Abstract
Significance: Calcium (Ca2+) hypothesis of Alzheimer's disease (AD) gains popularity. It points to new signaling pathways that may underlie AD pathogenesis. Based on calcium hypothesis, novel targets for the development of potential AD therapies are identified.
Recent Advances: Recently, the key role of neuronal store-operated calcium entry (nSOCE) in the development of AD has been described. Correct regulation of nSOCE is necessary for the stability of postsynaptic contacts to preserve the memory formation. Molecular identity of hippocampal nSOCE is defined. Perspective nSOCE-activating molecule, prototype of future anti-AD drugs, is described.
Critical Issues: Endoplasmic reticulum Ca2+ overload happens in many but not in all AD models. The nSOCE targeting therapy described in this review may not be universally applicable.
Future Directions: There is a need to determine whether AD is a syndrome with one critical signaling pathway that initiates pathology, or it is a disorder with many different signaling pathways that are disrupted simultaneously or one after each other. It is necessary to validate applicability of nSOCE-activating therapy for the development of anti-AD medication. There is an experimental correlation between downregulated nSOCE and disrupted postsynaptic contacts in AD mouse models. Signaling mechanisms downstream of nSOCE which are responsible for the regulation of stability of postsynaptic contacts have to be discovered. That will bring new targets for the development of AD-preventing therapies. Antioxid. Redox Signal. 29, 1176–1188.
Keywords: : Alzheimer's disease, ER calcium overload, nSOCE
Introduction
Alzheimer's disease (AD) is the age-related brain disorder that causes progressive neurodegeneration predominantly in the cortical and hippocampal brain regions. The major hallmarks of AD are the progressive impairment of memory storage and accumulation of fibrillary amyloid plaques in patient's brains. AD has two forms: sporadic AD (SAD) with currently unknown reasons for emergence and familial AD (FAD) caused by genetically inherited mutations in either amyloid precursor protein (APP), or presenilin 1 (PS1) or presenilin 2 (PS2) proteins (12, 49, 50, 59). The main risk factor for AD is the advanced age. First symptoms of FAD start to appear in patients ∼50 years old. This is in contrast to SAD cases, which emerge in much older age, ∼70 and later. FAD is a small portion of total AD cases—∼1–2%. Information about FAD-causing mutations is used for generation of transgenic animal models of AD. Since manifestation of SAD and FAD is similar, there is hope that successful treatment of FAD may lead to SAD-relevant therapeutics. Future investigations and clinical trials will shed light on this question.
There are many hypotheses of AD pathogenesis: the oldest one is the cholinergic hypothesis (11), the dominant one is the amyloidogenic hypothesis (51), and also popular is the tau hypothesis (67). Recently, amyloidogenic hypothesis has been transformed to the oligomer hypothesis or soluble beta-amyloid (Aβ) hypothesis (41). It differs from the classical amyloid hypothesis by positing that the proximal neurotoxins in AD are soluble oligomers of Aβ, rather than Aβ in the form of amyloid aggregates. However, so far none of these hypotheses has brought successful drugs to prevent the AD pathogenesis. Recently, calcium hypothesis of AD has started to gain popularity. It states that calcium signaling mishandling in neurons occurring at early disease stages is the key event triggering synaptic dysfunction and neurodegeneration (4). Development of new powerful and precise calcium imaging techniques enabled extensive research in this field, and new intriguing data recently appeared. This review is devoted to description of calcium signaling pathways disrupted during AD with particular emphasis on endoplasmic reticulum (ER) calcium channels and store-operated calcium entry. Based on the calcium hypothesis, novel targets for the development of AD-preventing therapies are suggested, and their applicability to the treatment of AD cases is discussed.
Ca2+ hypothesis of AD
The calcium (Ca2+) hypothesis of brain aging was first formulated in 1982. In 1989 and 2017, the hypothesis was revised to introduce new data and outline questions which needed to be answered in the future (4, 63). Calcium hypothesis of AD is connected with other hypotheses in the field since changes in calcium signaling are likely to be secondary to deleterious actions of Aβ oligomers in neurons, disruption of presenilin (PS) functions, defects in mitochondria dysfunction, and aging-related changes.
There is a growing body of evidence that dysregulation in signaling pathways that handle Ca2+ plays a major role in the initiation of AD pathogenesis. Ca2+ is a second messenger that is involved in many if not all cellular processes of neuronal life. Calcium can enter the neuron from extracellular space via membrane-embedded Ca2+-permeable channels. Among them are voltage-gated Ca2+ channels (VGCCs), nonspecific cation channels N-methyl-D-aspartate receptors (NMDARs), and transient receptor potential channels (TRPCs).
Neurons have intracellular Ca2+ stores such as ER and mitochondria. Ca2+ can be released from ER via inositol trisphosphate receptor (InsP3R) and ryanodine receptors (RyanR) (14). Mitochondria can shape intracellular calcium signaling, mainly via Ca2+ sequestering mechanism (97). Ca2+ uptake into mitochondria plays an important role in neuronal physiology by stimulating mitochondrial metabolism and increasing mitochondrial energy production. Excessive Ca2+ entry into mitochondria can lead to opening of a permeability transition pore (PTP) and may lead to apoptosis (111). How these calcium entry pathways affected during AD will be discussed later.
First symptoms start to appear in patients 70–80 years old for SAD. For genetically inherited familiar form of AD, first symptoms may appear already at 50 years of age. The human brain has protective mechanisms that fight with the disease until middle age or later. However, with age the capacitance of such mechanisms gets lower, and at certain moment brain is not able to resist AD anymore. Loss of ability to handle Ca2+ is one of the features of aging neurons. In AD experimental models, Ca2+ is accumulated inside of neurons, and intracellular Ca2+ concentration is increased (4, 15). Elevated calcium levels appear to be toxic to cells and trigger subsequent pathological processes, which drive AD pathogenesis. What are the reasons for the increase of Ca2+ in AD? Is there a main Ca2+ handling mechanism that is dysregulated in AD, or it is a consequence of events that lead to development of the AD? Are there any therapeutic agents that can normalize Ca2+ signaling system in AD? Calcium hypothesis of AD is aimed at answering these and many other related questions.
Familial forms of AD are caused by mutations in genes encoding APP, PS1, and PS2 proteins. For a long time, Aβ, the product of proteolytic cleavage of APP, has been considered the initial molecule that triggers AD. While there is a debate on whether the Aβ is a major toxic culpit in AD (55, 80), it plays a major role in the pathogenesis of AD and in calcium dysregulation as well. Other AD-related proteins are PSs, which form the catalytic subunit of gamma secretase. In amyloidogenic pathway (Fig. 1), gamma secretase is responsible for cleavage of APP at its transmembrane domain and produces toxic Aβ (60). In addition to gamma secretase function, PS1 plays the function of passive Ca2+ leak channel (84, 121), which is disrupted by many but not all FAD-associated mutations in PS1. The influence of mentioned proteins on Ca2+ signaling pathways during AD pathology is discussed below.
