Abstract
Invasive aspergillosis (IA) is the most serious mold infection encountered in patients with iatrogenic immunosuppression. IA is also a major cause of mortality and morbidity in individuals with primary immunodeficiency (PID). Although Aspergillus fumigatus is the most common etiologic agent of IA reported in PID patients, followed by A. nidulans, multiple poorly recognized Aspergillus species such as A. udagawae, A. quadrilineatus, A. pseudoviridinutans, A. tanneri, A. subramanianii, and A. fumisynnematus have been reported almost exclusively from patients with inborn defects in host antifungal defense pathways. Infection in PID patients exhibits patterns of disease progression distinct from those in iatrogenic immunosuppression. Specifically, the disease can be extrapulmonary and chronic with a tendency to disseminate in a contiguous manner across anatomical planes. It is also more refractory to standard antifungal therapy. This synopsis summarizes our understanding of emerging rare Aspergillus species that primarily affect patients with PIDs but not those with acquired immunodeficiencies.
Keywords: emerging non-fumigatus Aspergillus species, invasive aspergillosis, primary immunodeficiencies
Aspergillus species are one of the most ubiquitously found saprophytic molds in soil and decaying vegetations, with a potential to cause opportunistic disease primarily in patients with defective immune systems [1]. Reports of aspergillosis have increased, mainly due to the increasing number of individuals with immunodeficiency and advances in life support systems [2]. Although invasive aspergillosis (IA) is most commonly recognized among the population with acquired immunodeficiency (secondary immunodeficiency), the number of primary immunodeficiencies (PIDs) recognized as underlying conditions predisposing to mycoses is increasing, most often in children and young adults [3]. PIDs are rare congenital genetic disorders, mostly due to abnormalities in a single gene that enhance susceptibility to autoimmunity or infectious disease [3]. Consequently, patients may encounter recurrent, protracted, or severe infections due to pathogens including fungi [4]. Among the known congenital primary immunodeficiencies at risk for IA discussed here are chronic granulomatous disease (CGD), autosomal-dominant (AD) hyper-IgE syndrome (HIES; also known as Job syndrome), AD deficiency in GATA2 (also known as MonoMAC, monocytopenia syndrome, B-cell and NK-cell lymphopenias), AD or AR (autosomal-recessive) severe congenital neutropenia (SCN), LAD (AR type I leukocyte adhesion deficiency), also called CD18 deficiency, and caspase recruitment domain- containing protein 9 (CARD9) deficiency [3]. Aspergillus fumigatus is the primary cause of IA in individuals with PIDs, followed by A. nidulans, due to its propensity to cause infection in patients with CGD [5]. Other aspergilli also documented to infect PID patients include A. pseudoviridinutans [6], A. tanneri [7], A. udagawae [8], A. flavus [9], A. niger [10], A. calidoustus [11], A. quadrilineatus [12], A. fumisynnematus [13], and A. subramanianii. Without proper identification of the etiologic agents and adequate therapy, IA results in high mortality rates regardless of the species involved [14]. This paper focuses on the important mycological features of emerging non-fumigatus Aspergillus species reported from IA cases in PID patients.
DISTRIBUTION OF PATHOGENIC SPECIES IN DIFFERENT SECTIONS OF ASPERGILLUS
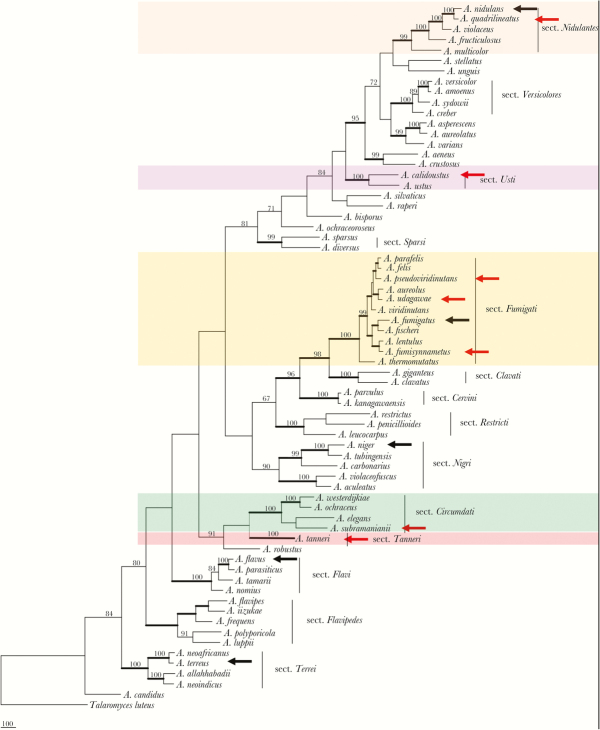
The genus Aspergillus contains more than 250 species, which are subdivided into 22 distinct sections. Of these, 13 sections, including Candidi, Circumdati, Flavipides, Fumigati, Nidulantes, Nigri, Ornati, Restricti, Tanneri, Terrei, Usti, Versicolores, and Warcupi, contain clinically relevant species (Figure 1) [15].
Figure 1.
Phylogenetic tree inferred from combined β-tubulin, Calmodulin, MCM7, and RPB2 genome sequences. A black arrow indicates 5 well-known pathogenic species of Aspergillus, and a red arrow identifies other emerging aspergilli reported primarily in patients with underlying primary immunodeficiency. Thick branches indicate >95% bootstrap support; numbers above nodes are Bayesian probabilities greater than 0.90. Abbreviations: MCM7, Minichromosome Maintenance Protein 7; RPB2, RNA polymerase II second largest subunit.
