Abstract
Infection with the parasite Toxoplasma gondii leads to the induction of a Th1-type response dominated by IFN-γ production and control of this pathogen. Cells of the innate immune system are essential in initiating this response both through the production of IL-12 as well as the presentation of parasite-derived Ags to MHC-restricted T cells. Although dendritic cells (DCs) have been implicated in these events, the contribution of individual DC populations remains unclear. Therefore, multiparameter flow cytometry was used to identify and characterize subsets of murine DCs during acute toxoplasmosis. This approach confirmed that infection leads to the expansion and activation of conventional DC (cDC) subsets. Unexpectedly, however, this analysis further revealed that plasmacytoid DCs are also expanded and that these cells up-regulate MHC class II and costimulatory molecules associated with their acquired ability to prime naive CD4+ T cells. Furthermore, T. gondii-activated plasmacytoid DCs produce high levels of IL-12 and both plasmacytoid DC maturation and cytokine production are dependent on TLR11. Together these studies suggest that pDCs are a prominent DC subset involved in the initial stages of T. gondii infection, presenting parasite Ags and producing cytokines that are important for controlling infection.
Protective immunity to the intracellular parasite Toxoplasma gondii is characterized by a strong Th1-type response dominated by the early production of IL-12, which in turn drives NK and T cells to secrete IFN-γ (1–3). Several innate cell populations have been implicated as the early source of this IL-12 with experimental evidence that neutrophils, macrophages, and dendritic cells (DCs)3 have prominent roles in these events (1, 4–6). In addition to the production of cytokines, the ability of APCs to process and present pathogen-derived peptides in the context of MHC molecules and appropriate costimulatory signals is required for the expansion of parasite-specific T cell responses. For many years, the macrophage was regarded as the major APC population during toxoplasmosis (7), but the identification of DCs and their unique biology suggest that DCs are more likely to perform this function. DCs sample exogenous Ags at sites of inflammation and traffic to lymphoid tissues where they prime naive T cells, while current models suggest that macrophages remain at sites of inflammation and are less efficient at T cell priming.
In recent years, there has been increasing evidence implicating DCs in resistance to T. gondii through the production of cytokines and as APCs for class I-restricted responses (6, 8, 9). For the activation of class II-restricted CD4+ T cell responses even less is known, although bone marrow-derived DCs infected with transgenic T. gondii-expressing OVA do have the ability to activate naive OVA-specific T cells (10). However, there is also evidence that DCs infected with T. gondii are unable to mature and present Ag (11). These apparently conflicting observations may be explained by the identification of different DC populations such as CD8α+, CD11b+, and plasmacytoid DCs (pDCs) that have been ascribed with distinct properties. The studies presented here reveal that several subsets of DCs are involved in the initial response to T. gondii, including, unexpectedly, plasmacytoid DCs (pDCs). Although pDCs have been primarily associated with antiviral responses, this subset expands, displays an activated phenotype as demonstrated by high levels of MHC class II and costimulatory molecules, and expresses cytokines in direct response to the parasite. Although immature pDCs cannot present peptide to naive T cells, pDCs from infected mice are capable of priming naive CD4+ T cells. Furthermore, a transgenic parasite expressing a model Ag that can be identified by a mAb when processed and presented on MHC class II demonstrates that these cells present parasite-derived Ags. Together, these findings suggest a previously unsuspected role for pDCs during the innate response to T. gondii and in the development of adaptive responses to nonviral pathogens.
Materials and Methods
Mice
Female 6- to 8-wk-old C57BL/6 and BALB/c mice were obtained from Jackson ImmunoResearch Laboratories. DO11.10 TCR-transgenic BALB/c mice (12) were originally obtained from Dr. P. Scott and bred within the University Laboratory Animal Resources facility of the University of Pennsylvania. The TLR11−/− mice were obtained from Dr. A. Sher (National Institutes of Health, Bethesda, MD) from stock originally provided by S. Ghosh (Yale University). All mice were maintained under specific-pathogen-free conditions in accordance with institutional guidelines.
Parasites
All parasite lines were maintained by serial passage in human foreskin fibroblast cell monolayers in DMEM medium (Invitrogen) containing 10% FBS as previously described (13). Before in vivo or in vitro infections, tachyzoites were purified from human foreskin fibroblast cells by needle passage, filtration through a 3.0-μm pore-size filter (Nucleopore) and washed in PBS. The EaRFP coding sequence was PCR amplified from a pTrcEaRFP plasmid obtained from Dr. M. K. Jenkins (University of Minnesota, Minneapolis, MN) (14) using primers as follows: sense, 5′-CCG GCCTAGGGCCCTTGAAGAATTTGCAAAG-3′ and antisense, 5′-CTAGCTAGCTCAATGATGATGATGATGATGGTCG-3′; this plasmid consists of aa 46–74 of the I-Eα chain from the I-Ed allele inserted upstream of the DsRed coding sequence. The PCR product was cloned into TOPO-TA vector (Invitrogen), sequenced, and subcloned via AvrII/NheI restriction digests in frame with the P30 signal sequence from the T. gondii SAG1 gene, in ptubP30-YFP-HA-dhfr, which is a Bluescript pKS+ based vector and contains: 1) the T. gondii TUB1 promoter (15), terminating at a BglII site upstream of the initiation codon; 2) P30 signal sequence from the T. gondii SAG1 gene terminating in an AvrII site, cloned in frame with YFP terminating in a NheI site, followed by a hemagglutinin tag terminating in a AflII site; 3) a 3′ untranslated region from the T. gondii dihydrofolate reductase-thymidylate synthase gene, terminating in a NotI site (16); and 4) a chloramphenicol acetyltransferase-selectable marker (17) expressed under the control of 5′ and 3′ untranslated regions derived from the T. gondii SAG1 gene.