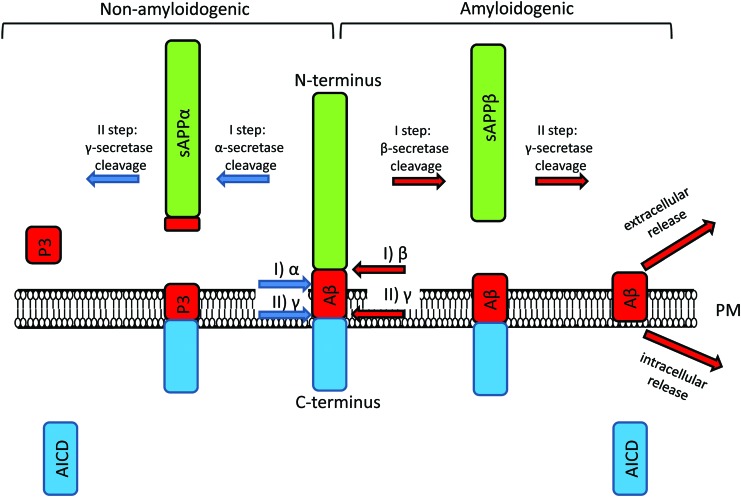
FIG. 1.
Two pathways mediate APP processing in neurons. APP is processed by α -, β-, and γ- secretases. In nonamyloidogenic pathway, α-secretase cleaves APP first, leading to the production of soluble APP fragment (sAPPα), P3 and AICD. Although role of P3 is not precisely studied, sAPPα and AICD play physiological roles in neurons (80). In amyloidogenic pathway, β-secretase cleaves APP first, producing soluble extracellular fragment of APP (sAPPβ) and transmembrane C-terminal fragment of APP (APP-CTF). This APP-CTF is further cleaved by γ- secretases to produce Aβ and AICD. Aβ, beta-amyloid; AICD, APP intracellular domain; APP, amyloid precursor protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Aβ and Neuronal Calcium Signaling
Aβ was initially recognized as the main toxic agent in AD (103). Currently, Aβ theory is under revision (55, 80). It is apparent that Aβ plays an important role in AD pathogenesis, but some other factors also contribute to AD pathology together with Aβ or may even precede the Aβ toxicity. Detrimental effect of Aβ oligomers on neurons has been extensively studied, and many publications demonstrated that Aβ aggregates promote the increase in neuronal cytosolic Ca2+ concentration (16, 34–37, 46, 68, 107, 124). The exact mechanism of Aβ-mediated disruption of Ca2+ homeostasis is under active investigation. Concerning the role of Aβ in Ca2+ dyshomeostasis during AD, it has been observed that Aβ can make Ca2+-permeable channels in plasma membrane by themselves (7) (Fig. 2).
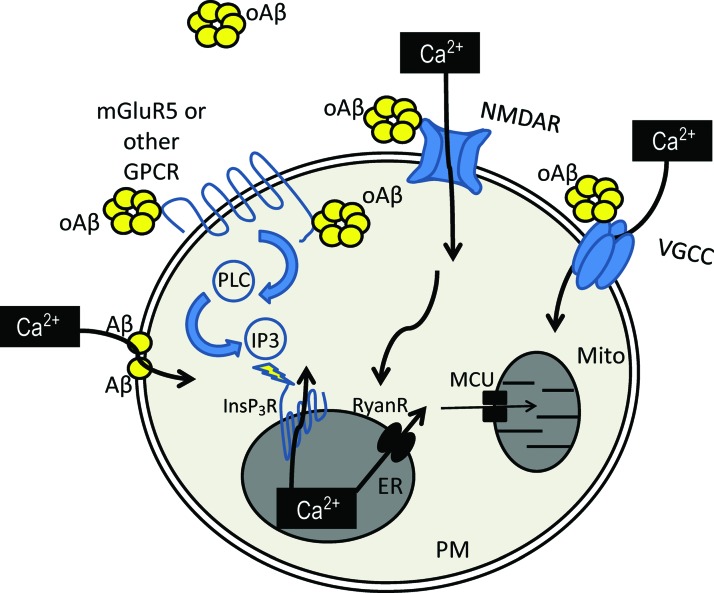
FIG. 2.
Aβ mediated increase of cytosolic Ca2+ concentration. Aβ has several interaction partners on the PM. Among them are NMDAR, VGCC, and mGluR5. Interaction with NMDAR and VGCC leads to influx of Ca2+ ions from extracellular space. Interaction with mGluR5 or other GPCR leads to production of IP3 that potentiates the release of Ca2+ via InsP3R from the ER to the cytosol. Moreover, Aβ is able to make Ca2+-permeable channels in PM by itself. Increase in the ER Ca2+ potentiates Ca2+-dependent calcium release via RyanR. This Ca2+ release from the RyanR plays a role in the Ca2+ entry to mitochondria (Mito) via MCU. Ca2+, calcium; ER, endoplasmic reticulum; GPCR, G-protein coupled receptor; IP3, inositol triphosphate; InsP3R, inositol triphosphate receptor; MCU, mitochondria channel uniporter; mGluR5, metabotropic glutamate receptor 5; NMDAR, N-methyl-D-aspartate receptor; oAβ, oligomeric beta-amyloid; PM, plasma membrane; RyanR, ryanodine receptor; VGCC, voltage-gated calcium channel. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Probably the most important Aβ targets are NMDA receptors. Activation of NMDA receptors is a key event in long-term potentiation phenomenon, which is thought to be the cellular basis of memory formation process. The effects of Aβ on NMDA receptors were extensively studied (43, 81, 134). Particularly, it has been shown that Aβ is able to increase the vulnerability of neurons to excitotoxicity, which is caused by excessive NMDAR activation with subsequent cell calcium overload (77, 78). Some data indicate that Aβ in its oligomeric form may directly bind and modulate activity of NMDA receptors (30, 69, 108, 117). There is indication that NMDARs are required for synaptic targeting of Aβ oligomers, but they do not appear to comprise the actual binding sites for Aβ oligomers (32). Various deleterious effects of Aβ on NMDAR were reported. It was reported that in early disease stages, Aβ activates NMDAR and induces rapid Ca2+ elevation in neurons (40, 87, 99, 134). Usage of Aβ oligomers at sublethal concentrations induces prolonged Ca2+ signaling via NMDAR. These Ca2+ signals trigger redox-sensitive stimulation of RyanR-mediated Ca2+ release from the ER, decreased RyanR2 protein expression, mitochondrial fragmentation, and prevented RyanR-mediated spine remodeling (89).
Detrimental effect of oligomeric Aβ on RyanR-mediated Ca2+ signaling in ER was also observed in glia, particularly in cultured astrocytes (2). In the study performed by Gavello et al., the oligomeric Aβ42 differently regulated RyanR, NMDAR, and VGCCs by increasing Ca2+ release through RyaRs, and inhibiting Ca2+ influx through NMDARs and VGCCs. According to this study, the overall increased intracellular Ca2+ concentration caused stimulation of K+ current carried by big conductance Ca2+activated potassium (BK) channels and inhibition of hippocampal network firing (44). Application of oligomeric Aβ species in vivo causes fast rise in resting Ca2+ levels which depend on NMDARs activation and triggers dendritic spines loss (6). Treatment with aducanumab (anti-Aβ antibody) restores calcium homeostasis in Tg 2576 mice (61). The treatment effect was connected to restoration of NMDAR function rather than to restoration of intracellular Ca2+ signaling. In addition, it was reported that Aβ induces reduction in NMDAR expression and enhances its endocytosis (109), impairs NMDAR-dependent long-term potentiation (LTP) (29) and reduces NMDAR-mediated calcium influx into active spines (104).