Aspergillus fumigatus in the section Fumigati is the primary etiologic agent of IA, and its inhaled conidia can cause multiple diseases depending on the host immune status. The spectrum of disease includes invasive pulmonary infection, disseminated disease, fungus ball (aspergilloma), and various hypersensitivity diseases such as allergic bronchopulmonary aspergillosis, allergic asthma, and hypersensitivity pneumonitis [16]. However, aspergillosis caused by emerging poorly recognized species morphologically similar to A. fumigatus in patients with PID may exhibit differences in the clinical presentation, kinetics of tissue invasion, and susceptibility to antifungal therapy [17].
HUMAN PIDS ASSOCIATED WITH INVASIVE ASPERGILLOSIS
There are more than 200 different forms of PIDs, which are classified into 9 subclasses, depending on their underlying immunologic defect or predominant manifestations. Globally, a total of ~77 000 patients are registered as having a specific PID [18]. Table 1 summarizes the PIDs in which IA has been documented.
Table 1.
Category of PIDs Associated With Aspergillosis
| Category | Disease | Genetic Defect/Presumed Pathogenesis | Inheritance | Affected Immune Cells | Affected Immune Function Associated With Susceptibility to Aspergillosis | Susceptibility to Aspergillosis | Susceptibility to Other Fungal Infections | Reported Aspergillus Species | |
|---|---|---|---|---|---|---|---|---|---|
| Congenital defects of phagocyte numbers and/or function | Defects of phagocyte function (respiratory burst) | (a) CGD-gp91phox deficiency | Mutation in CYBB: electron transport protein (gp91phox) | XL | N+M | Killing (defective superoxide production) | High | Other mold infections (occasionally) Invasive candidiasis, (rarely) |
A. calidoustus, A. flavus, A. fumigatus, A. fumisynnematus, A. quadrilineatus, A. nidulans, A. niger, A. pseudoviridinutans, A. tanneri, A. udagawae, A. terreus |
| (b) CGD-p22phox deficiency | Mutation in CYBA: electron transport protein (p22phox) | AR | N+M | Killing (defective superoxide production) | High | Other mold infections (occasionally), invasive candidiasis (rarely) |
|||
| (c) CGD-p47phox deficiency | Mutation in NCF1: adapter protein (p47phox) | AR | N+M | Killing (defective superoxide production) | High | Other mold infections (occasionally), Invasive candidiasis (rarely) |
|||
| (d) CGD-p67phox deficiency | Mutation in NCF2: activating protein (p67phox) | AR | N+M | Killing (defective superoxide production) | High | Other mold infections (occasionally) Invasive candidiasis (rarely) |
|||
| (e) CGD-p40phox deficiency | Mutation in NCF4: activating protein (p40phox) | AR | N+M | Killing (defective superoxide production) | High | Other mold infections (occasionally), invasive candidiasis (rarely) |
|||
| Defects of neutrophil numbers (congenital neutropenias) | (a) SCN1 (elastase deficiency) | Mutation in ELANE: misfolded protein response, increased apoptosis | AD | N | Myeloid differentiation | Rarely | Invasive candidiasis (rarely) | Aspergillus sp. | |
| (b) SCN3 (Kostmann disease) | Mutation in HAX1: control of apoptosis | AR | N | Myeloid differentiation | Rarely | Invasive candidiasis (rarely) | Aspergillus sp. | ||
| Defects of phagocyte motility | Leukocyte adhesion deficiencies type 1 (LAD1) | Mutation in ITGB2: B chain for adhesion proteins CD18 | AR | N+M+L+NK | Adherence, chemotaxis, endocytosis, T/NK cytotoxicity | Rarely | Invasive candidiasis (rarely) | Aspergillus sp. | |
| Defects of phagocyte number | GATA2 deficiency (Mono MAC syndrome) | Mutation in GATA2: loss of stem cells | AD | N+monocytes+peripheral DC+NK+B | Multilineage cytopenias | ~20% | Histoplasmosis, cryptococcal meningitis |
Aspergillus sp., A. udagawae |
|
| Combined immunodeficiencies with associated or syndromic features | AD-HIES (Job syndrome) | Dominant-negative heterozygous mutations in signal transducer and activatorof transcription STAT3 | AD, often de novo (spontaneous) defect | T cells, B cells |
Structural lung disease as a result of prior pulmonary bacterial infections | High in patients with lung cavities | Histoplasmosis, cryptococcosis (rarely), mucosal candidiasis (~80%) |
Aspergillus sp., A. psudoviridinutans |
|
| Defects in intrinsic and innate immunity | CARD9 deficiency | Mutations of CARD9 | AR | N | Impaired neutrophil recruitment | Occasionally | Invasive candidiasis, specifically involving the central nervous system (high), deep dermatophytosis (high), phaeohyphomycosis | A. fumigatus | |
Abbreviations: AD, autosomal dominant inheritance; AR, autosomal recessive inheritance; B, B-lymphocytes; CGD, chronic granulomatous disease; CYBA, cytochrome b alpha subunit; CYBB, cytochrome b beta subunit; DC, dendritic cells; ELANE, elastase neutrophil-expressed; GATA2, GATA binding protein 2; HAX1, HLCS1-associated protein X1; HIES, hyper-IgE syndromes; ITGB2, integrin beta-2; L, lymphocytes; LAD1, leukocyte adhesion deficiencies type 1; M, monocytes–macrophages; Mono MAC syndrome, syndrome of monocytopenia, B cell, and NK cell lymphopenias; N, neutrophils; NCF1, neutrophil cytosolic factor 1; NCF2, neutrophil cytosolic factor 2; NCF4, neutrophil cytosolic factor 4; NK, natural killer cells; SCN, severe congenital neutropenias; T, T-lymphocytes; XL, X-linked inheritance.
