Abstract
As few studies have examined the relationship between the apolipoprotein E (APOE) gene and clinical outcomes after military-related traumatic brain injury (TBI), we aimed to determine whether the ε4 allele of the APOE gene influences neuropsychiatric symptoms in veterans with a history of mild-to-moderate TBI. Participants included 133 veterans (TBI = 79; military controls [MC] = 54) who underwent APOE genotyping and were divided into ε4+ (TBI = 18; MC = 15) and ε4– (TBI = 61; MC = 39) groups. All participants underwent evaluation of psychological distress using the Beck Depression Inventory-II, Beck Anxiety Inventory, and PTSD Checklist-Military Version. Two-way analyses of variance were conducted to examine the effect of group (TBI vs. MC) and APOE-ε4 status (ε4+ vs. ε4–) across symptom measures. There was a significant main effect of group across all symptom measures (TBI > MC; all p values <0.001), no main effect of ε4 genotype (p = 0.152–0.222), and a significant interaction of group by ε4 genotype across all measures (p = 0.027–0.047). Specifically, for TBI participants, ε4+ veterans demonstrated significantly higher symptom scores across all measures when compared to ε4– veterans (p = 0.007–0.015). For MC participants, ε4 status had no effect on the severity of psychiatric symptom scores (p = 0.585–0.708). Our results demonstrate that, in our well-characterized sample of veterans with history of neurotrauma, possession of the ε4 allele conveys risk for increased symptomatology (i.e., depression, anxiety, and post-traumatic stress disorder), even well outside of the acute phase of injury. Findings suggest a meaningful relationship between APOE genotype and psychiatric distress post-TBI, and they suggest that there is a brain basis for the complex neuropsychiatric presentation often observed in this vulnerable population. Future longitudinal studies are needed in order to further our understanding of how genetic factors influence response to TBI.
Keywords: : APOE gene, genetics, military veterans, psychiatric distress, traumatic brain injury
Introduction
Research regarding clinical outcomes in the aftermath of traumatic brain injury (TBI) has burgeoned considerably, and it has become well established that military-related TBI is often coupled with high levels of psychiatric distress.1–5 Post-traumatic stress disorder (PTSD) has undoubtedly been the most widely studied comorbid condition associated with TBI, but other mental health diagnoses have also been linked with TBI, including, but not limited to, depression and anxiety.3,6–9 For example, in a study of Afghanistan and Iraq-era veterans with positive TBI screens, Carlson and colleagues7 documented that over 80% of these service members also had at least one clinician-diagnosed psychiatric disorder. Interestingly, in this study, veterans who screened positive for TBI were 3 times more likely to have been diagnosed with PTSD compared to those who screened negative for TBI.7 These findings not only highlight the prevalence of psychiatric distress in this unique population, but also raise the question of why such high rates of mental health symptoms—especially PTSD—are observed in service members with a history of TBI relative to those without TBI.
A number of theories have been proposed to account for the increased rates of psychiatric distress and symptomatology in veterans who have experienced a TBI. Notably, though, the occurrence of psychiatric distress post-TBI is not specific to military-related TBI, given that civilian outcome studies have also reported high rates of mental health symptoms post-TBI.10–12 Explanations for these observed comorbidities broadly fall within the realm of environmental versus biological contributions. Environmental considerations primarily relate to the context under which the TBI was sustained. For example, the presence or degree of combat exposure,9,13 mechanism of injury (such as blast or blunt force),14–16 and severity of the injury event14,17 have all been hypothesized to at least partially explain the development of persisting symptoms and/or psychiatric distress post-TBI. In contrast, postulated biological mechanisms accounting for the high rates of psychiatric distress include pathophysiological changes associated with TBI,18–20 as well as the influence of genetic predispositions.21–23
Our understanding of the associations between specific genetic polymorphisms and TBI susceptibility and outcome is still in its infancy. Several candidate genes have been explored thus far, but at present, the gene encoding apolipoprotein E (apoE) has been the most widely studied gene with respect to its role in recovery and outcome post-TBI.24–26 ApoE is a lipoprotein that transports and metabolizes lipids (such as cholesterol) within the central nervous system (CNS),27,28 and it is primarily involved in neuronal maintenance, growth, and repair.28–30 ApoE is encoded by the apolipoprotein E (APOE) gene, located on chromosome 19, which is comprised of three alleles—APOE ε2, APOE ε3, and APOE ε4—for a total of six genotypes (three homozygous: ε2/ε2, ε3/ε3, ε4/ε4, and three heterozygous: ε2/ε3, ε2/ε4, and ε3/ε4).31 The properties of the ε2, ε3, and ε4 alleles result in differential capacities for cell maintenance and repair/regrowth.28,32 For instance, whereas the ε3 allele facilitates neurite outgrowth, the ε4 allele inhibits neurite outgrowth.33–35 The ε4 allele has also been implicated in other neuropathological processes, including mitochondrial dysfunction, inflammation, increased amyloid β (Aβ) production/accumulation, and altered Aβ peptide clearance.26,28,32 Thus, the ε4 allele is considered to be a risk factor for possible neuropathology post-CNS compromise.24,28,36
The APOE gene was initially studied in the context of aging, and it has consistently been found to be a risk factor for Alzheimer's disease (AD; for a review, see Verghese and colleagues37 and Kim and colleagues38). More recently, there are now several lines of research suggesting that the presence of the ε4 allele is associated with unfavorable outcome post-TBI.23–25,31 For example, TBI ε4 carriers, relative to non-ε4 carriers, have been found to demonstrate 1) worse global and functional outcomes,39–43 2) poorer neuropsychological performance post-injury,44–47 and 3) increased risk for developing AD or other dementia.48–50 However, despite rapid expansion of research related to the APOE gene and clinical outcome post-TBI, to our knowledge, no studies have examined the relationship between the APOE gene and psychiatric symptom distress in the context of military TBI. Therefore, in the present study, we aimed to determine whether the ε4 allele of the APOE gene influences neuropsychiatric symptoms in military veterans with and without mild-to-moderate TBI. We hypothesized that among veterans with a history of TBI, ε4+ participants would experience greater psychiatric symptoms relative to ε4– participants. In contrast, we hypothesized that ε4 allele status would not influence psychiatric symptoms in military controls (MCs).
Methods
Participants and procedures
Participants were 79 veterans with a history of TBI (n = 69 mild, n = 10 moderate) and 54 MCs without a history of TBI who were predominantly involved in the Iraq and Afghanistan conflicts (i.e., Operation Enduring Freedom [OEF], Operation Iraqi Freedom [OIF], and Operation New Dawn [OND]). Veterans were recruited from the VA San Diego Healthcare System (VASDHS) through outpatient clinics (e.g., a TBI specialty clinic), recruitment flyers posted within the VASDHS, and word of mouth. Participants were administered several questionnaires, which included completion of self-report measures of psychiatric distress, as well as select modules of the Mini-International Neuropsychiatric Interview (M.I.N.I.; i.e., Major Depressive Episode and Posttraumatic Stress Disorder [PTSD]). The present study was reviewed and approved by local institutional review boards, and informed consent was obtained from all participants before research participation.
An initial screening interview was conducted to determine participant eligibility. TBI history was assessed using a clinical interview adapted from the VA Semi-Structured Clinical Interview for TBI.51 The interview was comprised of questions pertaining to the nature of previously sustained TBIs. Specifically, the following information was gathered for each TBI reported: injury-severity characteristics (presence and duration of loss of consciousness [LOC], post-traumatic amnesia [PTA], and alteration of consciousness [AOC]), the context under which the TBI was sustained (military vs. nonmilitary event), the mechanism of injury (blast-related vs. blunt/mechanical force), and when the TBI occurred. This information was used to determine the “worst” or “most significant” TBI ever experienced by each service member, which was, in turn, used to classify injury severity for the present study. Additionally, the interview was used to determine the total number of lifetime TBIs sustained by each participant (a TBI was counted if it met criteria as defined below).
