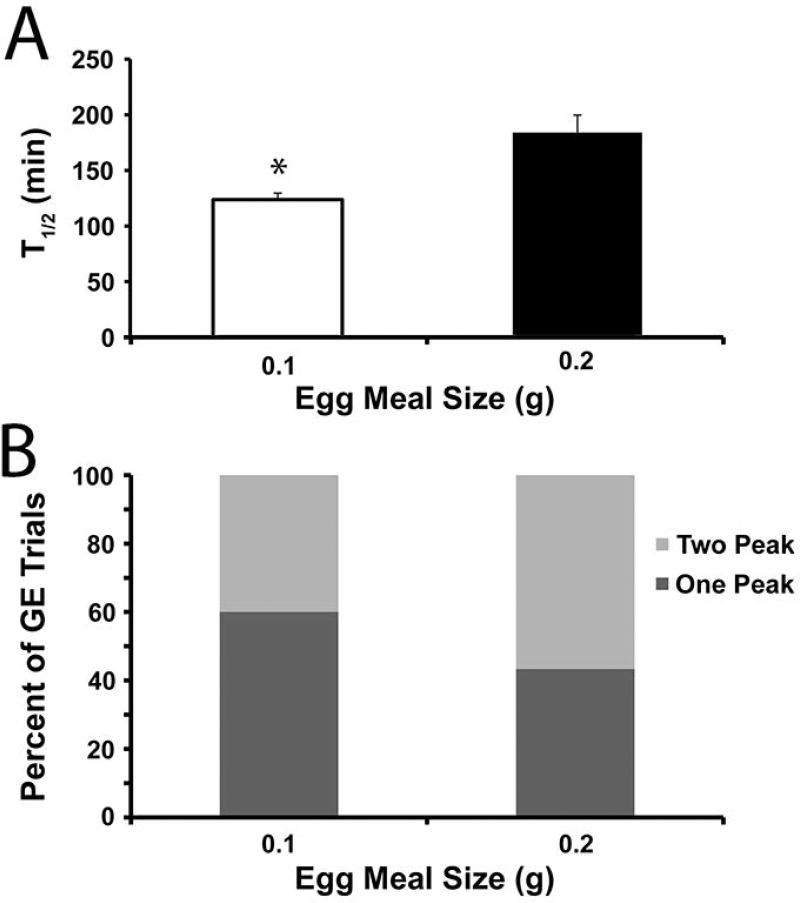
In an attempt to reduce the presence of 2-peak gastric emptying curves for C57Bl/6J mice, the size of the egg meal was reduced to 0.1 g. 30 gastric emptying tests were analyzed from nine C57Bl/6J mice for each of the 0.2 and 0.1 g egg meals. Gastric emptying was accelerated with the 0.1 g egg meal (T1/2= 124 ± 6 min) compared to the 0.2 g meal (T1/2= 184 ±16 min, P<0.001, unpaired T test; Figure 5A). In addition, the time to reach the first peak (Tlag) was faster with the 0.1 g egg meal (Table 4). The smaller meal size did not significantly reduce the proportion of 2-peak curves because 12 curves (40%) with the 0.1 g egg meal and 17 curves (57%) with the 0.2 g egg meal were better fit by the GVS2 model (P>0.05, Fisher’s Exact Test; Figure 5B). These results suggest that while reduced meal size may accelerate the rate of gastric emptying closer to that of Balb/cJ mice, 2-peak curves may be a fundamental part of emptying in some C57BL/6J mice.
Figure 5.
Decreased meal size accelerates solid gastric emptying in C57Bl6 mice but does not alter the proportion of tests that are better fit with GVS2 model. A) The mean T1/2 values (± SEM) for C57Bl/6J mice (N=30 tests) for egg meal sizes of 0.1g (open bars) and 0.2g (closed bars). *P<0.001 T-test compared to 0.2g meal size. B) A similar proportion of the gastric emptying curves were better fit by the GVS2 model (light gray; 2 peak) than the GVS model (dark gray; 1 peak) in C57BL/6J mice offered a 0.1g versus a 0.2g meal. P>0.05, Fishers Exact Test.
Table 4.
