Abstract
Cadmium (Cd) has been linked to a variety of cancers, including breast cancer; however, the molecular mechanism of its carcinogenic activity is not fully understood. To this end, the present study investigated the roles of ferroportin (FPN), a prognostic marker of breast cancer, in Cd-induced stimulation of cell proliferation and cell migration. Triple-negative MDA-MB-231 cells were treated with 1–3 μM Cd. The cells exhibited significant reduction in FPN expression and concomitant increase in iron concentration. Cells treated with Cd for 8 weeks displayed elevated proliferative and migratory activities which were inversely related with FPN expression. Reduced FPN expression also resulted in EMT as indicated by an increase in the expression of E-cadherin, and a decrease in the expression of N-cadherin, Twist and Slug. Further investigation revealed that Cd suppressed FPN expression at least partially by activating TGF-ß, a known regulator of FPN expression. Taken together, these results indicate that Cd-induced stimulation of MDA-MB-231 cell proliferation, EMT, and migration is brought about by suppression of FPN expression and associated disruption of iron homeostasis.
Keywords: Cadmium, Iron, Ferroportin, Breast cancer, EMT
Introduction
Cadmium (Cd) is one of the top ten hazardous substances with known health effects on multiple organs and tissues, owing partly to its low excretion rate with elimination half-time of 10–30 years (Jarup and Akesson, 2009; Faroon et al., 2012). The principal sources of Cd exposure in the general population are contaminated food, water, and tobacco smoke (Marano et al., 2012). Postulated mechanisms of Cd-induced injury are oxidative damage, DNA damage, suppression of DNA repair, and apoptosis (Templeton and Liu, 2010; Asara et al, 2013; Rani et al, 2014). Cd is also a carcinogen and is implicated in a variety of cancers, including breast cancer (Jouybari et al, 2018) which is the leading type of cancer among women worldwide (Hiatt and Brody, 2018). Our laboratory has studied the association between Cd and breast cancer cell growth and metastatic progression. Song et al (2015) and Wei et al (2015) reported that Cd stimulated cell proliferation in both hormone-positive MCF 7 and triple-negative HCC 1937 breast cancer cells. Also, prolonged treatment with low micromolar concentration of Cd transformed the non-tumorigenic breast epithelial MCF10A cells to a more mesenchymal-like morphology, suggesting a potential role of Cd in promoting metastasis (Wei et al, 2018). Furthermore, Cd enhanced the migratory ability of metastatic MDA-MB-231 cells (Wei and Shaikh, 2017).
Mechanistic investigations have revealed that the Cd-induced breast cancer cell growth may be mediated through activation of multiple signal transduction pathways. For instance, in estrogen receptor-positive MCF-7 and T47D cells, Cd exerted estrogen-like behavior and activated estrogen receptor ERα (Zang et al., 2009; Siewit et al., 2010). In ER-negative SKBR3 cells, G protein coupled receptor 30 was activated in response to Cd treatment (Yu et al., 2010). Moreover, activation of integrin stimulated metastasis-associated phenotype in triple-negative MDA-MB-231 cells (Wei and Shaikh, 2017). It is noteworthy that TGF-ß, an epithelial-mesenchymal transition (EMT) inducer, is known to play a significant role in late stages of cancer progression. Chen et al. (2016) have reported that Cd can activate TGF-ß signaling in MCF-7 cells.
Iron is an essential element for all forms of life and plays crucial roles in providing metabolic needs and fulfilling specialized functions. Maintenance of a fine-tuned systemic iron homeostasis is critical as both iron deficiency and overload cause adverse health effects (Ganz and Nemeth, 2011; Evstatiev and Gasche, 2012). Ferroportin (FPN) is the sole known mammalian iron exporter which plays a central role in cellular iron homeostasis. FPN expression is predominantly localized in cells that transfer iron into the blood plasma and includes enterocytes, hepatocytes, and macrophages (Ganz and Nemeth, 2011). Tumor cells express FPN (Neves et al., 2017), however, the FPN level is significantly reduced in malignant breast tumors relative to non-cancerous tissues (Zhang et al., 2014). Whereas FPN down regulation enhances tumor growth, it’s up regulation only marginally impedes tumor growth (Chen et al., 2015). The FPN levels are reported to have prognostic significance in breast cancer patients (Zhang et al., 2014).
Cd can influence FPN expression in macrophages, which supply iron for erythropoiesis by recycling iron from senescent erythrocytes (Weiss, 2002). Whether Cd can also influence FPN expression and iron levels in breast cancer cells has not yet been investigated. Thus, here we aimed to study whether Cd affected FPN expression and iron homeostasis in breast epithelial MDA-MB-231 cells and, if so, did it impact cell proliferation, EMT, and cell migration.
Materials and Methods
Materials
DMEM, PBS, trypsin and penicillin/streptomycin were purchased from Gibco (Grand Island, NY). Halt protease inhibitor cocktail, RIPA Buffer, BCA Protein Assay Kit and supersignal west femto maximum sensitivity substrate were from Thermo Scientific (Pittsburgh, PA). Cd chloride was obtained from Sigma-Aldrich (Dallas, TX). TRIzol reagent, acrylamide/Bis 19:1 and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Flowery Branch, GA). FPN siRNA (siGENOME Human SLC40A1), non-targeting siRNA and DharmaFECT transfection reagent were purchased from GE Healthcare Dharmacon (Chicago, IL). High capacity cDNA reverse transcription Kits and PowerUp SYBR Green Master Mix were purchased from Applied Biosystems (Foster City, CA). Primary antibodies against phospho-Smad 2/3 and GAPDH and HRP-conjugated secondary antibody were from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal FPN antibody was from Novus Biologicals (Littleton, CO). Transwell inserts were from EMD Millipore (Billerica, MA). Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies (Rockville, MD). ICP-MS Calibration Standard was obtained from Ultra Scientific (North Kingstown, RI). Restriction enzymes were from New England BioLabs (Ipswich, MA).
Cell culture and treatment
Breast cancer-derived MDA-MB-231 cells were purchased from the American Type Culture Collection (Manassas, VA). The cells were maintained in DMEM supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 U/mL) in a humidified atmosphere of 5% CO2 at 37 °C. Authenticity of the cell line was confirmed by ATCC’s cell authentication testing service to be 100% MDA-MB-231 cells. Mycoplasma was monitored by using the MycoAlert kit (Lonza, Allendale, NJ) and the cell cultures were found to be free of mycoplasma. For short-term Cd treatment (up to 24 h), the cells were cultured in DMEM without FBS, and for long-term Cd treatment (up to 8 weeks), DMEM contained 10% FBS.
RNA isolation, reverse transcription and qPCR
RNA from the cell extracts was purified using the TRIzol reagent. Total RNA (2 μg) was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit. Gene expression was analyzed on a qPCR machine (Bio-Rad) using the PowerUp SYBR Green Master Mix. GAPDH was used as the internal control. Primer sequences for the qPCR analysis are listed in Table 1. Data was calculated using the 2−ΔΔCt method (Arocho et al., 2006).
