Abstract
Transcranial electrical stimulation (tES) refers to a group of non-invasive brain stimulation techniques to induce changes in the excitability of cortical neurons in humans. In recent years, studies in animal models have been shown to be essential for disentangling the neuromodulatory effects of tES, defining safety limits, and exploring potential therapeutic applications in neurological and neuropsychiatric disorders. Testing in animal models is valuable for the development of new unconventional protocols intended to improve tES administration and optimize the desired effects by increasing its focality and enabling deep-brain stimulation. Successful and controlled application of tES in humans relies on the knowledge acquired from studies meticulously performed in animal models.
Keywords: transcranial electrical stimulation, tDCS, tACS, tRNS, brain stimulation, neuromodulation, animal models, plasticity
Introduction
Transcranial electrical stimulation (tES) typically consists of several minutes of non-invasive application of weak electrical currents to the scalp through strategically positioned electrodes [1,2]. The technique began to gain popularity at the beginning of this century, when Nitsche and Paulus demonstrated its neuromodulatory effects by applying weak electrical currents over the human motor cortex [1,3]; however, the first systematic experimental studies exploring the effects of prolonged DC electric field exposition over neuronal activity in animal models began to be published in the mid-1960s [4–6]. Those pioneering studies administered DC currents through epidural electrodes in anesthetized animals, establishing the importance of current polarity (anodal or cathodal) [4,6] and duration of the intervention in determining the short- and long-term effects of stimulation [5]. The seminal work of Nitsche and Paulus [1,3] promoted a new interest in studying the effects of weak DC currents in humans and also in new animal models developed for tES studies.
Present animal models employed for tES studies include in-vitro preparations using brain slices and in-vivo investigations in both anesthetized and alert animals (see [7] for a review). Our current understanding of the basic mechanisms mediating tES effects arose from animal models, showing the importance of orientation and morphological aspects of stimulated neurons, the impact on synaptic events [8,9], the involvement of glial cells [10], and the role of receptors such as NMDA [11], mGluR5 [12], AMPA [13] or adenosine [14], and the implication of BDNF [11,15]. Animal models have also been used over the past decade to define the safety limits of cathodal [16] and more recently anodal [17] tDCS in rats, suggesting that the current densities typically applied in humans are two orders of magnitude below the calculated lesion threshold. On the other hand, as animal models represent a versatile test field for computational models, they are the perfect complement for validating in-silico predictions and obtaining relevant physiological data to be implemented in designing tES models. Two main aspects of tES have been recently modeled: the distribution of the electric field in the brain [14,17,18] and the impact of the exogenous electric field on the single neuron [8,19] or on neuronal assemblies [20]. Moreover, exploring the possibility to enhance brain function has shown that tES is capable of modulating sensory processing [14,20,21], associative learning [14], spatial memory [22,23] and movement accuracy [24] in alert, behaving animals. Finally, the examination of tES effects (mainly tDCS) in different neurological and neuropsychiatric disorders has benefited from animal-based studies. Thus, animal models of acute cerebral ischemia, chronic inflammatory pain, addiction, epilepsy, Alzheimer’s disease, and Parkinson’s disease, among others, have helped to identify potential therapeutic applications of tES [7].
To overcome the limitations of this non-invasive stimulation technique and deepen our knowledge of the way exogenous electric fields interact with the neuronal network, a series of animal models has been employed in recent years that differ from popular tDCS protocols, in an effort to explore unconventional ways of applying electric fields to the brain. Exploring new tES paradigms and optimizing the effectiveness of classical ones is of highest importance for the future application of tES in humans. This review aims to summarize the main tES protocols generally applied in animals, commenting on key recent studies that have used animal models to propose new tES-based stimulation paradigms. Finally, we discuss limitations and potential challenges that require urgent attention in improving animal models.