Another calcium-permeable plasma membrane channels are presynaptic VGCC. It has been observed that Aβ oligomers decrease synaptic transmission between hippocampal neurons, most likely via depression of Ca2+ flux through P/Q-type calcium channels (85). However, in HEK293 cells that overexpress recombinant P/Q-type calcium channels the increase in P/Q-type currents by Aβ oligomers has been observed (54). Authors explain such differences by the fact that ion channels can be bidirectionally regulated by the same molecule. For example, potassium channel blocker k-conotoxin PVIIA both enhances and reduces potassium currents depending on its activation state (65). In contrast, the authors report that block of postsynaptic L-type calcium channels by 10 μM nimodipine did not reverse Aβ42-induced deficits, indicating that Aβ oligomer pathology is specifically mediated via presynaptic ion channels. In contrast to the results mentioned above, there are data on age-dependent upregulation of L-type VGCC currents in Cornu Ammonis area 1 region of hippocampus in 3 × TgAD (triple transgenic mouse model of Alzheimer's disease) mice (125). It was reported that antagonists of L-VGCC can protect neurons, and preserve synaptic function in animal models of aging and AD (5, 73, 88, 98, 122).
Beside actions of Aβ on the plasma membrane-embedded calcium channels, it was shown that both extracellular and intracellular Aβ applications alter activity of ER-resident calcium channels—RyanR and InsP3R (42, 57). RyanR- and InsP3R-mediated Ca2+ responses were induced by application of Aβ (25–35) and Aβ40 on cultured cortical neurons. It was shown that Aβ42-induced Ca2+ release from the ER in intact human neuroblastoma cells was just partially mediated by InsP3R, while the greater part of Ca2+ elevation was induced by an alternative mechanism (57). Interestingly, it was reported that lowering of RyanR-mediated Ca2+ release leads to the reduction of both intracellular and extracellular Aβ load in APP(swe)-expressing (Tg2576) mice (86). According to Briggs et al., it is an example of proposed pathogenic feed-forward cycle in which elevated Ca2+ levels triggered by Aβ further facilitate production of Aβ (17). The link between extracellular Aβ and intracellular channels is still elusive, but several possible mechanisms of action have been proposed. It was shown that Aβ oligomers induce InsP3 production through stimulation and dimerization of synaptic metabotropic glutamate receptor 5 (mGluR5) receptors (96). Another possible link is that in dendritic spines, Ca2+ release by RyanR can be triggered by Aβ-facilitated Ca2+ influx through NMDARs (17, 45, 89). Studies performed by SanMartin et al. demonstrated that Aβ oligomers promote RyanR2-mediated Ca2+ release, mitochondrial Ca2+ entry, ROS generation, and fragmentation of the mitochondrial structural network. It was further shown that RyanR2 knockdown as well as usage of antioxidants reduces Ca2+-mediated noxious effects of Aβ oligomers on mitochondrial function (101, 102). Some AD models demonstrated intracellular Aβ accumulation, which may also take part in ER calcium signaling destabilization (75). Intracellular application of Aβ oligomers into Xenopus oocytes stimulates G-protein-mediated InsP3 production and consequent cytotoxic Ca2+ release from the ERs (35). Another study demonstrated that InsP3Rs were not required for Aβ42-stimulated Ca2+ release from ER in DT40 chicken B-lymphocyte line permeabilized cells, revealing an additional direct effect of Aβ42 upon the ER (57).
Role of PSs in Ca2+ Homeostasis
PSs act as a catalytic subunit of gamma secretase. FAD-associated mutations disrupt gamma secretase function, leading to amyloidogenic processing of APP and production of toxic Aβ species (Fig. 1) (60). Whether FAD mutations cause gain of function or loss of gamma secretase function is the subject of debate (123, 127, 128). Development of gamma secretase modulators as potential anti-AD therapeutics is complicated due to essential role of gamma secretase in Notch processing (31). Calcium signaling effects of Aβ were discussed above. APP intracellular domain also affects ER Ca2+ release by regulating the expression of genes involved in Ca2+ homeostasis (71).
Significant body of research suggests that AD-bearing PS mutants cause Ca2+ dysregulation independently of its gamma secretase function and Aβ accumulation, and due to changes in activity of RyanR and InsP3R (17, 25, 39, 93). Upregulation of RyanR-mediated Ca2+ release and increased levels of RyanR expression among different PS-mutation bearing AD models were reported (17, 21, 23, 26, 33, 45, 113). It was proposed that PSs alter RyanR gating through direct protein–protein interaction mediated by N-terminal cytosolic domain of PSs (52, 91, 100). RyanR gating effects of PS1 and PS2 are isoform specific (91). Increase of the PS2 to PS1 ratio was reported for normal aging mice in both cerebellum and forebrain, which correlates to loss of spatial memory, learning, and motor function (58). Such homologue misbalance is proposed to contribute to age-dependent cytosolic Ca2+ level increase (17). Based on these findings, it was proposed that excessive Ca2+ release from ER and elevated cytosolic Ca2+ concentrations observed during AD may be a result of altered RyanR interaction with PSs (91). Changes in RyanR function have been suggested to be responsible for alterations in synaptic activity induced by PSs (126).
Sensitivity of InsP3R to its agonist InsP3 significantly increased in cell expressing mutant PSs (27, 28). Suppression of InsP3R expression normalized exaggerated Ca2+ signals observed in cortical and hippocampal neurons in PS1-M146 V knock-in and 3 × Tg AD mice models, indicating that it might be a potential therapeutic strategy (106). Recent research using data-based computational modeling provided deeper insight into InsP3R gating in the presence of mutated PSs (76). This model predicted that that the gain-of-function enhancement is sensitive to both InsP3 and Ca2+, and that very small amount of InsP3 is required to stimulate InsP3R channels in the presence of FAD-causing mutant PS. Therefore, significant activity of the InsP3R at resting InsP3 concentration should lead to spontaneous Ca2+ signals in cells (76). Using computational model, the same research group suggested that mutation in PSs increases the open probability of mitochondrial PTP, which in turn triggers pathological processes and may induce cell death (119). It was proposed that mutated PSs enhance Ca2+ release through InsP3R into a cytoplasmic microdomain formed by neighboring cluster of a few InsP3R channels and mitochondria channel uniporter, and therefore facilitate mitochondrial calcium uptake (119). This investigation proposes direct link between Ca2+ disruptions and impaired mitochondrial function, as observed in AD.
Additional gamma secretase-independent function of PSs was suggested. PS1 and PS2 were reported to act as passive ER calcium leak channels (84, 121, 131). This idea was initially controversial (105), but it was supported by unbiased screen for ER Ca2+ leak channels (10). This function of PS1 is altered by many but not all FAD-associated mutations. For example, extensively studied M146 V mutation is a classic example of PS1 mutation that causes disruption of Ca2+ leak function (121). However, the deletion of Exon 9 in PS1 is a pathological mutation that acts as a gain of function for ER Ca2+ leak activity (121). A correlation between patient clinical phenotypes and effects of FAD mutations on ER Ca2+ leak function was observed (82). Site-directed mutagenesis approach was used to map potential ion conduction pore of PS1 (83). It was demonstrated that D385 but not D257 residue is important for channel function of PSs (83, 121). Interestingly, PSs share the fold with chloride channels (118), and the high-resolution crystal structure of archaeal PS homologue, PSH1, has a hole that traverses through the entire protein and is large enough to allow passage of Ca2+ ions (74). This hole was however not apparent when structure of γ-secretase complex was solved (9). Proteolytically cleaved PS does not form ER Ca2+ leak channels (121), which may explain lack of obvious ion conduction pathway in mature γ-secretase complex.