Prugniaud T. gondii tachyzoites were transfected as previously described (10), selected for chloramphenicol resistance, and cloned by limiting dilution. Multiple independent clonal lines were screened by microscopy to assess red fluorescent protein (RFP) expression levels and subcellular compartmentalization. Generation of control RFP parasites and OVA-transgenic parasites has been previously described (10, 11).
To study the processing and presentation of T. gondii-derived EaRFP in vivo, C57BL/6 mice were inoculated i.p. with 200 μl of PBS containing 104 control Prugniaud parental parasites, Prugniaud RFP parasites (RFP), or Prugniaud-expressing P30EaRFP (EaRFP).
Cell preparations and flow cytometry
Spleens and lymph nodes (mesenteric, periaortal, and mediastinal) were injected with 0.75 mg/ml Liberase (Roche) and incubated at 37°C for 20 min. Single-cell suspensions were washed with FACS buffer (PBS containing 1% BSA, 1 mM EDTA, and 0.05%sodium azide) and RBCs were lysed with ACK (BioWhittaker). Cells (5 × 106) were washed, blocked, and stained at 4°C in a 100-ml final volume containing 24G2 and 1 μg/ml normal rat and 1 μg/ml mouse serum (Caltag Laboratories). Abs were purchased from the indicated vendors or purified and labeled in our laboratory. The FITC-labeled Abs used were: anti-CD19, anti NK1.1, CD3 (BD Biosciences). PE-labeled Abs: CD11c (BD Biosciences), CD40, CD80, CD83, and MHC class II I-Ab (AF6; BD Biosciences), KJ126 (Caltag Laboratories), and IL-12 (BD Biosciences). PerCP-Cy5.5 conjugated CD8α/Ly-2 (BD Biosciences). The PE-Cy7-labeled Abs used were: CD8α (eBioscience) and GR-1 (RB6-8C5; eBioscience). Allophycocyanin- and biotin-labeled anti-mPDCA-1 were purchased from Miltenyi Biotec. Allophycocyanin-Cy7 B220, CD11b Pacific Blue, streptavidin-conjugated Pacific Blue, and streptavidin-PE-Cy7 were obtained from eBioscience. Fc-RII/III (24G2; American Type Culture Collection) and biotinylated and allophycocyanin-conjugated Y-Ae were prepared using standard methods in our laboratory. For intracellular IL-12 staining, surface-stained samples were washed, fixed (Cytofix; BD Biosciences) and then permeabilized with 0.01% saponin in FACS buffer before intracellular staining. Analysis was performed on a dual-laser FACSCalibur (BD Biosciences) or a four-laser 10-color LSRII (BD Biosciences). Flow cytometry was analyzed using FlowJo 8.5 (Tree Star). DCs were sorted on a FACsCanto or FACSDiva sorter.
Preparation of bone marrow-derived and ex vivo-sorted DCs
CD11b+ bone marrow-derived DCs were prepared using GM-CSF (PeproTech) (18) while bone marrow-derived pDCs were prepared using Flt-3L (18, 19). Bone marrow-derived PDCA+ pDCs were coated with anti-mPDCA-1 MACS beads (Miltenyi Biotec) and pDCs were selected using magnetized MACS columns according to the manufacturer’s protocol. Anti-mPDCA-1 MACS and Pan-DC MACS beads were also used to isolate pDCs or whole DC populations from liberase-treated spleens for analysis (purity 79–84% by FACS analysis).
In vitro cultures of ex vivo or bone marrow-derived DC subsets
Bone marrow-derived or ex vivo FACS-sorted DC subset cultures were plated at 5 × 105 cells/well in a 96-well plate. For cytokine production assays, cells were stimulated for 16 h (in the presence of 3 mM monensin (Golgi Stop; BD Biosciences) for intracellular cytokine staining) or for 3 days for ELISA with 50 ng/ml soluble Toxoplasma Ag (STAg), 10 μg/ml, CpG DNA or infected 1:1 with Prugniaud parasites in a 200-μl final volume. Supernatants from cultures were assayed for IL-12p40 and IFN-α (20) by ELISA. Proliferation assays were conducted as previously described (10).
Adoptive cell transfer and T. gondii infection
Naive CD3+ T cells were purified from DO11 mice. Single-cell suspensions from spleens and lymph nodes were RBC lysed and T cells were isolated using CD3+-specific columns (R&D Systems.), as described by the manufacturer. For depletion experiments, naive DO11 T cells were labeled with CFSE (Molecular Probes) as previously described (10). Each BALB/c recipient animal received 2 × 106 CFSE-labeled cells in 0.2 ml of PBS by retro-orbital i.v. injection. For depletion experiments, animals were treated on days −1, 1, and 3 with 200 mg of anti-GR-1 or 250 mg of rat IgG.