Gastric emptying parameters for meal size offered to C57BL/6J mice
| Egg Meal Size | T1/2 (min) | Tlag (min) | Tpeak2 (min) | R (×10−3) (min−1) |
|---|---|---|---|---|
| 0.1 g | 124 ± 6 | 70 ± 6 | 140 ± 9 | 6.0 ± 0.3 |
| 0.2 g | 184 ± 16b | 91 ± 7a | 163 ± 8 | 3.8 ± 0.2b |
P<0.001,
P<0.001, compared to Balb/cJ group, paired T-test.
Effect of PYY on Gastric Emptying in Balb/cJ Mice
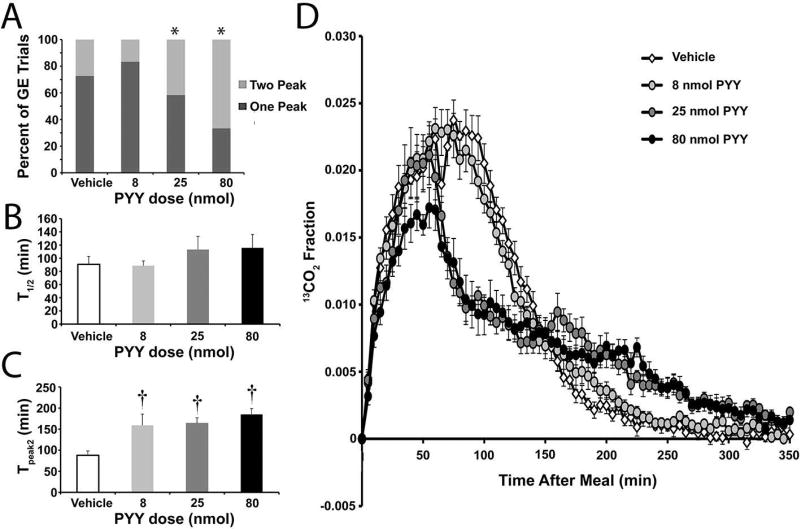
Two peak gastric emptying may involve intestinal feedback to temporarily pause gastric emptying. The hormone PYY, a suppressor of upper gut motility involved in the ileal brake,36–39 was used to test the potential of pharmacologically inducing two-peak curves. PYY, delivered 60 min after the egg meal to a cohort of 12 Balb/cJ mice, caused a dose-dependent difference in the frequencies of two-peak curves (P<0.05, Chi-square; Figure 6A). The proportion of 2-peak curves from vehicle-treated (27%) and 8nmol PYY-treated (17%) tests were similar to untreated Balb/cJ mice (above). The higher doses of PYY, 25 nmol and 80 nmol, increased the proportion of 2-peak curves (42% and 67%, respectively) in Balb/cJ mice to levels consistent with C57BL/6J mice (above).
Figure 6.
Administration of PYY following egg meal ingestion increases the occurrence of two-peak gastric emptying tests, but does not alter the mean T1/2 values in Balb/c mice. A) The proportion of the gastric emptying curves better fit by the GVS2 model (light gray; 2 peak) or the GVS model (dark gray; 1 peak) in Balb/c mice treated with saline or 8nmol, 25nmol or 80nmol PYY. *P<0.05, Chi Square. B) Mean T1/2 values (± SEM) obtained for solid gastric emptying for Balb/c mice treated with saline (open bars; N=22) or 8nmol (light gray bars; N=24), 25nmol (dark gray bars; N=12) or 80nmol (closed bars; N=12) PYY. P>0.05 ANOVA. C) Mean Tpeak2 values (± SEM) obtained for solid gastric emptying for Balb/c mice treated with saline (open bars; N=6) or 8nmol (light gray bars; N=4), 25nmol (dark gray bars; N=5) or 80nmol (closed bars; N=8) PYY where the GVS2 model was the better fit. †P<0.05 ANOVA with Neuman Keuls Multiple Comparisons Test compared to saline-treated controls. D) The mean molar percent of 13C (± SEM; N=11) for each average five-minute time point plotted over time generates average solid gastric emptying curves for Balb/c mice treated with saline (open symbols) or 8nmol (light gray symbols), 25nmol (dark gray symbols) or 80nmol (closed symbols) PYY. P<0.0001 2-way ANOVA. The text describes the time points at which there was a significant difference between treatment groups.