Present tES protocols
With the aim to replicate results from human experiments, studies performed in animal models highlight the mechanisms that mediate induced effects and explore new therapeutic applications. Animal studies usually apply tES in the most common forms of stimulation, i.e., tDCS and, to a lesser extent, tACS and tRNS (Fig. 1A). In humans, tDCS is achieved by applying a constant current between a pair of electrodes (an anode or cathode electrode and a reference electrode) for the specified amount of time (10 to 30 min) [1,2]. In anodal and cathodal transcranial stimulation, the respective electrode is placed on the scalp over the region of interest, whereas the reference electrode is typically placed over the contralateral supraorbital, the mastoid, or the shoulder [9]. Two new protocols, tACS and tRNS, similar in stimulating electrode configuration and stimulus duration, apply tES with alternating and random-noise currents, respectively. Both techniques have demonstrated the potential to interact with ongoing cortical oscillations to enhance or reduce specific electrocortical frequencies and their related physiological functions [25].
Figure 1.
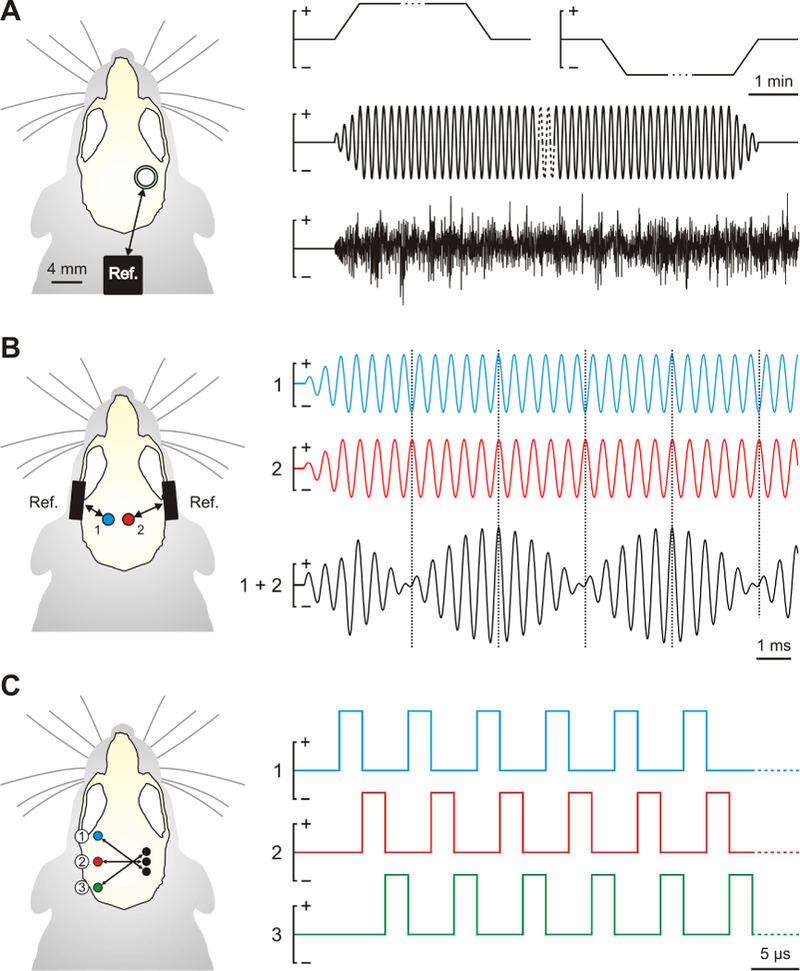
Comparison of tES protocols applied to animal models. On the left, schematic rodent skull with the location and relative size of the electrodes used for the different protocols. On the right, schematic shape of the electric current applied to the skull. A) Conventional tES protocols (from top to bottom, traces corresponding to tDCS, tACS and tRNS). An active electrode is placed over the region of interest, with a distant reference electrode, for the application of long-lasting weak electric fields. B) Temporal interference (TI) protocol. High-frequency electric fields are applied at strategic locations; frequencies differ in the neural firing range. Upper blue and red traces represent electric current applied to electrode 1 and 2 (left panel). The lower black trace represents the sum of both signals in the stimulated region, showing a slow amplitude modulation resulting from the progressive phase shift between the original electric currents. In this example, signals from 2.2 and 2 kHz waves are used to facilitate the visual interpretation of the protocol. Vertical dotted lines indicate when the two signals are in antiphase or phase. C) Intersectional short pulse (ISP) protocol. Temporal application of pulses to the three pairs of electrodes, shown schematically on the left, is depicted at microsecond scale.