Results
Characterization of DCs after T. gondii infection
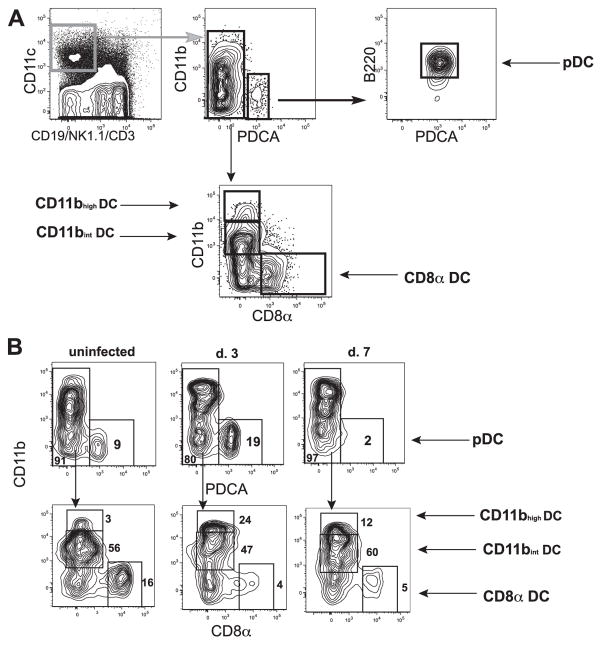
To gain a better understanding of whether there is preferential activation or expansion of different DC subsets during toxoplasmosis, the numbers and activation status of the three major blood-derived CD11c+ DC populations were examined. CD8α+ (lymphoid), CD11b+ (myeloid), and pDCs can be differentiated from each other based on the expression of cell surface markers (see Table I). CD8α+ DCs are CD11c+, CD8α+, CD11b−, B220−, and PDCA−; CD11b+ DCs are CD11c+, CD8α−, CD11b−, B220−, and PDCA−; and pDCs are CD11clow, CD8α+/−, CD11b−, B220+, and PDCA+. The gating strategy used to identify these different DC subsets is shown in Fig. 1A. To facilitate identification of the relevant subsets, Ab-stained cells were first subjected to live/dead exclusion using 4′-diamidino-2-phenylindole and doublets were excluded using SSC-W by SSC-A. CD3+ T cells, CD19+ B cells, and NK1.1+ NK cells were then excluded by negative gating. Macrophages, which are CD11c−, were not examined in this study. CD11c+ DC populations were then further subdivided based on the expression of the levels of CD11b, PDCA, CD8α, and B220 (Fig. 1A). Using this gating strategy, a fourth subset of CD11c+CD11bhigh cells emerged that was recruited to the lymphoid tissue after infection. These cells appear to be monocyte-derived DCs previously described in inflamed secondary lymphoid tissue in other systems (21–23), yet which are distinct from the CD11c−F4/80+ monocytes described by Sibley and colleagues (24). This gating strategy was then used to determine the kinetics of DC activation and expansion after T. gondii infection in the lymphoid tissues (Fig. 1B). In an uninfected animal (Fig. 1B, left panels), there were small populations of pDCs, CD8α, and CD11bhigh DCs (Fig. 1B, top and bottom left panels), yet CD11b+ DCs are the primary population (Fig. 1B, bottom left panel). By 3 days postinfection, the numbers of pDCs (Fig. 1B, top panel, center), CD11b+, and CD11bhigh DCs increased while the CD8α+ population did not (Fig. 1B, bottom panel, center). The kinetics of these populations differ and while pDCs in the lymphoid tissue peak by day 3, by day 7 postinfection the numbers were reduced to below those of an uninfected animal (Fig. 1B, top panel, center and right). These kinetics were recapitulated with the virulent RH strain of T. gondii and following oral infection with the ME49 strain (data not shown).
Table I.
DC subset phenotype
| DC Subsets | Surface Phenotype
|
|||||
|---|---|---|---|---|---|---|
| CD11c | CD8α+ | CD4 | Class II | CD11b | B220 | |
| Blood-derived | ||||||
| CD8α+ | + | + | − | Intermediate | − | − |
| CD11bhigh | + | − | + | Intermediate | ++ | − |
| Plasmacytoid | Low | +/− | +/− | Low | − | + |
| CD11b+CD4+ | + | − | + | Intermediate | + | − |
| Tissue-derived | ||||||
| Langerhans | + | − | − | ++ | + | − |
| Interstitial | + | −/low | − | + | +/− | − |
FIGURE 1.
Several populations of CD11c+ DCs expand after infection with T. gondii. A, The gating strategy used to identify DC subpopulations. After exclusion of doublets by SSC-A/SSC-W and dead cells by 4′-diamidino-2-phenylindole, CD19, NK1.1, and CD3, negative cells that are CD11C+ are DCs. pDCs are first gated on PDCA+CD11b− cells and then B220+ cells are selected. CD11b+PDCA− cells are then examined for CD8α expression. CD8α− cells are divided into two populations, CD11bint and CD11bhigh. CD8α+CD11b− cells are CD8α DCs. B, Kinetics of DC subsets. Using the above-described gating strategy, the kinetics of the four different subsets were examined. pDCs expand by day 3 (top panel, center) and then are diminished by day 7 while the CD8α DC subset is diminished within days of infection and begins to increase again by day 7 postinfection.