Despite the appearance of two peaks, the average T1/2 for the vehicle, 8 nmol, 25 nmol, and 80 nmol dosage groups were not different between the groups (T1/2 ± SEM, P>0.05, 1-way ANOVA Neuman-Keuls Comparisons test; Figure 6B, Table 5). The time to reach the second peak, however, was significantly different between vehicle and the 8, 25, and 80 nmol dosage groups (P<0.001, 1-way ANOVA Neuman-Keuls Multiple Comparisons test; Figure 6C, Table 5). The average molar percent of 13C for every five minute time point was averaged for each mouse in a particular dose group to create an overall gastric emptying curve for each of the four dosage groups (Figure 6D). There was no significant difference between the vehicle and 8 nmol group for any of the time points (P>0.05, 2-way ANOVA Bonferroni Multiple Comparisons test). The 25 nmol group was different from vehicle at 70–130 (P<0.01) and 180 min (P<0.05) and the 80 nmol was different form vehicle at 30 min (P<0.05), between 60 and 125 min (P<0.05), and at 215 (P<0.01) and 225 min (P<0.001, 2-way ANOVA Bonferroni Multiple Comparisons test).
Table 5.
Gastric emptying parameters for PYY doses with Balb/cJ Mice
| PYY (nmol/kg) |
T1/2 (min) | Tlag (min) | Tpeak2 (min) | R (×10−3) (min−1) |
|---|---|---|---|---|
| Vehicle | 91 ± 12.2 | 65 ± 5.0 | 88 ± 10 | 9.6 ± 0.6 |
| 8 | 89 ± 7.3 | 64 ± 4.5 | 159 ± 27b | 9.0 ± 0.7 |
| 25 | 113 ± 20.0 | 44 ± 2.4a | 164 ± 13b | 9.0 ± 1.7 |
| 80 | 115 ± 20.2 | 52 ± 6.5 | 185 ± 14b | 8.1 ± 0.8 |
P<0.05,
P<0.001, compared to Vehicle, One-way ANOVA with Repeated Measures, Neuman-Keuls Multiple Comparisons Test.
Discussion
In this study, the [13C]-octanoic acid breath test in mice was validated using the high throughput and high temporal resolution OA-ICOS analyzer. Implementing its use of OA-ICOS rather than gas chromatography or conventional infrared spectroscopy provides an increased sensitivity that generates reproducible and easy to fit data. The MIU feature, which automates the sampling process, increases the productive capacity (12 mice) of the assay. Using this system, Balb/cJ mice analyzed between interventions until 54 weeks of age maintained a stable T1/2 for gastric emptying of a solid egg meal. Furthermore, OA-ICOS detected accelerated and delayed gastric emptying of both liquids and solids using pharmacological or physiological tools.
Gastric emptying curves that contain a second peak (i.e. more than one phase), although rare, has been observed in previous literature in both mice and humans with the solid meal.2 However, a mathematical expression that was versatile enough to fit these data has only recently been described.20 Putting to use our increased temporal resolution and analysis tool, the potential physiological bases for the presence of these two-peak curves were investigated. We began by considering if complex curves were more prevalent in mice with diabetic gastroparesis. After development of diabetes, a proportion of mice will develop delayed gastric emptying,14, 15 which is evident by a slowing of GE or increase in T1/2. It was hypothesized that there would be an increase in the proportion of two-peak curves for delayed mice when compared to non-delayed groups. The T1/2 values obtained for two mouse models of diabetes using the new analysis tools revealed comparable results to previously measured values.14, 15 Likewise, the new analysis of these data revealed that Tlag and R values were also slower in the Delayed groups compared to the Resistant and Non-Diabetic groups. Less consistent were changes in two-peak gastric emptying curves during diabetes. For NOD/ShiLtJ mice, two-peak curves were present for approximately 17% of the mice and did not vary with diabetes or the rate of gastric emptying. For streptozotocin-treated B6;C3Fe a/a-Csf1<+> mice, the frequency of two-peak curves increased in the delayed group, but the representative curves and lack of difference in the Tpeak2 values between the Resistant and Delayed groups, suggest this increased frequency may reflect an increased ability to resolve the second phase when it constitutes a greater proportion of the entire meal. Therefore, the rate of gastric emptying reflected in the T1/2, Tlag and R values is the dysfunction that occurs in a subset of diabetic mice rather than phase changes in individual mice.