To determine whether veterans met criteria for having sustained a TBI, the VA/DoD (Department of Defense) Clinical Practice Guideline for Management of Concussion/Mild TBI52 definition was applied; these guidelines indicate that in order for an event to be classified as a TBI, the individual must have experienced at least one of the following: 1) LOC, 2) AOC, 3) PTA, 4) neurological deficits (such as weakness, loss of balance, etc.), and 5) intracranial lesion.52 For the purpose of this study, information pertaining to neurological deficits and intracranial lesions was not available for all participants; thus, classification of TBI was based on participant self-reported duration of LOC, AOC, and PTA. A mild TBI was defined as experiencing LOC <30 min, AOC up to 24 h, and/or PTA <24 h; a moderate TBI was defined as experiencing LOC >30 min and <24 h, AOC >24 h, and/or PTA >24 h but <7 days.52 The interviews were conducted face to face by either post-baccalaureate research assistants or graduate students under the supervision of a neuropsychologist. If participants did not report a history of TBI (as defined above), these veterans were classified as MC participants.
Exclusion criteria for the TBI group included the following: 1) history of severe TBI (defined as LOC ≥24 h, AOC >24 h, and/or PTA ≥7 days); 2) having the “worst” or “most significant” TBI occur before the age of 18; 3) history of a neurological disorder or serious medical illness (e.g., epilepsy, multiple sclerosis, stroke, myocardial infarction, etc.); 4) history of bipolar disorder, schizophrenia, or another psychotic disorder as per Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition, Text Revision (DSM-IV-TR) criteria53; 5) current (within the past 30 days) substance/alcohol abuse or dependence as per DSM-IV-TR criteria; 6) a positive toxicology screen (measured by the Rapid Response 10-drug Test Panel); and 7) suboptimal effort as determined by the Test of Memory Malingering (TOMM)54 or the California Verbal Learning Test-II (CVLT-II)55 Forced Choice Recognition subset. Exclusion criteria for the MC group included the following: 1) history of TBI (regardless of severity level) and 2) meeting criteria 3–7 as defined above for the TBI sample. Inclusion criteria for both the TBI and MC groups required that veterans provide a DNA sample that was successfully analyzed for their APOE genotype.
Laboratory procedures
Participants' DNA was collected by buccal sample; specifically, participants swabbed the inside of their cheek to obtain a saliva sample that could be used for APOE genotyping. The APOE genotype for each participant was determined by using two Taqman® single-nucleotide polymorphism (SNP) assays for the SNPs, APOE112 (rs429358) and APOE158 (rs7412). Participants were genotyped using a method based on polymerase chain reaction identical to that of Saunders and colleagues.56 APOE genotyping results for the overall sample were as follows: ε2/ε2 (n = 0; 0%), ε2/ε3 (n = 12; 9.0%), ε2/ε4 (n = 4; 3.0%), ε3/ε3 (n = 88; 66.2%), ε3/ε4 (n = 26; 19.5%), and ε4/ε4 (n = 3; 2.3%). Based on these observed frequencies, participants were divided into two groups—veterans with one or two copies of the ε4 allele were classified as “ε4 present” (ε4+) and veterans with no copies of the ε4 allele were classified as “ε4 absent” (ε4–). Veterans were not informed of their APOE genotype.
Primary outcome measures
Beck Depression Inventory-II
The Beck Depression Inventory-II (BDI-II)57 is a 21-item self-report measure assessing depressive symptomatology. Each item on the BDI-II is comprised of four statements related to a particular symptom of depression. Participants are instructed to select the statement in each item that best describes how they have been feeling over the past 2 weeks. The statements correspond to values ranging from 0 to 3, with higher values representing more-severe depressive symptomatology. A total score was calculated by adding together the ratings for the 21 items (possible range, 0–63). The psychometric properties of the BDI-II are well established.57,58
Beck Anxiety Inventory
The Beck Anxiety Inventory (BAI)59 is a 21-item self-report measure assessing generalized anxiety. Each item corresponds to a common symptom of anxiety and participants are asked to rate the extent to which they were bothered by each symptom during the past week using a 4-point rating scale ranging from 0 (“Not at all”) to 3 (“Severely—I could barely stand it”). Higher scores represent more-severe anxiety. A total score was calculated by aggregating the individual responses from each item (possible range, 0–63). Similar to the BDI-II, the BAI has sound psychometric properties.59–62
PTSD Checklist – Military Version
The PTSD Checklist – Military Version (PCL-M)63 is a 17-item self-report measure designed to assess DSM-IV-TR diagnostic criteria for PTSD. Each item on the PCL-M corresponds to a DSM symptom of PTSD, and participants are asked to rate the extent to which they have been bothered by each symptom over the past month using a 1–5 scale, with 1 indicating “Not at all” and 5 indicating “Extremely.” Higher scores represent more-severe PTSD symptomatology. A total score was calculated by summing the selected values from each item (possible range, 17–85). The psychometric properties of the PCL-M have also been well established.64–67
Participants were also administered a measure of pre-morbid intellectual functioning—the Reading subtest of the Wide Range Achievement Test 4,68 as well as measures of effort, including the TOMM54 and the CVLT-II Forced Choice Recognition subtest.55 As noted above, participants who demonstrated performances below clinical cut-offs on one or both of these tasks were removed from the analyses.
Statistical analyses
Descriptive statistics were run on the overall sample, and TBI and MC participants were compared to determine whether there were any differences between groups with regard to basic demographic characteristics. Independent-samples t-tests were used to evaluate continuous data, and chi-square analyses were used to evaluate categorical data. Within the TBI sample, participants were divided into mild and moderate TBI groups and were compared across demographic and injury severity characteristics. TBI participants were also divided into groups based on the presence or absence of an ε4 allele (ε4+ vs. ε4–), and allele groups were compared across the same demographic and injury severity characteristics. Two-way analyses of variance (ANOVAs) were conducted in order to examine the effect of group (TBI vs. MC) and ε4 status (ε4+ vs. ε4–) across self-report measures of psychiatric distress. Two-way ANOVAs were also conducted to determine whether results would differ after removal of moderate TBI participants from the analyses. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS; version 24; SPSS IBM, New York, NY).
Results
Demographic and injury-related characteristics
The overall sample included 133 military veterans (79.7% male) who were, on average, 32.35 years old (standard deviation [SD] = 7.08; median [Mdn] = 30.00; range = 21–53) and who completed 14.33 years of education (SD = 1.74; Mdn = 14.00; range = 12–18). Approximately half of the participants self-identified as white (51.1%), followed by Hispanic/Latino (27.1%), black (9.0%), Asian/Pacific Islander (8.3%), and Other (4.5%). The majority of the participants (89.5%) served in OEF/OIF/OND, and 24.8% of the overall sample had at least one ε4 allele.
Participant demographic characteristics for the TBI (n = 79) and MC (n = 54) groups are presented in Table 1. Overall, groups were well matched, given that there were no differences between TBI and MC participants with respect to age, sex, marital status, employment status, branch of service, OEF/OIF/OND veteran status, and APOE ε4 allele status. As expected, a greater proportion of participants in the TBI group (68.4%) were exposed to combat compared to the MC group (37.0%). Groups also differed on ethnicity; 66.7% of MCs identified as white compared to 40.5% of TBI participants. Finally, education significantly differed between groups (p = 0.007), with the MC group having, on average, 0.8 more years of education than the TBI group. However, the groups did not differ on a measure of pre-morbid intellectual functioning (WRAT4 Reading subtest, p > 0.05).
Table 1.
