Abstract
Plant and animal life forms evolved mechanisms for sensing and responding to gravity on Earth where homeostatic needs require responses. The lack of gravity, such as in the International Space Station (ISS), causes acute, intra-generational changes in the quality of life. These include maintaining calcium levels in bone, maintaining muscle tone, and disturbances in the vestibular apparatus in the ears. These problems decrease work efficiency and quality of life of humans not only during microgravity exposures but also after return to higher gravity on Earth or destinations such as Mars or the Moon. It has been hypothesized that lack of gravity during mammalian development may cause prenatal, postnatal and transgenerational effects that conflict with the environment, especially if the developing organism and its progeny are returned, or introduced de novo, into the varied gravity environments mentioned above. Although chicken and frog pregastrulation development, and plant root development, have profound effects due to orientation of cues by gravity-sensing mechanisms and responses, mammalian development is not typically characterized as gravity-sensing. Although no effects of microgravity simulation (MGS) on mouse fertilization were observed in two reports, negative effects of MGS on early mammalian development after fertilization and before gastrulation are presented in four reports that vary with the modality of MGS. This review will analyze the positive and negative mammalian early developmental outcomes, and enzymatic and epigenetic mechanisms known to mediate developmental responses to simulated microgravity on Earth and microgravity during spaceflight experiments. We will update experimental techniques that have already been developed or need to be developed for zero gravity molecular, cellular, and developmental biology experiments.
Keywords: : microgravity, protein kinase, embryogenesis
Introduction: Gravity Has Instructive Roles in Early Development, But Microgravity May Be Stressful
Mammalian gametes and embryos—between fertilization in the oviduct and implantation into the uterus—are “free-living” in an avascular low-oxygen state for ∼4.5 days [1] (Fig. 1). They can also be removed from the lumen of the oviduct or uterus and cultured, such as during typical in vitro fertilization (IVF) procedures, and then cryopreserved or transferred back into the uterine lumen. After implantation into the endometrium, the embryos establish the placenta and invade into the uterus to establish paracrine and endocrine interactions with maternal organs; developmental events that are aimed at obtaining nutrition for exponential placental and embryonic growth [2].
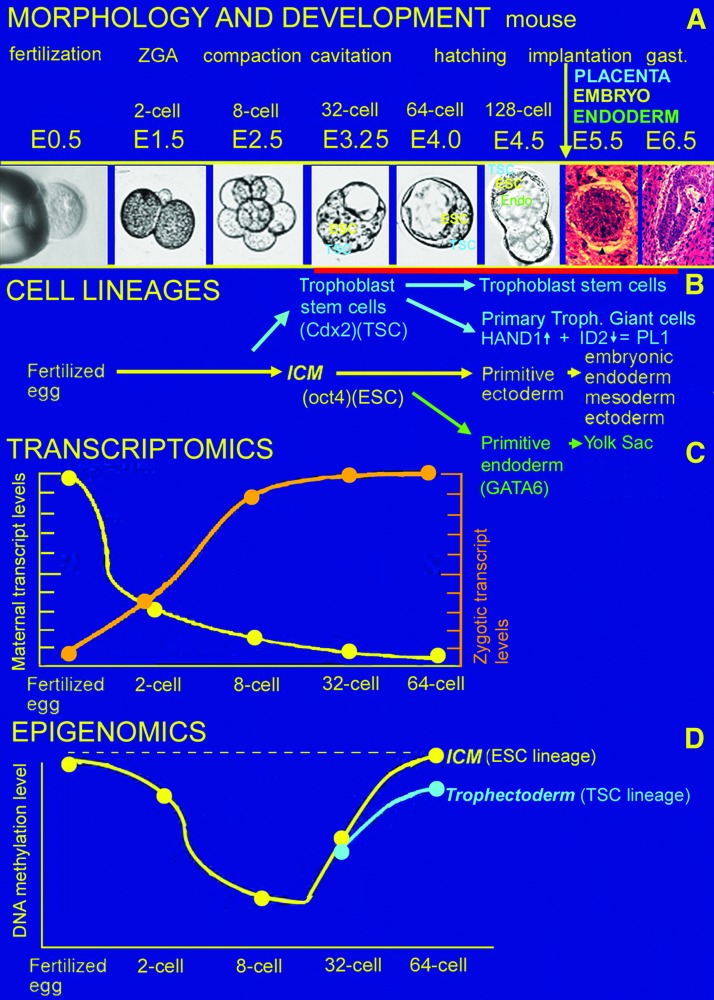
FIG. 1.