In recent years, animal models attempting to mimic neuromodulatory protocols originally applied in humans have applied diverse technical approaches [7,8] in brain slices, anesthetized animals, and awake (even behaving) animals. Each of these experimental preparations is suited to explore tES effects at different complexity levels, with specific advantages and disadvantages in each case.
In-vitro experiments with brain slices can test the effects of electric fields on cellular activity, allowing stable and high-quality recordings at the network [26], cellular [27], and sub-cellular [19] level. In addition, pharmacological or electrical manipulation can be performed with greater precision in brain slices, compared to whole-brain preparations. However, any comparison of obtained results with outcomes from human experiments should be made with caution, as in-vitro tissue slices usually show homogeneous extracellular solution concentrations, altered neural activity, and a lack of interconnected networks with other parts of the brain [19].
Experimental preparations with anesthetized animals facilitate in-vivo recording [28] and manipulation [29] of cellular activity and allow for procedures that would cause pain or stress in the awake animal, such as higher tES intensities [17] or ischemia induction [30]. Nevertheless, considering that final effects of tES depend on the animal’s brain state [31,32], results obtained in anesthetized animals should be carefully interpreted when compared to human results.
The in-vivo awake animal experiment offers the approach closest to human tES studies, enabling behavioral tests with simultaneous electrophysiological recording (EEG, local field potential [LFP], or extracellular unitary recordings) [14], as well as systemic or pharmacological manipulations during and after tES [18]. The combination of these tools in the alert animal brings up the possibility to test the effectiveness of different tES protocols and provide information about the neuronal mechanisms involved. However, the use of behaving animal models can be limited due to electrical artifacts associated to tES or animal movements, as well as to restrictions required to minimize animal stresses, such as tES duration or current intensity.
Although most tES studies performed in animal models apply an active electrode over the region of interest and a distant reference electrode to pass electrical current (commonly DC), a variety of technical solutions can be found in the literature [32]. In slices, two parallel silver–silver chloride electrodes are placed for stimulation on the surface of the artificial cerebrospinal fluid [8], whereas in whole-brain preparations small stimulating electrodes (including cup-shaped, plastic tubes filled with saline solution, multiple silver balls, and square rubber electrodes) are being used in combination with larger reference electrodes (including rubber plate and needle electrodes placed on the back, neck, or ventral region of the animal). Diversity in the shape and size of stimulating electrodes used in animals (depending on skull shape and size) has led to a wide range of current densities (defined as intensity/electrode surface area) from tens to hundreds of A/m2 applied directly to the skull, compared to 0.28 to 2.0 A/m2 commonly applied to the human scalp [9,32]. The most conventional tES protocol, tDCS, has been applied successfully in the three previously described experimental preparations (see [7] for a recent review) (Fig. 1A). For example, DC modulatory effects on the neuron’s transmembrane potential, as well as the importance of various neuronal features, have been determined in brain slices [8]. In-vitro experiments have decisively contributed to our understanding of plasticity mechanisms associated to induced long-term effects [12,33,34]. Applied in anesthetized animals, tDCS has demonstrated the modulatory effects of DC on the corticospinal tract, whereas the polarity-specific impact of tDCS on more complex brain functions such as sensory [14,20,21], learning or memory processes [14,22,23,38] has been determined in alert animals.
In contrast to the great number of studies employing tDCS performed in animal models, tACS effects have been less explored in animals. Most of what we know about tACS mechanisms has been obtained from slices and anesthetized animals, where a few pioneering studies showed that the sensitivity of hippocampal neurons to AC fields in brain slices drops as an exponential decay function of frequency [39], and that low-frequency tACS (0.8–1.7 Hz) is capable of entraining neurons in cortical areas of anesthetized rats Additionally, ultimate effects associated to low-frequency tACS (2–6 Hz) observed in anesthetized rats could be explained through a model of cortical excitation/inhibition balance However, alert animals provide a more appropriate physiological condition for testing, which could expand our knowledge of the underlying mechanisms of tACS. Indeed, the application of tACS in alert animals has demonstrated that the sub- or supra-threshold nature of associated effects depends on the frequency of the current used, evoking motor potentials and tactile sensations when administered to the motor or somatosensory cortices, respectively, at specific frequencies [42]. In addition, low-frequency tACS (0.05–10 Hz) applied in alert animals modulates sensory evoked potentials [42], sleep-dependent memory consolidation [23], and sensory adaptation to visual stimuli [43].
