Summary
This study was designed to investigate the HPA‐axis impairment in the streptozotocin (STZ)‐diabetic gerbils (Gerbillus gerbillus). Twenty‐six male gerbils (body weight ~27 g) were divided into 3 groups: vehicle control (n = 10), 2 days of diabetes (n = 09) and 30 days of diabetes (n = 07). The latter 2 groups received an intraperitoneal injection of STZ (150 mg/kg of body weight). At 2 and 30 days of diabetes, streptozotocin‐diabetic gerbils underwent a retro‐orbital puncture for assessment of biochemical and hormonal parameters. Subsequently the animals were decapitated and the adrenal glands were removed, weighed and processed for light microscopy and stereology. Nondiabetic control gerbils that had been injected with citrate buffer were examined as a comparison. At 2 days of diabetes, STZ gerbils exhibited symptoms that are characteristic of human diabetes type 1. The adrenal gland showed significant increase in weight, associated with a larger cortex layer, hypertrophy of the fasciculate cells and a significant decrease in the nucleocytoplasmic index. These changes were associated with higher plasma ACTH and cortisol concentrations compared to nondiabetic controls. At 30 days postdiabetes, ACTH levels remained elevated, whereas cortisol levels decreased compared to the early stage of diabetes. Histological analysis revealed the existence of a band of connective tissue (collagen) that separates the cortical and medullary zones and is not present in humans or laboratory rodents, which represents a striking change seen throughout the disease. STZ‐induced diabetes mellitus in Gerbillus gerbillus resulted in hyperactivation of the HPA axis in the early stages of diabetes mellitus which did not persist into the final stages of the disease, suggesting a possible reduction in adrenocortical sensitivity over time.
Keywords: adrenal gland, cortisol, Gerbillus gerbillus, HPA axis, streptozotocin, type 1 diabetes
1. INTRODUCTION
Patients with poorly controlled diabetes mellitus exhibit altered endocrine and neuroendocrine function. In particular, responsiveness of the hypothalamic‐pituitary‐adrenal (HPA) axis is disrupted in insulin‐dependent diabetic patients.1, 2 Various neurohumoral systems are involved in the development and progressive advancement of diabetes mellitus as well as its complications, including activation of the HPA axis. Elevated glucocorticoid levels, which are a hallmark of increased activation of the HPA axis, have been reported in diabetic patients by many research groups.1, 3, 4, 5
There have been several previous reports on STZ‐diabetic rats.2, 3, 6, 7, 8 Tkachuk9, 10 demonstrated changes in the HPA axis at multiple levels such as increased hippocampal mineralocorticoid receptor (MR) mRNA expression, increased hypothalamic corticotropin‐releasing hormone (CRH) mRNA expression and elevated circulating ACTH levels, all of which may contribute to hypersecretion of corticosterone in diabetes. The fact that pituitary‐adrenal activity remained elevated in the face of increased corticosteroid receptor expression led the authors to conclude that glucocorticoid negative feedback is reduced in STZ‐diabetic animals. The latter was confirmed using a dexamethasone suppression test.6
In the STZ‐diabetic mouse,11 at 2 and 11 days of diabetes, the HPA axis readily reached a new set point characterized by high circulating corticosterone, low ACTH levels and enhanced adrenocortical sensitivity. Surprisingly, ACTH levels were never elevated after diabetes onset even though hypercorticosteronaemia was present. These results are consistent with a study by Dallman et al12 showing that in response to food deprivation, corticosterone levels increase independent of an ACTH surge, suggesting an acute rise in adrenal sensitivity.
These alterations have mainly been attributed to effects exerted at higher levels of the HPA axis, and several authors suggest this phenomenon may be a consequence of the disease at the level of the adrenal gland.1, 6, 8, 11 In addition, these changes were mainly described in rodent models with shorter duration of diabetes, and little work has been done to characterize the morphological and functional changes of the system in later stages of the disease.
According to Poulet,13 the adrenal glands of the gerbils are much closer to the adrenals of the human species, due to their high concentration of ascorbic acid and also by their high secretion of cortisol.
Therefore, the objective of this study was to compare morphological and functional changes in the adrenal gland in short and longer duration of diabetes with assessment of hormonal changes in relation to the HPA axis, using the STZ‐diabetic gerbil as a model of type 1 diabetes.
2. METHODS
2.1. Animals
Wild male gerbils (n = 26), with an initial body weight of ~27 g, were captured from the region of Béni Abbès, Béchar, Algeria, and then transported to our laboratory where they were allowed to acclimate for a period of 2 weeks before the start of experimentation.