Postfertilization, preimplantation mouse development encompasses changes in (A) MORPHOLOGY, allocation of embryonic and placental stem (B) ESC (yellow, marked by Oct4 transcription) and TSC (blue, requiring Cdx2 TF) CELL LINEAGES arise from totipotent cells from the 8-cell to 32-cell stage and then yolk sac extraembryonic endoderm (green, requiring GATA6 TF) arises from the 32–64 cell stage blastocyst, changes in (C) TRANSCRIPTOMICS at about the 2-cell stage ∼80% of maternal transcripts are destroyed (yellow line) and during zygotic genome activation ∼4,000 new transcripts are expressed at the 2-cell stage and another ∼5,000 new transcripts are expressed at the 8-cell stage (orange line), and changes in (D) EPIGENOMICS with global demethylation by the 8-cell stage and remethylation by the 64-cell blastocyst stage (yellow line indicates that remethylation is higher than in the embryonic than the placental trophoblast lineage—blue line) although a small set of 199–200 parentally imprinted genes are not demethylated under nonstress conditions (broken yellow line). ESCs, embryonic stem cells; TF, transcription factor; TSC, trophoblast stem cells. Color images available online at www.liebertpub.com/scd
During the preimplantation period, mouse embryos undergo morphogenetic changes, during which an epithelium forms and is polarized at the eight-cell stage and the outer cells pump ions, glucose, and amino acids to form a blastocoele cavity by the blastocyst stage (Fig. 1). Transcriptomic reprogramming involve a large erasure or the maternal mRNA program and turn on their own zygotic mRNA program (∼9,000 mRNA types) at two-cell (ie, zygotic genome activity) and eight-cell stages [2,3] (Fig. 1); at the two-cell stage embryo in mouse, the maternal mRNA is about 80% erased and the first zygotic genome activation about 4,000 new genes are transcribed, with a further 5,000 new genes transcribed at the eight-cell stage embryo. It should be noted that bovine and human embryos have major zygotic genome activation at 4–16 cell stage [4–6] and other events described for mouse in Figure 1A occur at different times and stages in other mammalian species.
The 8-cell mouse embryo erases many of its epigenetic programs by DNA demethylation and then reprograms them by remethylaton at the 32-cell blastocyst stage [7], to make the major two types of stem cells that enable embryonic and placental development [2]. There are also ∼100–200 methylated imprinted genes that are not de- and re-methylated. In the well-characterized mouse model in unstressed embryos [8], these are the pluripotent embryonic stem cells (ESCs) that make the ∼250 or more adult cell types, and the placental trophoblast stem cells (TSCs) that make the estimated 10 differentiated lineages in the placenta that supports the embryo [2].
However, in stressed embryos during culture in poor media loss of parental imprinting through abnormal demethylation occurs [9,10]. This would potentially be a target of microgravity or microgravity simulation (MGS) stressors as would be growth of stem cells and correct induction of the new mRNA programs in two-cell stage and eight-cell stage embryos.
Physical forces could also affect the preimplantation embryo through changes in relative buoyancy of cells and blastocoelic fluid or loss of gravity-dependent convection of fluids adjacent to our inside the embryo.
Within days after implantation into the uterus, a key event in embryonic development occurs; gastrulation. During gastrulation, derivatives of the ESC lineage from the preimplantation embryo inner cell mass allocate into the three “germ layers” (stem cell classes) that make up the ectoderm (ecto = outer; such as skin and nerves), mesoderm (meso = middle; such as bone and muscle), and endoderm (endo = inner; such as the gut tube and derivatives; like the lung and liver). During gastrulation, the three body axes, head-tail, dorsal-ventral, and left-right, are established.
The role and gravity-sensing of normal gravity in instructive preparation for gastrulation in chicken and frog compared with gravity-sensing in mouse
The studies by Eyal-Giladhi and colleagues in the 1970s [11] and Gerhart and colleagues in the 1980s [12–14] showed that an incorrect 1G vector (ie, turning the embryo to an incorrect angle) before gastrulation misdirects formation of body axes in chick and frog, respectively. Gravity sensing is between tissues of differential density (Chick) or macromolecules of differential density in the oocyte (frog) during postfertilization “cortical” rotation. Effects of misdirected 1G vector or microgravity on gastrulation have apparently not yet been directly tested in mammals.