In the present study, strain-dependent differences in gastric emptying were found between Balb/cJ and C57Bl/6J mice. The T1/2 values of C57Bl/6J mice were double those observed in Balb/cJ mice. A transit study using RITC-Dextran confirmed this delay and demonstrated that at four hours, a large proportion of the solid egg meal had completely passed through the small intestine for the Balb/cJ mice but remained in the stomach and small bowel in C57Bl/6J mice. There were a significantly higher proportion of two-peak curves for the C57Bl/6J mice than Balb/cJ mice. The time of the first peak (Tlag) and the time of the second peak (Tpeak2) were both significantly faster in Balb/cJ mice compared to C57Bl/6J mice, suggesting that rather than just the presence of a second peak, there are likely fundamental differences in the physiology of gastric emptying between the two mouse strains. There are well recognized differences between Balb/cJ and C57Bl/6J mice especially in the immune40, 41 and nervous systems.42, 43 C57Bl/6J mice are known to have a greater stress response than Balb/cJ mice.31–33 Furthermore, most stressors, except cold stress, cause a delay in gastric emptying.44, 45 Serum corticosterone levels were higher in C57Bl/6J mice immediately following a gastric emptying test compared to naïve C57Bl/6J mice; this is consistent with the stress of an overnight fast.46, 47 However, post-gastric emptying corticosterone levels were not different between C57Bl/6J and Balb/cJ mice and there was no correlation between corticosterone levels and simultaneously obtained T1/2 values. Therefore, it seems unlikely that strain-related difference in stress responses could account for the strain-related differences in gastric emptying.
To further explore potential mechanisms for the differences in gastric emptying between Balb/cJ and C57Bl/6J mice, single strains were subjected to conditions to try and recapitulate what is observed in the opposite strain. Reducing the meal size in C57Bl/6J mice significantly accelerated gastric emptying. However, the gastric emptying rate in C57Bl/6J mice given 0.1g meal was not as fast as Balb/cJ mice given 0.2 g egg, and there was no difference in the proportion of two-peak curves in these mice offered the 0.1g meal compared to the 0.2g meal. Likewise, the time of the second peak was not affected by the meal size, suggesting that the occurrence of two-peak curves may just be fundamentally more prevalent in C57Bl/6J mice.
Intestinal feedback to the stomach can inhibit gastric emptying.48, 49 The most dramatic example of this is the physiological phenomenon known as the ileal break. The intestinal hormone PYY, secreted by enteroendocrine L cells is a known suppressor of upper gut motility and proposed to contribute to the ileal brake.50–52 By delivering PYY to Balb/cJ mice during the gastric emptying test, we tested if we could create the complexity (increase proportion of two peak curves) we observe in the C57Bl/6J mice. PYY concentration-dependently increased the proportion of two-peak curves of Balb/cJ mice compared to saline-treated controls. Likewise, the time of the second peak in PYY-treated mice were double those of the controls and similar to the time of the second peak observed in C57Bl/6J mice. These data suggest that two-peak curves may reflect intestinal feedback, which may occur with greater frequency in C57Bl/6J mice. However, the overall T1/2 was not significantly different for any of the PYY dose groups when compared to vehicle. This suggests that although PYY can be used to induce a second peak during gastric emptying in Balb/cJ mice, it does not recapitulate the gastric emptying of the C57BL/6J mouse.