During this time, they were housed under standard laboratory conditions: constant temperature of 26‐28°C, a 12‐hour light cycle (lights on between 07:00 and 19:00 hours) and ad libitum access to food (salad, carrot and some barley).
Gerbils were divided into the following groups: nondiabetic (control, n = 10), 2 days of diabetes (acute, n = 09) and 30 days of diabetes (chronic, n = 07).
Throughout all experimental procedures, the animals were maintained in an animal research facility according to institutional guidelines for animals' care (Directive 2010/63/EU).
2.2. Ethical approval
This article does not contain any study with human participants. All procedures performed in this study involving animals were in accordance with the ethical standards of Institutional Animal Care and Use Committee of Bab Ezzouar University (Algiers, Algeria; permit number for the present research project: D01N01UN160420130021) and have been achieved according to the Executive Decree no. 10–90 completing the Executive Decree no. 04–82 of the Algerian Government, establishing the terms and modalities of animal welfare in animal facilities.
2.3. Preparation of streptozotocin (STZ) and induction of diabetes mellitus
Streptozotocin (Sigma S0130 (PROCHIMA‐SIGMA, Tlemcen, Algeria)) was dissolved in sodium citrate buffer (0.1 mol/L, pH 4.4) prepared freshly in the morning for immediate administration within 5 minutes.
The diabetogenic agent was injected intraperitoneally at a dose of 150 mg/kg body weight. Control gerbils received an intraperitoneal injection of an equivalent volume of citrate buffer vehicle.
2.4. Experimental protocol
Twenty‐four to forty‐eight hours postinjection, gerbils with blood glucose levels greater than 2.5 g/L were considered diabetic. At 2 and 30 days of diabetes, animals were weighed and urine volume was measured. Blood samples were taken from retro‐orbital venous plexus, into heparin and EDTA tubes; then, the gerbils were sacrificed by decapitation. Plasma samples were separated, aliquoted into storage tubes and stored at −20°C for analysis of biochemical parameters (triglyceride and total cholesterol) and at −80°C for hormones (ACTH, cortisol and insulin).
2.5. Analysis of biochemical and hormone parameters
Blood glucose was measured using the Accu‐Chek Compact glucometer (Roche Diagnostics, Mannheim, Germany). Serum triglyceride (TG) levels and total cholesterol (TC) were determined spectrophotometrically using enzymatic colorimetric assay kits (Spinreact, Spain). Plasma insulin, ACTH and cortisol concentrations were measured by chemiluminescence immunoassay, using immunoassay analyzer Cobas e411 (Roche diagnostics).
2.6. Histological and morphometric analyses
The adrenal glands were quickly removed, weighed and dissected from fat tissue under sterile conditions. The left adrenal gland from each animal was used for histology and morphometric analysis. Three glands from each treatment group were used for morphometric examination. These adrenals were fixed in Bouin's solution and embedded in paraffin. Subsequently, they were serially sectioned (5 μm) and stained with haematoxylin and eosin for stereology. In addition, we used the Masson's trichrome stain for histological examination.
Stereological analysis of every 3 sections from one half of each gland was performed using AxioVision ver. 4.6. Cortex, fasciculate zone, connective band and lipid droplet length were measured in each single section from 6 fields. The area of fasciculate cells and nucleus was measured from 3 fields of each section.
The nucleocytoplasmic index (NCindex) was calculated using the formula:
2.7. Statistical analysis
All results are expressed as mean ± standard deviation. Statistical differences were evaluated using Kruskal‐Wallis test followed by Mann‐Whitney post hoc analysis for hormone responses and Student's t test for biochemical analysis and stereological evaluations. Differences are considered statistically significant for P < 0.05.
3. RESULTS
3.1. Clinical symptoms and organ weight
After 2 days of diabetes, STZ‐ animals showed a significant increase in blood glucose (P < 0.001), food consumption (P < 0.01) and urine volume (P < 0.001) accompanied by a significant decrease in body weight (P < 0.01) compared to nondiabetic control animals (Table 1). At 30 days of diabetes, these clinical symptoms still persisted. The weight of the adrenal glands—both the absolute and the relative weight to the body weight (BW)—exhibited a significant increase in the STZ‐treated group at both stages of the disease compared to controls (Table 1).
Table 1.