Gravity sensing in individual cells in mammals has also not yet been defined. Unlike frog, where free-living embryos arise from oocytes with maternally differentially-deposited nutritional heavy yolk, mammalian embryos rely on maternal nutrition after implantation and have little yolk. Thus, mouse embryos are not thought to sense gravity by macromolecular (yolk) distribution sensed by the cytoskeleton, as reported in frog [15]. The best-described gravity-sensing mechanism in single cells is the positive geotropic, gravity-sensing columella cells in plant root tips where dense amyloplasts are sensed by the cytoskeleton [16,17]. This mediates downward root growth by mechanisms discussed in these reports.
Open questions are whether cells in the early mammalian embryo sense microgravity (ie, the absence of gravity), what outcomes arise if they do, and how this sensing might occur? Our goal in this review is to analyze reports on the effects of MGS and microgravity on early postfertilization development in the context of developmental processes and mechanisms ongoing during this period.
Microgravity Activated Negative Outcomes in Models of Early Mammalian Development
MGS creates morbidity or slows growth of the postfertilization, preimplantation mouse embryo
Using different methods to MGS, four labs showed MGS caused morbidity or slowed embryonic development for 4 days after fertilization and before uterine implantation [18–21]. These alternate MGS modalities are reviewed elsewhere [22,23]. The major mechanism for slowed growth and morbidity caused by MGS was apparently the activation of the mitogen-activated protein kinase (MAPK8/9) subfamily known as stress-activated protein kinase (SAPK, also known as jun kinases [JNKs]) [19] which is reviewed below. In the postfertilization period, totipotent 2-cell embryos develop to the 32-cell stage blastocyst where both pluripotent embryonic and multipotent placental TSCs (ESCs and TSCs) arise (Fig. 1) [1]. Interestingly, it has been reported that microgravity on the space shuttle increases potency of ESCs and makes them more resistant to differentiation [24,25].
About 2/3rd of human embryos are lost before birth and most of the loss occurs soon after implantation [26]. Two of the reports found no negative effects on oogenesis or fertilization [20,21], Interestingly, Wakayama and colleagues using a clinostat, and Wang et al. using a rotating wall vessel (RWV) found that MGS slowed growth in the embryo, however Wakayama localized diminished growth solely to the outer epithelium of the TSC lineage [19,20]. Both groups calculated their MGS protocol created “milligravity” (ie, 10−3 g) and Wang and colleagues also calculated there was only 1% of the shear stress that was created by a previous report that used a slightly different MGS protocol in the same RWV [27]. Both shear stress, which occurred only in outer TSC as indicated by Fos protein induction [27], and MGS-stress required SAPK, which was activated in all cells [19], to cause pathogenic effects.
Perhaps the clinostat and random positioning machine [28] do create some shear and this was detected by Wakayama in addition to MGS effects, which may be more pronounced in outer cells. Thus, postfertilization is a difficult period and adding microgravity stress should affect preimplantation embryos. The Decadal survey on Biological and Physical Sciences in Space from the Space studies board at the National Academy of Sciences (https://www.nap.edu/catalog/12944/life-and-physical-sciences-research-for-a-new-era-of-space-exploration) and NASA's Space Biology SciPlan (https://www.nasa.gov/pdf/541222main_10-05-17%20FSB%20Sci%20Plan-Signed_508.pdf) have stated the high significance of early mammalian development and the maintenance of pluripotency and high epigenetic quality of stem cells in the embryo.
The Epigenetic Effects of Microgravity and Its Simulation
There are MGS-sensitive developmental events in two-cell stage embryos
This is a period of maximal DNA demethylation and maternal mRNA erasure and is the most sensitive preimplantation period for MGS effects [19]. As mentioned above, there are two waves of new transcription with ∼9,000 new mRNA types (Fig. 1). The DNA in the male and female germ cells are differentially DNA methylated at over 100 differentially methylated regions in imprinted genes [29–31]. After fertilization, DNA methylation on both the maternal and paternal chromosomes is rapidly erased by the TET-family of proteins within 2–3 cell divisions [32,33], except at the imprinted loci that maintain their germline DNA methylation profile.
Imprinted loci have DNA methylation patterns that differ between the maternal and paternal chromosomes to allow parent-of-origin differences in gene expression [34,35]. DNA methylation erasure during embryogenesis is highest at the eight-cell stage and de novo methylation is restored by the blastocyst stage. During implantation, the primordial germ cells (ie, that later form sperm and eggs) in the embryo undergo the most extensive DNA methylation erasure of the lifespan, in which even imprinted loci and transposons are demethylated [31,36,37]. Shortly after the erasure of the DNA methylation in the primordial germ cells, the DNA is remethylated such that female embryos have female imprints established and male embryos have male imprints established in the primordial germ cells.