Demographic Characteristics (N = 133)
| TBI group (n = 79) | MC group (n = 54) | ||||
|---|---|---|---|---|---|
| Variables | M | SD | M | SD | p valuea |
| Age | 32.43 | 7.05 | 32.22 | 7.19 | 0.869 |
| Education (years) | 13.99 | 1.57 | 14.83 | 1.86 | 0.007 |
| WRAT4 Reading SS | 100.96 | 10.92 | 103.28 | 9.95 | 0.218 |
| N | % | N | % | p valueb | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 67 | 84.8 | 39 | 72.2 | 0.076 |
| Female | 12 | 15.2 | 15 | 27.8 | |
| Ethnicity | |||||
| White | 32 | 40.5 | 36 | 66.7 | 0.002 |
| Hispanic | 28 | 35.4 | 8 | 14.8 | |
| Black | 9 | 11.4 | 3 | 5.6 | |
| Asian/Pacific Islander | 9 | 11.4 | 2 | 3.7 | |
| Other | 1 | 1.3 | 5 | 9.3 | |
| Married/cohabitating | |||||
| Yes | 29 | 36.7 | 21 | 38.9 | 0.887 |
| No | 48 | 60.8 | 33 | 61.1 | |
| Missing | 2 | 2.5 | 0 | 0 | |
| Currently employed | |||||
| Yes | 36 | 45.6 | 31 | 57.4 | 0.204 |
| No | 42 | 53.2 | 23 | 42.6 | |
| Missing | 1 | 1.3 | 0 | 0 | |
| Branch of service | |||||
| Air Force | 6 | 7.6 | 5 | 9.3 | 0.731 |
| Army | 19 | 24.1 | 10 | 18.5 | |
| Marines | 28 | 35.4 | 16 | 29.6 | |
| Navy | 25 | 31.6 | 21 | 38.9 | |
| Other | 1 | 1.3 | 0 | 0 | |
| Missing | 0 | 0 | 2 | 3.7 | |
| OEF/OIF/OND veteran | |||||
| Yes | 74 | 93.7 | 45 | 83.3 | 0.056 |
| No | 5 | 6.3 | 9 | 16.7 | |
| Combat exposure | |||||
| Yes | 54 | 68.4 | 20 | 37.0 | 0.002 |
| No | 25 | 31.6 | 30 | 55.6 | |
| Missing | 0 | 0 | 4 | 7.4 | |
| APOE ε4 allele status | |||||
| ε4 Present (ε4+) | 18 | 22.8 | 15 | 27.8 | 0.513 |
| ε4 Absent (ε4–) | 61 | 77.2 | 39 | 72.2 |
Independent-samples t-tests were used to determine whether there were group differences for age, education, and WRAT4 Reading.
Chi-square analyses were used to determine whether there were group differences for sex, ethnicity, marital status, employment status, military branch of service, OEF/OIF/OND status, combat exposure, and APOE ε4 allele status.
WRAT4, Wide Range Achievement Test 4; SS, standard score; OEF, Operation Enduring Freedom; OIF, Operation Iraqi Freedom; OND, Operation New Dawn; TBI, traumatic brain injury; MC, military control; M, mean; SD, standard deviation.
Among the TBI participants, 69 (87.3%) were classified as having a mild TBI, and 10 (12.7%) were classified as having a moderate TBI. Table 2 compares TBI participants by injury severity (mild vs. moderate TBI) across demographic variables and injury-related characteristics. The average time from injury to assessment was 76.32 months (∼6 years) across the TBI sample. There were no significant differences between mild and moderate TBI groups on any of the demographic variables, and results were similar when nonparametric statistics were performed (all p > 0.05). By definition, mild and moderate groups differed on injury severity characteristics (refer to Table 2).
Table 2.
TBI Participants: Sample Characteristics by TBI Severity (N = 79)
| Mild TBI group (n = 69) | Moderate TBI group (n = 10) | ||||
|---|---|---|---|---|---|
| Variables | M | SD | M | SD | p valuea |
| Age | 32.00 | 7.02 | 35.40 | 6.88 | 0.155 |
| Education (years) | 13.91 | 1.61 | 14.50 | 1.18 | 0.270 |
| WRAT4 Reading SS | 100.75 | 11.19 | 102.40 | 9.28 | 0.658 |
| Time (months) from most recent TBI to testing | 65.13 | 48.96 | 64.50 | 46.50 | 0.970 |
| Time (months) from most sig. TBI to testing | 75.56 | 51.75 | 81.50 | 57.70 | 0.739 |
| Age at most sig. TBI | 26.06 | 6.16 | 28.50 | 6.85 | 0.251 |
| Lifetime number of TBIs | 2.36 | 1.33 | 2.50 | 1.72 | 0.769 |
| N | % | N | % | p valueb | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 59 | 85.5 | 8 | 80.0 | 0.650 |
| Female | 10 | 14.5 | 2 | 20.0 | |
| Ethnicity | |||||
| White | 29 | 42.0 | 3 | 30.0 | 0.257 |
| Hispanic | 24 | 34.8 | 4 | 40.0 | |
| Black | 9 | 13.0 | 0 | 0 | |
| Asian/Pacific Islander | 6 | 8.7 | 3 | 30.0 | |
| Other | 1 | 1.4 | 0 | 0 | |
| Married/cohabitating | |||||
| Yes | 28 | 40.6 | 1 | 10.0 | 0.053 |
| No | 39 | 56.5 | 9 | 90.0 | |
| Missing | 2 | 2.9 | 0 | 10.0 | |
| Currently employed | |||||
| Yes | 30 | 43.5 | 6 | 60.0 | 0.347 |
| No | 38 | 55.1 | 4 | 40.0 | |
| Missing | 1 | 1.4 | 0 | 0 | |
| Branch of service | |||||
| Air Force | 5 | 7.2 | 1 | 10.0 | 0.647 |
| Army | 18 | 26.1 | 1 | 10.0 | |
| Marines | 25 | 36.2 | 3 | 30.0 | |
| Navy | 20 | 29.0 | 5 | 50.0 | |
| Other | 1 | 1.4 | 0 | 0 | |
| OEF/OIF/OND veteran | |||||
| Yes | 66 | 95.7 | 8 | 80.0 | 0.057 |
| No | 3 | 4.3 | 2 | 20.0 | |
| Combat exposure | |||||
| Yes | 49 | 71.0 | 5 | 50.0 | 0.182 |
| No | 20 | 29.0 | 5 | 50.0 | |
| LOC (most sig. TBI) | |||||
| Yes | 39 | 56.5 | 10 | 100.0 | 0.008 |
| No | 30 | 43.5 | 0 | 0 | |
| AOC (most sig. TBI) | |||||
| Yes | 30 | 43.5 | 0 | 0 | 0.008 |
| No | 39 | 56.5 | 10 | 100.0 | |
| PTA (most sig. TBI) | |||||
| Yes | 33 | 47.8 | 10 | 100.0 | 0.009 |
| No | 30 | 43.5 | 0 | 0 | |
| Unsure | 5 | 7.2 | 0 | 0 | |
| Missing | 1 | 1.4 | 0 | 0 | |
| APOE ε4 allele status | |||||
| ε4 Present (ε4+) | 15 | 21.7 | 3 | 30.0 | 0.561 |
| ε4 Absent (ε4–) | 54 | 78.3 | 7 | 70.0 |
Independent-samples t-tests were used to determine whether there were group differences for age, education, WRAT4 Reading, time from most recent TBI to testing, time from most significant TBI to testing, age at most significant TBI, and lifetime number of TBIs.
Chi-square analyses were used to determine whether there were group differences for sex, ethnicity, marital status, employment status, military branch of service, OEF/OIF/OND status, combat exposure, presence of LOC, presence of AOC, presence of PTA, and APOE ε4 allele status.