Using molecular dynamics approach, a dynamic all-atom model of mature PS1 embedded into the membrane has been published recently (110). It is important to note that PS1 undergoes post-translational modifications, particularly autoendo-proteolysis. As many other post-translational modifications, autoproteolysis is suggested to be essential for the change of PS1 from inactive to active state (110). Authors have confirmed previously published gating mechanism for PS1 (64). In agreement with previously published data, they have observed that Exon 9 plays a role of a “plug” that closes or opens the “doors” to the catalytic pocket of the PS1 depending on the activation state. Although not modeled in this article, these data suggest that deletion of Exon 9 permanently opens the interior chamber of PS1, consistent with superleaky pore phenotype of PS1ΔE9 mutant (121).
In conclusion, mutations in PSs are shown to enhance calcium release via both ER-resident channels—RyanR and InsP3R. In addition, PSs themselves play a role of ER Ca2+ leak channels. Excessive Ca2+ release from ER contributes to AD pathology. Modifying Ca2+ release from ER is a promising therapeutic strategy to reduce toxic cytosolic calcium elevations.
ER calcium overload in AD hippocampal neurons
ER Ca2+ concentration is increased in experimental models of AD including transgenic mice. It has been observed that InsP3-evoked calcium release from the ER is upregulated in PC12 cells and in fibroblasts that express mutant PS1 (48, 70). Similar effects were observed in neurons in brain slices taken from mutant PS1-M146 V, 3 × TgAD, and APPSweTauP301 L mice (112–114). Stutzmann et al. suggest that enhanced Ca2+ release from the ER observed in these studies occurs due to upregulation of RyanR function (112–114).
Another possible mechanism responsible for these effects is that mutations in PS1 disrupt its function as ER calcium leak channel. In addition, it has been suggested that PSs may potentiate the activity of sarco/endoplasmic reticulum Ca2+-adenosine triphosphatase (ATPase) (SERCA) pump via direct protein–protein interactions (46). Aβ can indirectly increase ER calcium content. As discussed above, Aβ potentiates Ca2+ entry via plasma membrane channels. Aβ can also act on SERCA pump that sequesters cytosolic Ca2+ (Fig. 3). To compensate the ER Ca2+ overload, neurons may upregulate the calcium-induced Ca2+ release from the ER via RyanR.
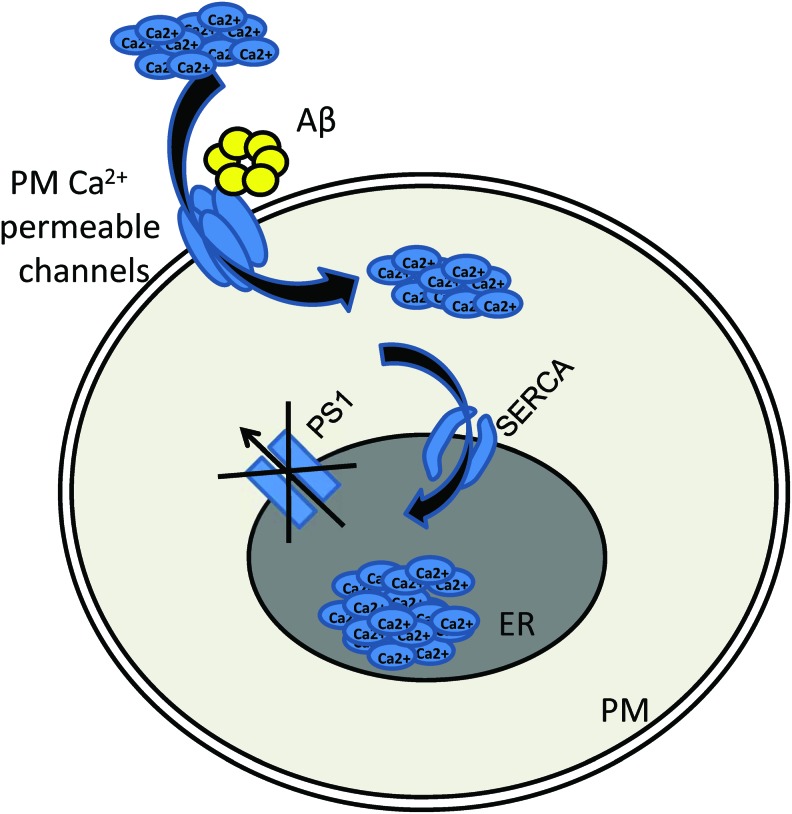
FIG. 3.
Intracellular signaling pathways involved in ER calcium overload at AD. Possible mechanisms involved in ER Ca2+ overload in AD. (i) FAD-associated mutations cause disruption of passive Ca2+ leak function of PSs, thus causing accumulation of Ca2+ in the ER lumen. (ii) Aβ potentiates plasma membrane Ca2+-permeable channels, leading to the increase of cytosolic and ER Ca2+ content. (iii) PS1 may interact with SERCA pump via direct protein–protein interaction, thus potentiating its Ca2+ pumping activity. AD, Alzheimer's disease; ATPase, adenosine triphosphatase; FAD, familial form of Alzheimer's disease; PS, presenilin; PS1, presenilin 1; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Indeed, changes in expression of RyanR have been described in human AD cases and in patients with mild cognitive impairment (18, 62). It is important to note that there are three subtypes of RyanR—1, 2, and 3. RyanR2 and 3 subtypes are expressed in the brain. It has been observed that RyanR2 is upregulated at early stages and is downregulated in advanced stages of AD in human postmortem samples (18, 62). Concerning RyanR3 subtype, it has been observed that its protein (89) and mRNA expression (18) is upregulated in late stages of the disease, suggesting that upregulation of RyanR3 might be a compensatory response to decreased function of RyanR2. Increase in RyanR2 expression and enhanced Ca2+ release have been reported in presymptomatic AD mice (21, 62, 113, 131). It has been shown that muscle relaxant dantrolene that targets RyanR exerts neuroprotective effects in mouse models of AD (24, 86, 92). Disadvantage of usage of dantrolene in the treatment of AD is that it does not have specificity to neuronal type of RyanR and may lead to side-effects. Moreover, there are data that long-term treatment with dantrolene can worsen AD pathology (131).
Neuronal Store-Operated Calcium Entry Is a Potential Therapeutic Target
Neuronal store-operated calcium entry (nSOCE) is a unique mechanism that refills ER calcium store in response to its depletion (95). For a long time, it has been believed that SOCE exists only in nonexcitable cells where it is the main mechanism to refill intracellular stores (79). However, there is a growing body of evidence that SOCE exists in neurons (8, 13, 47, 66, 94, 115, 129, 130). nSOCE is composed of two parts. The first one is plasma membrane proteins from ORAI and TRPC families that are able to make calcium-permeable channels. Second one is ER membrane protein that has calcium-sensitive domain inside of the ER. There are two ER proteins that participate in functioning of SOCE: STIM1 and STIM2. Stromal interacting molecule (STIM) 2 is predominantly expressed in hippocampus (115, 132). When calcium concentration drops inside of the ER, calcium-sensitive domain sends signal to the STIM to oligomerize. When it is in oligomerized form, it goes to ER–plasma membrane junctions to bind ORAI and TRPC proteins to form nSOC channels (Fig. 4) (132).