MGS sensitivity has not yet been studied in the PGCs. Thus, both large changes in mRNA expression and DNA methylation occur, and are potentially influenced by stress, during periods of embryonic development affected by MGS [19] and should be studied on the ISS.
Where it has been studied, MGS sensitivity, but also shear stress sensitivity, is highest during the 24 h following the eight-cell stage mouse embryo [19,27]. This is the stage when the embryo creates its first outer epithelium that is essential for survival. Gene expression and methylation status are affected by stresses such as IVF culture [9,38,39] or improper O2 levels [40,41]. Interestingly, the amount of stress caused by different culture media as measured by SAPK activation [42], unpublished data] is proportional to the amount of loss of imprinting by DNA demethylation by embryos in those media [9]. It will be important to assay mRNA and epigenetic changes at the 4-cell, 16-cell, and late-blastocyst embryo stages to assay for the effects during the three periods of development affected by MGS as previously reported [19] and to assay for the effects of SAPK activity during the MGS response.
The Enzymatic Signal Transduction Effects of Microgravity and Its Simulation
The mechanism for slowed growth and morbidity for embryos from the 2-cell stage through 32-cell blastocyst stage caused by MGS was SAPK [19]. Two SAPK inhibitors rescued the negative effects of MGS by the Synthecon rotational wall vessel cell culture system (RCCS1). In this system, free fall cancels out G-forces and thereby simulates microgravity conditions. In static culture SAPK has also been reported to enable cultured embryos to respond to poor medium [42,43], benzopyrene and dioxin [44], and hyperosmotic stress [45] and to meet the needs of normal development [46]. Thus, SAPK mediates sensing and response to a wide range of stressors through a wide range of sensors at the plasmalemma (cell membrane), cytosol, and nucleus.
These studies do not offer clues into how microgravity is sensed by SAPK or by the cells in the postfertilization, preimplantation embryos. However, shear stress also causes SAPK-dependent morbidity in preimplantation embryos [27] so MGS was performed to maximally reduce shear stress that would obfuscate causes of the morbidity [19]. But the amount of shear in different MGS modalities is an important confounding variable.
SAPK also mediates stress-induced potency loss of Oct4 and Rex1 transcription factors (TFs) in ESCs and increased differentiation through Hand1 TF in TSCs [2,19,42,43,45,47–53]. Hand1 is necessary to activate the promoter of the first placental hormone (ie, Placental lactogen 1/PL1) in the first differentiated lineage arising from mouse TSCs soon after implantation (Fig. 1) [2]. Protein kinase inhibitors (PKIs) have been used to screen about 10% of the protein kinome (50/500 protein kinases in the shared mouse and human kinomes) for their roles in potency loss and differentiation increase in ESCs, TSCs from the blastocyst and also two-cell embryos, and blastocysts themselves [2,3]. These PKI screens identified SAPK and AMPK as key kinases that control stress-induced potency loss and differentiation increase in addition to controllers of the anabolic/catabolic metabolism balance and cell cycle regulators.
Although SAPK mediates shear stress, and pipeting shear stress effects in preimplantation blastocyts [27,51], it also mediates negative effects of MGS on blastocysts where there is little shear stress in the Synthecon rotating cell culture system (RCCS1) designed by NASA to emulate microgravity [19].
When microgravity is compounded by other reproductive problems in spaceflight
Microgravity and cosmic radiations pose significant health hazards to astronauts working in outer space or during spaceflights, and are of special concern for the prospective exploration of outer space. Radiation outside the Earth's magnetosphere is particularly worrisome because it includes ionizing radiation from cosmic galactic events. In a study [54] we performed using simulated solar/cosmic particles, we demonstrated that oocytes from both cows and mice are susceptible to the irradiation effects of linear energy transfer (LET) irradiants (Krypton-78 and ultraviolet-B).
Interestingly, significant differences exist in the radiation sensitivity between the two species. Murine oocytes died by necrosis following massive activation of caspases; bovine oocytes, on the other hand, activated a physiological indeterminate state of partial cell death, attributed to rapid induction of DNA repair and low activation of caspases.
Our LET studies demonstrated that mammalian germ cells are equipped to respond in an adaptive way to the deleterious effects of exposure to radiation, but that not all species have an identical capacity to adapt to these types of environmental influences. It therefore remains to be investigated how human oocytes, embryos, and stem cells will respond to those environmental challenges. In addition, we will need to consider the potential additive or synergistic effects of both microgravity and radiation. Microgravity tests on birds, sea urchins, amphibians, and fish have proven inconclusive [22]. If there was any damage, the researchers were unable to detect it.