Table 1.
Oligonucleotide primers used in the present study.
| Primer | Sequence(5’−3’) |
|---|---|
| RT Twist F | GACAAGCTGAGCAAGATTCAGACC |
| RT Twist R | AGACCGAGAAGGCGTAGCTGAG |
| RT E-Cadherin F | CAGAAGGTTCACCCAGCACCTTGC |
| RT E-Cadherin R | CAGCCTGGCCGATAGAATGAGACC |
| RT N-Cadherin F | ACCGGTGCCATCATTGCCATCC |
| RT N-Cadherin R | CAGCTGGCTCAAGTCATAGTCCTG |
| RT FPN F | ACCTCGCTGGTGGTACAGAATG |
| RT FPN R | AGCAGGAAGTGAGAACCCATCC |
| RT GAPDH F | ATGGCACCGTCAAGGCTGAGAAC |
| RT GAPDH R | CATGGTGGTGAAGACGCCAGTG |
| RT Slug F | ACTACCGCTGCTCCATTCCACG |
| RT slug R | GGCTTTCTGAGCCACTGTGGTC |
| EGFP-FPN-F | CCGGAATTCATGACCAGGGCGGGAGATCAC |
| EGFP-FPN-R | CCGCTCGAGAACAACAGATGTATTTGCTTG |
Bold nucleotides represent the locations of restricted endonucleases
Determination of cellular Cd and iron concentrations
Quantification of the cellular Cd and iron concentrations was performed using an XSeries ICP- MS system (Thermo Electron Corporation, Madison, WI). For this procedure, cells were washed three times with PBS containing 2 mM EGTA and once with PBS without the EGTA. The washed cells were lysed using the RIPA lysis buffer, diluted 1:2 with 65% ultrapure nitric acid and digested on a heat block at 60 °C for 2 h. Prior to ICP-MS analysis, the samples were centrifuged, and the supernatant was further diluted with Milli-Q water to an acid concentration of 2%. The multi-element standard solution was serially diluted to the range of 1 to 50 μg/L to prepare the Cd and iron calibration curves. The metal concentrations in the cell lysates were expressed as per mg protein.
Western blot analysis
After treatment with Cd, the cells were washed with PBS and harvested with ice-cold RIPA lysis buffer supplemented with the Halt Protease Inhibitor Cocktail. The cell lysates were centrifuged for 15 min at 14,000×g and the supernatant were used for further analysis. Protein concentration was determined using the Micro BC A Protein Assay Kit. An aliquot containing 20 μg protein was subjected to 12% SDS-PAGE. Western blot analysis was performed as described previously (Liu et al., 2016) and detected by enhanced chemiluminescence technique. Antibodies used were anti-GAPDH (1:1000), phospho-Smad2/3 (1:300), and FPN (1:300).
Cytotoxicity and cell proliferation assay
Both cytotoxicity and cell proliferation were measured by utilizing CCK-8. Briefly, MDA-MB-231 cells were seeded into 96-well plates at a density of 5×103 cells/well in DMEM supplemented with 1% FBS. For the cytotoxicity assay, 24 h later the cells were treated with 1–10 μM Cd for 24 h. at which point 10 μL CCK 8 reagent was added to each well. Cytotoxicity was assessed by measuring the absorbance at 450 nm using a plate-reader (Bio-Rad). DMEM (100 μL) was used as a blank. For the cell proliferation assay, the cells were treated with 1 or 3 μM Cd for 8 weeks, followed by treatment with control siRNA, FPN siRNA, EGFP, or EGFP-FPN vector for 2 days. The cells were cultured for 72 h and CCK 8 reagent was used to measure relative cell proliferation.
Transfection with FPN siRNA
The MDA-MB-231 cells were plated in a 6-well plate and transiently transfected with either siRNA against human FPN or non-specific siRNA (control) using the DharmaFECT reagent following manufacturer’s instructions. Briefly, transfection mixture containing 2 μL siRNA (20 μM) and 2 μL DharmaFECT reagent was incubated for 20 min prior to adding to the cells. After incubation for 48 h, the cells were washed with PBS and used for further studies.
EGFP-FPN plasmid construction and transfection
To construct an FPN expression plasmid for transfection experiments, coding region of FPN was amplified with EGFP-FPN-F and EGFP-FPN-R primers (as shown in Table 1). The FPN fragment was then cloned in the Kpn I/Xho I sites of EGFP vector. Transient transfection of plasmids, EGFP-FPN and EGFP (control), was carried out using Lipofectamine 2000 reagent. Briefly, 2 μg plasmid and 3 μL Lipofectamine 2000 were separately diluted in 100 μL DMEM and incubated for 5 min at room temperature. Subsequently, the diluted plasmids and Lipofectamine 2000 were mixed and incubated for 20 min before adding to the cells cultured in a 6-well plate.
Cell migration assay
The cell migration assay was performed using Transwell inserts (12 μM pore size) coated with 2 μg/mL collagen, 2 μg/mL fibronectin, 8 μg/mL vitronectin, and 8 μg/mL laminin. A total of 5×104 cells were seeded into the upper part of the Transwell chamber in 300 μL serum-free DMEM. The lower chamber was loaded with 500 μL DMEM containing 10% FBS. After incubation for 48 h, cells on the upper chamber side of the membrane were carefully scraped off with cotton swabs. Migrated cells at the bottom of the membrane were then fixed in 70% ethanol, stained with 0.5 % crystal violet, and imaged using an EVOS microscope. Finally, the cells were de-stained in 20% methanol to quantify cell migration relative to control by measuring the absorbance of the de-staining solution at 450 nm.
Statistical analysis
All experiments were repeated independently at least three times and the results were averaged. Independent-samples t-test (Fig. 4A) and One-way ANOVA followed by Tukey’s post hoc test were used to analyze the experimental data. Results were plotted as mean±SE. Statistical significance was determined at p< 0.05.
Results
Cytotoxicity of Cd in MDA-MB-231 cells
To determine the concentration of Cd that caused minimal to no cytotoxicity, the cells were treated with up to 10 μΜ Cd for 24 h and cell viability was measured using the Cell Counting Kit-8. As shown in Fig. 1, there was no significant effect on viability when the cells were treated with up to 3 μΜ Cd, however, the relative viability was reduced by 38% at 10 μΜ Cd. Furthermore, concentrations of Cd up to 3 μΜ had no significant effect on either total protein levels or GAPDH levels (data not shown). Based on these results, concentrations of 1 and/or 3 μΜ Cd were chosen for subsequent studies.
Fig. 1.