CGD
Ten Aspergillus species including Aspergillus fumigatus, A. nidulans, A. calidoustus, A. flavus, A. niger, A. terreus, A. udagawae, A. pseudoviridinutans, A. quadrilineatus, and A. tanneri have been documented from IA cases in CGD patients [3]. A. fumigatus is the primary etiologic agent (estimated ~55%), followed by A. nidulans (~35%), a species that is almost exclusively associated with IA in CGD but not encountered in patients with iatrogenic immunosuppression [5]. The reasons for the unique association of A. nidulans with CGD patients are not completely understood. It has been suggested that A. nidulans is less virulent than A. fumigatus due to the lower content of N-acetyl-galactosamine (GalNAc) in the galactosaminogalactan (GAG), an exopolysaccharide bound to the cell wall. GalNAc mediates adherence and is required for full virulence. The lower content of GalNAc in GAG was due to the low expression of the Uge3 gene, which encodes glucose epimerase [19]. This may partly explain why A. nidulans rarely infects patients without CGD. However, the lower virulence level of A. nidulans does not explain the reason for the unique association with CGD and not with other PIDs.
In CGD patients, aspergillosis is almost always subacute to chronic, regardless of the etiologic agent involved, and does not spread hematogenously because of the lack of angio-invasion. Unlike in patients with acquired immunosuppression, dissemination of Aspergillus to the lung, to the chest wall, and into the ribs or vertebrae is common [5].
AD and AR SCN Syndromes
IA caused by unspeciated Aspergillus has been reported in SCN patients harboring heterozygous mutations in the ELA2 gene. Although profound neutropenia is the highest risk factor for aspergillosis in patients with acquired immunosuppression, fungal infection in general and aspergillosis in particular has rarely been documented for SCN patients [20], indicating that mononuclear phagocytes may compensate for the lack of neutrophils in these patients.
AR Type I LAD (CD18 Deficiency)
Though rare, IA due to unspeciated Aspergillus has been reported in patients with LAD type I. Aspergillosis has also been associated with LAD type II (genetic defect undetermined) [21], in which adherence and migration of granulocytes are diminished due to a carbohydrate fucosylation defect.
AD GATA2 Deficiency (MonoMAC Syndrome)
The incidence of IA in this population has been estimated to be ~17%, but the species distribution of the etiologic agents remains unknown [22].
AD Hyper-IgE Syndrome (AD-HIES)
In a recent French national survey of AD-HIES patients, 13 of 60 patients in the cohort (22%) had invasive aspergillosis and/or colonization [23]. Similarly, 25% of AD-HIES patients from the National Institutes of Health (NIH) cohort [24], particularly those with underlying structural lung disease, developed IA. The Aspergillus species causing infection in these cohorts of patients were not specified [24]. Recently, A. fumisynnematus was identified in a patient with underlying STAT3 haploinsufficiency caused by a novel STAT3 splice site mutation. The patient had elevated IgE, allergic rhinitis, eosinophilic esophagitis, and fatal IA due to this species [13].
CARD9 Deficiency
In 2016, Rieber et al. [25] reported extrapulmonary A. fumigatus infection in 2 unrelated patients age 8 and 18 years with CARD9 deficiency. The younger patient died at age 12 with progressive intra-abdominal aspergillosis, whereas the second developed central nervous system IA at age 18 and intra-abdominal aspergillosis at age 25. Neither patient presented with pulmonary infection, and their neutrophils failed to migrate to the extrapulmonary site of infection. The mutations in these patients were identified as homozygous M1I CARD9 and homozygous Q295X CARD9, respectively. Lack of pulmonary infection by A. fumigatus in these patients with PID is noteworthy as the primary disease caused by A. fumigatus in patients with secondary immunodeficiency is invasive pulmonary infection [2].
Cystic Fibrosis
Although cystic fibrosis is not in the general category of PID, it is worth mentioning that cystic fibrosis patients are predisposed to allergic bronchopulmonary aspergillosis and their respiratory secretions often contain various common Aspergillus species. Cystic fibrosis is a genetic disorder caused by mutations in the gene encoding the transmembrane conductance regulator protein [26].