Pathophysiological measures of STZ‐diabetic gerbils compared to controls
| Treatment | Blood glucose (g/L) | Food consumption (Kcal) | Body weight (g) | Urine volume (μL) | Adrenal absolute weight (mg) | Adrenal relative weight mg/10 g BW | |
|---|---|---|---|---|---|---|---|
|
Control (n = 10) |
1.12 ± 0.08 | 6.63 ± 0.97 | 27.83 ± 1.02 | 33.37 ± 7.26 | 5.36 ± 0.49 | 2.17 ± 0.22 | |
| Days of diabetes | 2 days (n = 09) | 4.47 ± 0.44*** | 10.11 ± 0.66** | 24.98 ± 1.22* | 132.5 ± 13.8*** | 10.15 ± 1*** | 4.12 ± 0.39 *** |
| 30 days (n = 07) | 3.38 ± 0.57** | 13.05 ± 0.22*** | 21.64 ± 0.78*** | 87.77 ± 17.71** | 9.36 ± 1.56** | 4.02 ± 0.76** | |
Data for body weight, blood glucose, food consumption, urine volume and adrenal glands' absolute and relative weights to the body weight (BW) in control and STZ‐diabetic gerbils in the acute (2 days) and chronic stages of diabetes (30 days).
Data are expressed as mean ± SEM; n = 7‐10.
P < 0.05.
P < 0.01.
P < 0.001 vs controls.
Diabetes onset is according to the first measured hyperglycaemia after STZ injection.
3.2. Blood hormonal and biochemical parameters
Plasma ACTH levels in the STZ‐ group were significantly higher (P < 0.05) than in the control group (Figure 1A). At 30 days of the disease, ACTH levels remained elevated (P < 0.05). Within 2 days after the onset of diabetes, STZ‐treated gerbils showed significant increases in plasma cortisol levels (P < 0.01), whereas at 30 days of the disease, cortisol levels decreased significantly (P < 0.05) from the 2‐day time point, but still remained significantly elevated (P < 0.05) than those of the control group (Figure 1A).
Figure 1.
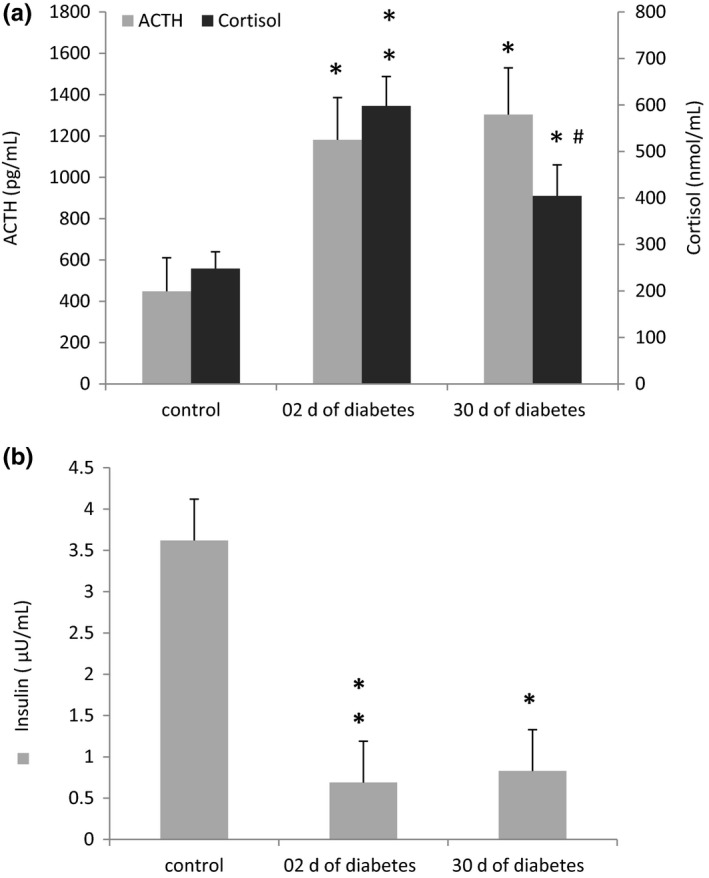
Plasma ACTH and cortisol (A) and insulin (B) levels in control and diabetic animals (48 h and 30 d of diabetes). Data are expressed as means ± SEM; n = 3‐5.*P < 0.05 vs control, **P < 0.01 vs controls and # P < 0.05 between the acute and chronic stages of diabetes
In the early stage of diabetes (Figure 1B), insulin levels in STZ‐treated gerbils were significantly lower (P < 0.01) than those of control gerbils. These low levels persisted to the 30‐day time point of our experiment (P < 0.05).
Serum triglyceride (TG) levels in the STZ‐treated group increased ~4‐fold within 48 hours after the onset of diabetes (Figure 2). This was significantly higher (P < 0.001) than that in the control group. The serum TG levels still remained significantly higher (P < 0.001) at 30 days of diabetes compared to controls. However, these levels were significantly lower (P < 0.001) compared to the previous period of experimentation. Total cholesterol (TC) was similar between diabetic and nondiabetic control animals.
Figure 2.