Finally, the most recent protocol used in human studies (normally implemented in human tES-devices) is tRNS, in which electrical currents are applied between active and reference electrodes, following a random-noise frequency pattern (Fig. 1A). Surprisingly, although tRNS has been shown to increase human cortex excitability [44] and enhance learning and performance in children with mathematical learning disabilities [45], this protocol has not yet been published in an animal model. One study has tried to shed light on the basic mechanisms underlying tRNS, using optogenetic tools to explore the effects of noise photostimulation in anesthetized mice; the study found that tRNS amplifies the signal-to-noise ratio of sensory evoked potentials [46].
It is clear from the available data that using animal models in tES studies, which allows invasive recordings and manipulations offers a great advantage in disentangling the neuromodulatory effects associated to this type of stimulation. Furthermore, animal-based investigations provide a valuable testing environment for the development of new protocols intended to improve tES administration.
Exploring new protocols and overcoming tES limitations
Experimental results acquired by present tES methods, mainly tDCS, tACS and tRNS, are highly variable and often contradictory across studies [47]. The high potential of these methods in brain neuromodulation is reason enough to explore new stimulation paradigms aiming for higher focality and allowing deep-brain stimulation in order to optimize the desired effects. These new protocols differ from the conventional application of tES in two ways: 1) in the waveform and duration of the injected current and 2) in the number of electrodes used for electric current application.
Due to the decay of the electric field intensity with increasing distance, present tES approaches primarily focus on the stimulation of superficial brain structures. Nevertheless, it has been reported that in-vivo tDCS produces a lasting effect on deep-brain structures such as the hippocampus or the striatal nucleus [48–50]. Several studies have demonstrated the modulation of remote brain structures with respect to the stimulation electrode [37,50]. This indirect modulation could be caused by changes in the input activity, reflecting a nearly straightforward but attenuated copy of the direct effects at the cortical stimulation site [49]. In addition, it is known that tDCS [38] and tACS [23] can modify LFP power and coherence, inducing an indirect modulation of the oscillations that functionally connect distant brain regions. In any case, it is not clear whether reported tDCS-associated effects in deeper structures are due to the stimulation of superficial areas that project to these structures [51,52], or to direct effects of exogenous electric fields in deep local populations of neurons. It is important to note that animal studies typically use density currents at one or two orders of magnitude higher than human tDCS experiments [32]. To address this issue, we need to carefully model the electric fields to better estimate the strength of the applied electric field and design experiments that more faithfully mimic what we expect to see in the human (electric fields of the order of 1 V/m [9]).
With the aim of overcoming limitations faced by present tDCS protocols, in which direct effects are theoretically restricted to superficial brain regions, a new approach combining different high-frequency oscillatory electric fields, called temporal interference (TI) stimulation, has been recently proposed. This unconventional concept consists in delivering multiple electric fields at specific frequencies (around 2 kHz) thought to be too high to interfere with neural activity, but which together generate a complex new wave of an amplitude modulated within the dynamic range of neural firing (10 Hz) [53] (Fig. 1B). Thus, the brain region where the original waves interfere is stimulated by the electric field envelope of that new complex wave.
This novel method is capable of modulating hippocampal neurons without affecting the overlying tissue, as well as of evoking different motor patterns. However, as the neuronal mechanisms that allow filtering for constant but not for variable amplitudes in high-frequency signals remain unknown, the translational potential of this technique is limited. A better understanding of neuronal filtering would be necessary to develop computational models that predict optimal frequencies and electrode locations of the human scalp optimizing stimulation focality and neuronal response [54].