MYCOLOGY
Poorly Recognized Aspergillus Species Documented From Cases of IA in Patients With PIDs
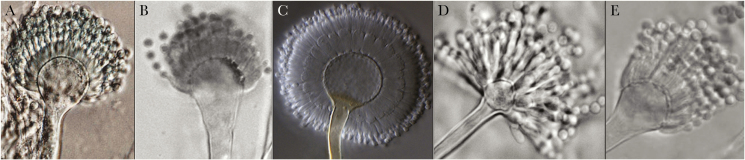
The exact Aspergillus epidemiology in patients with PIDs is not known because of infrequent culture isolation compounded by the lack of proper species identification. In many cases, the etiologic agents are reported simply as “Aspergillus.” However, it is clear that A. fumigatus and A. nidulans are the most frequently reported Aspergillus species in patients with PIDs [3]. These 2 species and their pathogenicity have been exhaustively reviewed. Aspergillus flavus, A. terreus, and A. niger are also well-characterized species that cause IA both in patients with PIDs and in those with iatrogenic immunosuppression [27]. The present discussion focuses largely on emerging, poorly recognized, non-fumigatus Aspergillus reported from patients with PIDs. In Figure 1, these species are marked with red arrows in the sections Fumigati, Nidulantes, Usti, Circumdati, and Tanneri, and Figure 2 shows conidial structures of the 5 least studied species. The well-known species are marked with black arrows. Table 2 summarizes the major characteristics and the outcome of IA caused by these species.
Figure 2.
Micromorphology of emerging rare Aspergillus species associated with primary immunodeficiencies. A, A. fumisynnematus (adopted from Samson et al. with permission). B, A. pseudoviridinutans (adopted from Sugui et al. with permission). C, A. subramanianii (adopted from Visagie et al. with permission). D, A. tanneri (adopted from Sugui et al. with permission). E, A. udagawae (adopted from Vinh et al. with permission).
Table 2.
Characteristics and Outcome of Primary Immunodeficiency Patients With Emerging Rare Aspergillus Invasive Aspergillosis
| Case | Causative Agent | Underlying Disease | Sex | Age | Clinical Signs During Course of Illness | Signs of Disease in Autopsy | Antifungal Used | Surgery | Duration of Course of Illness | Outcome (Alive/Died) |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. fumisynnematus | STAT3 haploinsufficiency | M | 37 | Sinuses, orbital apex, cavernous sinus, temporal lobe | - | AmB, VRC, POS, MFG, TRB, | Sinus surgeries, orbital and cavernous sinus tissue debridements | 1 y | Died | [13] |
| 2 | A. pseudoviridinutans | CGD (p47phox) | M | 14 | Liver abscesses, pneumonia, right middle lobe nodule, mediastinal lymphadenopathy mediastinal with necrosis |
- | ITC, VRC, VRC+CAS, POS, POS+CAS | - | 6 y | Alive | [6] |
| 3 | A. pseudoviridinutans | AD-HIES | M | 8 | Bilateral pulmonary lesions, left pleural mass and effusion, and a right upper lung cavity with nodule, Guillain-Barré syndrome, progressive respiratory failure with aspiration of abscess cavity content |
Extensive fungal abscesses in the lungs and left pleural space that extended into the airways, diaphragm, and spleen | VRC, AmB, POS+CAS+5FC, granulocyte infusions, adjunctive granulocyte colony stimulating factor, interferon-γ | Debridements of lung, pleural, diaphragmatic, and ubpulmonic abscesses, left lower lobe segmentectomy | <1 y | Died | [6] |
| 4 | A. tanneri | CGD (gp91phox) | M | 17 | Osteomyelitis, recurrent pneumonias, with pleural effusion, mediastinal abscess, and extension to liver and spleen, massive pulmonary infection |
Innumerable abscesses in lung, liver, mediastinum, and abdominal cavity specimens, with multiple adhesions, intense inflammatory infiltrates, and necrosis |
Gamma interferon, AmB, ITC, VRC, CAS, granulocyte transfusions |
Appendectomy, excision of liver abscess and splenectomy | 1 y | Died | [7] |
| 5 | A. tanneri | CGD (gp91phox) | M | 19 | Mediastinal adenopathy, pneumonia of the right upper lobe, cervical adenopathy, hepatosplenic abscesses |
Bilateral fungal pneumonia with cavities, ocular vein involvement, hepatomegaly, periaortic and mesenteric lymphadenopathy |
VRC, VPOS, AmB, MFG, L-AmB, TBF |
Dilation of a ureter and stent placement | 2 y | Died | [7] |
| 6 | A. subramanianii | Work-up ongoing | M | 49 | Thoracic spine, mediastinum | - | VRC, POS | Several thoracic spine laminectomies | 10 y | Alive | Present study |
| 7 | A. udagawae | CGD (gp91phox) | M | 27 | Cough, hemoptysis, left lower lobe nodule, pneumonia | - | AmB, VRC, CAS | Partial lobectomy, thoracoplasty, vertebral resection with rod lacement | <1 y | Alive | [8] |
| 8 | A. udagawae | CGD (p47phox) | F | 36 | Cough, dyspnea, lingular consolidation, scattered nodules, persistent mediastinal lymphadenopathy | - | ITC, VRC, POS | - | <1 y | Alive | [8] |
| 9 | A. udagawae | CGD (gp91phox) | M | 29 | Diffuse pulmonary interstitial thickening, infiltrates in the left pulmonary apex and right lower lobe, apical consolidation involving the pleura, lingula, fissure and left lower lobe, thyroid lesions, neurological deficits, and bilateral cerebral hypodensities |
Septated bulbous hyphae were identified diffusely in the lungs with multiple pleural adhesions, in the left ventricular and septal myocardium with fibrinous pericardium along the tricuspid valve, in both thyroid lobes, and in the brain |
L-AmB, POS, VRC+CAS, AmB, TRB, 5-FC |
Mediastinal and hilar lymphadenopathy |
14 y | Died | [8] |
Abbreviations: 5-FC: flucytosine; AFG, anidulafungin; AmB, amphotericin B; CAS, caspofungin; CGD, chronic granulomatous disease; ITC, itraconazole; L-AmB, liposomal amphotericin B; POS, posaconazole; TRB: terbinafine; VRC, voriconazole.