Collectively, the present study demonstrates the utility of a high temporal resolution [13C]-octanoic acid breath test to analyze gastric emptying. The test can measure accelerated or delayed gastric emptying of either solid or liquid meals. Furthermore, the temporal resolution allows accurate determination of two-phase gastric emptying curves. These two-phase curves are more prevalent in streptozotocin-induced diabetic mice with delayed gastric emptying, and in C57Bl/6J mice compared to Balb/cJ. The mechanisms contributing to differences in the physiology of the stomach between C57Bl/6J and Balb/cJ mice requires further study. The occurrence of a second peak in gastric emptying curves likely reflects intestinal feedback to the stomach; however, the rate of gastric emptying is not influenced by the presence or absence of two peaks. Therefore, intestinal feedback, manifest as two-phase gastric emptying, may not contribute to diabetic gastroparesis, but may contribute to delayed gastric emptying in other conditions, for example following pancreatoduodectomy.53
Key Points Summary.
High temporal resolution gastric emptying breath tests reveal differences in gastric physiology between mouse strains.
Meal size affects the rate of solid gastric emptying but does not alter the complexity of breath excretion curves.
Systemic delivery of the enteroendocrine hormone PYY during a solid gastric emptying test, dose-dependently induces two-phase gastric emptying suggesting that high temporal resolution gastric emptying breath tests may measure intestinal feedback.
Acknowledgments
This work was supported by NIH grants DK68055, DK58185 and DK106011. The authors are grateful to Ms. Kristy Zodrow for her assistance preparing the manuscript.
Author Contributions
KEM performed analyses and wrote the manuscript
ZB wrote software and performed analyses
SSH performed the experiments and analyses
JEP performed the experiments
SS performed the experiments
AEW performed the experiments and analyses
GC performed the experiments
SJG helped design and interpret analyses
JHS helped design and interpret analyses and edited the manuscript
GF helped design and interpret analyses and edited the manuscript
TO designed experiments, interpreted analyses and edited the manuscript
DRL conceived of study, designed experiments, interpreted analyses and edited the manuscript
References
- 1.Symonds E, Butler R, Omari T. Noninvasive breath tests can detect alterations in gastric emptying in the mouse. Eur J Clin Invest. 2002;32:341–4. doi: 10.1046/j.1365-2362.2002.00991.x. [DOI] [PubMed] [Google Scholar]
- 2.Symonds EL, Butler RN, Omari TI. Assessment of gastric emptying in the mouse using the [13C]-octanoic acid breath test. Clin Exp Pharmacol Physiol. 2000;27:671–5. doi: 10.1046/j.1440-1681.2000.03318.x. [DOI] [PubMed] [Google Scholar]
- 3.Osinski MA, Seifert TR, Cox BF, Gintant GA. An improved method of evaluation of drug-evoked changes in gastric emptying in mice. J Pharmacol Toxicol Methods. 2002;47:115–20. doi: 10.1016/s1056-8719(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 4.Yeung CK, McCurrie JR, Wood D. A simple method to investigate the inhibitory effects of drugs on gastric emptying in the mouse in vivo. J Pharmacol Toxicol Methods. 2001;45:235–40. doi: 10.1016/s1056-8719(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang SC, Lu KY, Chen SM, Young TK. Gastric emptying and intestinal transit of liquid and solid markers in rats with chronic uremia. Chin J Physiol. 2001;44:81–7. [PubMed] [Google Scholar]
- 6.El-Salhy M. Gastric emptying in an animal model of human diabetes type 1: relation to endocrine cells. Acta Diabetol. 2001;38:139–44. doi: 10.1007/s005920170011. [DOI] [PubMed] [Google Scholar]
- 7.Bennink RJ, De Jonge WJ, Symonds EL, et al. Validation of gastric-emptying scintigraphy of solids and liquids in mice using dedicated animal pinhole scintigraphy. J Nucl Med. 2003;44:1099–104. [PubMed] [Google Scholar]
- 8.Habraken JB, de Bruin K, Shehata M, et al. Evaluation of high-resolution pinhole SPECT using a small rotating animal. J Nucl Med. 2001;42:1863–9. [PubMed] [Google Scholar]