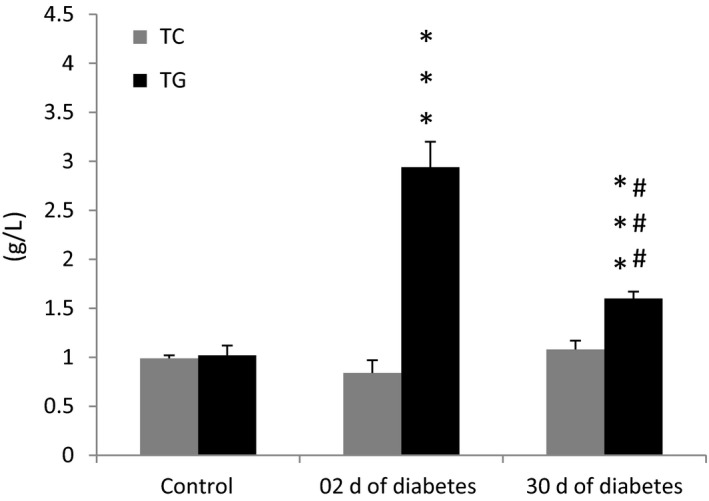
Total cholesterol (TC) and triglyceride (TG) levels in control and diabetic animals (after 2 and 30 d of diabetes). Data are expressed as means ± SEM; n = 7‐10. ***P < 0.001 vs controls, and ### P < 0.001 between acute and chronic stages of diabetes
3.3. Adrenal gland histology
In the early stages of diabetes (Figure 3B,E), the fasciculate zone exhibited a significant increase in length (P < 0.01), with more cellular hypertrophy (Table 2), compared to controls. There were scattered light and dark regions of extensive lipid content and granular cytoplasm (Figure 3A,D) indicative of uneven active storage, synthesis and release of glucocorticoids, and these results corroborate the findings of Wexler and Lutmer14 on STZ‐treated rats' adrenal glands. The length of the fasciculate zone was still significantly (P < 0.001) increased in the later stages of the disease with fat infiltration and interstitial fibrosis (Figure 3C,F; Table 2) compared to controls.
Figure 3.
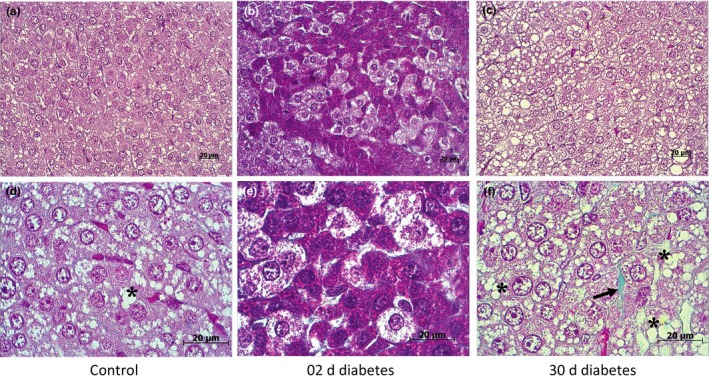
Histological characterization of the fasciculate zone of Gerbillus gerbillus adrenal gland using Masson's trichrome staining (collagen fibres stained by green). Control adrenal gland (A,D): zona fasciculata shows an equal distribution of lipid droplets (*) and granular cytoplasm. In the acute stage of diabetes (B,E), the zona fasciculata shows a dark and a light region with hypertrophic cells. At higher magnification (E), the light regions of the zona fasciculata show clear cells, field with lipid, whereas the dark regions show eosinophilic cells with plenty of granular cytoplasm. In the chronic stage of diabetes (C,F), the zona fasciculata manifests an extensive adrenocortical hyperplasia with extensive fat infiltration (*) and interstitial fibrosis (black arrow) [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Stereological study of the STZ‐diabetic gerbils' adrenal gland compared to controls
| Control | Days of diabetes | ||
|---|---|---|---|
| 2 d | 30 d | ||
| Cortex length (μm) | 436.38 ± 16.82 | 545.29 ± 19.04 *** | 700.71 ± 11.28 *** |
| Connective band length (μm) | 25.32 ± 2.41 | 175.91 ± 22.15 *** | 122.58 ± 9.10***/ ## |
| Fasciculate zone length (μm) | 330.51 ± 14.35 | 377.72 ± 5.51** | 498.82 ± 7.4*** |
| Fasciculate zone cell surface area (μm2) | 161.88 ± 8.83 | 250.31 ± 17.24*** | 189.83 ± 6.21* |
| Nucleocytoplasmic index of fasciculate cells (NCI) | 0.340 ± 0.01 | 0.268 ± 0.02* | 0.285 ± 0.01** |
| Lipid droplet length in the connective band (μm) | 8.71 ± 0.55 | 39.22 ± 3*** | No droplets |
Morphometric analysis of adrenal cortex, fasciculate zone and connective band in control and STZ‐treated gerbils at the acute and chronic stages of diabetes.