Aspergillus calidoustus
Aspergillus calidoustus belongs in the Aspergillus section Usti and is a relatively widespread species isolated from the indoor environment [28], although it is rarely reported from clinical cases. The species can be differentiated from other related species such as A. ustus or A. granulosus by its growth at ≥37ºC and from A. pseudodeflectus by its low susceptibility to antifungal drugs [29].
A. calidoustus caused cerebral aspergillosis in an X-linked 17-year-old CGD patient who also underwent allogeneic hematopoietic stem cell transplantation (HSCT) [11]. The patient was treated with liposomal amphotericin B, but the brain abscess recurred; the patient died from a brain hemorrhage after a second operation. His underlying CGD combined with acquired immunodeficiency due to HSCT contributed to the susceptibility to this rare pathogen. Interestingly, A. calidoustus has been increasingly isolated from transplant populations, especially those who have had lung transplantation [28].
Aspergilus fumisynnematus
Aspergillus fumisynnematus, a member of section Fumigati, was first isolated from soil with the maximum growth temperature of 45ºC [30]. The species is morphologically similar to other members of the section Fumigati, and identification requires a molecular method. However, synnemata production in aged culture on malt extract (ME) agar medium can be its unique feature within the section Fumigati.
Isolation of A. fumisynnematus from the bronchoalveolar lavage of a patient was tested for its susceptibility to antifungal agents, but patient information was lacking [31]. Recently, A. fumisynnematus was recovered from 2 PID patients with aspergillosis. In 1 case, a 37-year-old man with STAT3 haploinsufficiency caused by a STAT3 splice site mutation had progressive infection of sinuses, orbital apex, and cavernous sinus [13]. The patient was treated with a combination of voriconazole, amphotericin B, posaconazole, micafungin, and terbinafine, in addition to surgical removal of the infected portions of the sinuses. However, the patient died 1 year after surgery due to uncontrollable brain invasion by the species [13]. The second case, a 4-year-old X-linked CGD patient hospitalized at the NIH, suffered from A. fumisynnematus infection in his spine and pelvis, with a probable primary lung lesion and possible dissemination to the brain. Radiographic evidence of scarring in his lung was suggestive of initial pulmonary infection. Amphotericin B therapy failed to arrest the disease, but aggressive therapy with voriconazole and granulocyte transfusions stabilized the brain lesion. A. fumisynnematus is susceptible to triazoles but is significantly more resistant to amphotericin B than A. fumigatus (Table 3).
Table 3.
Antifungal Susceptibility Profile of Non-fumigatus Aspergillus Species Causing Invasive Aspergillosis in Patients With Primary Immunodeficiencies, Using CLSI Broth Microdilution Method
| Aspergillus spp. | MIC/MEC, μg/mL | |||
|---|---|---|---|---|
| AmB | VRC | ITC | POS | |
| A. calidoustus | 2 | 8 to 16 | 4 to 16 | 8 to16 |
| A. fumigatus | 0.5 | 0.5 | 0.5 | 0.125 |
| A. fumisynnematus | 4 | 0.25 | 0.5 | 0.25 |
| A. nidulans | 2 | 0.25 | 0.5 | 0.25 |
| A. pseudoviridinutans | 2 to 8 | 1 to 8 | 1 to 16 | <0.016 to 0.5 |
| A. quadrilineatus | 0.5 | 0.5 | 0.125 | 0.25 |
| A. subramanianii | 2 | 0.5 | 0.25 | 0.25 |
| A. tanneri | >16 | 4 | 4 | 0.5 |
| A. udagawae | 0.5 to 2 | 1 to 4 | 1 to 4 | 0.25 to 0.5 |
Abbreviations: AFG, anidulafungin; AmB, amphotericin B; CAS, caspofungin; MIC, minimum inhibitory concentration; MEC, minimum effective concentration; ITC, itraconazole; POS, posaconazole; VRC, voriconazole.
Aspergillus pseudoviridinutans
Aspergillus viridinutans belongs to Aspergillus section Fumigati, first isolated from rabbit dung in Australia and subsequently documented in human clinical isolates around the world [32]. Recently, significant genetic diversity was found among strains of A. viridinutans, and some clinical strains have been reclassified to 4 closely related new cryptic species, A. pseudoviridinutans, A. felis, A. pseudofelis, and A. parafelis [33]. Among these 4 clinically relevant species, only A. pseudoviridinutans has been reported from patients with PID; 1 from a CGD patient and the other from a patient with AD-HIES syndrome [6]. Both cases were reported in 2009 using the old name of A. viridinutans [6]. The clinical manifestation of A. pseudoviridinutans infection was similar to that of A. udagawae and distinct from A. fumigatus. The infection was chronic and disseminated in a contiguous spread and appeared to be refractile to antifungal therapy. A. pseudoviridinutans grows at 37ºC, with an intermediate growth rate between A. fumigatus (fast) and A. viridinutans (slow). The A. psuedoviridinutans colonies attain a diameter of 5.5 to 6.5 cm on ME agar, as well as on Czapek’s solution agar, in 5 days. Colonies on ME agar are white with dense aerial hyphae and scant conidiation with yellow colony reverse. The A. pseudoviridinutans growth patterns at 42ºC and 37ºC are similar, and it fails to grow at 50ºC [15]. The species has decreased susceptibility in vitro to both amphotericin B and azoles compared with A. fumigatus (Table 3) [6].