On the other hand, optimization of present tES protocols implies improving focality of the exogenously applied electric currents that interact with neuronal networks at the desired location, thereby minimizing effects in surrounding brain regions. Considering that most of the published tDCS studies performed in humans use large sponge electrodes (25 – 35 cm2) [2], a great margin for improvement could be expected. Nevertheless, a substantial reduction in size of the transcranial stimulating sponge electrodes without decreasing the current delivered necessarily implies an increase in current density, which could provoke discomfort in the subjects and other undesired effects.
New tES protocols have emerged that use several small-size electrodes to administer the stimulation to the desired brain region from a “multifocal” perspective. This technique has been effective in both human [55,56] and animal [14,42] studies. Recently, a new spatially focused tES protocol, tested in humans and rodents, has been proposed, consisting in the sequential application of very short pulses that converge at specific brain regions [57]. In this method, called “intersectional short pulse” (ISP) stimulation, several electrode pairs strategically located over the scalp are sequentially activated in a spatio-temporal rotating pattern, focusing the strongest modulatory effects in a spatially confined region (Fig. 1C). Unlike the previously cited multifocal approach, current injection in ISP stimulation can reach higher intensities (while keeping charge density and sensations at the scalp surface relatively low) through the activation of each pair of electrodes for just a few microseconds. In this way, modulation of the neuronal activity can be spatially focused since neurons perform a temporal integration of electrical gradients in the order of milliseconds. Although this stimulation paradigm holds great potential, the conclusions made by the authors, supported by measurements in rodents and cadavers, may not be applicable to future studies performed in living humans. The recording of electric field strengths in living humans is still required to determine optimal protocols and intensities, which is essential to translationality of the technique [58–60].
Finally, unconventional tES protocols using a transcranial electric field with different waveforms constrained in the temporal domain have also been employed. The application of these protocols, administering AC [42] and Gaussian [61] waveforms in the millisecond range (100 and 50 ms, respectively), induced tactile perception to natural stimuli during an associative learning paradigm [42] and resulted in reduced spike-and-wave episodes in response to seizure-triggered feedback in epileptic rats [61]. Therefore, tES shows potential as a valuable non-invasive tool for closing the loop in computer-to-brain communication feedback.
Conclusions
Although animal models differ from human experiments in important parameters, including brain size, simplified cortical geometry, brain state and higher current densities, that may limit translational utility, these potential problems are beginning to be attenuated by the increasing number of species involved (including mice, rats, rabbits, cats or monkeys) and improved control of the density of injected currents. Accordingly, animal models offer a unique opportunity to disentangle the mechanisms underlying the effects of well-established tDCS protocols and to explore new therapeutic approaches. Although the number of tDCS studies using animal models has exponentially increased in recent years, experiments focused on tACS and tRNS are scarce in animal-based research, in part as a consequence of introduced artifacts in the recordings based on stimulation protocols. Resolving these technical issues will be important in future efforts to disclose basic mechanisms triggered by new methods such as tACS and tRNS.
Animal models are an excellent choice to explore unconventional protocols of non-invasive stimulation, eliminating limitations of current protocols, such as low focality and insufficient stimulation of deeper structures. Thus, the future of non-invasive tES relies on both an improved understanding of underlying mechanisms and the development of new protocols that reduce current limitations by applying recently acquired knowledge.
Highlights.
Animal models play a key role disclosing the mechanisms underlying tES effects.
Novel strategies for tES are overcoming major limitations of current protocols.
Translationality of animal-based tES studies to applications in humans is increasing.
Acknowledgements:
The authors acknowledge the editorial help of Elaine M. Lilly, PhD. This work was supported by grants from the Spanish MINECO-FEDER (BFU2014-53820-P and BFU2017-89615-P) and from the US National Institutes of Health (RF1MH114269) to JMR.