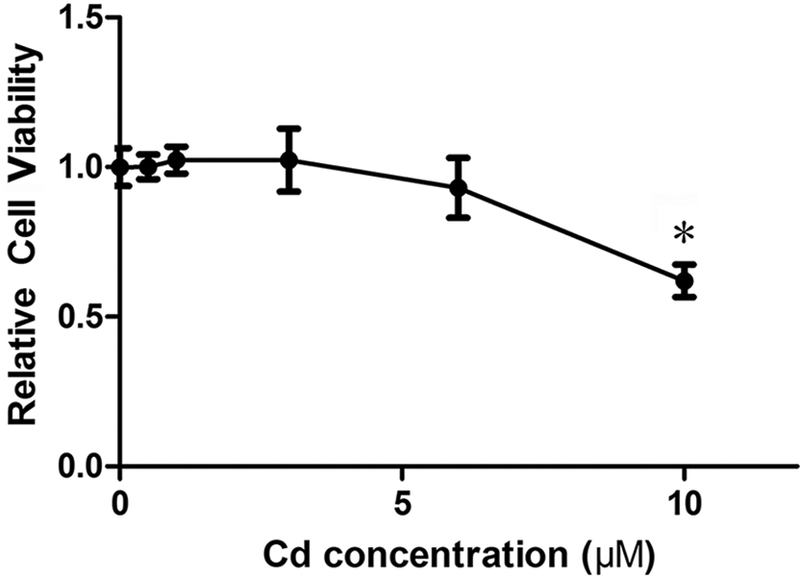
Cytotoxicity of Cd in MDA-MB-231 cells. The cells were treated with up to 10 μΜ Cd for 24 h and the cytotoxicity was assessed by CCK-8. Results are expressed as mean±SE (n = 10). *Significantly different from the control (p < 0.05).
Expression of FPN in MDA-MB-231 cells
To investigate the effect of Cd on the expression of FPN, the mRNA levels in both short-term and long-term Cd-treated MDA-MB-231 cells were measured and normalized to the control. Treatment with 3 μΜ Cd significantly decreased the expression of FPN mRNA by 35% within 12 h and maintained it thereafter (Fig. 2A). A similar pattern was observed in the long-term Cd-treated cells, where 3 μΜ Cd showed a 27 and 36% decreased in the FPN mRNA levels at 4 and 8 weeks, respectively (Fig. 2C). To confirm that reductions in the mRNA levels were also reflected in the protein levels, the FPN levels were measured by Western blot analysis. As depicted in Fig. 2B, the FPN protein levels followed the mRNA levels and were reduced by 27 and 36% at 12 and 24 h, respectively. Similarly, in cells treated with Cd for 4 and longer, the FPN levels were about half of the control levels (Fig. 2D). Since these results demonstrated a direct relationship between the FPN mRNA and protein levels, in subsequent experiments only the mRNA levels were measured.
Fig. 2.
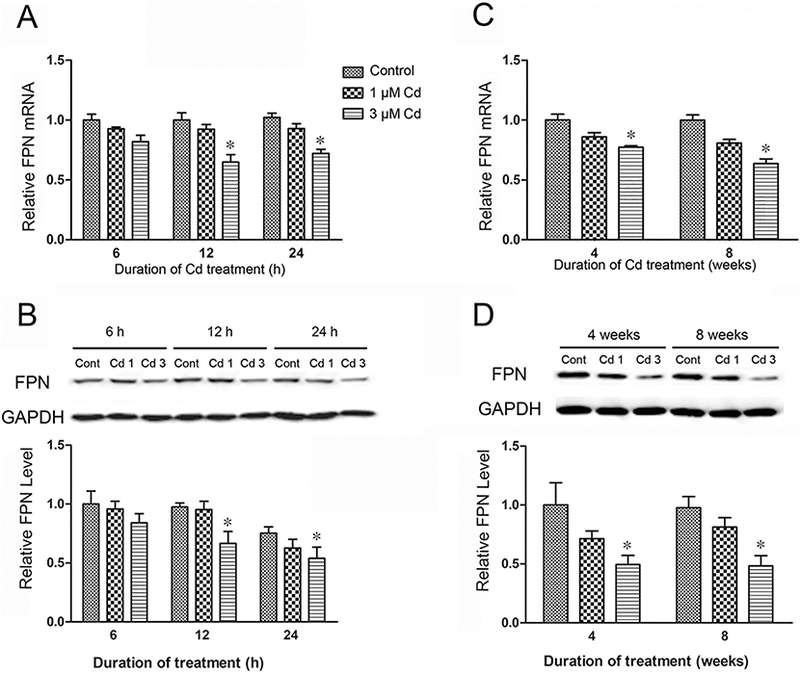
Effect of Cd on FPN mRNA and protein levels in MDA-MB-231 cells. The FPN mRNA levels were determined by qPCR after (A) short-term (6, 12 and 24 h), and (C) long-term (26 and 56 days) treatment with Cd. The respective FPN protein levels (B and D) were determined after Western blot analysis of the cell extracts. Results are expressed as mean±SE (n = 4). *Significantly different from the respective control (p < 0.05).
Effect of Cd treatment on cellular iron levels
Whole cell Cd and iron concentrations were measured in the control MDA-MB-231 cells and cells treated with 3 μΜ Cd for 1, 28 and 56 days. The Cd concentration in the control cells was 0.5 ng/mg protein or less at all time points (Fig. 3A). In the Cd-treated cells, the Cd concentration rose rapidly during the first 24 h to 217±16 ng/mg protein and steadily thereafter, reaching 890±41 ng/mg protein at 56 days (Fig. 3A). The iron concentration in the control cells at time zero was 55±8 ng/mg protein, with no significant change during the 56-day period (Fig. 3B). In the Cd-treated cells, however, it increased in a manner like Cd such that the concentration was 91±8 after 24 h and increased steadily to 205±28 ng/mg protein after 56 days.
Fig. 3.
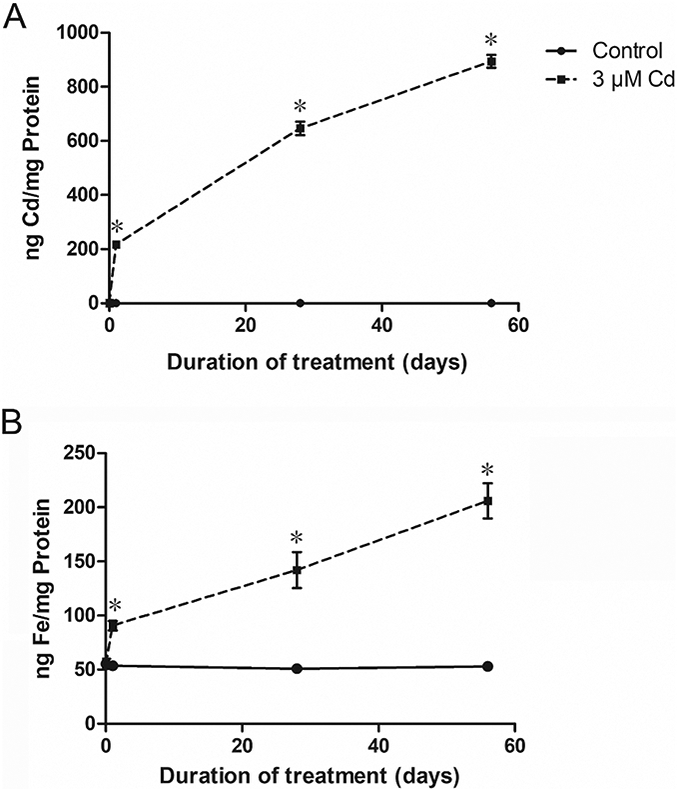
Accumulation of Cd and iron in MDA-MB-231 cells following treatment with Cd. The cells were treated with 3 μΜ Cd for 1, 28, and 56 days and the metal concentrations were measured by ICP-MS. Results are expressed as mean±SE (n = 6). *Significantly higher than the untreated cells at the same time point. (p < 0.05).