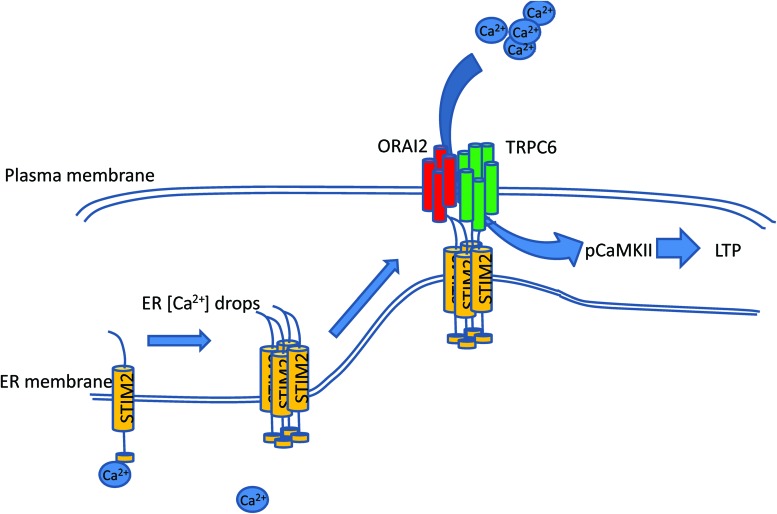
FIG. 4.
Physiological regulation of nSOCE in hippocampal neurons. nSOCE has two partners at different cellular compartments: ER-resident protein STIM2 and plasma membrane proteins ORAI2 and TRPC6. STIM2 has intraluminal domain that senses changes in ER Ca2+ concentration. When ER Ca2+ drops, Ca2+ dissociates from N-terminal calcium-sensitive domain. That leads to conformational change of STIM2, which is oligomerization. In oligomerized form, STIM2 travels to ER–PM junctions where it binds with plasma membrane partners of nSOCE–ORAI2 and TRPC6 proteins. This binding allows opening of nSOC channels and Ca2+ entry into the neuron. We propose that this Ca2+ entry is necessary to maintain pCaMKII levels, and that is essential to maintain LTP. LTP, long-term potentiation; nSOCE, neuronal store-operated calcium entry; ORAI2, calcium release-activated calcium channel protein 2; pCaMKII, phosphorylated calcium/calmodulin-activated protein kinase II; STIM, stromal interacting molecule. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recently, cellular nSOCE-dependent signaling pathway has been described in hippocampal neurons (115). It has been shown that neurons downregulate STIM2 expression in response to ER Ca2+ overload, resulting in drop in the amount of Ca2+ ions that enter neurons via nSOCE channels. STIM2 is downregulated in cultured hippocampal neurons and in hippocampus in animal models of AD, as well as in human AD brain samples (94, 115, 133). Cleavage of STIM proteins by PSs was suggested as a potential mechanism involved in these effects (120). nSOCE channels constitute ternary complex made by STIM2 at the ER part, and calcium release-activated calcium channel protein 2 (ORAI2) and TRPC6 at the plasma membrane part (132) (Fig. 4). In other studies, a role of ORAI1 in supporting SOCE in hippocampal and cortical neurons was demonstrated (47, 66). Knockdown of TRPC6 expression abolished nSOCE in hippocampal neurons. Overexpressed TRPC6 or pharmacological activators of TRPC6 channels restored nSOCE and spine loss in AD neurons (132). The mice that overexpress TRPC6 in the brain display enhanced cognitive performance and increased formation of excitatory synapses (135).
What is a physiological role of nSOCE in hippocampal neurons? It has been shown that nSOCE participates in regulation of stability of mature mushroom spines (Fig. 5) (94, 115, 133). Mushroom spines are sites of strong synapses that are necessary for formation and storage of memories. It has been proposed that downstream target for nSOCE is pCaMKII (phosphorylated calcium/calmodulin-activated protein kinase II), molecule that participates in LTP (Fig. 4). LTP is the best studied physiological mechanism of making participating synapses stronger and is essential for preservation of memories. It has been suggested that nSOCE is active in resting neurons (115), and is the main supplier of Ca2+ ions for CaMKII at rest. CaMKII is necessary for LTP performance. Shifting a balance from CaMKII to CaN is detrimental to synapses, leading to their instability and consequently causing memory dysfunction (93).
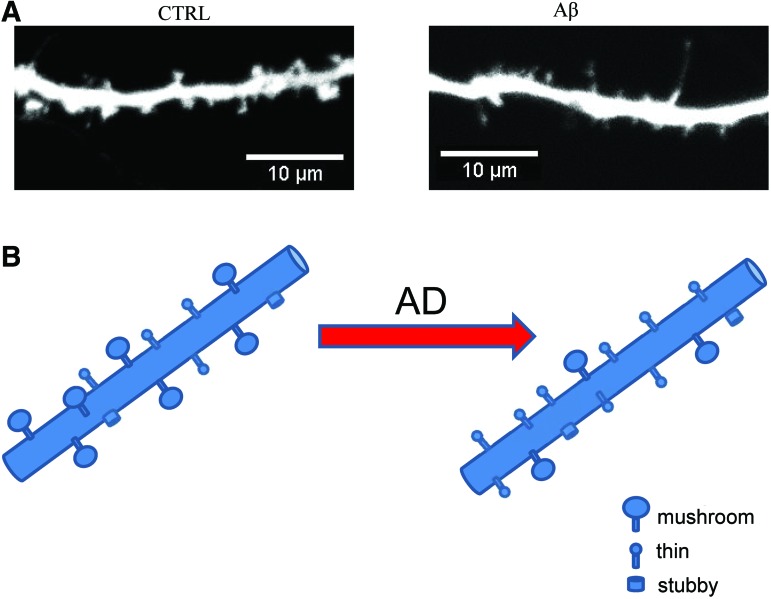
FIG. 5.
Loss of stable postsynaptic contacts in AD. (A) Confocal images of DIV14-fixed hippocampal neurons in culture. Primary hippocampal neurons were transfected with TD-Tomato plasmid at DIV7 and left untreated (control, CTRL) or treated for 3–4 days with synthetic oligomeric Aβ (Aβ). (B) Cartoon representation of the synaptic loss observed in amyloid-beta-induced synaptotoxic model of AD. Usually postsynaptic contacts are divided into three morphological groups. Mushroom spines have thin neck and big head, thin spines have thin neck and small head, barely distinguishable from neck and stubby spines that do not have head and more or less look like protrusions on dendritic shafts. Due to big head size, mushroom spines able to make strong synapses that participate in memory formation and storage. Mushroom spines are selectively lost in AD models, and proportion of thin spines is increased. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
From this discussion, TRPC6 appears to be an attractive target for development of AD-preventing therapies. There are two molecules that are able to activate TRPC6 channels—hyperforin and NSN21778 (Fig. 6) (132). Hyperforin is a natural compound that activates TRPC6 channels (72). Beneficial effects of hyperforin and its derivatives in animal models of AD have been demonstrated (20, 38, 56). In double transgenic APPswe/PSEN1DE9 mice, derivative of hyperforin–tetrahydrohyperforin improves memory and prevents the impairment of synaptic plasticity in a dose-dependent manner, inducing a recovery of LTP (56). It has also been reported that tetrahydrohyperforin is able to enhance autophagic clearance of APP (19). In hippocampal neurons, TRPC6-dependent downstream signaling was connected with activation of the RAS/MEK/ERK, PI3K, and CAMKIV pathways (53, 116).