WRAT4, Wide Range Achievement Test 4; SS, standard score; TBI, traumatic brain injury; sig., significant; OEF, Operation Enduring Freedom; OIF, Operation Iraqi Freedom; OND, Operation New Dawn; LOC, loss of consciousness; AOC, alteration of consciousness; PTA, post-traumatic amnesia; M, mean; SD, standard deviation.
Table 3 compares TBI participants by ε4 status (ε4+ vs. ε4–) across demographic and injury-related characteristics, as well as across lifetime diagnosis of psychiatric disorders (i.e., Major Depressive Disorder [MDD], PTSD). Eighteen participants (22.8%) were classified as ε4+ and 61 (77.2%) were classified as ε4–. APOE ε4+ and ε4– participants did not differ across any of the demographic or injury-related characteristics, nor did the groups differ on presence of psychiatric disorders.
Table 3.
TBI Participants: Sample Characteristics by ε4 Allele Group (N = 79)
| ε4 allele present (ε4+) (n = 18) | ε4 allele absent (ε4–) (n = 61) | ||||
|---|---|---|---|---|---|
| Variables | M | SD | M | SD | p valuea |
| Age | 33.56 | 7.94 | 32.10 | 6.81 | 0.445 |
| Education (years) | 13.50 | 1.30 | 14.13 | 1.62 | 0.134 |
| WRAT4 Reading SS | 100.17 | 8.42 | 101.20 | 11.63 | 0.727 |
| Months since most recent TBI | 56.72 | 37.96 | 67.55 | 51.08 | 0.408 |
| Months since most sig. TBI | 67.11 | 42.48 | 79.08 | 54.78 | 0.397 |
| Age at most sig. TBI | 28.22 | 7.35 | 25.82 | 5.86 | 0.154 |
| Number of TBIs sustained | 2.17 | 1.34 | 2.44 | 1.39 | 0.457 |
| N | % | N | % | p valueb | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 15 | 83.3 | 52 | 85.2 | 0.843 |
| Female | 3 | 16.7 | 9 | 14.8 | |
| Ethnicity | |||||
| White | 6 | 33.3 | 26 | 42.6 | 0.439 |
| Hispanic | 7 | 38.9 | 21 | 34.4 | |
| Black | 4 | 22.2 | 5 | 8.2 | |
| Asian/Pacific Islander | 1 | 5.6 | 8 | 13.1 | |
| Other | 0 | 0 | 1 | 1.6 | |
| Married/cohabitating | |||||
| Yes | 6 | 33.3 | 23 | 37.7 | 0.819 |
| No | 11 | 61.1 | 37 | 60.7 | |
| Missing | 1 | 5.6 | 1 | 1.6 | |
| Currently employed | |||||
| Yes | 7 | 38.9 | 29 | 47.5 | 0.481 |
| No | 11 | 61.1 | 31 | 50.8 | |
| Missing | 0 | 0 | 1 | 1.6 | |
| Branch of service | |||||
| Air Force | 0 | 0 | 6 | 9.8 | 0.277 |
| Army | 3 | 16.7 | 16 | 26.2 | |
| Marines | 6 | 33.3 | 22 | 36.1 | |
| Navy | 9 | 50.0 | 16 | 26.2 | |
| Other | 0 | 0 | 1 | 1.6 | |
| OEF/OIF/OND veteran | |||||
| Yes | 18 | 100.0 | 56 | 91.8 | 0.209 |
| No | 0 | 0 | 5 | 8.2 | |
| Combat exposure | |||||
| Yes | 10 | 55.6 | 44 | 72.1 | 0.184 |
| No | 8 | 44.4 | 17 | 27.9 | |
| TBI severity | |||||
| Mild | 15 | 83.3 | 54 | 88.5 | 0.561 |
| Moderate | 3 | 16.7 | 7 | 11.5 | |
| LOC (most sig. TBI) | |||||
| Yes | 9 | 50.0 | 40 | 65.6 | 0.232 |
| No | 9 | 50.0 | 21 | 34.4 | |
| AOC (most sig. TBI) | |||||
| Yes | 9 | 50.0 | 21 | 34.4 | 0.232 |
| No | 9 | 50.0 | 40 | 65.5 | |
| PTA (most sig. TBI) | |||||
| Yes | 10 | 55.6 | 33 | 54.1 | 0.423 |
| No | 8 | 44.4 | 22 | 36.1 | |
| Unsure | 0 | 0 | 5 | 8.2 | |
| Missing | 0 | 0 | 1 | 1.6 | |
| Lifetime diagnosis: MDDc | |||||
| Yes | 8 | 50.0 | 20 | 39.2 | 0.445 |
| No | 8 | 50.0 | 31 | 60.8 | |
| Lifetime diagnosis: PTSDc | |||||
| Yes | 8 | 53.3 | 23 | 45.1 | 0.574 |
| No | 7 | 46.7 | 28 | 54.9 |
Independent-samples t-tests were used to determine whether there were group differences for age, education, WRAT4 Reading, time from most recent TBI to testing, time from most significant TBI to testing, age at most significant TBI, and lifetime number of TBIs.
Chi-square analyses were used to determine whether there were group differences for sex, ethnicity, marital status, employment status, military branch of service, OEF/OIF/OND status, combat exposure, TBI severity, presence of LOC, presence of AOC, presence of PTA, lifetime diagnosis of MDD, and lifetime diagnosis of PTSD.
Lifetime diagnosis of MDD and PTSD were gathered through one-on-one interview using the Mini-International Neuropsychiatric Interview (M.I.N.I.; Sheehan and colleagues, 2006). Further, M.I.N.I. data were not available for all participants; MDD diagnostic information was available for 67 of the 79 TBI participants, and PTSD diagnostic information was available for 66 of the 79 TBI participants.
WRAT4, Wide Range Achievement Test 4; SS, standard score; TBI, traumatic brain injury; sig., significant; OEF, Operation Enduring Freedom; OIF, Operation Iraqi Freedom; OND, Operation New Dawn; LOC, loss of consciousness; AOC, alteration of consciousness; PTA, post-traumatic amnesia; MDD, Major Depressive Disorder; PTSD, post-traumatic stress disorder; M, mean; SD, standard deviation.
Psychiatric distress
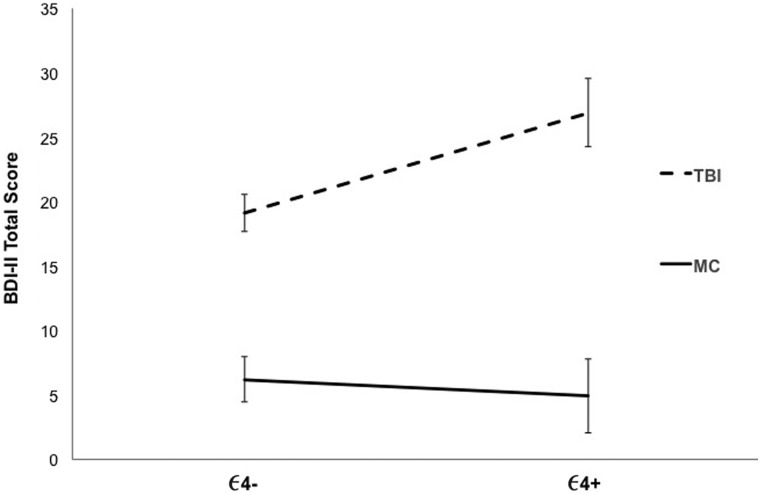
When examining the BDI-II total score, a main effect of group was found (F(1, 129) = 59.60; p < 0.001; ηp2 = 0.316), such that the total score was significantly greater for TBI participants (mean [M] = 20.89; SD = 12.87) than for MCs (M = 5.85; SD = 8.65). Although the main effect of ε4 allele status was not significant (F(1, 129) = 2.07; p = 0.152; ηp2 = 0.016), the interaction between group and ε4 allele status was significant (F(1, 129) = 4.01; p = 0.047; ηp2 = 0.030). Specifically, as depicted in Figure 1, TBI-ε4+ veterans had higher BDI-II scores than TBI-ε4– veterans (F(1, 142) = 6.77; p = 0.010; ηp2 = 0.050). In contrast, there were no significant group differences by APOE-ε4 status for MCs (F(1, 129) = 0.14; p = 0.708; ηp2 = 0.001).