- 9.Weber DA, Ivanovic M. Ultra-high-resolution imaging of small animals: implications for preclinical and research studies. J Nucl Cardiol. 1999;6:332–44. doi: 10.1016/s1071-3581(99)90046-6. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz R, Kaspar A, Seelig J, Kunnecke B. Gastrointestinal transit times in mice and humans measured with 27Al and 19F nuclear magnetic resonance. Magn Reson Med. 2002;48:255–61. doi: 10.1002/mrm.10207. [DOI] [PubMed] [Google Scholar]
- 11.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–64. 64 e1–2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KM, Gibbons SJ, Sha L, et al. Interleukin 10 Restores Gastric Emptying, Electrical Activity, and Interstitial Cells of Cajal Networks in Diabetic Mice. Cell Mol Gastroenterol Hepatol. 2016;2:454–67. doi: 10.1016/j.jcmgh.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–409. 409 e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KM, Zhu J, Stoltz GJ, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–45. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani G, Gibbons SJ, Verhulst PJ, et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol. 2016;2:40–7. doi: 10.1016/j.jcmgh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creedon CT, Verhulst PJ, Choi KM, et al. Assessment of gastric emptying in non-obese diabetic mice using a [13C]-octanoic acid breath test. J Vis Exp. 2013:e50301. doi: 10.3791/50301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi Y, Toyomasu Y, Saravanaperumal SA, et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology. 2017;153:521–35. e20. doi: 10.1053/j.gastro.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izbeki F, Asuzu DT, Lorincz A, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J Physiol. 2010;588:3101–17. doi: 10.1113/jphysiol.2010.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashyap PC, Choi KM, Dutta N, et al. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G1013–9. doi: 10.1152/ajpgi.00069.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajzer Z, Gibbons SJ, Coleman HD, Linden DR, Farrugia G. A gamma variate model that includes stretched exponential is a better fit for gastric emptying data from mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G162–70. doi: 10.1152/ajpgi.00280.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Bourg E. Hormetic effects of repeated exposures to cold at young age on longevity, aging and resistance to heat or cold shocks in Drosophila melanogaster. Biogerontology. 2007;8:431–44. doi: 10.1007/s10522-007-9086-6. [DOI] [PubMed] [Google Scholar]
- 22.Shushimita S, Grefhorst A, Steenbergen J, et al. Protection against renal ischemia-reperfusion injury through hormesis? Dietary intervention versus cold exposure. Life Sci. 2016;144:69–79. doi: 10.1016/j.lfs.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–84. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulie B, Tack J, Maes B, Geypens B, De Roo M, Janssens J. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am J Physiol. 1997;272:G902–8. doi: 10.1152/ajpgi.1997.272.4.G902. [DOI] [PubMed] [Google Scholar]
- 25.Houghton LA, Fowler P, Keene ON, Read NW. Effect of sumatriptan, a new selective 5HT1-like agonist, on liquid gastric emptying in man. Aliment Pharmacol Ther. 1992;6:685–91. doi: 10.1111/j.1365-2036.1992.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Xue L, Camilleri M, Locke GR, 3rd, et al. Serotonergic modulation of murine fundic tone. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1180–6. doi: 10.1152/ajpgi.00224.2006. [DOI] [PubMed] [Google Scholar]
- 27.Xue L, Locke GR, Camilleri M, et al. Effect of modulation of serotonergic, cholinergic, and nitrergic pathways on murine fundic size and compliance measured by ultrasonomicrometry. Am J Physiol Gastrointest Liver Physiol. 2006;290:G74–82. doi: 10.1152/ajpgi.00244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyasaka K, Ohta M, Kanai S, et al. Enhanced gastric emptying of a liquid gastric load in mice lacking cholecystokinin-B receptor: a study of CCK-A,B, and AB receptor gene knockout mice. J Gastroenterol. 2004;39:319–23. doi: 10.1007/s00535-003-1297-2. [DOI] [PubMed] [Google Scholar]
- 29.Uchida M, Endo N, Shimizu K. Simple and noninvasive breath test using 13C-acetic acid to evaluate gastric emptying in conscious rats and its validation by metoclopramide. J Pharmacol Sci. 2005;98:388–95. doi: 10.1254/jphs.fp0050153. [DOI] [PubMed] [Google Scholar]