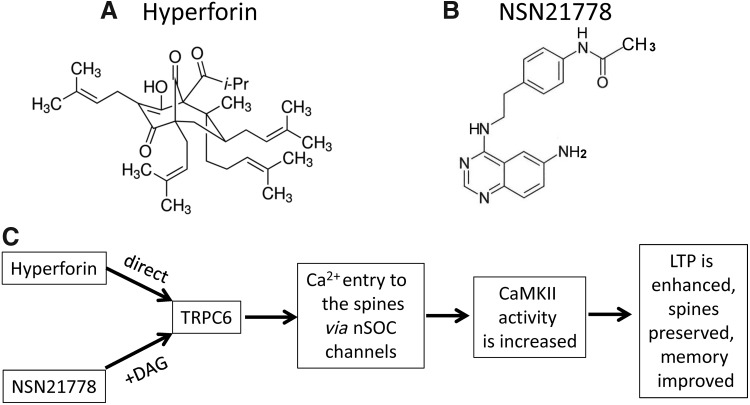
FIG. 6.
Chemical structures of hyperforin and NSN21778, and mechanism of their action in hippocampal neurons. (A, B) Structures of hyperforin and NSN21778. Structure of hyperforin is adopted from Sigma-Aldrich Web site. The structure of NSN21778 is adopted from a previous study (132). (C) The schema demonstrates neuroprotective mechanisms of hyperforin and NSN21778. Both these compounds activate TRPC6 channels. However, hyperforin is a direct activator of TRPC6, while NSN21778 needs/or modulates DAG-dependent activation of TRPC6 channels. Due to the activation of TRPC6 channels, Ca2+ enters the postsynaptic contacts and supports the functionality of CaMKII, which is necessary for LTP performance and preservation of spines and memory. DAG, diacylglycerol.
NSN21778 compound was recently discovered as a positive modulator of nSOC (132). It is important to note that NSN21778 is different from hyperforin in the mechanism of TRPC6 activation. It has been shown that hyperforin is a direct activator of TRPC6 while NSN facilitates OAG-induced Ca2+ influx through TRPC6 channels in conditions of partially depleted intracellular stores (132). The neuroprotective mechanism of NSN that is currently proposed is that NSN activates TRPC6 channels in diacylglycerol (DAG)-dependent manner. Following activation of TRPC6 channels, Ca2+ enters spines and activates CaMKII. All these events lead to spine and memory preservation and protection from AD (132) (Fig. 6). Future studies will be needed to establish utility of NSN21778 and its derivatives for treatment of AD.
Conclusions
Calcium hypothesis of AD is gaining popularity (4) since it points to new intracellular signaling pathways that are dysregulated in neurons, and more importantly it brings new targets for the development of AD-preventing therapies. AD is a multifactorial brain disorder (3) that manifests itself as a loss of memory. Modern therapeutical interventions should be based on understanding the mechanisms of memory loss in AD. Multiple lines of evidence suggest that Ca2+ signaling dysregulation plays an important role in synaptic pathology in AD. We propose that downregulation of nSOCE is one of the mechanisms responsible for synaptic and memory loss in AD, and that activators of TRPC6 channels should exert beneficial effects on AD. Future studies will be needed to test these ideas.
Abbreviations Used
- 3 × TgAD
triple transgenic mouse model of Alzheimer's disease
- Aβ
beta-amyloid
- AD
Alzheimer's disease
- AICD
APP intracellular domain
- APP
amyloid precursor protein
- ATPase
adenosine triphosphatase
- Ca2+
calcium
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- FAD
familial form of Alzheimer's disease
- InsP3R
inositol trisphosphate receptor
- LTP
long-term potentiation
- mGluR5
metabotropic glutamate receptor 5
- NMDAR
N-methyl-D-aspartate receptors
- nSOCE
neuronal store-operated calcium entry
- ORAI2
calcium release-activated calcium channel protein 2
- pCaMKII
phosphorylated calcium/calmodulin-activated protein kinase II
- PS1
presenilin 1
- PS2
presenilin 2
- PS
presenilin
- PTP
permeability transition pore
- RyanR
ryanodine receptor
- SAD
sporadic form of Alzheimer's disease
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- STIM
stromal interacting molecule
- TRPC
transient receptor potential channels
- VGCC
voltage-gated calcium channels
Acknowledgments
Ilya Bezprozvanny is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer's Disease Research. This work was supported by the National Institutes of Health Grant R01NS080152 (I.B.) (chapter: Ca2+ hypothesis of Alzheimer disease), Russian Science Foundation Grant 14-25-00024-II (I.B.) (chapter: Beta-amyloid and neuronal calcium signaling), by the state Grant 17.991.2017/4.6 (I.B.) (chapter: Role of PS1 in Ca2+ homeostasis), supported in part by RFBR grant (Project No. 17-04-00710\18) (E.P.) (chapter: ER calcium overload in AD hippocampal neurons) and by the grant of President of Russian Federation 14.Y30.17.1043-MK (E.P.) (chapter: nSOCE is a potential therapeutic target).
References
- 1. This reference has been deleted.
- 2.Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, and Matute C. Ca(2+) - dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer's disease. Aging Cell 12: 292–302, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Alkadhi K. and Eriksen J. The complex and multifactorial nature of Alzheimer's disease. Curr Neuropharmacol 9: 586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer's Association Calcium Hypothesis W. Calcium Hypothesis of Alzheimer's disease and brain aging: a framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 13: 178–182 e17, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Anekonda TS, Quinn JF, Harris C, Frahler K, Wadsworth TL, and Woltjer RL. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer's disease. Neurobiol Dis 41: 62–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbel-Ornath M, Hudry E, Boivin JR, Hashimoto T, Takeda S, Kuchibhotla KV, Hou S, Lattarulo CR, Belcher AM, Shakerdge N, Trujillo PB, Muzikansky A, Betensky RA, Hyman BT, and Bacskai BJ. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol Neurodegener 12: 27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arispe N, Rojas E, and Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A 90: 567–571, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, and Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci 23: 7737–7741, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, and Shi Y. An atomic structure of human gamma-secretase. Nature 525: 212–217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandara S, Malmersjo S, and Meyer T. Regulators of Calcium Homeostasis Identified by Inference of Kinetic Model Parameters from Live Single Cells Perturbed by siRNA. Sci Signal 6: ra56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartus RT, Dean RL, 3rd, Beer B, and Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Bergmans BA. and De Strooper B. gamma-secretases: from cell biology to therapeutic strategies. Lancet Neurol 9: 215–226, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, and Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal 2: ra67, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ. Calcium hypothesis of Alzheimer's disease. Pflug Arch 459: 441–449, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med 15: 89–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bezprozvanny I. and Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci 31: 454–463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs CA, Chakroborty S, and Stutzmann GE. Emerging pathways driving early synaptic pathology in Alzheimer's disease. Biochem Biophys Res Commun 483: 988–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, and Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 33: 1001.e1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavieres VA, Gonzalez A, Munoz VC, Yefi CP, Bustamante HA, Barraza RR, Tapia-Rojas C, Otth C, Barrera MJ, Gonzalez C, Mardones GA, Inestrosa NC, and Burgos PV. Tetrahydrohyperforin inhibits the proteolytic processing of amyloid precursor protein and enhances its degradation by Atg5-dependent autophagy. PLoS One 10: e0136313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerpa W, Hancke JL, Morazzoni P, Bombardelli E, Riva A, Marin PP, and Inestrosa NC. The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer's mouse model. Curr Alzheimer Res 7: 126–133, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Chakroborty S, Goussakov I, Miller MB, and Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci 29: 9458–9470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. This reference has been deleted.