Data from the effects of microgravity on various biological systems including embryos and adult stem cells have generated conflicting results [22]. This disparity is most probably due to the variety of methodologies used to simulate the microgravity environment. Most recently, results of experiments performed with mouse sperm previously held in the ISS demonstrated that although space radiation can damage sperm DNA, it did not affect the production of viable offspring after at least 9 months of storage on the ISS [55]. The authors speculated that most probably the oocyte was able to repair the sperm DNA damage.
The fact that microgravity did not apparently affect the sperm capabilities for fertilization is not totally unexpected. It is highly probable that these cells have the capability to respond by undergoing micro-rotations, and therefore are less subjected to shear stress under gravitational forces. We speculate that all the preimplantation embryo developmental stages nullify effects of microgravity by undergoing micro-rotations. However, upon implanting, the embryo would be most susceptible to the effects of microgravity. In the implantation stages, the embryo becomes fixed and would have limited ability to rotate; hence shear stress due to gravitational (and centrifugal) forces would be more sustained and detrimental.
Moreover, the uterine tissues at the site of implantation would also be similarly restricted in movements and would be subjected to the sustained microgravity forces. Hence, the resultant is the induction of stress signals by both the embryonic cells and placental counter parts, and could lead to induction of cell death or total embryonic mortality. These theories are partially supported by Wakayama et al., since their data demonstrated that in vitro culture under microgravity caused slower development and fewer trophectoderm cells than in 1G controls but did not affect polarization of the blastocyst.
Those results suggested that fertilization can occur normally under microgravity environment in a mammal, but normal preimplantation embryo development might require 1G. However, it is unclear whether the adverse effects on implantation were caused by microgravity or as a side effect of the culturing system.
What Should Be Done Next?
Clearly, it is important to test whether microgravity on ISS causes immediate harmful effects in postfertilization, preimplantation development. The four reports reporting deleterious effects in MGS modalities predict a hypothesis that the preimplantation embryo through enzymatic sensors in its stem cells senses and responds to MGS with stress responses caused by other stressors. In addition, to the four reports for MGS there was one report for effects on preimplantation mouse embryos in spaceflight. None of 59 two-cell stage mouse embryos on Columbia STS-80 developed beyond the two-cell stage whereas ∼20% of control embryos reached late blastocyst stage and ∼75% underwent cell division but did not reach compaction at the eight-cell stage (Fig. 1A; http://asgsb.indstate.edu/programs/1998/92.html).
This sensing appears to be unrelated to sensing of gravity cues to enable the three-dimensional (3D) address code during gastrulation in frogs and chicks. Although these MGS modalities may all have some amount of shared or unique confounding variables, the similarities of slowed growth and morbidity suggest a hypothesis for negative effects that should be testing using the microgravity of the ISS where confounding variables are minimized.
The ISS was launched in 1998, so this is the 20th anniversary of its mission. However, despite biological research being a key program in the ISS panoply, cell, developmental, and molecular biology approaches are still in their infancy at the ISS. The Feinberg laboratory recently began to fill this void by asking and answering several basic questions in MGS conditions. They used the NASA KC-135A aircraft in parabaloid flight including free-fall (also known as the Vomit Comet) to determine whether standard laboratory techniques, such as pipetting and aliquoting liquids, can be done in zero G conditions [56,57]. They found, contrary to their own expectations that liquids can easily be pipetted into tubes and that liquids of different densities remain separated at zero G.
In fact, they found that such liquids are even easier to manage in zero G than in one G in many respects, because inverting the tubes does not cause the liquids to spill out or mix, as would occur under normal laboratory conditions. They also proposed several adaptations to standard molecular biology instruments, such as tube holders with clamps and snaps to hold pipet boxes open. The fact that these basic questions were only addressed in 2016 demonstrates how far we need to go before sophisticated molecular and developmental biology experiments can be done on the ISS.
Many aspects of biomedical research are improving on the ISS including isolation of mRNA and multiplexing study of transcript expression in small numbers of cells [58] such as embryos. Finally, key factors of embryonic or stem cell stress are epigenetic anomalies, which may not only be transmitted to progeny of the postnatal generation but also into future generations. However, conducting such studies would require the development of even more techniques and approaches, such as rearing mice for several generations in the ISS.