To further establish that the increase in iron levels following treatment with Cd was indeed due to reduced FPN expression, the FPN mRNA was knocked down by siRNA to 32% of control (Fig. 4A). As expected, the reduction in the FPN mRNA level had no effect on the accumulation of Cd (Fig. 4B). However, it significantly increased the accumulation of iron in both the control (1.3-fold) and Cd-treated (1.4-fold) cells (Fig. 4C). These results confirmed that indeed the Cd-induced increase in iron accumulation was due to reduced FPN expression. Further, the results indicated that FPN was not involved in the transport of Cd.
Fig. 4.
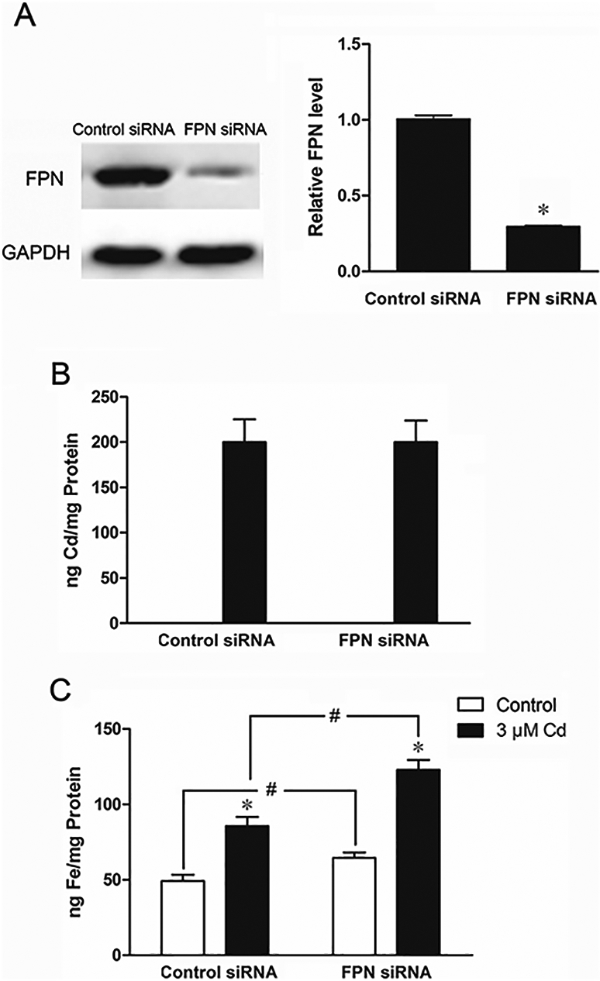
Effect of treatment with FPN siRNA on FPN levels and on Cd and iron accumulation. The cells were transiently transfected with either control or FPN siRNA for 2 days and then treated with 3 μΜ Cd for 24 h. (A) Suppression of FPN expression by the FPN siRNA. (B and C) Cellular Cd and iron concentrations measured by ICP-MS. Results are expressed as mean±SE (n = 6). *Significantly different from the respective control.#Significantly different from the cells treated with control siRNA. (p < 0.05).
Effect of FPN on cell proliferation
The proliferation of MDA-MB-231 cells following treatment with 1 and 3 μΜ Cd for 8 weeks was investigated in cells transfected with either the control or the FPN siRNA. As shown in Fig. 5A, in cells treated with control siRNA, prolonged treatment with 1 and 3 μΜ Cd increased cell proliferation by 13 and 16 %, respectively. Suppression of FPN mRNA level by the specific siRNA further increased cell proliferation in both control as well as the Cd-treated cells by 11, 22, and 26%, respectively.
Fig. 5.
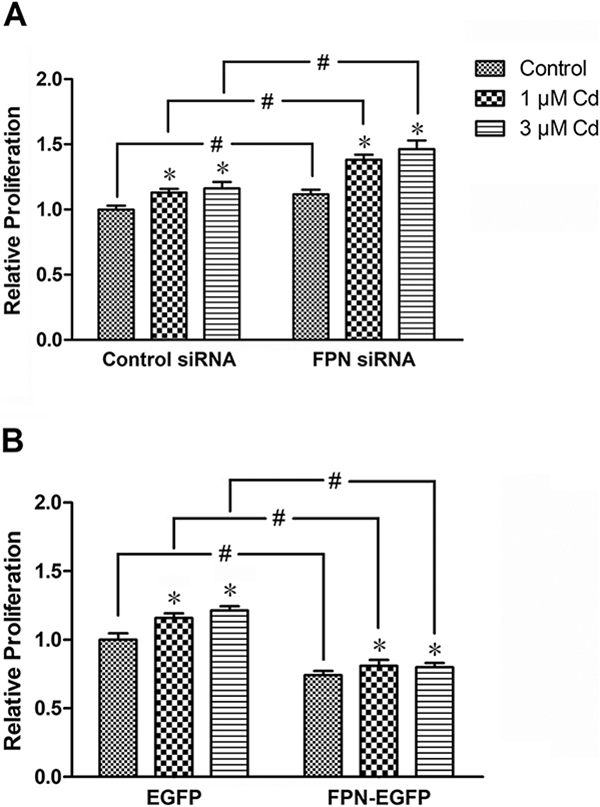
Effect of suppression of FPN expression on the proliferation of cells treated with Cd. The cells were treated with 1 or 3 μΜ Cd for 8 weeks. Control cells were cultured in DMEM alone. (A) The cells were transiently transfected with either control or FPN siRNA, or (B) EGFP or EGFP-FPN vector for 2 days, and then seeded into 96-well plates at equal density. After 72 h, cell proliferation was assessed by CCK-8. Results are expressed as mean±SE (n = 10). *Significantly different from the respective control.#Significantly different from the respective control siRNA- or EGFP-treated cells, (p < 0.05).