Data are expressed as mean ± SEM.
P < 0.05.
P < 0.01.
P < 0.001 vs controls.
P < 0.01 between acute and chronic stages of the disease.
Compared to controls, at 2 days after diabetes onset, the adrenal glands of STZ‐treated gerbils exhibited a dramatic infiltration of collagen (Figure 4) that was highlighted by a significant increase in the thickness of the connective band (Table 2) that separates the cortisol and medullar zones. This includes fibrous connective tissue with numerous large lipid droplets, accompanied by abundant fibroblasts and lymphocyte infiltration and blood vessels. This suggests fibrosis that occurs in early diabetes and distorts the architecture of the gland.
Figure 4.
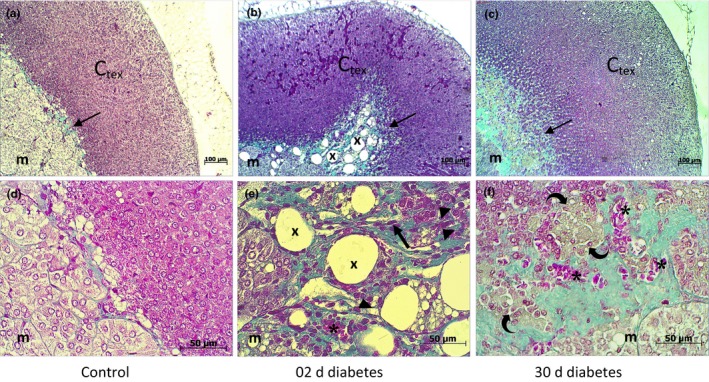
Histological characterization of Gerbillus gerbillus adrenal gland, treated with vehicle (A,D) or streptozotocin at 2 d of diabetes (B,E) and at 30 d of diabetes (C,F) by Masson's trichrome, for demonstrating collagen fibres. Control adrenal gland (A,D) shows a thin line of collagen fibres, stained by green (arrow) lying between the cortex (Ctex) and medulla (m). In the acute stage of diabetes (B,E), the connective band exhibits an increase in collagen fibres, large lipid droplets (X) with abundant blood vessels (sinusoids *), lymphocyte infiltration (arrowhead) and increased fibrous and scattered fibroblast (thick arrow) content. In the chronic stage of diabetes (C,F), the connective band shows focal fibrosis resulting in thickening, and distortion, which likely indicates macrophage infiltration (curved arrow) and dilated blood vessels (*); at this stage, lipid droplets disappeared completely [Colour figure can be viewed at http://wileyonlinelibrary.com]
In the later stages of the disease, the thickness of the connective band decreased significantly (P < 0.01; Table 2) compared to the previous period of the disease, but it still remained thicker (P < 0.001) than in the control group. This was composed of dense collagen with blood vessels and macrophage infiltration (Figure 4).
4. DISCUSSION
The HPA axis plays an important role in maintaining homoeostasis. It is a part of the neuroendocrine system and regulates glucocorticoid production and release in response to environmental as well as physiological and psychological stressors. Activation of the HPA axis requires concomitant interaction between the sympathetic nervous system, neurons in the paraventricular nucleus of the hypothalamus (PVN) and peptidergic mediators in the anterior pituitary resulting in release of glucocorticoids from the adrenal glands into the circulation. In healthy individuals, cortisol levels are regulated by a negative feedback mechanism where cortisol acts on both mineralocorticoid and glucocorticoid receptors located in the brain and pituitary gland to suppress corticotrophin‐releasing hormone (CRH) and AVP (arginine vasopressin) release. Together, these actions inhibit ACTH release. Hyperactivity of the HPA axis leads to increased plasma glucocorticoids levels in diabetic patients which can adversely affect glycaemic control.1, 2, 3, 5 However, the mechanism(s) underlying hyperactivation of the HPA axis in diabetes remain unclear. Hence, the objective of this study was to evaluate the effect of diabetes on pituitary‐adrenocortical function in the STZ‐diabetic gerbil by assessing morphological changes in the adrenal gland and changes in hormones in both the early and late stages of diabetes mellitus.
Compared to controls, a single intraperitoneal injection of 150 mg/Kg of streptozotocin in the gerbil resulted in the major clinical symptoms of type 1 diabetes melitus including hyperglycaemia, polyuria, cachexia, hyperphagia and hypoinsulinaemia which are consistent with other reports in STZ‐diabetic animals16, 17, 32, 33.