The inherent stressfulness of human space flight and working in outer space environment affects many physiological systems, and the evidence suggests that the magnitude of some cellular and molecular changes may increase with prolonged exposure in space. Inevitably, many cellular and molecular events remain to be investigated, given microgravity, cosmic radiations, and sustained differences in buoyancy and density of stem cells near and adjacent water-filled cavities like the blastocoele (Fig. 1) and amniotic cavity. The studies should cover mammalian developmental stages from preimplantation embryos through implantation and gastrulation stages.
The importance of spatial relationship of cells in all the stages is important to factor in as this may modify the induction of stress mechanisms. It is highly likely that the most mass dense microstructures in a cell would be the most likely to sustain mass effects of microgravity and sustained centrifugal force. Hence, there is the possibility that a preimplantation embryo in the uterine-tube has the mechanism to detect microgravity through its organelles or cytoskeletal entities. Because of their mass content, these organelles and cytoskeleton would be susceptible to microdisplacements or microshifts or microtranslocation, and the cells would respond by rotational movements to reorient themselves and thus nullify mild effects of microgravity below a certain threshold of centrifugal forces.
However, above a sustained centrifugal force of a huge magnitude (above the threshold), the efforts of the cells would be futile and expression of stress molecules would be triggered [22]. Thus, at the molecular level, there is need to characterize response of cytoskeleton and organelles in every cell, polarization and functionality of organelles, and gene expression during sustained centrifugal force given a certain level of microgravity.
Since sustained high magnitude centrifugal force above a certain threshold is speculated to induce stress on cells of both uterine-tube (mucosa and muscularis mucosa) and preimplantation embryo, a 3D in vitro system would be ideal for studying microgravity effects; here the importance of spatial relationship between cells and tissues at all stages is the drive.
Acknowledgments
Funding: D.A.R. and E.E.P. from the Office of the Vice President for Research at Wayne State University and an R03 to D.A.R. 1R03HD061431, an R41 to D.A.R. 1R41ES028991-01, and from the REI fellows' fund (A.B.) and from the funding of the Kamran Moghissi Endowed Chair.
Authors Disclosure Statement
The authors have no commercial associations that would cause a conflict of interest in connection with this review and that all funding sources supporting the work and all institutional or corporate affiliations are acknowledged.
References
- 1.Nagy A, Gertsenstein M, Vintersten K. and Behringer RR. (2003). Manipulating the Mouse Embryo. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2.Puscheck EE, Awonuga AO, Yang Y, Jiang Z. and Rappolee DA. (2015). Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol 843:77–128 [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Awonuga AO, Zhou S, Puscheck EE. and Rappolee DA. (2011). Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol 287:43–95 [DOI] [PubMed] [Google Scholar]
- 4.Graf A, Krebs S, Heininen-Brown M, Zakhartchenko V, Blum H. and Wolf E. (2014). Genome activation in bovine embryos: review of the literature and new insights from RNA sequencing experiments. Anim Reprod Sci 149:46–58 [DOI] [PubMed] [Google Scholar]
- 5.Braude P, Bolton V. and Moore S. (1988). Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332:459–461 [DOI] [PubMed] [Google Scholar]
- 6.Telford NA, Watson AJ. and Schultz GA. (1990). Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol Reprod Dev 26:90–100 [DOI] [PubMed] [Google Scholar]
- 7.Reik W, Dean W. and Walter J. (2001). Epigenetic reprogramming in mammalian development. Science 293:1089–1093 [DOI] [PubMed] [Google Scholar]
- 8.Peters J. (2014). The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet 15:517–530 [DOI] [PubMed] [Google Scholar]
- 9.Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM. and Bartolomei MS. (2004). Selective loss of imprinting in the placenta following preimplantation development in culture. Development 131:3727–3735 [DOI] [PubMed] [Google Scholar]
- 10.Doherty AS. (2000). Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 62:1526–1535 [DOI] [PubMed] [Google Scholar]
- 11.Kochav S. and Eyal-Giladi H. (1971). Bilateral symmetry in chick embryo determination by gravity. Science 171:1027–1029 [DOI] [PubMed] [Google Scholar]