- 23.Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, and Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J Neurosci 32: 8341–8353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, and Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J Neurosci 32: 8341–8353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakroborty S. and Stutzmann GE. Calcium channelopathies and Alzheimer's disease: insight into therapeutic success and failures. Eur J Pharmacol 739: 83–95, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Chan SL, Mayne M, Holden CP, Geiger JD, and Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275: 18195–18200, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, and Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal 3: ra22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, and Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP(3) receptor channel gating. Neuron 58: 871–883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danysz W. and Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine – searching for the connections. Br J Pharmacol 167: 324–352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, and Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem 282: 11590–11601, 2007 [DOI] [PubMed] [Google Scholar]
- 31.De Strooper B. Lessons from a failed gamma-secretase Alzheimer trial. Cell 159: 721–726, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, Epstein AL, De Felice FG, Jerusalinsky D, and Ferreira ST. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-beta peptide oligomers. J Neurochem 115: 1520–1529, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Del Prete D, Checler F, and Chami M. Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9: 21–21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demuro A, Mina E, Kayed R, Milton SC, Parker I, and Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280: 17294–17300, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Demuro A. and Parker I. Cytotoxicity of intracellular abeta42 amyloid oligomers involves Ca2+ release from the endoplasmic reticulum by stimulated production of inositol trisphosphate. J Neurosci 33: 3824–3833, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demuro A, Parker I, and Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem 285: 12463–12468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshpande A, Mina E, Glabe C, and Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 26: 6011–6018, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinamarca MC, Cerpa W, Garrido J, Hancke JL, and Inestrosa NC. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits. Mol Psychiatry 11: 1032–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Elena Popugaeva EP, Zhang H, Vlasova O, and Bezprozvanny I. STIM2 protects mushroom spines from amyloid synaptotoxicity. Mol Neurodegener 10: 37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, and Rego AC. Amyloid beta peptide 1–42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium 51: 95–106, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Ferreira ST. and Klein WL. The Abeta oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease. Neurobiol Learn Mem 96: 529–543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreiro E, Oliveira CR, and Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res 76:872–880, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Foster TC, Kyritsopoulos C, and Kumar A. Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer's disease. Behav Brain Res 322: 223–232, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Gavello D, Calorio C, Franchino C, Cesano F, Carabelli V, Carbone E, and Marcantoni A. Early Alterations of Hippocampal Neuronal Firing Induced by Abeta42. Cereb Cortex 28: 433–446, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Goussakov I, Miller MB, and Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. J Neurosci 30: 12128–12137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, and LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol 181: 1107–1116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruszczynska-Biegala J. and Kuznicki J. Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. J Neurochem 126: 727–738, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Guo Q, Sopher BL, Furukawa K, Pham DG, Robinson N, Martin GM, and Mattson MP. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci 17: 4212–4222, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem 110: 1129–1134, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Hardy J. and Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Hardy JA. and Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 256: 184–185, 1992 [DOI] [PubMed] [Google Scholar]
- 52.Hayrapetyan V, Rybalchenko V, Rybalchenko N, and Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium 44: 507–518, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Heiser JH, Schuwald AM, Sillani G, Ye L, Muller WE, and Leuner K. TRPC6 channel-mediated neurite outgrowth in PC12 cells and hippocampal neurons involves activation of RAS/MEK/ERK, PI3K, and CAMKIV signaling. J Neurochem 127: 303–313, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Hermann D, Mezler M, Muller MK, Wicke K, Gross G, Draguhn A, Bruehl C, and Nimmrich V. Synthetic Abeta oligomers (Abeta(1–42) globulomer) modulate presynaptic calcium currents: prevention of Abeta-induced synaptic deficits by calcium channel blockers. Eur J Pharmacol 702: 44–55, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18: 794–799, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Inestrosa NC, Tapia-Rojas C, Griffith TN, Carvajal FJ, Benito MJ, Rivera-Dictter A, Alvarez AR, Serrano FG, Hancke JL, Burgos PV, Parodi J, and Varela-Nallar L. Tetrahydrohyperforin prevents cognitive deficit, Abeta deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1DeltaE9 model of Alzheimer's disease: a possible effect on APP processing. Transl Psychiatry 1: e20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen LE, Bultynck G, Luyten T, Amijee H, Bootman MD, and Roderick HL. Alzheimer's disease-associated peptide Aβ(42) mobilizes ER Ca(2+) via InsP(3)R-dependent and -independent mechanisms. Front Mol Neurosci 6: 36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaja S, Sumien N, Shah VV, Puthawala I, Maynard AN, Khullar N, Payne AJ, Forster MJ, and Koulen P. Loss of spatial memory, learning and motor coordination during normal aging is accompanied by changes in brain presenilin 1 and 2 expression levels. Mol Neurobiol 52: 545–554, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karch CM, Cruchaga C, and Goate AM. Alzheimer's disease genetics: from the bench to the clinic. Neuron 83: 11–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karran E. and De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem 139 (Suppl. 2): 237–252, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Kastanenka KV, Bussiere T, Shakerdge N, Qian F, Weinreb PH, Rhodes K, and Bacskai BJ. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J Neurosci 36: 12549–12558, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelliher M, Fastbom J, Cowburn RF, Bonkale W, Ohm TG, Ravid R, Sorrentino V, and O'Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer's disease neurofibrillary and beta-amyloid pathologies. Neuroscience 92: 499–513, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Khachaturian ZS. Calcium, membranes, aging, and Alzheimer's disease. Introduction and overview. Ann N Y Acad Sci 568: 1–4, 1989 [DOI] [PubMed] [Google Scholar]
- 64.Knappenberger KS, Tian G, Ye X, Sobotka-Briner C, Ghanekar SV, Greenberg BD, and Scott CW. Mechanism of gamma-secretase cleavage activation: is gamma-secretase regulated through autoinhibition involving the presenilin-1 exon 9 loop? Biochemistry 43: 6208–6218, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Koch ED, Olivera BM, Terlau H, and Conti F. The binding of kappa-Conotoxin PVIIA and fast C-type inactivation of Shaker K+ channels are mutually exclusive. Biophys J 86: 191–209, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korkotian E, Oni-Biton E, and Segal M. The role of the store-operated calcium entry channel Orai1 in cultured rat hippocampal synapse formation and plasticity. J Physiol 595: 125–140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosik KS, Joachim CL, and Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A 83: 4044–4048, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, and Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59: 214–225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, and Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci 27: 796–807, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, and LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol 149: 793–798, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Muller U, Saftig P, De Strooper B, Wolfe MS, Golde TE, and LaFerla FM. A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc Natl Acad Sci U S A 99: 4697–4702, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, and Muller WE. Hyperforin–a key constituent of St. John's wort specifically activates TRPC6 channels. FASEB J 21: 4101–4111, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Levere TE. and Walker A. Old age and cognition: enhancement of recent memory in aged rats by the calcium channel blocker nimodipine. Neurobiol Aging 13: 63–66, 1992 [DOI] [PubMed] [Google Scholar]
- 74.Li X, Dang S, Yan C, Gong X, Wang J, and Shi Y. Structure of a presenilin family intramembrane aspartate protease. Nature 493: 56–61, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Lina J, Xi Z, Weijie L, Qing Z, and Zichun H. Intracellular Aβ and its Pathological role in Alzheimer's disease: lessons from cellular to animal models. Curr Alzheimer Res 13: 621–630, 2016 [DOI] [PubMed] [Google Scholar]
- 76.Mak DOD, Cheung KH, Toglia P, Foskett JK, and Ullah G. Analyzing and quantifying the gain-of-function enhancement of IP(3) receptor gating by familial Alzheimer's disease-causing mutants in presenilins. PLoS Comput Biol 11: e1004529, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature 430: 631–639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, and Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci 12: 376–389, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, and D'Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front Cell Neurosci 9: 153, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris GP, Clark IA, and Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun 2: 135, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mota SI, Ferreira IL, and Rego AC. Dysfunctional synapse in Alzheimer's disease - A focus on NMDA receptors. Neuropharmacology 76(Pt A): 16–26, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Nelson O, Supnet C, Liu H, and Bezprozvanny I. Familial Alzheimer's disease mutations in presenilins: effects on endoplasmic reticulum calcium homeostasis and correlation with clinical phenotypes. J Alzheimers Dis 21: 781–793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson O, Supnet C, Tolia A, Horre K, De Strooper B, and Bezprozvanny I. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J Biol Chem 286: 22339–22347, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, and Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest 117: 1230–1239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, and Bruehl C. Amyloid beta oligomers (A beta(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci 28: 788–797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F, Benfenati F, and Chami M. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32: 11820–11834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parameshwaran K, Dhanasekaran M, and Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp Neurol 210: 7–13, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Paris D, Bachmeier C, Patel N, Quadros A, Volmar CH, Laporte V, Ganey J, Beaulieu-Abdelahad D, Ait-Ghezala G, Crawford F, and Mullan MJ. Selective antihypertensive dihydropyridines lower Abeta accumulation by targeting both the production and the clearance of Abeta across the blood-brain barrier. Mol Med 17: 149–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paula-Lima AC, Adasme T, SanMartin C, Sebollela A, Hetz C, Carrasco MA, Ferreira ST, and Hidalgo C. Amyloid beta-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid Redox Signal 14: 1209–1223, 2011 [DOI] [PubMed] [Google Scholar]
- 90. This reference has been deleted.