To further investigate the role of FPN in cell proliferation, the FPN mRNA was over expressed. The cells were transfected with either EGFP (control), or FPN-EGFP vector to stimulate the expression of FPN, and then treated with 1 and 3 μΜ Cd. As shown in Fig. 5B, in cells transfected with the EGFP control vector treatment with 1 and 3 μΜ Cd increased cell proliferation by 16 and 21%, respectively. In comparison, overexpression of FPN by FPN-EGFP vector significantly reduced cell proliferation in the control as well as the Cd-treated cells by 26, 30, and 34%, respectively. Taken together, these results indicated that the stimulatory effect of Cd on the proliferation of MDA-MB-231 cells was inversely related to the expression of FPN.
Effect of FPN on cell migration
To assess whether reduction in FPN expression had a functional effect on cell migration, the MDA-MB-231 cells treated with 3 μΜ Cd for 8 weeks were transfected with either control or FPN siRNA and the migratory ability of the cells was assessed by the Transwell assay. A composite photograph of the lower surface of the membranes of Transwell inserts depicting relative cell migration is shown in Fig. 6. Also shown is a quantitative plot of the relative cell migration. As expected, treatment with Cd increased cell migration by 41% in cells transfected with the control siRNA. Reduction in FPN levels by transfection with the siRNA further enhanced cell migration in both the control and Cd-treated cells by 32 and 37 %, respectively. These results indicated that a reduction in FPN expression and associated iron accumulation promoted the migration of MDA-MB-cells in both control and Cd-treated cells.
Fig. 6.
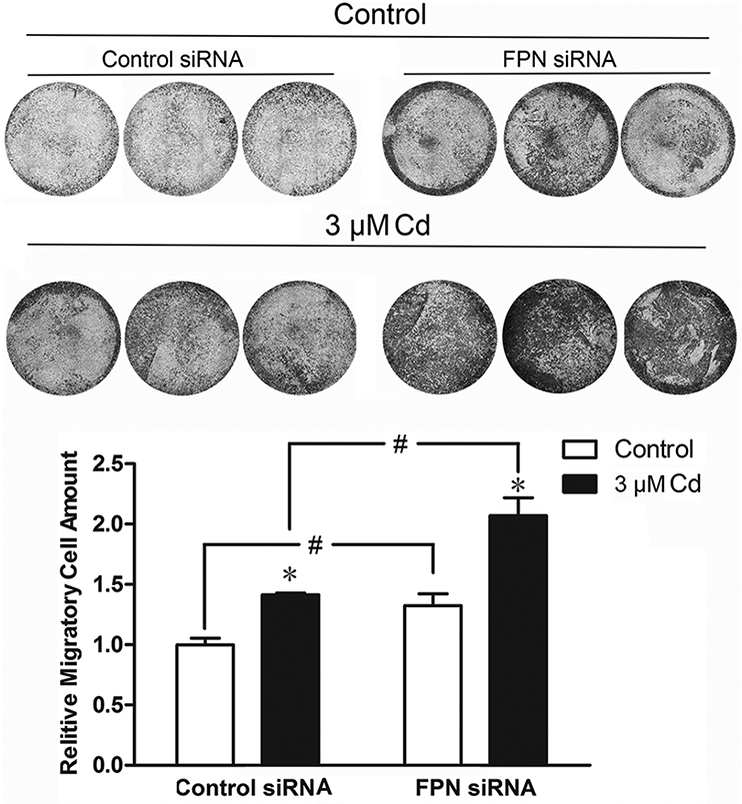
Effect of suppression of FPN expression on the migration of cells treated with Cd. The cells were treated with 3 μM Cd for 8 weeks. Control cells were cultured in DMEM alone. Cells were transiently transfected with either control or FPN siRNA for 2 days and seeded in Transwell inserts. After 48 h, migrated cells were imaged after staining with crystal violet. Relative migration ability was quantified by measuring the absorbance of the de-staining solution. Results are expressed as mean±SE (n=3). *Significantly different from the respective control.#Significantly different from the cells treated with control siRNA. (p < 0.05).
Effect of FPN on EMT
The promotion of cell migration by reducing the FPN level suggested that increased iron levels apparently contribute to EMT, a metastasis-related property. To explore this possibility, the expressions of a few representative EMT markers was studied. As shown in Fig. 7, in cells transfected with the control siRNA, treatment with 3 μM Cd for 12 h reduced the mRNA level of the epithelial marker E-cadherin by about half, while increasing the mRNA levels of the mesenchymal markers N-cadherin by 36%, Twist by 40%, and Slug by 38%. Reducing the FPN level with the siRNA further enhanced the down-regulation of E-cadherin as well as the up-regulation of N-cadherin, Twist, and Slug in both the control and Cd-treated cells. This observation further solidified the pivotal role of FPN in regulating the Cd-stimulated EMT.
Fig. 7.
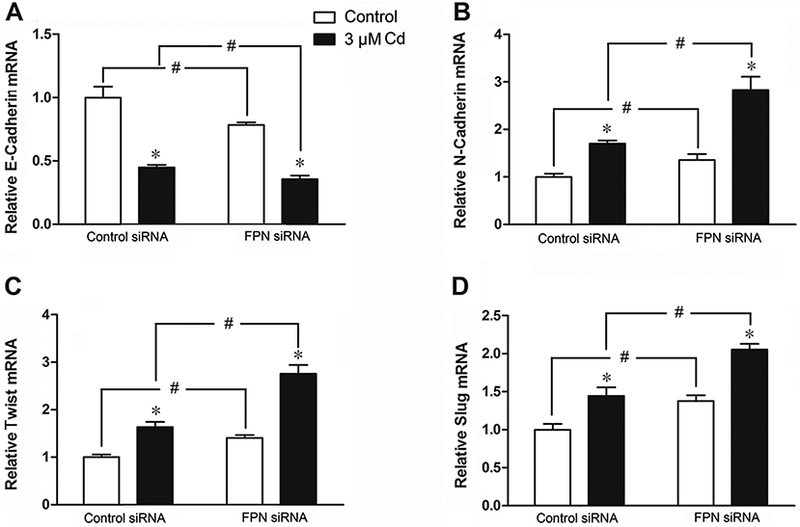
Effect of suppression of FPN expression on the expression of EMT markers in cells treated with Cd. The cells were transiently transfected with either control or FPN siRNA for 2 days and treated with 3 μM Cd for 12 h. (A) E-Cadherin, (B) N-Cadherin, (C) Twist, and (D) Slug mRNA were determined by qPCR. Results are expressed as mean±SE (n = 4). *Significantly different from the respective control. #Significantly different from the control siRNA (p < 0.05).
Activation of TGF-ß pathway by Cd
TGF-ß signaling pathway is implicated in cell proliferation and migration in breast cancer cells (Zarzynska, 2014). To examine whether treatment with Cd activated the TGF-ß pathway, the cells were treated with 1 or 3 μΜ Cd, or 1 ng/mL TGF-ß (positive control). As shown in Fig. 8, TGF caused nearly 4-fold elevation in Smad2/3 phosphorylation at 6 and 12 h. Similarly, 1 and 3 μM Cd increased Smad2/3 phosphorylation about 2- and 3-fold, respectively. These results indicated that indeed treatment with Cd activated the TGF-ß pathway.