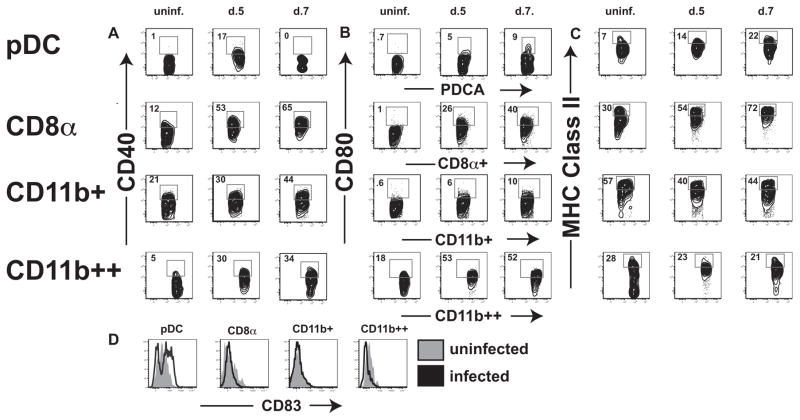
To examine the activation status of DCs in response to infection with T. gondii, the kinetics of expression of MHC class II and the costimulatory molecules CD80 and CD40 were examined. The Siglec family member CD83 was also examined as a marker of maturation. Before infection, pDCs from uninfected animals displayed low levels of MHC class II, CD40, CD80, CD83, and CD8 (Fig. 2, A and B). All four subsets of DCs up-regulated CD40 and CD80, yet only the pDC and CD8α+ DC populations concurrently up-regulated MHC class II (Fig. 2C). These data suggest a role for pDCs and CD8α+ DCs in the priming of the CD4+ T cell response, yet does not exclude the other subsets from a role in resistance to T. gondii. Furthermore, only pDCs express increased levels of CD83 after infection (Fig. 2D), further implicating these cells in the initial response to infection. Since previous studies have associated pDC activation predominantly with viral infections, the observation that this population expanded during toxoplasmosis was unexpected and was therefore the focus of the subsequent studies presented here.
FIGURE 2.
Several subsets of DC subsets are activated during T. gondii infection. The activation status of pDCs (top row), CD8α (second row), CD11b intermediate (third row), ad Cd11bhigh DCs are evaluated in uninfected (left panel), day 5 (center panel), and day 7 populations (right panel) postinfection with T. gondii. A, CD40 is up-regulated in pDC, CD8α, and CD11bhigh populations by day 5. pDCs quickly become CD40low by day 7 while the three other populations continue to up-regulate the costimulatory molecule. B, CD80 is highly up-regulated in all four populations after infection. C, MHC class II is up-regulated in pDC and CD8α populations by 5 days postinfection while the CD11b populations do not change their MHC class II profiles. D, CD83 is up-regulates on pDCs only. uninf., Uninfected.
Ag presentation by pDCs
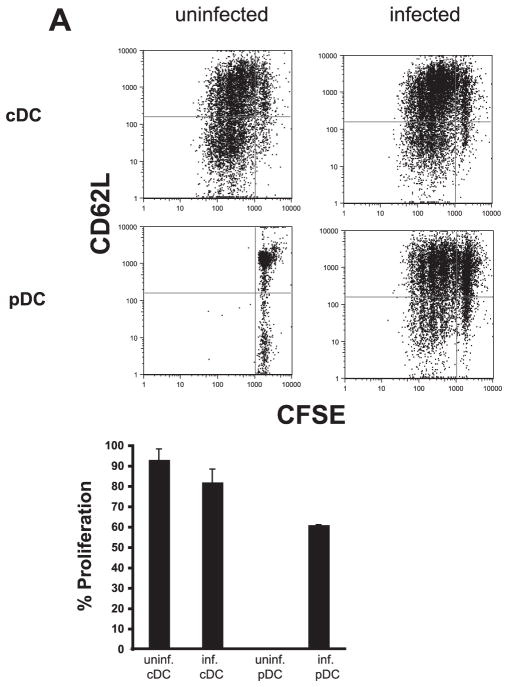
The observation that challenge with T. gondii leads to an expansion and maturation of pDCs suggests a function for these cells in T cell activation. Previous reports have established that resting pDCs are incapable of priming naive T cells due to their low levels of MHC and costimulatory molecules. After activation, however, pDCs were able to induce CD4+ T cell activation through the up-regulation of these molecules (25, 26). To test whether the DC populations induced by T. gondii in vivo are able to activate naive T cell proliferation, pDCs and conventional CD8α+ DCs were FACS sorted from uninfected or infected animals (day 3 postinfection) and their ability to activate DO11 TCR-transgenic CD4+ T cells was tested. In these experiments, T cells were CFSE labeled and cultured with the DC populations and OVA peptide. Naive T cells cultured with CD8α+ cDCs (regardless of their source) in the presence of OVA peptide proliferated and displayed an activated phenotype as detected by decreased CD62L levels (Fig. 3A, top panel). In agreement with previous reports, naive CD4+ T cells incubated with pDCs from uninfected animals in the presence of OVA peptide did not divide or become activated. However, when pDCs from infected animals were used, these naive T cells were able to proliferate and become activated (Fig. 3A, lower panel). Thus, challenge with T. gondii leads to the emergence of a population of pDCs that is capable of Ag presentation and provides the costimulatory signals required for T cell activation.