- 91.Payne AJ, Kaja S, and Koulen P. Regulation of ryanodine receptor-mediated calcium signaling by presenilins. Receptors Clin Invest 2: e449, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, and Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516: 274–279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popugaeva E, Pchitskaya E, and Bezprozvanny I. Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity? Biochem Biophys Res Commun 483: 998–1004, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Popugaeva E, Pchitskaya E, Speshilova A, Alexandrov S, Zhang H, Vlasova O, and Bezprozvanny I. STIM2 protects hippocampal mushroom spines from amyloid synaptotoxicity. Mol Neurodegener 10: 37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Putney JW., Jr. Capacitative calcium entry in the nervous system. Cell Calcium 34: 339–344, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL, Triller A.Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 66: 739–754, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rizzuto R, De Stefani D, Raffaello A, and Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Roberts-Lewis JM, Savage MJ, Marcy VR, Pinsker LR, and Siman R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 14: 3934–3944, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rönicke R, Mikhaylova M, Rönicke S, Meinhardt J, Schröder UH, Fändrich M, Reiser G, Kreutz MR, and Reymann KG. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging 32: 2219–2228, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Rybalchenko V, Hwang SY, Rybalchenko N, and Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol 40: 84–97, 2008 [DOI] [PubMed] [Google Scholar]
- 101.SanMartin CD, Adasme T, Hidalgo C, and Paula-Lima AC. The antioxidant N-acetylcysteine prevents the mitochondrial fragmentation induced by soluble amyloid-beta peptide oligomers. Neurodegener Dis 10: 34–37, 2012 [DOI] [PubMed] [Google Scholar]
- 102.SanMartin CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, Garcia A, Hartel S, Hidalgo C, and Paula-Lima AC. RyR2-Mediated Ca2+ Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid beta Peptide Oligomers. Front Mol Neurosci 10: 115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selkoe DJ. and Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8: 595–608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, and Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27: 2866–2875, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shilling D, Mak DO, Kang DE, and Foskett JK. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem 287: 10933–10944, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shilling D, Muller M, Takano H, Mak DO, Abel T, Coulter DA., Foskett JK. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer's disease pathogenesis. J Neurosci 34: 6910–6923, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simakova O. and Arispe NJ. The cell-selective neurotoxicity of the Alzheimer's Abeta peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Abeta toxicity. J Neurosci 27: 13719–13729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sinnen BL, Bowen AB, Gibson ES, and Kennedy MJ. Local and use-dependent effects of beta-amyloid oligomers on NMDA receptor function revealed by optical quantal analysis. J Neurosci 36: 11532–11543, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, and Greengard P. Regulation of NMDA receptor trafficking by amyloid-[beta]. Nat Neurosci 8: 1051–1058, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Somavarapu AK. and Kepp KP. Loss of stability and hydrophobicity of presenilin 1 mutations causing Alzheimer's disease. J Neurochem 137: 101–111, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Spat A, Szanda G, Csordas G, and Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium 44: 51–63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stutzmann GE, Caccamo A, LaFerla FM, and Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24: 508–513, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, and Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci 26: 5180–5189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, and Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann N Y Acad Sci 1097: 265–277, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL, 3rd, and Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82: 79–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, and Wang Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci 121: 2301–2307, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Texido L, Martin-Satue M, Alberdi E, Solsona C, and Matute C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium 49: 184–190, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Theobald DL. Presenilin adopts the ClC channel fold. Protein Sci 25: 1363–1365, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toglia P. and Ullah G. The gain-of-function enhancement of IP3-receptor channel gating by familial Alzheimer's disease-linked presenilin mutants increases the open probability of mitochondrial permeability transition pore. Cell Calcium 60: 13–24, 2016 [DOI] [PubMed] [Google Scholar]
- 120.Tong BC, Lee CS, Cheng WH, Lai KO, Foskett JK, and Cheung KH. Familial Alzheimer's disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci Signal 9: ra89, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, and Bezprozvanny I. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer's disease. Cell 126: 981–993, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Veng LM, Mesches MH, and Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res 110: 193–202, 2003 [DOI] [PubMed] [Google Scholar]
- 123.Veugelen S, Saito T, Saido TC, Chavez-Gutierrez L, and De Strooper B. Familial Alzheimer's Disease Mutations in Presenilin Generate Amyloidogenic Abeta Peptide Seeds. Neuron 90: 410–416, 2016 [DOI] [PubMed] [Google Scholar]
- 124.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, and Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Wang Y. and Mattson MP. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol Aging 35: 88–95, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu B, Yamaguchi H, Lai FA, and Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc Natl Acad Sci U S A 110: 15091–15096, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xia D, Kelleher RJ, 3rd, and Shen J. Loss of Abeta43 production caused by presenilin-1 mutations in the knockin mouse brain. Neuron 90: 417–422, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J, and Kelleher RJ., 3rd Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer's disease. Neuron 85: 967–981, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, and Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron 27: 561–572, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, and Bezprozvanny I. Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer's disease. J Alzheimers Dis 45: 561–580, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang H, Sun S, Herreman A, De Strooper B, and Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci 30: 8566–8580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Fon Tacer K, and Bezprozvanny I. Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer's disease treatment. J Neurosci 36: 11837–11850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, and Bezprozvanny I. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer's disease. J Neurosci 35: 13275–13286, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, Li P, Feng J, and Wu M. Dysfunction of NMDA receptors in Alzheimer's disease. Neurol Sci 37: 1039–1047, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, Ding Y, and Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 11: 741–743, 2008 [DOI] [PubMed] [Google Scholar]