Abbreviations:
- AC
alternating current
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotrophic factor
- DC
direct current
- EEG
electroencephalography
- ISP
intersectional short pulse
- LFP
local field potential
- mGluR5
metabotropic glutamate receptor 5
- NMDA
N-methyl-D-aspartate
- tACS
transcranial alternating-current stimulation
- tDCS
transcranial direct-current stimulation
- tES
transcranial electrical stimulation
- TI
temporal interference
- tRNS
transcranial random-noise stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Nitsche MA, Paulus W: Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000, 527 Pt 3:633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio BS, Fregni F, et al. : Transcranial direct current stimulation: State of the art 2008. Brain Stimul 2008, 1:206–23. [DOI] [PubMed] [Google Scholar]
- [3].Nitsche MA, Paulus W: Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57:1899–901. [DOI] [PubMed] [Google Scholar]
- [4].Creutzfeldt OD, Fromm GH, Kapp H: Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol 1962, 5:436–52. [DOI] [PubMed] [Google Scholar]
- [5].Bindman LJ, Lippold OC, Redfearn JW: The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 1964, 172:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Purpura DP, McMurtry JG: Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 1965, 28:166–85. [DOI] [PubMed] [Google Scholar]
- [7].Sánchez-León CA, Ammann C, Medina JF, Márquez-R uiz J: Using Animal Models to Improve the Design and Application of Transcranial Electrical Stimulation in Humans. Curr Behav Neurosci Reports 2018, 5:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bikson M, Reato D, Rahman A: Cellular and network effects of transcranial direct current stimulation: insights from animal models and brain slice. In Transcranial Brain Stimulation Edited by Miniussi C, Paulus W, Rossini PM. CRC Press; 2012:55–92. [Google Scholar]
- [9].Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, Bikson M: Animal models of transcranial direct current stimulation: Methods and mechanisms. Clin Neurophysiol 2016, 127:3425–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y: Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 2016, 7:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B: Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010, 66:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun Y, Lipton JO, Boyle LM, Madsen JR, Goldenberg MC, Pascual-Leone A, Sahin M, Rotenberg A: Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol 2016, 80:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stafford J, Brownlow ML, Qualley A, Jankord R: AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiol Learn Mem 2017, [DOI] [PubMed]
- [14].Márquez-Ruiz J, Leal-Campanario R, Sánchez-Cam pusano R, Molaee-Ardekani B, Wendling F, Miranda PC, Ruffini G, Gruart A, Delgado-García JM: Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A 2012, 109:6710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ranieri F, Podda M V, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C: Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol 2012, 107:1868–80. [DOI] [PubMed] [Google Scholar]
- [16].Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA: Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol 2009, 120:1161–7. [DOI] [PubMed] [Google Scholar]
- [17]●.Jackson MP, Truong D, Brownlow ML, Wagner JA, McKinley RA, Bikson M, Jankord Safety parameter considerations of anodal transcranial Direct Current Stimulation in rats. Brain Behav Immun 2017, 64:152–61. This study considers safety parameters by using different epicranial electrode montages and computational brain current-density models in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]●.Opitz A, Falchier A, Yan CG, Yeagle EM, Linn GS, Megevand P, Thielscher A, Deborah AR, Milham MP, Mehta AD, et al. : Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep 2016, 6:31236 This work uses stereotactic EEG electrode arrays in monkeys and in patients with epilepsy to show crucial information about spatial and temporal characteristics of the electric field generated by tES. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M: Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013, 591:2563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Molaee-Ardekani B, Márquez-Ruiz J, Merlet I, Leal-Campanario R, Gruart A, Sánchez-Campusano R, Birot G, Ruffini G, Delgado-García JM, Wendling F: Effects of transcranial Direct Current Stimulation (tDCS) on cortical activity: a computational modeling study. Brain Stimul 2013, 6:25–39. [DOI] [PubMed] [Google Scholar]
- [21].Schweid L, Rushmore RJ, Valero-Cabre A: Cathodal transcranial direct current stimulation on posterior parietal cortex disrupts visuo-spatial processing in the contralateral visual field. Exp Brain Res 2008, 186:409–17. [DOI] [PubMed] [Google Scholar]