FIGURE 3.
Activated pDCs can induce naive CD4+ T cell proliferation. Sorted cDCs from either uninfected or infected animals cultured with 1 μg/ml OVA peptide are capable of inducing proliferation of naive CFSE-labeled CD4+ T cells (top panels, left and right). As previously described, pDCs from an uninfected animal (bottom, left-hand panel) are not capable of priming naive CD4+ T cells, even in the presence of 1 μg/ml OVA peptide. After infection (bottom, right-hand panel), pDCs up-regulate co-stimulatory molecules and MHC class II, allowing them to stimulate naive CD4+ T cells when pulsed with 1 μg/ml OVA peptide as seen by the extensive dilution of CFSE and down-regulation of CD62L.
In vivo Ag presentation by pDCs
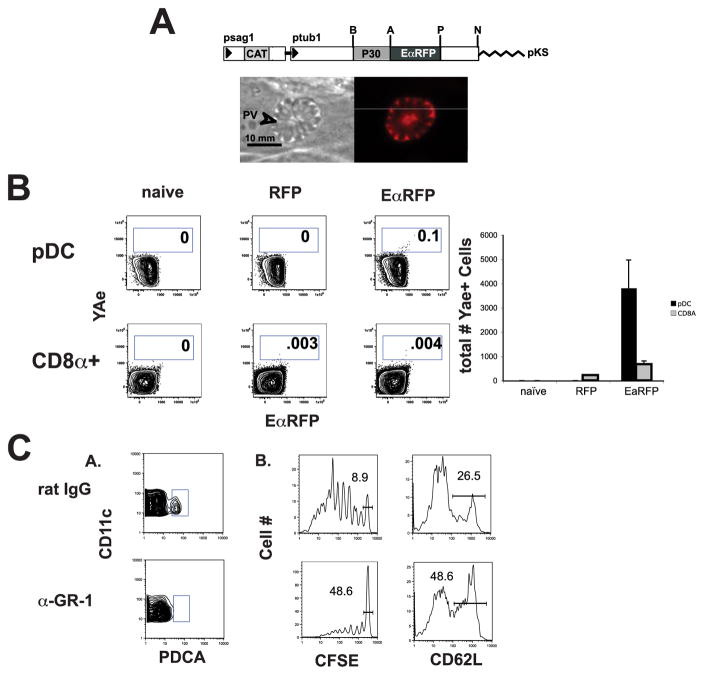
To determine whether pDCs are able to present parasite-derived peptides in vivo, tachyzoites were engineered to express the EaRFP Ag, a 32-kDa protein consisting of aa 46–74 of the I-Ed α MHC class II subunit at the N terminus and red fluorescent protein DsRed at the C terminus (14). When this model Ag is processed by APCs, it generates a class II-Eα peptide complex that can be visualized using the Y-Ae mAb. Since previous studies indicated that secreted Ags are preferentially recognized by CD4+ T cells, the SAG1 (P30) signal peptide was fused upstream of the EaRFP-coding sequence to direct expression via the dense granule secretory pathway into the parasitophorous vacuole (Fig. 4A, top panel). Expression of this chimeric protein was controlled by the T. gondii α-tubulin gene promoter which resulted in strong constitutive expression (15). Multiple independent clonal lines were screened by immunofluorescence to assess EaRFP expression levels and subcellular compartmentalization. Immunofluorescent images of infected cells demonstrated that the EaRFP protein is secreted by the parasites into the parasitophorous vacuole and was not detected in the host cell cytoplasm (Fig. 4A, lower panel).
FIGURE 4.
pDCs present parasite-derived Ag early in infection. Parasites were generated to secrete the EaRFP protein into the parasitophorous vacuole under the control of the tubulin promoter (A). Three days after injection, only splenic pDCs demonstrated a significant population of Y-Ae+ cells (n = 3) while a very small population of CD8α+ DCs presented pEa:I-Ab (B, right column and graph). C, Depletion of pDCs leads to diminished proliferation and activation of CD4+ T cells.
To assess pDC Ag presentation in vivo, mice were infected with the control RFP parasites or the EaRFP parasites and spleens were harvested 3 days later. DCs were enriched using Pan-DC beads (Miltenyi Biotec) and magnetic bead sorting and the gating strategies described above were used to analyze DC populations for presentation of pEa:I-Ab using the Y-Ae Ab. Although the numbers of CD8α Y-Ae+ DCs in the EaRFP-infected animals were not significantly above those of the RFP control, there was a small, yet significant pDC population presenting pEa:I-Ab complexes (Fig. 4B). Infection with a RFP control yields no Y-Ae+ pDCs, whereas 3 days after infection with EaRFP parasites there are ~4 × 103 Y-Ae+ pDCs (SD = 1.16 × 103). These results indicate that in this time point early in infection, the majority of cells displaying pEa: I-Ab complexes were pDCs.