Aspergillus quadrilineatus
Aspergillus quadrilineatus (syn. = Emericella quadrilineata) is a soil fungus commonly isolated in tropical countries [34]. It belongs to the Aspergillus section Nidulantes and is a sibling species of A. nidulans. A. quadrilineatus grows faster on both Czapek’s and ME agar media at 30°C and 37°C than at 25°C. Conidial structures of A. nidulans and A. quadrilineatus can not be distinguished, but the length of conidiophores tends to be slightly shorter in A. quadrilineatus. The 2 species can be differentiated with certainty by their number of ascospore crests depicted in scanning electron microscopy: 4 crests in A. quadriliniatus vs 2 crests in A. nidulans ascospores. The 2 species can also be readily distinguished by DNA sequencing (Figure 1) [12]. Despite the close morphologic and genetic relatedness, the 2 species differ significantly in their in vitro susceptibility to amphotericin B and caspofungin (Table 3). However, the triazoles are active in vitro against both species [12].
Although A. nidulans causes IA primarily in CGD patients, infection due to A. quadrilineatus has been documented in both CGD and cancer patients [12, 34]. In fact, IA caused by A. quadrilineatus in 2 of 3 CGD patients was originally reported as A. nidulans based only on the conidial structures [12].
Aspergillus subramanianii
Aspergillus subramanianii belongs to the Aspergillus section Circumdati and produces yellow to ochre colonies owing to the yellow-colored conidia and sclerotia that do not turn black. The section Circumdati members can be distinguished from each other based on morphological characteristics, along with DNA sequences and extrolite profiles [35]. Unlike some species in the section Circumdati, A. subramanianii produces ochratoxin A inconsistently and/or only in trace amounts. It has been isolated from house dust samples in Mexico, air from Panama, and shelled Brazil nuts in Canada [35].
Aspergillus subramanianii produces faster-growing colonies on Czapek’s yeast autolysate agar at 37°C (4.5–5 cm in diameter after 7 days) compared with its phylogenetically close species in the section Circumdati. On ME agar, the colony surface is floccose to somewhat velutinous, and mycelial areas are white with light yellow sporulated spots and light brown colony reverse [35].
IA caused by A. subramanianii was diagnosed in a 49-year-old man without CGD or CARD9 deficiency but with a PID yet to be precisely identified (NIH–Fungal Pathogenesis Lab, unpublished data, 2018). The patient presented with chronic disseminated infection of the thoracic spine and adjacent mediastinal tissue. Several thoracic spinal laminectomies, followed by therapy with voriconazole and posaconazole, gradually resolved the infection over a 10-year period (Eric Meissner, MD, unpublished data, 2018; initial identification of A. subramanianii by Nathan Wiederhold, Pharm D, 2018).
Aspergillus tanneri
Aspergillus tanneri was described in 2012 [7], based on 2 strains isolated from fatal cases of IA in X-linked CGD patients treated at the NIH. Both patients came from the southwestern part of the United States, California and New Mexico, but the specific environmental source of the species remains unknown.
Aspergillus tanneri was originally classified as a non-ochratoxin-producing member of the Aspergillus section Circumdati [7]. Subsequently, its unique attributes have led to creation of a new Aspergillus section Tanneri to accommodate the species [36]. A. tanneri is a slow-growing species at 37°C and requires 2 weeks to attain a colony size of 3 cm and 4–5 cm on Czapek’s agar and ME agar, respectively. Growth is slightly faster at 30°C, and colonies are white at the beginning but grow yellow due to the maturation of conidia with age. A. tanneri has significantly lower in vitro susceptibility to most antifungals than other pathogenic Aspergillus species (Table 3).
Unlike A. fumigatus, A. tanneri causes chronic disease with primary pulmonary foci but has disseminated to almost every organ of the body [7]. Both patients with A. tanneri died despite intensive antifungal therapy supplemented by gamma interferon and granulocyte transfusions.
Aspergillus udagawae
Aspergillus udagawae (teleomorph = Neosartorya udagawae), a member of Aspergillus section Fumigati, was first isolated from the soil of a sugar cane plantation in Brazil [37]. Like A. fumigatus, A. udagawae is heterothallic, but A. udagawae produces the teleomorph much more readily on conventional mycological media, whereas strains of A. fumigatus require particular growth conditions to complete the sexual life cycle. A. udagawae fails to grow at a temperature above 42°C, whereas A. fumigatus grows at ≥45°C [8]. The 2 species also differ in their minimal and optimal growth temperatures. A. udagawae grows slowly at 10°C and optimally at 30–35°C, but A. fumigatus fails to grow at 10°C and grows optimally at 37°C [38]. Because A. udagawae is more resistant than A. fumigatus to various antifungals, the A. udagawae infections documented are primarily refractory to standard antifungal therapy [38].
Cases of aspergillosis due to Aspergillus udagawae have been reported sporadically in CGD patients [8], in a patient with myelodysplastic syndrome [8], and in a patient with diabetes mellitus [39]. The 3 cases of IA caused by A. udagawae reported in CGD patients were all chronic disease and disseminated in a contiguous manner [38]. The median duration of infection was approximately 7 times longer (35 weeks vs 5.5 weeks) than that typically observed for IA caused by A. fumigatus in CGD.