Fig. 8.
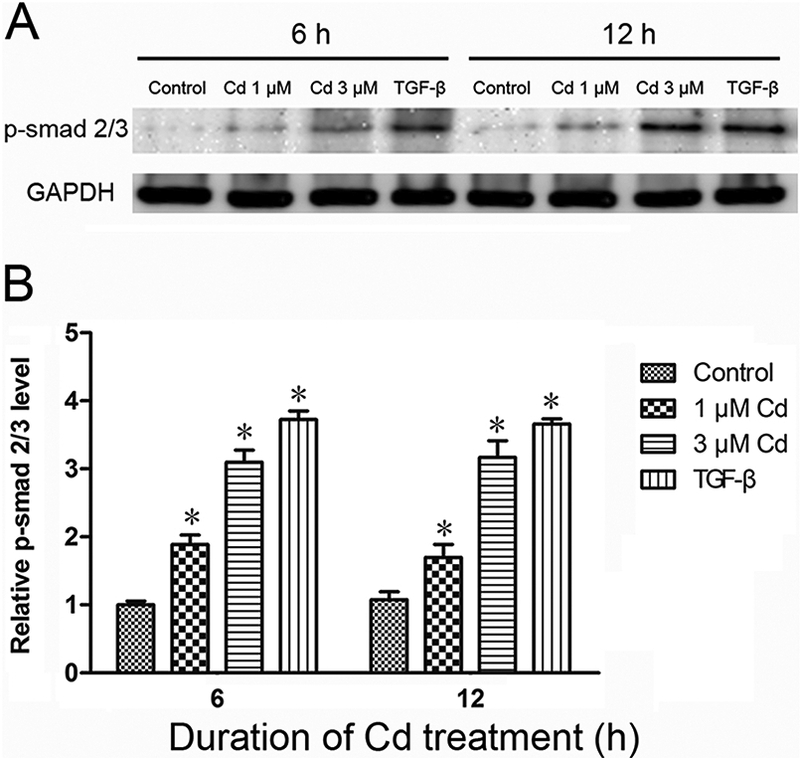
Activation of Smad 2/3 by Cd. The cells were treated with 1 and 3 μΜ Cd and p-Smad 2/3 was analyzed in cell extracts after 6 and 12 h. TGF-ß was used as a positive control. (A) Representative Western blot of p-Smad 2/3, and (B) Relative p-Smad levels. Density relative to the control is expressed as mean±SE (n = 3). *Significantly different from the respective control. (p < 0.05).
Regulation of FPN expression through the TGF-ß pathway
Whether down-regulation of FPN by Cd was mediated through the TGF-ß pathway was examined next. Treatment of cells with 3 μM Cd or 1 ng/mL TGF-ß reduced the expression of FPN by 33 and 46%, respectively (Fig. 9A). Combined treatment with both Cd and TGF-ß had an even greater effect (60% reduction) on FPN expression. Although inhibition of TGF-ß with SB431542 significantly alleviated the effect of Cd on FPN expression, it did not completely block it (Fig. 9B). Taken together, these results suggested that reduced expression of FPN by Cd was partially mediated through TGF-ß signaling.
Fig. 9.
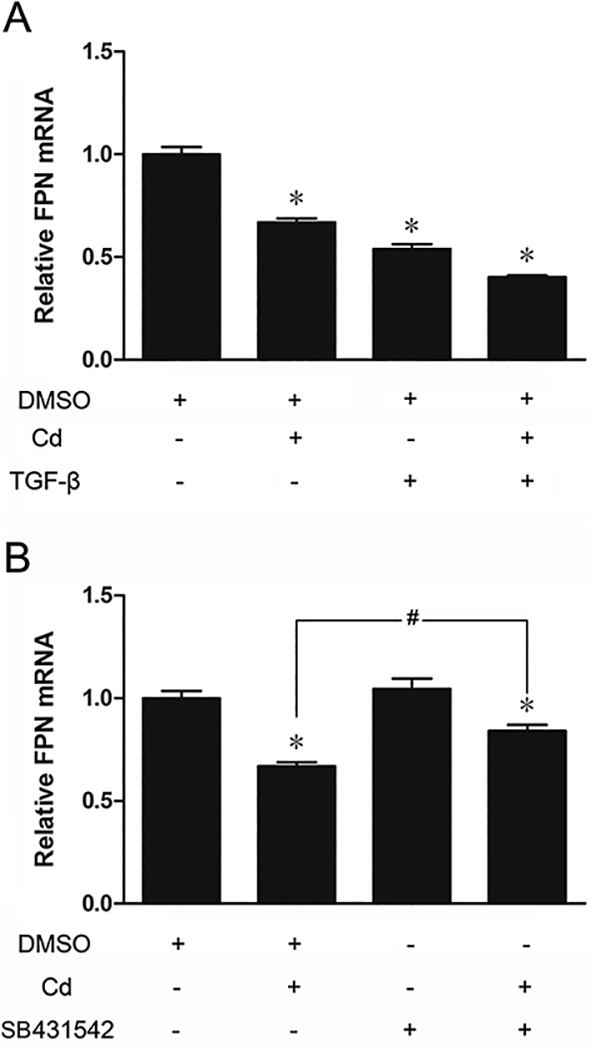
Effect of TGF-ß and its inhibitor on the expression of FPN. (A) The cells were treated with DMSO, 3 μΜ Cd, 1 ng/mL TGF-ß, or both Cd and TGF-ß for 12 h. (B) The cells were treated with DMSO, 3 μΜ Cd, 1 μΜ SB431542, or both Cd and SB431542 for 12 h. The FPN mRNA expression was measured by qPCR. Results are expressed as mean±SE (n = 4). *Significantly different from the respective DMSO control. #Significantly different from Cd alone. (p < 0.05).
Discussion
Iron is very important for maintaining normal cellular growth and biological functions, including DNA replication, energy generation and heme synthesis (Gozzelino et al., 2010; Sheftel et al., 2010). With respect to mammalian iron homeostasis, hepcidin-FPN axis was recently uncovered as the controller of iron absorption, export, storage and utilization (Ganz, 2011; Evstatiev and Gasche, 2012). Dysregulation of iron homeostasis is implicated in a spectrum of diseases, such as hereditary hemochromatosis and ß-thalassemia (Ganz and Nemeth, 2011). Numerous studies also reveal that accumulated body iron may contribute to the risk of cancer occurrence and progression (Nawrot et al., 2006; Zhou et al., 2012). However, the origin of elevated body iron burden in breast cancer patients is not well understood.
Although it has been recognized that oncogenic effects of Cd on breast tissue may be due to systemic disorder of iron metabolism, the biological mechanism underlying Cd-induced imbalances of iron homeostasis has not been elucidated. In the present study, we provide evidence demonstrating the inverse relationship between the iron exporter FPN and the stimulatory effect of Cd on iron accumulation and malignant progression of breast cancer cells. Clearly, both short- and long-term treatments with Cd augmented cellular iron levels in the MDA-MB-231 cells (Fig. 2 A, B). According to Park and Chang (2009) overproduction of reactive oxygen species by Cd could also disturb iron metabolism.