At both the acute and chronic stages of diabetes, striking morphological changes occurred at the level of the adrenal gland. These changes were highlighted by significant increases in the relative and absolute weights of the adrenals which are similar to previous reports in STZ‐diabetic rats and mice 2, 10, 11, 18, 31. Of particular interest is that the histology of the gerbil's adrenal gland shows the existence of a fibrous tissue layer that separates the cortex from the medullar zone. This layer is not present in laboratory rodents nor in humans. This connective band is mostly collagenous and varies much in thickness. This layer may be similar to that found in the adrenal of Saharan animals, such as the camel.19
Compared to controls, in the early stages of diabetes, significant changes in the connective band were observed, which deform the morphological structure of the organ. This layer was much broader and had plenty of lipid droplets which are indicative of active lipid storage and may help explain changes in the lipid profile that we observed as there were significant increases in triglyceride levels, but not in total cholesterol after onset of diabetes. This was described by Gylling et al20 associated with a high absorption and low synthesis of marker sterols, which is characteristic of type I diabetes in humans. These changes were marked by an abundant increase in vascularization, lymphocyte infiltration and fibrosis, and these findings are similar to those found in the adrenal medulla (lymphocytic infiltration and fibrosis) of patients with type 1 diabetes.21 In the later stages of diabetes, the connective band shows severe fibrosis with abundant and dilated blood vessels and likely macrophage infiltration, which could indicate an inflammatory response in the adrenal gland. These cells are likely macrophages as they are present throughout the adrenal cortex particularly in the deeper layers,22 and they are reported to extend long projections between the endocrine cells and localizing close to blood vessels.23 Macrophages have a central role in initiating and perpetuating inflammation in response to injury.24 Diabetes promotes an inflammatory macrophage phenotype as macrophages isolated from mouse models of type 1 diabetes exhibit an inflammatory phenotype.25
In both the earlier and later stages of diabetes, stereological analysis showed a significant increase in the cortex and zona fasciculata length. The latter showed cellular hypertrophy with a significant decrease in the nucleocytoplasmic index indicative of cell hyperactivity which is observed in the adrenal glands of STZ rats and mice.2, 10, 11 These alterations reflect prolonged exposure to high ACTH levels as described by several laboratories8, 26, 27, 28, 29 which may explain the significant increase in cortisol secretion found in these animals. Our data are consistent with the concept of HPA hyperactivation as evidenced by increased plasma ACTH and cortisol and adrenal gland hypertrophy that accompany experimental diabetes and have been well described in humans.1, 2, 7, 11
Thirty days after the onset of diabetes, our data show a constant hyperfunctioning of pituitary‐adrenal activity as indicated by significantly elevated plasma ACTH and cortisol compared to controls, which is in agreement with findings in STZ‐diabetic rats6, 7 indicating that chronic exposure to elevated glucocorticoid levels reduces the sensitivity of HPA axis to negative feedback effects of these hormones. Chan et al6, 7 suggested that in STZ‐diabetic rats, this may be an adaptation which helps reduce exposure to high glucocorticoid levels. Likewise, hyperactivation of the HPA axis in diabetic patients may be attributable, in part, to decreased glucocorticoid negative feedback sensitivity.7
Despite progressive and persistent adrenocortical hyperplasia in the later stages of the disease and despite lipid infiltration in the fasciculate zone, the adrenal cortex of the diabetic animals was unable to maintain the initial high level of cortisol production, as evidenced by the significant decrease in plasma cortisol concentrations by 30 days (P < 0,05) compared to the early stage of the disease. The reduction occurred despite continually elevated plasma ACTH concentrations. This is a novel observation that may explain why we do not always see elevated cortisol in patients with type 1 diabetes.11, 15 Our findings of a decrease in cortisol levels in STZ‐diabetic gerbils are consistent with those reported by De Nicola et al in STZ‐Wistar rats.4 The decrease in cortisol at 30 days of diabetes may be influenced by increased oxidative stress as indicted by Repetto et al,8 who reported that long‐term diabetes led to increased endogenous nitric oxide (NO) production which can inhibit steroid production. Similar results were reported by Astort et al,15 who showed that long‐term exposure to hyperglycaemia increased oxidative stress and decreased steroid production in adrenal cells. Cymeryng et al30 suggested that steroidogenesis in zona fasciculata adrenal cells may be negatively modulated by L‐arginine‐derived nitric oxide. Nevertheless, decreases in adrenal sensitivity to ACTH in the later stages of diabetes could be established, as was reported in STZ‐diabetic rats where the response to an ACTH challenge was reduced.6, 8 These mechanisms will have to be tested in the future.