Species Identification of Non-Fumigatus Aspergillus Species Primarily Associated With PIDs
Species identification of those poorly recognized Aspergillus isolated from patients with PID can be challenging. Because of their decreased susceptibility to widely used antifungal agents compared with A. fumigatus, identification at the species level is critical for proper patient management. Identification schemes include culture followed by polymerase chain reaction (PCR) sequencing of representative genes. Although ITS and partial ß-tubulin have been most commonly amplified and sequenced genes for species identification, the value of ITS sequencing is minimal for the speciation of emerging non-fumigatus Aspergillus species. The ß-tubulin gene, by using the primers currently reported, may amplify the benA or the tubC alleles, and hence could yield different results between tests [40]. For a diagnostic laboratory to complete its mission in a timely and accurate manner, we view caM as the best gene choice for PCR sequencing. It is sufficiently variable to detect several haplotypes in a species, is relatively easy to amplify, and produces a clean, easy to read sequence. In addition, the caM sequence of nearly every Aspergillus species is now accessible, thus making this locus very effective in assessment of species identity.
Innate Resistance of Pathogenic Aspergillus Species to Antifungal Agents
Table 3 summarizes the antifungal susceptibility profile of the rare Aspergillus species recovered from PID patients with IA. The antifungal triazoles are preferred agents for the treatment and prevention of IA in immunocompromised patients [14]. However, with wide application of azoles in clinical practice and agriculture, the emergence of triazole resistance in A. fumigatus due to mutations in the Cyp51A gene is a global public health concern [17].
Recent changes in the taxonomy of Aspergillus have also had major implications for our understanding of drug susceptibility profiles. For instance, the new cryptic species of Aspergillus section Fumigati exhibits significantly different drug susceptibilities than A. fumigatus in vitro, except for those that have acquired resistance against azoles [17]. A. calidoustus has decreased susceptibility to all triazoles, and Aspergillus tanneri is a species notorious for its decreased susceptibility to amphotericin B and triazoles (Table 3). Using the CLSI broth microdilution method, we also tested the antifungal susceptibility of A. subramanianii and A. fumisynnematus isolates recovered from PID patients. Both isolates showed elevated minimum inhibitory concentrations for amphotericin B but were susceptible to azole drugs.
CONCLUDING REMARKS
The present work summarizes the clinical, diagnostic, mycological, and treatment features of emerging Aspergillus species, which are almost exclusively reported from cases of IA in patients with various congenital immune defects. The species discussed here have not been well recognized, partly due to their rarity in clinical settings compounded by taxonomic changes of the species, but more importantly, due to the lack of species identification in diagnosis. In many reviews or case reports of aspergillosis in patients with PIDs, the etiologic agent is often listed as “Aspergillus.” Because these species cause subacute to chronic infection in patients with PID and are generally more difficult to treat due to their lower susceptibility to available antifungal agents than A. fumigatus, correct and prompt identification at the species level is critical for appropriate therapy to improve patient outcomes. Notably, DNA sequence-based species identification is more accurate than using morphological characteristics to differentiate the emerging Aspergillus species. It is critical that physicians who care for PID patients with aspergillosis are aware of these emerging non-fumigatus Aspergillus species and their unique features. It is also critical to develop new drugs and/or treatment strategies that will be effective against these species.
Acknowledgments
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Potential conflicts of interest. The authors declare no conflicts of interest related to this publication. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus–what makes the species a ubiquitous human fungal pathogen?PLoS Pathog 2013; 9:e1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marr KA, Carter RA, Boeckh M, et al. . Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358–66. [DOI] [PubMed] [Google Scholar]
- 3. Beauté J, Obenga G, Le Mignot L, et al. ; French PID Study Group CEREDIH Epidemiology and outcome of invasive fungal diseases in patients with chronic granulomatous disease: a multicenter study in France. Pediatr Infect Dis J 2011; 30:57–62. [DOI] [PubMed] [Google Scholar]
- 4. Segal BH, Holland SM. Primary phagocytic disorders of childhood. Pediatr Clin North Am 2000; 47:1311–38. [DOI] [PubMed] [Google Scholar]
- 5. Henriet S, Verweij PE, Holland SM, Warris A. Invasive fungal infections in patients with chronic granulomatous disease. Adv Exp Med Biol 2013; 764:27–55. [DOI] [PubMed] [Google Scholar]
- 6. Vinh DC, Shea YR, Jones PA, et al. . Chronic invasive aspergillosis caused by Aspergillus viridinutans. Emerg Infect Dis 2009; 15:1292–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sugui JA, Peterson SW, Clark LP, et al. . Aspergillus tanneri sp. nov., a new pathogen that causes invasive disease refractory to antifungal therapy. J Clin Microbiol 2012; 50:3309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinh DC, Shea YR, Sugui JA, et al. . Invasive aspergillosis due to Neosartorya udagawae. Clin Infect Dis 2009; 49:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikincioğullari A, Dogu F, Solaz N, et al. . Granulocyte transfusions in children with chronic granulomatous disease and invasive aspergillosis. Ther Apher Dial 2005; 9:137–41. [DOI] [PubMed] [Google Scholar]