The present study showed that the expression of iron exporter FPN was significantly decreased in the breast cancer MDA-MB-231 cells treated with Cd. Similar results were obtained in the preneoplastic MCF 10A breast epithelial cells (unpublished data). Contrary to this observation, in macrophages Cd up-regulated FPN expression and depleted cellular iron availability (Sun et al, 2015). The different expression patterns of FPN upon Cd treatment might be due to the different functional properties of the cell types. The decreased expression of FPN and accumulation of iron in the MDA-MB-231 cells (Fig. 3), affirms that FPN is important in regulating iron homeostasis in these cells. Cd could affect iron homeostasis through directly competing with iron ions in iron transporters, such as DMT-1 (Li et al., 2006). It is, therefore, probable that elevated iron accumulation in MDA-MB-231 cells results from the competition between Cd with iron for FPN. To test this possibility, we determined Cd and iron concentrations in cells after knocking down FPN expression. Our studies demonstrated that a decrease in FPN only influenced the iron level and had no effect on the Cd concentration (Fig. 4B, C). Likewise, Mitchell et al. (2014) reported that functional expression of FPN in Xenopus oocytes could stimulate transport of zinc and cobalt but not Cd, copper, or manganese. Our study supports the notion that Cd-induced accumulation of iron in breast cancer cells is derived from the reduced expression of iron exporter FPN and not from competition between Cd and iron for FPN.
The present study further demonstrated that treatment of MDA-MB-231 cells with Cd for 8 weeks stimulated proliferation (Fig. 5 A, B). Similar proliferative properties were also reported in A549 cells upon treatment with Cd for more than 8 weeks (Fujiki et al., 2017). FPN affects the proliferative ability of breast cancer cells through governing intracellular iron concentration. (Zhang et al., 2014). Diminished FPN levels promote breast cancer growth (Zhang et al., 2014; Chen et al., 2015), and its expression has prognostic significance in breast cancer patients (Pinnix et al., 2010).
Our laboratory reported previously that long-term treatment with Cd promoted metastasis-associated phenotypes, such as cell migration (Wei and Shaikh, 2017). In the present study, reduced FPN expression further accelerated cell migration in Cd-treated cells (Fig. 6). As previously reported, Cd could induce EMT-like characteristics in human epithelial cells (Person et al., 2013; Wei et al., 2018) and FPN played a pivotal role in modulating EMT (Guo et al., 2015). The present study revealed that suppression of FPN expression by Cd modulated the expression of EMT markers, N-cadherin, E-cadherin, Twist and Slug (Fig. 7). Thus, FPN appears to play a pivotal role in the stimulation of metastasis-associated phenotype by Cd in breast cancer cell, apparently through controlling cellular iron concentration.
TGF-ß signaling is connected with diverse biological processes including development, fibrosis, cell growth, tissue regeneration and EMT (Tatler and Jenkins, 2012; Fujiki et al., 2014; Woods et al, 2015). Although TGF-ß strongly suppresses cell growth during early stages of tumorigenesis, it plays a significant role in promotion of metastasis in breast cancer (Imamura et al, 2012). Considering the relative but opposite roles of TGF-ß signaling and FPN in malignant progression of breast cancer cells, it is plausible that TGF-ß signaling might function in the regulation of FPN expression in Cd-treated MDA-MB-231 cells. Indeed, in the present study, Cd increased the phosphorylation of Smad2/3 (Fig. 8) that is indicative of active TGF-ß signaling (Bassey-Archibong et al, 2016). TGF-ß signaling is also responsive to Cd in HepG2 and placental trophoblast cells (Urani et al, 2015; Adebambo et al, 2018). We demonstrated here that TGF-ß promotes Cd-induced downregulation of FPN (Fig. 9 A) and that the TGF-ß inhibitor SB-431542 partially blocked the effect of Cd on FPN expression (Fig. 9B). These findings suggest that TGF-ß signaling is at least partially responsible in regulating FPN expression in Cd-treated cells.
In summary, this study demonstrates that reduced FPN expression and disordered iron homeostasis are responsible for the Cd-stimulated cell proliferation, EMT, and migration in the triple-negative MDA-MB-231 breast cancer cells.
Highlights.
Treatment with cadmium decreases ferroportin expression in MDA-MB-231 cells.
Decrease in ferroportin expression increases accumulation of iron in the cells.
Suppression of ferroportin expression by cadmium stimulates cell proliferation, EMT, and migration.
The down regulation of ferroportin expression by cadmium involves the activation of TGF-ß.
Acknowledgement
This research was made possible by use of the RI-INBRE core facility support by NIH grant # P20GM103430 from NIGMS.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adebambo OA, Shea D, Fry RC, 2018. Cadmium disrupts signaling of the hypoxia-inducible (HIF) and transforming growth factor (TGF-beta) pathways in placental JEG-3 trophoblast cells via reactive oxygen species. Toxicol. Appl. Pharmacol 342, 108–115. [DOI] [PubMed] [Google Scholar]
- Arocho A, Chen B, Ladanyi M, Pan Q, 2006. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diag Mol. Pathol.: Am. J. Surg. Pathol., part B 15, 56–61. [DOI] [PubMed] [Google Scholar]
- Asara Y, Marchal JA, Carrasco E, Boulaiz H, Solinas G, Bandiera P, Garcia MA, Farace C, Montella A, Madeddu R, 2013. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int. J. Mol. Sci 14, 16600–16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey-Archibong BI, Kwiecien JM, Milosavljevic SB, Hallett RM, Rayner LG, Erb MJ, Crawford-Brown CJ, Stephenson KB, Bedard PA, Hassell JA, Daniel JM, 2016. Kaiso depletion attenuates transforming growth factor-beta signaling and metastatic activity of triple-negative breast cancer cells. Oncogene 5, e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Duan X, Li M, Huang C, Li J, Chu R, Ying H, Song H, Jia X, Ba Q, Wang H, 2016,. Systematic network assessment of the carcinogenic activities of cadmium. Toxicol. Appl. Pharmacol 310, 150–158. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang S, Wang X, Guo W, Wang L, Zhang D, Yuan L, Zhang Z, Xu Y, Liu S, 2015. Disordered signaling governing ferroportin transcription favors breast cancer growth. Cell. Signal 27, 168–176. [DOI] [PubMed] [Google Scholar]
- Evstatiev R, Gasche C, 2012. Iron sensing and signalling. Gut 61, 933–952. [DOI] [PubMed] [Google Scholar]
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman I, Rudisill C, 2012. Toxicological Profile for Cadmium. In: Atlanta (GA), Agency for Toxic Substances and Disease Registry (US); Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. [PubMed] [Google Scholar]