In further support of a role for pDCs in priming the CD4+ T cell response to T. gondii, pDCs were depleted in mice subsequently transferred with CFSE-labeled naive CD4+ DO11 T cells. Although GR-1 was expressed on several cell populations, the only GR-1+ population of cells that we observed presenting parasite-derived Ag at this early time point by Y-Ae staining were pDCs, and GR-1 depletes >90% of pDCs (Fig. 4C, panel A). Depleted animals and animals that received rat IgG were subsequently infected with T. gondii-expressing OVA and activation of KJ126+ DO11 T cells was assessed by CFSE dilution and L-selectin down-regulation. In animals that were treated with rat IgG, the majority of the cells proliferated and became activated, whereas in animals in which pDCs were depleted, ~5-fold fewer cells became activated (Fig. 4C, panel B), indicating a role for pDCs in initiating the CD4+ T cell response to T. gondii. Furthermore, specific depletion of neutrophils using anti-Ly6G (clone 1A8, 99% neutrophil depletion) did not alter DO11 CD4+ T cell proliferation (data not shown), demonstrating that the effect of GR-1 depletion is not simply a consequence of neutrophil-specific depletion.
Direct activation of pDCs by T. gondii leads to cytokine production
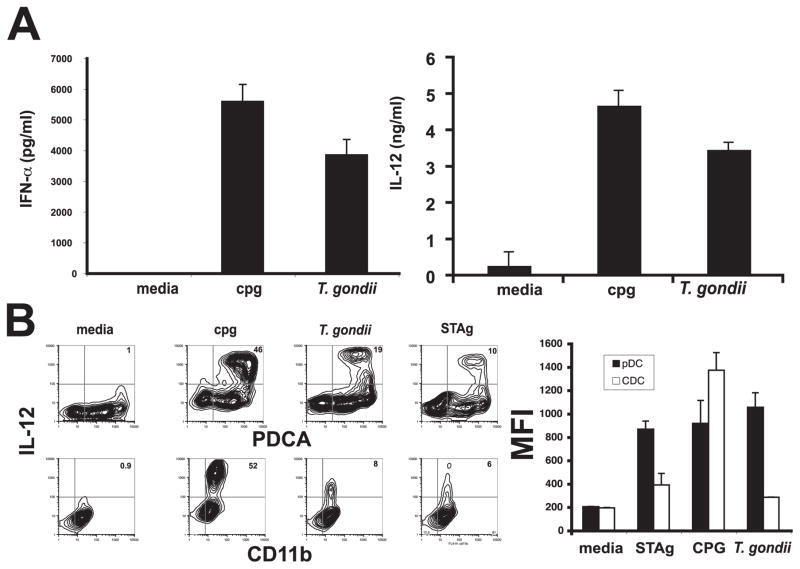
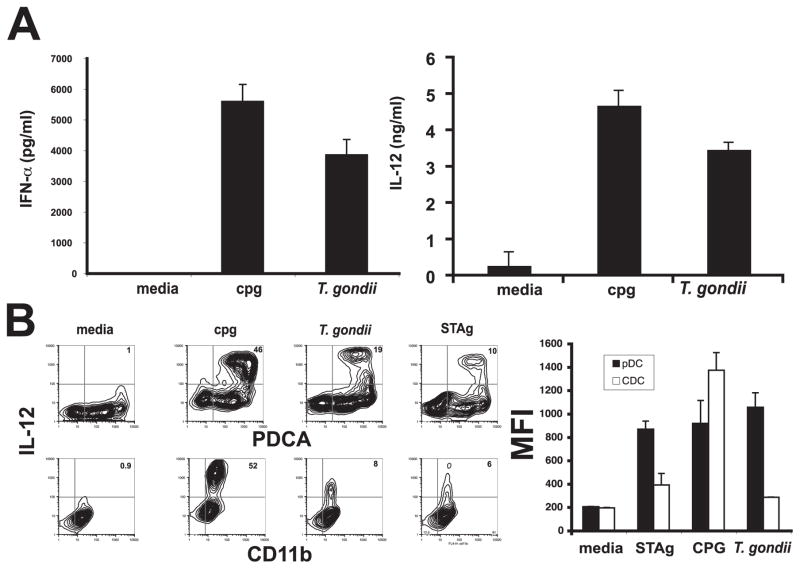
Mature pDCs are a potent source of IFN-α and mature murine pDCs have also been shown to produce IL-12 upon activation. To test whether T. gondii could directly activate pDCs, cultures of bone-marrow derived pDCs and cDCs were incubated with parasites and the supernatants from these cultures were assayed for IL-12p40 and IFN-α by ELISA or cytokine production was measured by intracellular staining. High levels of IFN-α and IL-12 were produced after stimulation with either CpG or parasites (Fig. 5A). In addition, intracellular staining of bead-sorted bone marrow-derived pDCs or cDCs revealed that these DC populations produce comparable levels of IL-12p40 in response to CpG. In contrast, pDCs express higher levels of IL-12p40 in response to parasites (mfi 1055 ± 125 SD) or STAg (mfi 867 ± 69 SD) than CD11b+ DCs (parasites mfi 285 ± 1.4, STAg mfi 390 ± 98) (Fig. 5B, left). Furthermore, a larger percentage of pDCs (19.5 ± 0.5% SD) are activated to produce IL-12 in response to infection than CD11b+ DCs (7.6 ± 1.0% SD) (Fig. 5B, right). The expression of IL-12 in response to parasites in these cultures is pDC specific since contaminating cells (PDCA−) do not produce IL-12 (Fig. 5B, right). Recent studies have demonstrated that TLR11 and the TLR adaptor protein MyD88 are important for resistance to T. gondii (27–29). Therefore, to get a better understanding of the pattern recognition receptors involved in the pDC response to T. gondii, experiments were conducted to examine IL-12 production from wild-type and TLR11−/− bone marrow-derived pDCs. Stimulation of these pDCs overnight with parasites or STAg revealed that TLR11−/− pDCs produced diminished levels of IL-12 in response to parasites and IL-12 production in response to STAg was completely abrogated (Fig. 6A). Together, these studies demonstrate that pDC production of IL-12 and in response to T. gondii is at least in part dependent upon TLR11.