- 12.Vincent JP. and Gerhart JC. (1987). Subcortical rotation in Xenopus eggs: an early step in embryonic axis specification. Dev Biol 123:526–539 [DOI] [PubMed] [Google Scholar]
- 13.Black SD. and Gerhart JC. (1985). Experimental control of the site of embryonic axis formation in Xenopus laevis eggs centrifuged before first cleavage. Dev Biol 108:310–324 [DOI] [PubMed] [Google Scholar]
- 14.Gerhart J, Ubbels G, Black S, Hara K. and Kirschner M. (1981). A reinvestigation of the role of the grey crescent in axis formation in xenopus laevis. Nature 292:511–516 [DOI] [PubMed] [Google Scholar]
- 15.Slack JMW. (1983). From Egg to Embryo: Determinative Events in Early Development. Cambridge University Press, Cambridge, Cambridgeshire; New York [Google Scholar]
- 16.Staehelin LA, Zheng HQ, Yoder TL, Smith JD. and Todd P. (2000). Columella cells revisited: novel structures, novel properties, and a novel gravisensing model. Gravit Space Biol Bull 13:95–100 [PubMed] [Google Scholar]
- 17.Hilaire E, Paulsen AQ, Brown CS. and Guikema JA. (1995). Effects of clinorotation and microgravity on sweet clover columella cells treated with cytochalasin D. Physiol Plant 95:267–273 [PubMed] [Google Scholar]
- 18.Zhang SQ, Gao SJ, Jiang QY, Feng DY. and Pen ZY. (2002). [Effects of simulated microgravity on preimplantation embryonic development of Kunming mouse in vitro]. Shi Yan Sheng Wu Xue Bao 35:54–57 [PubMed] [Google Scholar]
- 19.Wang Y, Xie Y, Wygle D, Shen HH, Puscheck EE. and Rappolee DA. (2009). A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK. Reprod Sci 16:947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakayama S, Kawahara Y, Li C, Yamagata K, Yuge L. and Wakayama T. (2009). Detrimental effects of microgravity on mouse preimplantation development in vitro. PLoS One 4:e6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima Y, Sasaki S, Kubota Y, Ikeuchi T, Hayashi Y. and Kohri K. (2000). Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil Steril 74:1142–1147 [DOI] [PubMed] [Google Scholar]
- 22.Andreazzoli M, Angeloni D, Broccoli V. and Demontis GC. (2017). Microgravity, stem cells, and embryonic development: challenges and opportunities for 3D tissue generation. Front Astron Space Sci. 10.3389/fspas.2017.00002 [DOI]
- 23.Klaus DM. (2001). Clinostats and bioreactors. Gravit Space Biol Bull 14:55–64 [PubMed] [Google Scholar]
- 24.Blaber E, Sato K. and Almeida EAC. (2014). Stem Cell Health and Tissue Regeneration in Microgravity. Stem Cells Dev 23:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaber EA, Finkelstein H, Dvorochkin N, Sato KY, Yousuf R, Burns BP, Globus RK. and Almeida EAC. (2015). Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem Cells Dev 24:2605–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross JC, Werb Z. and Fisher SJ. (1994). Implantation and the placenta: key pieces of the development puzzle. Science 266:1508–1518 [DOI] [PubMed] [Google Scholar]
- 27.Xie Y, Wang F, Zhong W, Puscheck E, Shen H. and Rappolee DA. (2006). Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod 75:45–55 [DOI] [PubMed] [Google Scholar]
- 28.Wuest SL, Stern P, Casartelli E. and Egli M. (2017). Fluid dynamics appearing during simulated microgravity using random positioning machines. PLoS One 12:e0170826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li E, Beard C. and Jaenisch R. (1993). Role for DNA methylation in genomic imprinting. Nature 366:362–365 [DOI] [PubMed] [Google Scholar]
- 30.Skinner MK, Haque CG, Nilsson E, Bhandari R. and McCarrey JR. (2013). Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS One 8:e66318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W. and Reik W. (2012). The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48:849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nettersheim D, Heukamp LC, Fronhoffs F, Grewe MJ, Haas N, Waha A, Honecker F, Waha A, Kristiansen G. and Schorle H. (2013). Analysis of TET expression/activity and 5mC oxidation during normal and malignant germ cell development. PLoS One 8:e82881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada Y, Yamagata K, Hong K, Wakayama T. and Zhang Y. (2010). A role for the elongator complex in zygotic paternal genome demethylation. Nature 463:554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haig D. (1993). Genetic conflicts in human pregnancy. Q Rev Biol 68:495–532 [DOI] [PubMed] [Google Scholar]
- 35.Haig D. (2004). Genomic imprinting and kinship: how good is the evidence? Annu Rev Genet 38:553–585 [DOI] [PubMed] [Google Scholar]
- 36.Farthing CR, Ficz G, Ng RK, Chan CF, Andrews S, Dean W, Hemberger M. and Reik W. (2008). Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet 4:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J. and Surani MA. (2002). Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 117:15–23 [DOI] [PubMed] [Google Scholar]