- Fujiki K, Inamura H, Matsuoka M, 2014. Detrimental effects of Notch1 signaling activated by cadmium in renal proximal tubular epithelial cells. Cell Death Dis. 5, e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki K, Inamura H, Miyayama T, Matsuoka M, 2017. Involvement of Notch1 signaling in malignant progression of A549 cells subjected to prolonged cadmium exposure. J. Biol. Chem 292, 7942–7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, 2011. Hepcidin and iron regulation, 10 years later. Blood 117, 4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E, 2011. Hepcidin and disorders of iron metabolism. Ann. Rev. Med 62, 347–360. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, Jeney V, Soares MP, 2010. Mechanisms of cell protection by heme oxygenase-1. Ann. Rev. Pharmacol. Toxicol 50, 323–354. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, Liu S, 2015. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim. Biophys. Sin 47, 703–715. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Brody JG, 2018. Environmental Determinants of Breast Cancer. Ann. Rev. Pub. Health 391, 113–133. [DOI] [PubMed] [Google Scholar]
- Imamura T, Hikita A, Inoue Y, 2012. The roles of TGF-beta signaling in carcinogenesis and breast cancer metastasis. Breast Cancer 19, 118–124. [DOI] [PubMed] [Google Scholar]
- Jarup L, Akesson A, 2009. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol 238, 201–208. [DOI] [PubMed] [Google Scholar]
- Jouybari L, Saei Ghare Naz M, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, Hasanpour Dehkordi A, 2018. Toxic elements as biomarkers for breast cancer: a meta-analysis study. Cancer Manag. Res 10, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen H, Huang X, Costa M, 2006. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1alpha) and HIF-regulated genes. Toxicol. Appl. Pharmacol 213, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhao J, Liu Y, Huang Y, Shen Y, Wang J, Sun W, Sun Y, 2016. A novel pentacyclic triterpenoid, Ilexgenin A, shows reduction of atherosclerosis in apolipoprotein E deficient mice. Int. Immunopharmacol 40, 115–124. [DOI] [PubMed] [Google Scholar]
- Marano KM, Naufal ZS, Kathman SJ, Bodnar JA, Borgerding MF, Garner CD, Wilson CL, 2012. Cadmium exposure and tobacco consumption: Biomarkers and risk assessment. Reg. Toxicol. Pharmacol. RTP 64, 243–252. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B, 2014. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. American journal of physiology. Am. J. Physiol., Cell Physiol. 306, C450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA, 2006. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 7, 119–126. [DOI] [PubMed] [Google Scholar]
- Neves J, Leitz D, Kraut S, Brandenberger C, Agrawal R, Weissmann N, Muhlfeld C, Mall MA, Altamura S, Muckenthaler MU, 2017. Disruption of the hepcidin/ferroportin regulatory system causes pulmonary iron overload and restrictive lung disease. EBio Med. 20, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Chung J, 2009. Cadmium increases ferroportin-1 gene expression in J774 macrophage cells via the production of reactive oxygen species. Nutr. Res. Prac 3, 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RJ, Tokar EJ, Xu Y, Orihuela R, Ngalame NN, Waalkes MP, 2013. Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol. Applied Pharmacol. 273, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnix ZK, Miller LD, Wang W, D’Agostino R Jr., Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, Torti SV, Torti FM, 2010. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med 2, 43ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani A, Kumar A, Lal A, Pant M, 2014. Cellular mechanisms of cadmium-induced toxicity: a review. Internat. J. Environ. Health Res 24, 378–399. [DOI] [PubMed] [Google Scholar]
- Sheftel A, Stehling O, Lill R, 2010. Iron-sulfur proteins in health and disease. Trends Endocrinol. Metabol 21, 302–314. [DOI] [PubMed] [Google Scholar]
- Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC, 2010. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERalpha and c- Jun. Mol. Endocrinol 24, 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Wei Z, Shaikh ZA, 2015. Requirement of ERalpha and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol. Appl. Pharmacol 287, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang L, Wang Z, He W, Zhang S, Guo W, Qian Y, Ji H, Rong H, Liu S, 2015. Cadmium depletes cellular iron availability through enhancing ferroportin translation via iron responsive element. Mol. Med. Rep 11, 3129–3133. [DOI] [PubMed] [Google Scholar]
- Tatler AL, Jenkins G, 2012. TGF-beta activation and lung fibrosis. Proc.Am. Thor. Soc 9, 130–136. [DOI] [PubMed] [Google Scholar]
- Templeton DM, Liu Y, 2010. Multiple roles of cadmium in cell death and survival. Chem. Int 188, 267–275. [DOI] [PubMed] [Google Scholar]
- Urani C, Melchioretto P, Bruschi M, Fabbri M, Sacco MG, Gribaldo L, 2015. Impact of cadmium on intracellular zinc levels in HepG2 cells: Quantitative evaluations and molecular effects. BioMed Res. Internat 2015, 949514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Shaikh ZA, 2017. Cadmium stimulates metastasis-associated phenotype in triple-negative breast cancer cells through integrin and beta-catenin signaling. Toxicol. Appl. Pharmacol 328, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Shan Z, Shaikh ZA, 2018. Epithelial-mesenchymal transition in breast epithelial cells treated with cadmium and the role of Snail. Toxicol. Appl. Pharmacol 344, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Song X, Shaikh ZA, 2015. Cadmium promotes the proliferation of triple-negative breast cancer cells through EGFR-mediated cell cycle regulation. Toxicol. Appl. Pharmacol 289, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, 2002. Iron and immunity: a double-edged sword. Eur. J. Clin. Investig 32 Suppl 1, 70–78. [DOI] [PubMed] [Google Scholar]
- Woods LT, Camden JM, El-Sayed FG, Khalafalla MG, Petris MJ, Erb L, Weisman GA, 2015. Increased expression of TGF-beta signaling components in a mouse model of fibrosis induced by submandibular gland duct ligation. PloS One 10, e0123641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Filardo EJ, Shaikh ZA, 2010. The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol. Appl. Pharmacol 245, 83–90. [DOI] [PubMed] [Google Scholar]
- Zang Y, Odwin-Dacosta S, Yager JD, 2009. Effects of cadmium on estrogen receptor mediated signaling and estrogen induced DNA synthesis in T47D human breast cancer cells. Toxicol. Lett 184, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzynska JM, 2014. Two faces of TGF-beta1 in breast cancer. Mediat. Inflamm 2014, 141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen Y, Guo W, Yuan L, Zhang D, Xu Y, Nemeth E, Ganz T, Liu S, 2014. Disordered hepcidin-ferroportin signaling promotes breast cancer growth. Cell. Signal 26, 2539–2550. [DOI] [PubMed] [Google Scholar]
- Zhou ZH, Lei YX, Wang CX, 2012. Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium. Toxicol. Sci 125, 412–417. [DOI] [PubMed] [Google Scholar]