FIGURE 5.
Toxoplasma gondii directly activates pDCs to produce cytokines. After in vitro infection, sorted pDCs infected for 48 h with T. gondii produce both IFN-α (A, left graph) and IL-12 (A, right graph). Intracellular staining of these populations further confirms this finding and demonstrates a large population of PDCA-positive, IL-12-positive populations after either T. gondii infection or in vitro culture with STAg (B, panels and graph).
FIGURE 6.
pDC activation is dependent upon TLR11. A, pDC IL-12 cytokine production is greatly diminished in TLR11 pDC infected with T. gondii (□) vs wild-type (WT) C57BL/6 pDCs (■). B, TLR11−/− cDCs up-regulate MHC class II after either in vitro infection or culture with STAg (top panel). However, TLR11−/− pDCs do not up-regulate MHC class II after either infection with parasites or in vitro culture with STAg (bottom panel), demonstrating their dependence upon TLR11 for activation.
Discussion
In the last 15 years, there has been a concerted effort to understand the innate events that initiate the development of protective immunity to T. gondii and attention has focused on the possible role of DCs in these events (30, 31). The present studies revealed a dynamic change in the numbers and activation status of several DC subsets during the early response to T. gondii. Perhaps most surprising was the observation that T. gondii activates immature pDCs both in vivo and in vitro to up-regulate MHC class II and costimulatory molecules. As a consequence, pDCs from infected animals acquired the ability to induce proliferation of naive Ag-specific CD4+ T cells. The use of transgenic parasites expressing Ag that can be detected once processed and presented on MHC class II supports a role for pDCs as the major subset involved in processing and presentation of parasite-derived Ag. This was surprising however because until quite recently there has been a consensus that pDCs are predominantly involved in antiviral responses. In contrast, the ability of these cells to present T. gondii-derived Ags and to innately respond to this pathogen and produce IL-12 and type I IFNs in a TLR11-dependent fashion provide the first evidence that pDCs are an important link between the innate and adaptive immune responses to an intracellular parasite.
A role for pDCs in Ag presentation has been debated since these cells were first described. Consistent with their low levels of co-stimulatory molecules and expression of MHC class I and II early studies suggested a limited role for these cells in TCR-mediated activation of T cell responses. However, several biological properties of pDCs are consistent with an ability to interact with T cells. Thus, they migrate to inflamed lymph nodes and are found surrounding the high endothelial venules in the T cell rich areas, an appropriate location to induce T cell priming (32, 33). Furthermore, numerous studies have demonstrated that upon activation by pattern recognition receptors, cytokines, or CD40L, pDCs increase the expression of the MHC molecules and costimulatory molecules necessary to prime naive T cells (34). Indeed, subsequent studies have ascribed these cells with the ability to present Ag to naive CD4 T cells (35) while others have indicated a qualified Ag-presenting ability for pDCs, activating only certain populations of T cells such as memory CD4+ T cells (36) or regulatory T cells (37, 38). Although the results presented here are more consistent with a role for pDCs in the activation of naive CD4+ T cells, one question that they raise is how these APCs acquire parasite-derived material. Although pDCs are not efficient phagocytes (32), they have been shown to endocytose HIV via receptor-mediated pathways (39) and thus may use endocytic processes to acquire exogenous parasite-derived Ags. An alternative scenario is that only pDCs that become infected present Ag, an idea supported by reports that T. gondii preferentially infects DCs in vivo, although specific subsets were not distinguished (9). Related to this possibility are reports that establish pDCs as efficient autophagic cells (40, 41) and that autophagy is involved in the ability of primed macrophages to kill T. gondii (42–45). Linked to this process is the p48GTPase family (41, 43) that is also up-regulated by pDCs exposed to live T. gondii (E. Tait and C. A. Hunter, unpublished observations). Whether autophagy is involved in the ability of pDCs to process parasite-derived materials for Ag presentation is the subject of ongoing research in this laboratory.