- [22].Dockery CA, Liebetanz D, Birbaumer N, Malinowska M, Wesierska MJ: Cumulative benefits of frontal transcranial direct current stimulation on visuospatial working memory training and skill learning in rats. Neurobiol Learn Mem 2011, 96:452–60. [DOI] [PubMed] [Google Scholar]
- [23].Binder S, Berg K, Gasca F, Lafon B, Parra LC, Born J, Marshall L: Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul 2014, 7:508–15. [DOI] [PubMed] [Google Scholar]
- [24].Faraji J, Gomez-Palacio-Schjetnan A, Luczak A, Metz GA: Beyond the silence: bilateral somatosensory stimulation enhances skilled movement quality and neural density in intact behaving rats. Behav Brain Res 2013, 253:78–89. [DOI] [PubMed] [Google Scholar]
- [25].Paulus W: Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil 2011, 21:602–17. [DOI] [PubMed] [Google Scholar]
- [26].Reato D, Rahman A, Bikson M, Parra LC: Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci 2013, 7:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reato D, Bikson M, Parra LC: Lasting modulation of in vitro oscillatory activity with weak direct current stimulation. J Neurophysiol 2015, 113:1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koo H, Kim MS, Han SW, Paulus W, Nitsche MA, Kim YH, Kim HI, Ko SH, Shin YI: After-effects of anodal transcranial direct current stimulation on the excitability of the motor cortex in rats. Restor Neurol Neurosci 2016, 34:859–68. [DOI] [PubMed] [Google Scholar]
- [29].Pikhovych A, Stolberg NP, Jessica Flitsch L, Walter HL, Graf R, Fink GR, Schroeter M, Rueger MA: Transcranial Direct Current Stimulation Modulates Neurogenesis and Microglia Activation in the Mouse Brain. Stem Cells Int 2016, 2016:2715196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu YH, Chan SJ, Pan H-C, Bandla A, King NKK, Wong PTH, Chen YY, Ng WH, Thakor NV, Liao LD: Integrated treatment modality of cathodal-transcranial direct current stimulation with peripheral sensory stimulation affords neuroprotection in a rat stroke model. Neurophotonics 2017, 4:45002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Silvanto J, Pascual-Leone A: State-dependency of transcranial magnetic stimulation. Brain Topogr 2008, 21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Márquez-Ruiz J, Leal-Campanario R, Wendling F, Ruffini G, Gruart A, Delgado-García JM: Chapter 5 - Transcranial Electrical Stimulation in Animals. In The Stimulated Brain Edited by Kadosh RC., San Diego: Academic Press; 2014:117– 44. [Google Scholar]
- [33].Kronberg G, Bridi M, Abel T, Bikson M, Parra LC: Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul 2017, 10:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lafon B, Rahman A, Bikson M, Parra LC: Direct Current Stimulation Alters Neuronal Input/Output Function. Brain Stimul 2017, 10:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bolzoni F, Pettersson L-G, Jankowska E: Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiol 2013, 591:3381–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cambiaghi M, Velikova S, Gonzalez-Rosa JJ, Cursi M, Comi G, Leocani L: Brain transcranial direct current stimulation modulates motor excitability in mice. Eur J Neurosci 2010, 31:704–9. [DOI] [PubMed] [Google Scholar]
- [37].Baczyk M, Jankowska E: Presynaptic actions of transcranial and local direct current stimulation in the red nucleus. J Physiol 2014, 592:4313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]●.Krause MR, Zanos TP, Csorba BA, Pilly PK, Choe J, Phillips ME, Datta A, Pack CC: Transcranial Direct Current Stimulation Facilitates Associative Learning and Alters Functional Connectivity in the Primate Brain. Curr Biol 2017, 27:3086–3096.e3. A study that shows the impact of tDCS on associative learning and functional connectivity between distant brain regions in non-human primates. [DOI] [PubMed] [Google Scholar]
- [39].Deans JK, Powell AD, Jefferys JGR: Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol 2007, 583:555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G: Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci 2010, 30:11476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khatoun A, Asamoah B, Mc Laughlin M: Simultaneously Excitatory and Inhibitory Effects of Transcranial Alternating Current Stimulation Revealed Using Selective Pulse-Train Stimulation in the Rat Motor Cortex. J Neurosci 2017, 37:9389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]●●.Márquez-Ruiz J, Ammann C, Leal-Campanario R, Ruffin i G, Gruart A, Delgado-García JM: Synthetic tactile perception induced by transcranial alternating-current stimulation can substitute for natural sensory stimulus in behaving rabbits. Sci Rep 2016, 6:19753 This study applies multifocal epicranial electrodes for delivering short tACS pulses in the rabbit to induce tactile sensations and motor outputs, showing that the sub- or supra-threshold nature of short-pulse tACS-associated effects depends on the frequency of the used current. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kar K, Duijnhouwer J, Krekelberg B: Transcranial Alternating Current Stimulation Attenuates Neuronal Adaptation. J Neurosci 2017, 37:2325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Terney D, Chaieb L, Moliadze V, Antal A, Paulus W: Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci 2008, 28:14147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Looi CY, Lim J, Sella F, Lolliot S, Duta M, Avramenko AA, Kadosh RC: Transcranial random noise stimulation and cognitive training to improve learning and cognition of the atypically developing brain: A pilot study. Sci Rep 2017, 7:4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huidobro N, Mendez-Fernandez A, Mendez-Balbuena I, Gutierrez R, Kristeva R, Manjarrez E: Brownian Optogenetic-Noise-Photostimulation on the Brain Amplifies Somatosensory-Evoked Field Potentials. Front Neurosci 2017, 11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Horvath JC, Carter O, Forte JD: Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Front Syst Neurosci 2014, 8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rohan JG, Carhuatanta KA, McInturf SM, Miklasevich MK, Jankord R: Modulating Hippocampal Plasticity with In Vivo Brain Stimulation. J Neurosci 2015, 35:12824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]●.Kim MS, Koo H, Han SW, Paulus W, Nitsche MA, Kim Y-H, Yoon JA, Shin Y-I: Repeated anodal transcranial direct current stimulation induces neural plasticity-associated gene expression in the rat cortex and hippocampus. Restor Neurol Neurosci 2017, 35:137–46. A study that demonstrates the altered subcortical expression of plasticity-associated genes after anodal tDCS in rats. [DOI] [PubMed] [Google Scholar]
- [50].Winkler C, Reis J, Hoffmann N, Gellner A-K, Munkel C, Curado MR, Furlanetti L, Garcia J, Döbrössy MD, Fritsch B: Anodal Transcranial Direct Current Stimulation Enhances Survival and Integration of Dopaminergic Cell Transplants in a Rat Parkinson Model. ENeuro 2017, 4:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Leffa DT, de Souza A, Scarabelot VL, Medeiros LF, de Oliveira C, Grevet EH, Caumo W, de Souza DO, Rohde LAP, Torres ILS: Transcranial direct current stimulation improves short-term memory in an animal model of attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 2016, 26:368–77. [DOI] [PubMed] [Google Scholar]
- [52].Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, Colussi C, Ripoli C, Grassi C: Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep 2016, 6:22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]●●.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk H-J, Cassara AM, Neufeld E, Kuster N, Tsai L-H et al. : Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169:1029–1041.e16. This work proposes a new non-invasive method tested in mice for deep-brain stimulation based on the interference between multiple high-frequency oscillatory electric fields. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dmochowski J, Bikson M: Noninvasive neuromodulation goes deep. Cell 2017, 169:977–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M: Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage 2013, 74:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A: Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage 2014, 89:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]●●.Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernandez-Ruiz A, Kozák G, Kincses ZT, Iványi B, Buzsáki G et al. : Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun 2018, 9:483 A study that combines experiments in rodents and humans proposing a new stimulating method based on the intersection of short pulses to increase tES focality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chhatbar P, Kautz S, Takacs I, Rowland NC, Revuelta GJ, George MS, Bikson M, Feng W: Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul 2018, 11:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ruhnau P, Rufener H, Heinze H, Zaehle T: Sailing in a sea of disbelief: in vivo measurements of transcranial electric stimulation in human subcortical structures. Brain Stimul 2018, 11:241–243. [DOI] [PubMed] [Google Scholar]
- [60].Vosskuhl J, Struber D, Herrmann C: Non-invasive brain stimulation: A paradigm shift in understanding brain oscillations. Front Hum Neurosci 2018, 12: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Berényi A, Belluscio M, Mao D, Buzsáki G: Closed-loop control of epilepsy by transcranial electrical stimulation. Science 2012, 337:735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