- 38.Giritharan G, Delle Piane L, Donjacour A, Esteban FJ, Horcajadas JA, Maltepe E. and Rinaudo P. (2012). In vitro culture of mouse embryos reduces differential gene expression between inner cell mass and trophectoderm. Reprod Sci 19:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, Chen HL, Hu JF, Behr B, Giudice L. and Hoffman AR. (2005). IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod 11:631–640 [DOI] [PubMed] [Google Scholar]
- 40.de Waal E, Mak W, Calhoun S, Stein P, Ord T, Krapp C, Coutifaris C, Schultz RM. and Bartolomei MS. (2014). In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies. Biol Reprod 90:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinaudo PF, Giritharan G, Talbi S, Dobson AT. and Schultz RM. (2006). Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril 86:1252–1265, 1265.e1–e36 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F. and Rappolee DA. (2005). Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril 83 Suppl 1:1144–1154 [DOI] [PubMed] [Google Scholar]
- 43.Xie Y, Puscheck EE. and Rappolee DA. (2006). Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced by the media. Mol Hum Reprod 12:217–224 [DOI] [PubMed] [Google Scholar]
- 44.Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE. and Rappolee DA. (2008). Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev 75:689–697 [DOI] [PubMed] [Google Scholar]
- 45.Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE. and Rappolee DA. (2007). Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod 13:473–481 [DOI] [PubMed] [Google Scholar]
- 46.Maekawa M, Yamamoto T, Tanoue T, Yuasa Y, Chisaka O. and Nishida E. (2005). Requirement of the MAP kinase signaling pathways for mouse preimplantation development. Development 132:1773–1783 [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Jiang Z, Bolnick A, Dai J, Puscheck EE. and Rappolee DA. (2017). Departure from optimal O2 level for mouse trophoblast stem cell proliferation and potency leads to most rapid AMPK activation. J Reprod Dev 63:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou S, Xie Y, Puscheck EE. and Rappolee DA. (2011). Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta 32:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdallah M, Xie Y, Puscheck EE, Rappolee DA. and Awonuga AO. (2009). Benzopyrene activates SAPK and induces HAND1 that favors differentiation of trophoblast stem cells. Fertil Steril 92:S136–S137 [Google Scholar]
- 50.Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE. and Rappolee DA. (2007). Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci 14:534–547 [DOI] [PubMed] [Google Scholar]
- 51.Xie Y, Wang F, Puscheck EE. and Rappolee DA. (2007). Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev 74:1287–1294 [DOI] [PubMed] [Google Scholar]
- 52.Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, Parker G, Huttemann M. and Rappolee DA. (2014). Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res 13:478–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awonuga AO, Zhong W, Abdallah ME, Slater JA, Zhou SC, Xie YF, Puscheck EE. and Rappolee DA. (2011). Eomesodermin, HAND1, and CSH1 proteins are induced by cellular stress in a stress-activated protein kinase-dependent manner. Mol Reprod Dev 78:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kujjo LL, Ronningen R, Ross P, Pereira RJ, Rodriguez R, Beyhan Z, Goissis MD, Baumann T, Kagawa W, et al. (2012). RAD51 plays a crucial role in halting cell death program induced by ionizing radiation in bovine oocytes. Biol Reprod 86:76. [DOI] [PubMed] [Google Scholar]
- 55.Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H, Shimazu T, Tada MN, Osada I, Nagamatsu A, et al. (2017). Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc Natl Acad Sci U S A 114:5988–5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntyre ABR, Rizzardi L, Yu AM, Alexander N, Rosen GL, Botkin DJ, Stahl SE, John KK, Castro-Wallace SL, et al. (2016). Nanopore sequencing in microgravity. NPJ Microgravity 2:16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rizzardi LF, Kunz H, Rubins K, Chouker A, Quiriarte H, Sams C, Crucian BE. and Feinberg AP. (2016). Evaluation of techniques for performing cellular isolation and preservation during microgravity conditions. NPJ Microgravity 2:16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parra M, Jung J, Boone TD, Tran L, Blaber EA, Brown M, Chin M, Chinn T, Cohen J, et al. (2017). Microgravity validation of a novel system for RNA isolation and multiplex quantitative real time PCR analysis of gene expression on the International Space Station. PLoS One 12:e0183480. [DOI] [PMC free article] [PubMed] [Google Scholar]