FIG. 1.
BDI-II total score across traumatic brain injury (TBI) and military control (MC) participants by ε4 genotype. Mean scores with standard errors are displayed. BDI-II, Beck Depression Inventory, Second Edition.
A main effect of group was also found for the BAI total score (F(1, 129) = 40.43; p < 0.001; ηp2 = 0.239), such that BAI scores were significantly greater for TBI participants (M = 13.43; SD = 11.18) than for MCs (M = 3.52; SD = 6.79). Additionally, although there was no main effect of ε4 allele status (F(1, 129) = 2.03; p = 0.157; ηp2 = 0.015), the interaction between group and ε4 allele status was significant (F(1, 129) = 4.99; p = 0.027; ηp2 = 0.037). For TBI participants, ε4+ veterans had higher BAI scores than ε4– veterans (F(1, 129) = 7.64; p = 0.007; ηp2 = 0.056); for MCs, ε4 status had no effect (F(1, 129) = 0.29; p = .590; ηp2 = 0.002; see Fig. 2).
FIG. 2.
BAI total score across traumatic brain injury (TBI) and military control (MC) participants by ε4 genotype. Mean scores with standard errors are displayed. BAI, Beck Anxiety Inventory.
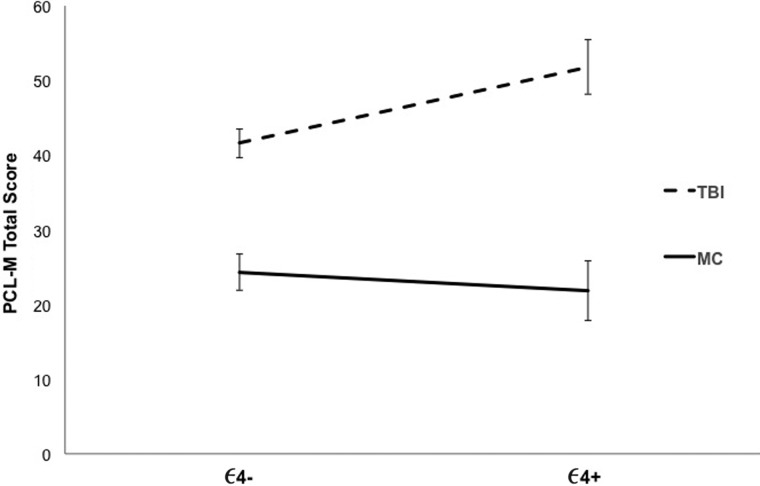
When examining PCL-M total score, a main effect of group was found (F(1, 129) = 57.43; p < 0.001; ηp2 < 0.308). Similar to the above results, PCL-M total score was significantly greater for TBI participants (M = 43.90; SD = 17.67) than for MCs (M = 23.65; SD = 11.99). There was no main effect of ε4 allele status (F(1, 129) = 1.51; p = 0.222; ηp2 = 0.012); however, the interaction between group and ε4 allele status was significant (F(1, 129) = 4.20; p = 0.042; ηp2 = 0.032). As seen in Figure 3, for TBI participants, ε4+ veterans had higher PCL-M scores than ε4– veterans (F(1, 129) = 6.13; p = 0.015; ηp2 = 0.045); for MCs, ε4 status had no effect (F(1, 129) = 0.30; p = 0.585; ηp2 = 0.002).
FIG. 3.
PCL-M total score across traumatic brain injury (TBI) and military control (MC) participants by ε4 genotype. Mean scores with standard errors are displayed. PCL-M, PTSD Checklist-Military Version.
Importantly, removal of participants with moderate TBI from the analyses revealed the same pattern of results. Specifically, there continued to be a significant main effect of group across all symptom measures (TBI > MC; all p values <0.001; ηp2 = 0.236–0.318) and no main effect of ε4 genotype (p = 0.147–0.345; ηp2 = 0.007–0.018). Group by ε4 genotype interaction remained significant for the BAI total score (p = 0.024; ηp2 = 0.042) and approached significance (trend) for the BDI-II total score (p = 0.058; ηp2 = 0.030) and PCL-M total score (p = 0.083; ηp2 = 0.025). APOE-ε4+ veterans with a history of mild TBI demonstrated significantly higher symptom scores on the BAI and BDI-II when compared to ε4– veterans with a history of mild TBI (BAI: p = 0.008; ηp2 = 0.058; BDI-II: p = 0.020; ηp2 = 0.045), and there was a trend in the same direction for the PCL-M (p = 0.054; ηp2 = 0.031). ε4 status had no effect on severity of psychiatric symptom scores for MC participants (p = 0.566–0.700; ηp2 = 0.001–0.003).
Secondary analyses
Given the observed differences in combat exposure and ethnicity between the TBI and MC samples as described above, analyses of covariance were conducted on the original sample. When controlling for combat exposure and ethnicity, a similar pattern of results was again observed—for TBI participants, ε4+ veterans demonstrated significantly higher symptom scores across all measures when compared to ε4– veterans (p = 0.008–0.015); for MC participants, ε4 status had no effect on the severity of psychiatric symptom scores (p = 0.488–0.682).
Discussion
To our knowledge, this study represents the first to explore associations between APOE genotype status and neuropsychiatric symptoms in the context of military TBI. As expected, consistent with several previous studies showing increased rates of psychiatric symptoms in military service members with TBI histories,1,3,4,7 our results showed that veterans with a reported history of TBI endorsed greater symptoms of depression, anxiety, and PTSD relative to MCs. Additionally, although a main effect was not found for ε4 genotype across veterans, a TBI by APOE-ε4 interaction was demonstrated, such that those with history of TBI and APOE-ε4 positivity showed the greatest level of psychiatric symptomatology across all measures administered. That is, among veterans with a history of neurotrauma, greater levels of anxiety, depression, and PTSD symptoms were found in those with an ε4 allele relative to those without an ε4 allele. Importantly, there was no effect of APOE-ε4 in those without a history of TBI. Given the proposed role of the APOE gene, and its purported mechanism of action post-CNS insult,28,32 these findings bolster the hypothesis that, in the aftermath of neurological insult (e.g., TBI), those with an ε4 allele are at risk for more adverse outcomes relative to ε4– individuals.24,25,31
Although, to our knowledge, the current study represents the first to examine the relationship between the APOE gene and neuropsychiatric sequelae in veterans with TBI, Lyons and colleagues69 demonstrated that those with a higher level of combat exposure and APOE-ε4 genotype status were at greatest risk for experiencing symptoms of PTSD. More recently, Kimbrel and colleagues70 found that the influence of the ε4 allele differed by level of combat exposure, such that those who experienced high levels of combat and ε4-positivity 1) were more likely to be diagnosed with PTSD and 2) demonstrated more-severe PTSD symptoms. Interestingly, when combat exposure was low, the ε4 allele had no effect on psychiatric outcome. Although these findings only held in non-Hispanic blacks as compared to non-Hispanic whites, the overall findings from Lyons and colleagues69 and Kimbrel and colleagues70 are generally consistent with the results of our study showing that ε4 genotype in the context of neurotrauma (e.g., TBI or combat exposure) may predispose individuals to increased psychiatric distress (e.g., PTSD, depression, anxiety, etc.).