5. CONCLUSION
Gerbillus gerbillus made diabetic with a single intraperitoneal injection of STZ, at a dose of 150 mg/kg, developed type 1 diabetes mellitus, characterized by hyperglycaemia, hypoinsulinaemia and striking loss of body weight. At early and late stages of diabetes, the STZ‐diabetic gerbil's adrenal glands manifest an important hypertrophy. Histological analysis showed an increase in the cortex and connective band length with fasciculate zone hyperfunction and lipid droplet invasion. The connective band shows signs of inflammation characterized by the presence of lymphocytes, abundant blood vessels and fibrosis in the early stages of diabetes, followed by macrophage infiltration and severe fibrosis in the later stages of the disease. With longer duration of diabetes, there is a decrease in glucocorticoid secretion despite higher plasma ACTH levels compared to the early stage of the disease that may be explained by a decrease in adrenocortical sensitivity. Together, these findings show that long‐term diabetes may elicit changes in the adrenal gland that demonstrate continual dysregulation of the HPA axis.
Future immunohistochemical evaluation of macrophage cells and ACTH and cortisol mRNA expression will corroborate our results.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank Professor Owen Chan for his review of the article and his advice. His help is greatly appreciated. The present study was approved by The National Committee for Evaluation and Programming of University Research (C.N.E.P.R.U.) of the Algerian Government (No. F00220130075).
Hammadi S, Chan O, Abdellali M, et al. Hyperactivation of the hypothalamo‐pituitary‐adrenocortical axis in streptozotocin‐diabetic gerbils (Gerbillus gerbillus). Int J Exp Path. 2018;99:172–179. 10.1111/iep.12284
REFERENCES
- 1. Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG. Diabetes and the hypothalamo‐pituitary‐adrenal (HPA) axis. Minerva Endocrinol. 2003;28:87‐102. [PubMed] [Google Scholar]
- 2. Marı′a MA, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci U S A. 2000;97(20):11056‐11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torres RC, Prevatto JP, Silva PMR, Martins MA, Carvalho VF. From type‐1 diabetes HPA axis to the disease complications. J Diabetes Metab 2013;12:1‐8. 10.4172/2155-6156.S12-002 24567847 [DOI] [Google Scholar]
- 4. De Nicola AF, Fridman O, Del Castillo EJ, Foglia VG. Influence of streptozotocin diabetes on adrenal function in male rats. Horm Metab Res. 1976;8:388‐392. [DOI] [PubMed] [Google Scholar]
- 5. Cameron OG, Kronfol Z, Grenden JF, Carroll BJ. Hypothalamic‐pituitary‐adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41:1090‐1095. [DOI] [PubMed] [Google Scholar]
- 6. Chan O, Inouye K, Vranic M, Matthews SG. Hyperactivation of the hypothalamo‐pituitary‐adrenocortical axis in streptozotocin‐diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143:1761‐1768. [DOI] [PubMed] [Google Scholar]
- 7. Chan O, Chan S, Inouye K, Vranic M, Matthews SG. Molecular regulation of the hypothalamo‐pituitary‐adrenal axis in streptozotocin‐induced diabetes: effects of insulin treatment. Endocrinology. 2001;142:4872‐4879. [DOI] [PubMed] [Google Scholar]
- 8. Repetto EM, Sanchez R, Cipelli J, et al. Dysregulation of corticosterone secretion in streptozotocin‐diabetic rats: modulatory role of the adrenocortical nitrergic system. Endocrinology. 2010;151(1):203‐210. [DOI] [PubMed] [Google Scholar]
- 9. Yurii T. Morpho‐functional changes of pituitary‐adrenal system in the long‐term course of experimental diabetes mellitus. J Educ Health Sport. 2016;6(4):375‐384. [Google Scholar]
- 10. Elahi‐Moghaddam Z, Behnam‐Rassouli M, Mahdavi‐Shahri N, Hajinejad‐Boshroue R, Khajouee E. Comparative study on the effects of type 1 and type 2 diabetes on structural changes and hormonal output of the adrenal cortex in male wistar rats. J Diabetes Metab Disord. 2013;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Revsin Y, Wijk DV, Saravia FE, Oitzl MS, De Nicola AF, De Kloet ER. Adrenal hypersensitivity precedes chronic hypercorticism in streptozotocin‐induced diabetes mice. Endocrinology. 2008;149(7):3531‐3539. [DOI] [PubMed] [Google Scholar]
- 12. Dallman MF, Akana SF, Bhatnagar S, et al. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015‐40123. [DOI] [PubMed] [Google Scholar]
- 13. Poulet S. (2004) La gerbille un nouveau rongeur de compagnie. Doctoral thesis. Thesis director; Jacques Ducos de lahitte. Touleuse 3. National school of veterinary. 4041.150 p.