Although the studies discussed above highlight the possible role of pDCs in immunity to T. gondii, they are also relevant to previous studies that identified a role for GR-1+ cells in resistance to T. gondii (4). Similar to the results presented here, treatment with anti-GR-1 result in increased susceptibility to T. gondii, although in those studies it was attributed to its ability to deplete neutrophils or certain macrophage populations (4). In the studies presented, there was a marked decrease in the ability of DO11 CD4+ T cells to expand after depletion with anti-GR-1 and this cannot be attributed to depletion of neutrophils, because neutrophil-specific depletion had no effect on CD4+ T cell proliferation. Whether this is a consequence of the contribution of pDCs to Ag presentation as well as cytokine production in vivo remains unclear. At present, we are unaware of any studies that use DCs from T. gondii-infected mice and show that they are capable of activating parasite-specific naive T cells to produce the same type of Th1 responses observed in vivo. Consequently, it is currently difficult to distinguish between these two possibilities but these findings suggest that there are additional factors that cooperate with the DCs during this infection to drive the potent Th1-type response in vivo. Also relevant to the current work are studies by Aliberti and colleagues (6) who used mice engineered to express the diptheria toxin receptor under the CD11c promoter, and treatment with diptheria toxin led to the depletion of all CD11C+ DCs and a decrease in IL-12 levels. However, recent studies by Sapoznikov et al. (35) revealed that in a comparable model, treatment with diptheria toxin does not lead to the depletion of all CD11C+ cells and that pDCs are not depleted in their experimental system. Nevertheless, our observation that conventional CD11chigh DCs were not presenting the Ea peptide, in conjunction with these findings, suggests a model in which IL-12 production and Ag presentation are performed by distinct compartments. Together with reports which revealed that T. gondii interferes with DC activation and the up-regulation of MHC class II in infected bone marrow-derived CD11b+ DCs (11), it is becoming clear that there are many aspects to how T. gondii interacts with different DC subsets. Current studies are focused on gaining a better understanding of the unique roles for distinct DC subsets in response to T. gondii.
Footnotes
This work was supported by National Institutes of Health Grants AI 071302 and 42334, the State of Pennsylvania, and the Mari Lowe Center (to C.A.H.).
Abbreviations used in this paper: DC, dendritic cell; pDC, plasmacytoid DC; STAg, soluble Toxoplasma Ag; cDC, conventional DC; RFP, red fluorescent protein; mfi, mean fluorescent intensity.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan IA, Ely KH, Kasper LH. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 3.Hunter CA, Subauste CS, Remington JS. The role of cytokines in toxoplasmosis. Biotherapy. 1994;7:237–247. doi: 10.1007/BF01878489. [DOI] [PubMed] [Google Scholar]
- 4.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Denkers EY, Gazzinelli RT, Hieny S, Caspar P, Sher A. Bone marrow macrophages process exogenous Toxoplasma gondii polypeptides for recognition by parasite-specific cytolytic T lymphocytes. J Immunol. 1993;150:517–526. [PubMed] [Google Scholar]
- 8.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect Immun. 2004;72:7240–7246. doi: 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlow thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 13.Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 14.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 15.Nagel SD, Boothroyd JC. The α- and β-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol Biochem Parasitol. 1988;29:261–273. doi: 10.1016/0166-6851(88)90081-3. [DOI] [PubMed] [Google Scholar]
- 16.Roos DS. Primary structure of the dihydrofolate reductase-thymidylate synthase gene from Toxoplasma gondii. J Biol Chem. 1993;268:6269–6280. [PubMed] [Google Scholar]
- 17.Kim K, Soldati D, Boothroyd JC. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 18.Speirs K, Lieberman L, Caamano J, Hunter CA, Scott P. Cutting edge: NF-κB2 is a negative regulator of dendritic cell function. J Immunol. 2004;172:752–756. doi: 10.4049/jimmunol.172.2.752. [DOI] [PubMed] [Google Scholar]
- 19.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 21.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 23.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukocyte Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 25.O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon αβ. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 28.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 29.Hitziger N, Dellacasa I, Albiger B, Barragan A. Dissemination of Toxoplasma gondii to immunoprivileged organs and role of Toll/interleukin-1 receptor signalling for host resistance assessed by in vivo bioluminescence imaging. Cell Microbiol. 2005;7:837–848. doi: 10.1111/j.1462-5822.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 30.Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, Aliberti J. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol Res. 2003;27:521–528. doi: 10.1385/IR:27:2-3:521. [DOI] [PubMed] [Google Scholar]
- 31.Sher A, Hieny S, Charest H, Scharton-Kersten T, Collazo C, Germain RN, Reis e Sousa C. The role of dendritic cells in the initiation of host resistance to Toxoplasma gondii. Adv Exp Med Biol. 1998;452:103–110. doi: 10.1007/978-1-4615-5355-7_12. [DOI] [PubMed] [Google Scholar]
- 32.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 34.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 35.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki A. Role of autophagy in innate viral recognition. Autophagy. 2007;3:354–356. doi: 10.4161/auto.4114. [DOI] [PubMed] [Google Scholar]
- 41.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 42.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subauste CS, Andrade RM, Wessendarp M. CD40-TRAF6 and autophagy-dependent anti-microbial activity in macrophages. Autophagy. 2007;3:245–248. doi: 10.4161/auto.3717. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by γ IFN-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75 doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]