Beyond establishing a specific relationship between the presence of the ε4 allele and neuropsychiatric sequelae, the results from the present study also lend support to the broader theory that genetic factors influence psychiatric distress post-TBI. However, it is likely that still other biological and environmental contributions may impact the development of symptoms in this population.71,72 For instance, pre-morbid levels of psychiatric distress,14,73 degree of resilience,73,74 personality factors,75–77 and intellectual functioning or cognitive reserve78,79 may also be important moderators or mediators in this relationship. Importantly, our results show that ε4+ and ε4– participants with TBI did not differ from one another with respect to either lifetime diagnosis of depression (i.e., MDD) or PTSD. Thus, our finding of an association between greater levels of self-reported psychiatric symptomatology in TBI veterans with TBI histories was not merely driven by greater overall psychiatric distress reflected by psychiatric disorder diagnoses in the ε4 group. The relationship between TBI and the emergence of psychiatric distress after brain injury is likely considerably complex, and the present findings provide a first attempt to better understand and elucidate a particular susceptibility gene that may be important in this multi-faceted relationship.
Although speculative, there are several potential neurobiological or brain-based mechanisms that could explain the observed interaction between neurotrauma and APOE-ε4 genotype on psychiatric distress. One possibility is that the ε4 allele may exert many of its effects through frontal subcortical regions, which are often impacted post-TBI and are heavily involved in affective states, emotion regulation, and psychiatric distress.20 Additionally, associations between APOE ε4 genotype and vascular risk factors on the development of depression have been proposed, such that ε4 positivity enhances risk for vascular disease, thereby increasing risk for depression (for a review, see Panza and colleagues80). Another consideration may be that there is a link between early neurodegenerative processes and APOE ε4 status (e.g., promoting deposition of abnormal amyloid and tau protein species) that influences neuropsychiatric distress through limbic insult. Whereas additional brain-based mechanisms may also be at play, these theories may help to explain why we observe increased rates of psychiatric distress in many veterans with a history of neurotrauma relative to those without trauma histories. We are pursuing these questions through ongoing research in our laboratory.
Taken together, results of our study show compelling evidence that APOE-ε4 positivity may be more deleterious for those who have experienced neurotrauma versus those without TBI histories. Study strengths include a relatively large, well-characterized sample of veterans with a history of TBI, examination of mental health symptoms beyond PTSD to better assess the magnitude of psychiatric distress in veterans, and examination of participants who are well outside of the acute stage of injury to increase understanding of the long-term effects of APOE polymorphisms on TBI outcome. However, there are some limitations of the study that should be noted. First, although our male/female ratios are higher than many existing military TBI studies, the majority of participants were male veterans (∼80%), thus reducing the generalizability to females. We also examined milder forms of TBI, and it is therefore unclear whether similar findings would be observed in individuals with more severe TBI. Additionally, longitudinal studies are needed in order to improve our understanding of the timeline associated with the detrimental effects of the ε4 allele (i.e., how soon after injury and for how long after injury are these effects present?). Moreover, we relied on participants' self-report to characterize TBI severity and history (e.g., timing of injuries, number of injuries sustained, etc.). A final limitation is our relatively small sample size; however, the number of participants analyzed in this study is comparable—and in some cases larger than—previously published studies examining the effect of APOE genotype in the context of TBI.44–46,81 Nevertheless, it will be important for these findings to be replicated using larger samples. Relatedly, given the low number of participants who were homozygous for the ε4 allele (in the overall sample of 133, only 3 participants were ε4/ε4), concordant with its low frequency in the population, we were not able to assess a dose-response type of influence of the ε4 allele, although this is a future direction for our research.
In conclusion, a growing body of literature has emerged over the past several years that has established an association between TBI and symptoms of psychiatric distress. Although many studies have begun to investigate potential etiologies of this relationship, few studies have examined the relationship between genetics and the development or maintenance of neuropsychiatric sequelae post-TBI. This study is the first to show that the APOE-ε4 allele is a risk factor for the development of neuropsychiatric symptoms in veterans with a history of TBI. Importantly, these findings do not appear to be attributed to fundamental differences in demographic or injury severity characteristics, given that groups were equivalent across these variables. Moreover, findings held even after removing veterans with moderate TBI from the analyses. Although the present study furthers our understanding of how the APOE ε4 allele contributes to the emergence of mental health symptoms post-TBI, future longitudinal studies are necessary in order to more fully understand the association of APOE genotype status and psychiatric distress in the context of TBI.
Acknowledgments
This work was supported by Veterans Affairs grants awarded to Drs. Delano-Wood (829-MR-NB-25860), Schiehser (CDA-2-065-10S), and Sorg (CDA-2-CX001508). This work was further supported by grants awarded by the Department of Defense (W81XWH-10-2-0169) to Dr. Delano-Wood and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (F31NS09870) to Ms. Clark.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bryan C.J., and Clemans T.A. (2013). Repetitive traumatic brain injury, psychological symptoms, and suicide risk in a clinical sample of deployed military personnel. JAMA Psychiatry 70, 686–691 [DOI] [PubMed] [Google Scholar]
- 2.Hesdorffer D.C., Rauch S.L., and Tamminga C.A. (2009). Long‐term psychiatric outcomes following traumatic brain injury: a review of the literature. J. Head Trauma Rehabil. 24, 452–459 [DOI] [PubMed] [Google Scholar]
- 3.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in US soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 4.Yurgil K.A., Barkauskas D.A., Vasterling J.J., Nievergelt C.M., Larson G.E., Schork N.J., Litz B.T., Nash W.P., and Baker D.G. (2014). Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry 71, 149–157 [DOI] [PubMed] [Google Scholar]
- 5.Rogers J.M., and Read C.A. (2007). Psychiatric comorbidity following traumatic brain injury. Brain Inj. 21, 1321–1333 [DOI] [PubMed] [Google Scholar]
- 6.Carlson K.F., Kehle S.M., Meis L.A., Greer N., MacDonald R., Rutks I., Sayer N.A., Dobscha S.K., and Wilt T.J. (2011). Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J. Head Trauma Rehabil. 26, 103–115 [DOI] [PubMed] [Google Scholar]
- 7.Carlson K.F., Nelson D., Orazem R.J., Nugent S., Cifu D.X., and Sayer N.A. (2010). Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment‐related traumatic brain injury. J. Trauma Stress 23, 17–24 [DOI] [PubMed] [Google Scholar]