- 10. Kaltenis P, Mudeniené V, Maknavicius S, Seinin D. Renal amyloidosis in a child with chronic granulomatous disease and invasive aspergillosis. Pediatr Nephrol 2008; 23:831–4. [DOI] [PubMed] [Google Scholar]
- 11. Hubka V, Kubatova A, Mallatova N, et al. . Rare and new etiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med Mycol 2012; 50:601–10. [DOI] [PubMed] [Google Scholar]
- 12. Verweij PE, Varga J, Houbraken J, et al. . Emericella quadrilineata as cause of invasive aspergillosis. Emerg Infect Dis 2008; 14:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Natarajan M, Hsu AP, Weinreich MA, et al. . Aspergillosis, eosinophilic esophagitis, and allergic rhinitis in STAT3 haploinsufficiency. J Allergy Clin Immunol. 2018; 142:993–997.e3. doi: [DOI] [PubMed] [Google Scholar]
- 14. Patterson TF, Thompson GR 3rd, Denning DW, et al. . Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugui JA, Kwon-Chung KJ, Juvvadi PR, et al. . Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med 2014; 5:a019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Denning DW. Invasive aspergillosis. Clin Infect Dis 1998; 26(4): 781–803; quiz 4–5. [DOI] [PubMed] [Google Scholar]
- 17. Seyedmousavi S, Verweij PE. Azole resistance in Aspergillus fumigatus: mechanisms, route of resistance selection, and clinical implications. In: Berghuis A, Matlashewski G, Wainberg MA, Sheppard D, eds. Handbook of Antimicrobial Resistance. New York: Springer New York; 2017: 403–21. [Google Scholar]
- 18. Modell V, Knaus M, Modell F, et al. . Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunol Res 2014; 60:132–44. [DOI] [PubMed] [Google Scholar]
- 19. Lee MJ, Liu H, Barker BM, et al. . The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog 2015; 11:e1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klein C, Grudzien M, Appaswamy G, et al. . HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet 2007; 39:86–92. [DOI] [PubMed] [Google Scholar]
- 21. Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med 2000; 343:1703–14. [DOI] [PubMed] [Google Scholar]
- 22. Lionakis MS. Genetic susceptibility to fungal infections in humans. Curr Fungal Infect Rep 2012; 6:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chandesris MO, Melki I, Natividad A, et al. . Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) 2012; 91:e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vinh DC, Sugui JA, Hsu AP, et al. . Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol 2010; 125:1389–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rieber N, Gazendam RP, Freeman AF, et al. . Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight 2016; 1:e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stevens DA, Moss RB, Kurup VP, et al. . Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37:S225–64. [DOI] [PubMed] [Google Scholar]
- 27. Blumental S, Mouy R, Mahlaoui N, et al. . Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis 2011; 53:e159–69. [DOI] [PubMed] [Google Scholar]
- 28. Egli A, Fuller J, Humar A, et al. . Emergence of Aspergillus calidoustus infection in the era of posttransplantation azole prophylaxis. Transplantation 2012; 94:403–10. [DOI] [PubMed] [Google Scholar]
- 29. Öz Y, Özdemir HG, Gökbolat E, et al. . Time-kill kinetics and in vitro antifungal susceptibility of non-fumigatus Aspergillus species isolated from patients with ocular mycoses. Mycopathologia 2016; 181:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horie Y, Miyaji M, Nishimura K, Taguchi H, Udagawa S. Aspergillus fumisynnematus, a new species from Venezuelan soil. A Transact Mycol Soc Japan 1993; 34(1):3–7. [Google Scholar]
- 31. Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, et al. . Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob Agents Chemother 2008; 52:1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coelho D, Silva S, Vale-Silva L, et al. . Aspergillus viridinutans: an agent of adult chronic invasive aspergillosis. Med Mycol 2011; 49:755–9. [DOI] [PubMed] [Google Scholar]
- 33. Sugui JA, Peterson SW, Figat A, et al. . Genetic relatedness versus biological compatibility between Aspergillus fumigatus and related species. J Clin Microbiol 2014; 52:3707–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Polacheck I, Nagler A, Okon E, et al. . Aspergillus quadrilineatus, a new causative agent of fungal sinusitis. J Clin Microbiol 1992; 30:3290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Visagie CM, Varga J, Houbraken J, et al. . Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud Mycol 2014; 78:1–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jurjevic Z, Kubatova A, Kolarık M, Hubka V. Taxonomy of Aspergillus section Petersonii sect. nov. encompassing indoor and soil-borne species with predominant tropical distribution. Plant Syst Evol 2015; (301):2441–62. [Google Scholar]
- 37. Horie Y, Miyaji M, Nishimura K, Franco MF, Coelho KI. Two new species of Neosartorya from Brazilian soil. Mycoscience 1995; 36:159–65. [Google Scholar]
- 38. Sugui JA, Vinh DC, Nardone G, et al. . Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus?J Clin Microbiol 2010; 48:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gyotoku H, Izumikawa K, Ikeda H, et al. . A case of bronchial aspergillosis caused by Aspergillus udagawae and its mycological features. Med Mycol 2012; 50:631–6. [DOI] [PubMed] [Google Scholar]
- 40. Hubka V, Kolarik M. β-tubulin paralogue tubC is frequently misidentified as the benA gene in Aspergillus section Nigri taxonomy: primer specificity testing and taxonomic consequences. Persoonia 2012; 29:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]