- 14. Wexler BC, Lutmer RF. Adrenal Glandular Lipids And Circulating Corticosterone In Severely Diabetic Rats. Int J Exp Pathol. 1975;56(4):299‐306. [PMC free article] [PubMed] [Google Scholar]
- 15. Astort F, Repetto EM, Martínez CC, et al. High glucose‐induced changes in steroid production in adrenal cells. Diabetes Metab Res Rev. 2009;25:477‐486. [DOI] [PubMed] [Google Scholar]
- 16. Aileen JFK. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2004;22:359‐370. [DOI] [PubMed] [Google Scholar]
- 18. Inouye K, Chan O, Riddell MC, Akirav E, Matthews SG, Vranic M. Mechanisms of impaired hypothalamic‐pituitary‐adrenal (HPA) function in diabetes: reduced counter regulatory responsiveness. Diabetes Nutr Metab. 2002;15(5):348‐355. [PubMed] [Google Scholar]
- 19. Abdalla MA, Ali AM. Morphometric and histological studies on the adrenal glands of the camel Camelus dromedarius. Acta Morphol Neerl Scand. 1988;26(4):269‐281. [PubMed] [Google Scholar]
- 20. Gylling H, Tuominen JA, Koivisto VA, Miettinen TA. Cholesterol metabolism in type 1 diabetes. Diabetes. 2004;53:2217‐2222. [DOI] [PubMed] [Google Scholar]
- 21. Florence MB, Smith AM, Longway S, Steven L. Adrenal medullitis in type I diabetes. J Clin Endocrinol Metab. 1990;71(6):1491‐1495. [DOI] [PubMed] [Google Scholar]
- 22. Almeida H, Ferriera J, Neves D. Macrophage of the adrenal cortex –a morphological study of the effects of Aging and dexamethasone administration. Ann N Y Acad Sci. 2004;1019:135‐140. [DOI] [PubMed] [Google Scholar]
- 23. Unanue ER. (2016). Macrophages in endocrine glands with emphasis on pancreatic islets. Microbiol Spectr, 4(6):1‐11. 10.1128/microbiolspec.MCHD-0048-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tacke F, Zimmermann h. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090‐1096. [DOI] [PubMed] [Google Scholar]
- 25. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl‐CoA synthetase 1. PNAS. 2012;109(12):715‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rebuffat P, Belloni AS, Malendowicz LK, Mazzocchi G, Meneghelli V, Nussdorfer GG. Effects of streptozotocin‐induced experimental diabetes on the morphology and function of the zona fasciculata of rat adrenal cortex. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:13‐19. [DOI] [PubMed] [Google Scholar]
- 27. De Nicola AF, Fridman O, Del Castillo EJ, Foglia VG. Abnormal regulation of adrenal function in rats. Horm. Metab. 1977;9(6):469‐473. [DOI] [PubMed] [Google Scholar]
- 28. Golden SH, Malhotra S, Wand GS, Brancati FL, Ford D, Horton K. Adrenal gland volume and dexamethasone‐suppressed cortisol correlate with total daily salivary cortisol in African‐American women. J Clin Endocrinol Metab. 2007;92(1358–1363):22. [DOI] [PubMed] [Google Scholar]
- 29. Miller WL. The Adrenal Cortex and its Disorders In: Brook CGD, Clayton PE, Brown RS, Savage MO, eds. Clinical Pediatric Endocrinology, 5th ed Oxford, UK: Blackwell Publishing Ltd;2005:293‐351. ch15 [Google Scholar]
- 30. Cymeryng CB, Dada LA, Colonna C, Mendez CF, Podesta EJ. Effects of L‐arginine in rat adrenal cells: involvement of nitric oxide synthase. Endocrinology. 1999;140:2962‐2967. [DOI] [PubMed] [Google Scholar]
- 31. Mahar Y, Shoro A, Naqvi A. The effect of l‐arginine and insulin on histological changes in streptozotocin treated rat adrenal gland. Pak J Med Health Sci. 2012;6(4):843‐847. [Google Scholar]
- 32. Sachin A, Shreesh KO, Vohora D. Characterisation of streptozotocin induced diabetes mellitus in Swiss albino mice. Global J Pharmacol. 2009;3(2):81‐84. [Google Scholar]
- 33. Wei M, Ong L, Smith MT, et al. The streptozotocin‐diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003;12:44‐50. [DOI] [PubMed] [Google Scholar]
- 34. Simůnková K, Vondra K. Adrenocortical insufficiency and diabetes mellitus type 1. Cas Lek Cesk. 2010;149(3):120‐124. [PubMed] [Google Scholar]