- 8.Vasterling J.J., Brailey K., Proctor S.P., Kane R., Heeren T., and Franz M. (2012). Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br. J. Psychiatry 201, 186–192 [DOI] [PubMed] [Google Scholar]
- 9.Morissette S.B., Woodward M., Kimbrel N.A., Meyer E.C., Kruse M.I., Dolan S., and Gulliver S.B. (2011). Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabil. Psychol. 56, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould K., Ponsford J., Johnston L., and Schönberger M. (2011). The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychol. Med. 41, 2099–2109 [DOI] [PubMed] [Google Scholar]
- 11.Warriner E.M., and Velikonja D. (2006). Psychiatric disturbances after traumatic brain injury: neurobehavioral and personality changes. Curr. Psychiatry Rep. 8, 73–80 [DOI] [PubMed] [Google Scholar]
- 12.Whelan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 13.Cooper D.B., Kennedy J.E., Cullen M.A., Critchfield E., Amador R.R., and Bowles A.O. (2011). Association between combat stress and post-concussive symptom reporting in OEF/OIF service members with mild traumatic brain injuries. Brain Inj. 25, 1–7 [DOI] [PubMed] [Google Scholar]
- 14.Ponsford J., Willmott C., Rothwell A., Cameron P., Kelly A.-M., Nelms R., Curran C., and Ng K. (2000). Factors influencing outcome following mild traumatic brain injury in adults. J. Int. Neuropsychol. Soc. 6, 568–579 [DOI] [PubMed] [Google Scholar]
- 15.Reid M.W., Miller K.J., Lange R.T., Cooper D.B., Tate D.F., Bailie J., Brickell T.A., French L.M., Asmussen S., and Kennedy J.E. (2014). A multisite study of the relationships between blast exposures and symptom reporting in a post-deployment active duty military population with mild traumatic brain injury. J. Neurotrauma 31, 1899–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneiderman A.I., Braver E.R., and Kang H.K. (2008). Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 167, 1446–1452 [DOI] [PubMed] [Google Scholar]
- 17.Hart T., Benn E.K., Bagiella E., Arenth P., Dikmen S., Hesdorffer D.C., Novack T.A., Ricker J.H., and Zafonte R. (2014). Early trajectory of psychiatric symptoms after traumatic brain injury: relationship to patient and injury characteristics. J. Neurotrauma 31, 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkhoudarian G., Hovda D.A., and Giza C.C. (2011). The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 30, 33–48 [DOI] [PubMed] [Google Scholar]
- 19.Bigler E.D. (2008). Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 14, 1–22 [DOI] [PubMed] [Google Scholar]
- 20.Vasterling J.j., Verfaellie M., and Sullivan K.D. (2009). Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin. Psychol. Rev. 29, 674–684 [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Arrastia R., and Baxter V.K. (2006). Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J. Head Trauma Rehabil. 21, 361–374 [DOI] [PubMed] [Google Scholar]
- 22.Dretsch M.N., Williams K., Emmerich T., Crynen G., Ait‐Ghezala G., Chaytow H., Mathura V., Crawford F.C., and Iverson G.L. (2016). Brain‐derived neurotropic factor polymorphisms, traumatic stress, mild traumatic brain injury, and combat exposure contribute to postdeployment traumatic stress. Brain Behav. 6, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAllister T.W. (2011). Genetic factors, in: Textbook of Traumatic Brain Injury, 2nd ed. Silver J.M., McAllister T.W., and Yudofsky S.C. (eds). American Psychiatric Publishing: Washington, DC, pps. 37–48 [Google Scholar]
- 24.Dardiotis E., Fountas K.N., Dardioti M., Xiromerisiou G., Kapsalaki E., Tasiou A., and Hadjigeorgiou G.M. (2010). Genetic association studies in patients with traumatic brain injury. Neurosurg. Focus 28, 1–12 [DOI] [PubMed] [Google Scholar]
- 25.Jordan B.D. (2007). Genetic influences on outcome following traumatic brain injury. Neurochem. Res. 32, 905–915 [DOI] [PubMed] [Google Scholar]
- 26.Lawrence D.W., Comper P., Hutchison M.G., and Sharma B. (2015). The role of apolipoprotein E episilon (ɛ)-4 allele on outcome following traumatic brain injury: a systematic review. Brain Inj. 29, 1018–1031 [DOI] [PubMed] [Google Scholar]
- 27.Mahley R.W., Nathan B., and Pitas R. (1996). Apolipoprotein E structure, function, and possible roles in Alzheimer's disease. Ann. N. Y. Acad. Sci. 777, 139–145 [DOI] [PubMed] [Google Scholar]
- 28.Mahley R.W., Weisgraber K.H., and Huang Y. (2006). Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 103, 5644–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blennow K., Hardy J., and Zetterberg H. (2012). The neuropathology and neurobiology of traumatic brain injury. Neuron 76, 886–899 [DOI] [PubMed] [Google Scholar]
- 30.Horsburgh K., Fitzpatrick M., Nilsen M., and Nicoll J.A. (1997). Marked alterations in the cellular localisation and levels of apolipoprotein E following acute subdural haematoma in rat. Brain Res. 763, 103–110 [DOI] [PubMed] [Google Scholar]
- 31.Wilson M., and Montgomery H. (2007). Impact of genetic factors on outcome from brain injury. Br. J. Anaesth. 99, 43–48 [DOI] [PubMed] [Google Scholar]
- 32.Mahley R.W., and Huang Y. (2012). Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron 76, 871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan B., Bellosta D., Weisgraber K., Mahley R., and Pitas R. (1994). Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Electroencephalogr. Clin. Neurophysiol. 264, 850–852 [DOI] [PubMed] [Google Scholar]
- 34.Bellosta S., Nathan B., Orth M., Dong L., Mahley R., and Pitas R. (1995). Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J. Biol. Chem. 270, 27063–27071 [DOI] [PubMed] [Google Scholar]
- 35.Finnoff J.T., Jelsing E.J., and Smith J. (2011). Biomarkers, genetics, and risk factors for concussion. PM R. 3, 10 Suppl. 2, S452–S459 [DOI] [PubMed] [Google Scholar]
- 36.Crawford F., Wood M., Ferguson S., Mathura V., Gupta P., Humphrey J., Mouzon B., Laporte V., Margenthaler E., and O'Steen B. (2009). Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience 159, 1349–1362 [DOI] [PubMed] [Google Scholar]
- 37.Verghese P.B., Castellano J.M., and Holtzman D.M. (2011). Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 10, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J., Basak J.M., and Holtzman D.M. (2009). The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang M.-F., Chang J.-G., and Hu C.-J. (2003). Association between apolipoprotein E genotype and outcome of traumatic brain injury. Acta Neurochir. 145, 649–654 [DOI] [PubMed] [Google Scholar]
- 40.Friedman G., Froom P., Sazbon L., Grinblatt I., Shochina M., Tsenter J., Babaey S., Yehuda A.B., and Groswasser Z. (1999). Apolipoprotein E-ɛ4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 52, 244–244 [DOI] [PubMed] [Google Scholar]
- 41.Teasdale G.M., Nicoll J.A., Murray G., and Fiddes M. (1997). Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 350, 1069–1071 [DOI] [PubMed] [Google Scholar]
- 42.Liaquat I., Dunn L.T., Nicoll J.A., Teasdale G.M., and Norrie J.D. (2002). Effect of apolipoprotein E genotype on hematoma volume after trauma. J. Neurosurg. 96, 90–96 [DOI] [PubMed] [Google Scholar]
- 43.Lichtman S., Seliger G., Tycko B., and Marder K. (2000). Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation. Neurology 55, 1536–1539 [DOI] [PubMed] [Google Scholar]
- 44.Ariza M., Pueyo R., del M Matarín M., Junqué C., Mataró M., Clemente I., Moral P., Poca M.A., Garnacho Á., and Sahuquillo J. (2006). Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 77, 1191–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford F., Vanderploeg R., Freeman M., Singh S., Waisman M., Michaels L., Abdullah L., Warden D., Lipsky R., and Salazar A. (2002). APOE genotype influences acquisition and recall following traumatic brain injury. Neurology 58, 1115–1118 [DOI] [PubMed] [Google Scholar]