- 30.Uchida M, Shimizu K. 13C-acetic acid is more sensitive than 13C-octanoic acid for evaluating gastric emptying of liquid enteral nutrient formula by breath test in conscious rats. Biol Pharm Bull. 2007;30:487–9. doi: 10.1248/bpb.30.487. [DOI] [PubMed] [Google Scholar]
- 31.Depke M, Breitbach K, Dinh Hoang Dang K, et al. Bone marrow-derived macrophages from BALB/c and C57BL/6 mice fundamentally differ in their respiratory chain complex proteins, lysosomal enzymes and components of antioxidant stress systems. J Proteomics. 2014;103:72–86. doi: 10.1016/j.jprot.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Kulesskaya N, Karpova NN, Ma L, Tian L, Voikar V. Mixed housing with DBA/2 mice induces stress in C57BL/6 mice: implications for interventions based on social enrichment. Front Behav Neurosci. 2014;8:257. doi: 10.3389/fnbeh.2014.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marandici A, Monder C. The fate of corticosterone and 11-deoxycorticosterone in C57BL/6 and BALB/c strains of mice: distribution and oxidative metabolism. J Steroid Biochem. 1984;21:579–83. doi: 10.1016/0022-4731(84)90334-0. [DOI] [PubMed] [Google Scholar]
- 34.Plourde V. Stress-induced changes in the gastrointestinal motor system. Can J Gastroenterol. 1999;13(Suppl A):26A–31A. doi: 10.1155/1999/320626. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KS. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen Comp Endocrinol. 2012;179:406–13. doi: 10.1016/j.ygcen.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Allen JM, Fitzpatrick ML, Yeats JC, Darcy K, Adrian TE, Bloom SR. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion. 1984;30:255–62. doi: 10.1159/000199117. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–16. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–73. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 39.Whited KL, Tso P, Raybould HE. Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. Endocrinology. 2007;148:4695–703. doi: 10.1210/en.2006-1665. [DOI] [PubMed] [Google Scholar]
- 40.Brown JK, Donaldson DS, Wright SH, Miller HR. Mucosal mast cells and nematode infection: strain-specific differences in mast cell precursor frequency revisited. J Helminthol. 2003;77:155–61. doi: 10.1079/JOH2002160. [DOI] [PubMed] [Google Scholar]
- 41.Darville T, Andrews CW, Jr, Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect Immun. 2001;69:7419–24. doi: 10.1128/IAI.69.12.7419-7424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–34. [PubMed] [Google Scholar]
- 43.Mogil JS, Wilson SG, Bon K, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 44.Martinez V, Wu SV, Tache Y. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology. 1998;139:3730–5. doi: 10.1210/endo.139.9.6195. [DOI] [PubMed] [Google Scholar]
- 45.Stengel A, Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–39. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garces LY, Kenny FM, Drash A, Taylor FH. Cortisol secretion rate during fasting of obese adolescent subjects. J Clin Endocrinol Metab. 1968;28:1843–7. doi: 10.1210/jcem-28-12-1843. [DOI] [PubMed] [Google Scholar]
- 47.Vance ML, Thorner MO. Fasting alters pulsatile and rhythmic cortisol release in normal man. J Clin Endocrinol Metab. 1989;68:1013–8. doi: 10.1210/jcem-68-6-1013. [DOI] [PubMed] [Google Scholar]
- 48.Lin HC. Abnormal intestinal feedback in disorders of gastric emptying. Dig Dis Sci. 1994;39:54S–5S. doi: 10.1007/BF02300372. [DOI] [PubMed] [Google Scholar]
- 49.Schulze-Delrieu K. The load-to-length principle in the inhibition of gastric emptying by intestinal feedback. Gastroenterology. 1990;98:1387–8. [PubMed] [Google Scholar]
- 50.Pironi L, Stanghellini V, Miglioli M, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–9. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 51.Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 1982;79:2514–8. doi: 10.1073/pnas.79.8.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen J, Phillips SF, Sarr MG, Kost LJ, Holst JJ. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am J Physiol. 1995;269:G945–52. doi: 10.1152/ajpgi.1995.269.6.G945. [DOI] [PubMed] [Google Scholar]
- 53.Barreto SG, Windsor JA. Does the Ileal Brake Contribute to Delayed Gastric Emptying After Pancreatoduodenectomy? Dig Dis Sci. 2017;62:319–35. doi: 10.1007/s10620-016-4402-0. [DOI] [PubMed] [Google Scholar]