- 46.Liberman J.N., Stewart W.F., Wesnes K., and Troncoso J. (2002). Apolipoprotein E ɛ4 and short-term recovery from predominantly mild brain injury. Neurology 58, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 47.Sundström A., Marklund P., Nilsson L.-G., Cruts M., Adolfsson R., Van Broeckhoven C., and Nyberg L. (2004). APOE influences on neuropsychological function after mild head injury within-person comparisons. Neurology 62, 1963–1966 [DOI] [PubMed] [Google Scholar]
- 48.Koponen S., Taiminen T., Kairisto V., Portin R., Isoniemi H., Hinkka S., and Tenovuo O. (2004). APOE-ɛ4 predicts dementia but not other psychiatric disorders after traumatic brain injury. Neurology 63, 749–750 [DOI] [PubMed] [Google Scholar]
- 49.Luukinen H., Jokelainen J., Kervinen K., Kesäniemi Y., Winqvist S., and Hillbom M. (2008). Risk of dementia associated with the ApoE ɛ4 allele and falls causing head injury without explicit traumatic brain injury. Acta Neurol. Scand. 118, 153–158 [DOI] [PubMed] [Google Scholar]
- 50.Nicoll J., Roberts G., and Graham D. (1996). Amyloid β‐protein, APOE genotype and head injury. Ann. N. Y. Acad. Sci. 777, 271–275 [DOI] [PubMed] [Google Scholar]
- 51.Vanderploeg R.D., Groer S., and Belanger H.G. (2012). Initial developmental process of a VA semistructured clinical interview for TBI identification. J. Rehabil. Res. Dev. 49, 545–556 [DOI] [PubMed] [Google Scholar]
- 52.The Management of Concussion/mTBI Working Group. (2009). VA/DoD clinical practice guideline for the management of concussion/mild traumatic brain injury (mTBI): Guideline summary. Department of Veterans Affairs and Department of Defense: Washington, DC [Google Scholar]
- 53.American Psyciatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. American Psychiatic Association: Washington, DC [Google Scholar]
- 54.Tombaugh T.N. (1996). Test of Memory Malingering: TOMM. Multi-Health Systems North: Tonawanda, NY [Google Scholar]
- 55.Delis D., Kramer J., Kaplan E., and Ober B. (2000). California Verbal Learning Test: Adult Version Manual (2nd ed.). Psychological Corporation: San Antonio, TX [Google Scholar]
- 56.Saunders A.M., Strittmatter W.J., Schmechel D., George-Hyslop P.S., Pericak-Vance M., Joo S., Rosi B., Gusella J., Crapper-MacLachlan D., and Alberts M. (1993). Association of apolipoprotein E allele ε4 with late‐onset familial and sporadic Alzheimer's disease. Neurology 43, 1467–1467 [DOI] [PubMed] [Google Scholar]
- 57.Beck A.T., Steer R.A., and Brown G. (1996). Manual for the Beck Depression Inventory Second Edition (BDI-II). Psychological Corporation: San Antonio, TX [Google Scholar]
- 58.Storch E.A., Roberti J.W., and Roth D.A. (2004). Factor structure, concurrent validity, and internal consistency of the Beck Depression Inventory—second edition in a sample of college students. Depress. Anxiety 19, 187–189 [DOI] [PubMed] [Google Scholar]
- 59.Beck A.T., Epstein N., Brown G., and Steer R.A. (1988). An inventory for measuring clinical anxiety: psychometrie properties. J. Consult. Clin. Psychol. 56, 893–897 [DOI] [PubMed] [Google Scholar]
- 60.Beck A.T., and Steer R.A. (1991). Relationship between the Beck Anxiety Inventory and the Hamilton Anxiety Rating Scale with anxious outpatients. J. Anxiety Disord. 5, 213–223 [Google Scholar]
- 61.Fydrich T., Dowdall D., and Chambless D.L. (1992). Reliability and validity of the Beck Anxiety Inventory. J. Anxiety Disord. 6, 55–61 [Google Scholar]
- 62.Leyfer O.T., Ruberg J.L., and Woodruff-Borden J. (2006). Examination of the utility of the Beck Anxiety Inventory and its factors as a screener for anxiety disorders. J. Anxiety Disord. 20, 444–458 [DOI] [PubMed] [Google Scholar]
- 63.Weathers F.W., Huska J.A., and Keane T.M. (1991). PCL-M for DSM-IV. National Center for PTSD-Behavioral Science Division: Boston, MA [Google Scholar]
- 64.Blanchard E.B., Jones-Alexander J., Buckley T.C., and Forneris C.A. (1996). Psychometric properties of the PTSD Checklist (PCL). Behav. Res. Ther. 34, 669–673 [DOI] [PubMed] [Google Scholar]
- 65.Bliese P.D., Wright K.M., Adler A.B., Cabrera O., Castro C.A., and Hoge C.W. (2008). Validating the primary care posttraumatic stress disorder screen and the Posttraumatic Stress Disorder Checklist with soldiers returning from combat. J. Consult. Clin. Psychol. 76, 272–281 [DOI] [PubMed] [Google Scholar]
- 66.Keen S.M., Kutter C.J., Niles B.L., and Krinsley K.E. (2008). Psychometric properties of PTSD Checklist in sample of male veterans. J. Rehabil. Res. Dev. 45, 465–474 [DOI] [PubMed] [Google Scholar]
- 67.Wilkins K.C., Lang A.J., and Norman S.B. (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety 28, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkinson G.S., and Robertson G. (2006). Wide Range Achievement Test 4 (WRAT4). Psychological Assessment Resources, Inc.: Lutz, FL [Google Scholar]
- 69.Lyons M.J., Genderson M., Grant M.D., Logue M., Zink T., McKenzie R., Franz C.E., Panizzon M., Lohr J.B., and Jerskey B. (2013). Gene‐environment interaction of ApoE genotype and combat exposure on PTSD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162, 762–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimbrel N.A., Hauser M.A., Garrett M., Ashley‐Koch A., Liu Y., Dennis M.F., Klein R.C., and Beckham J.C. (2015). Effect of the APOE ɛ4 allele and combat exposure on PTSD among Iraq/Afghanistan-era veterans. Depress. Anxiety 32, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iverson G.L. (2012). A biopsychosocial conceptualization of poor outcome from mild traumatic brain injury, in: PTSD and Mild Traumatic Brain Injury. R. Bryant, and Keane T.M. (eds). Guilford: New York, NY, pps. 37–60 [Google Scholar]
- 72.Wäljas M., Iverson G.L., Lange R.T., Hakulinen U., Dastidar P., Huhtala H., Liimatainen S., Hartikainen K., and Öhman J. (2015). A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J. Neurotrauma 32, 534–547 [DOI] [PubMed] [Google Scholar]
- 73.McCauley S.R., Wilde E.A., Miller E.R., Frisby M.L., Garza H.M., Varghese R., Levin H.S., Robertson C.S., and McCarthy J.J. (2013). Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J. Neurotrauma 30, 642–652 [DOI] [PubMed] [Google Scholar]
- 74.Merritt V.C., Lange R.T., and French L.M. (2015). Resilience and symptom reporting following mild traumatic brain injury in military service members. Brain Inj. 29, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 75.Kennedy J.E., Cooper D.B., Reid M.W., Tate D.F., and Lange R.T. (2015). Profile analyses of the Personality Assessment Inventory following military-related traumatic brain injury. Arch Clin. Neuropsychol. 30, 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tate R.L. (2003). Impact of pre-injury factors on outcome after severe traumatic brain injury: does post-traumatic personality change represent an exacerbation of premorbid traits? Neuropsychol. Rehabil. 13, 43–64 [DOI] [PubMed] [Google Scholar]
- 77.Sela-Kaufman M., Rassovsky Y., Agranov E., Levi Y., and Vakil E. (2013). Premorbid personality characteristics and attachment style moderate the effect of injury severity on occupational outcome in traumatic brain injury: another aspect of reserve. J. Clin. Exp. Neuropsychol. 35, 584–595 [DOI] [PubMed] [Google Scholar]
- 78.Kesler S.R., Adams H.F., Blasey C.M., and Bigler E.D. (2003). Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl. Neuropsychol. 10, 153–162 [DOI] [PubMed] [Google Scholar]
- 79.Rassovsky Y., Levi Y., Agranov E., Sela-Kaufman M., Sverdlik A., and Vakil E. (2015). Predicting long-term outcome following traumatic brain injury (TBI). J. Clin. Exp. Neuropsychol. 37, 354–366 [DOI] [PubMed] [Google Scholar]
- 80.Panza F., Frisardi V., Seripa D., D'Onofrio G., Santamato A., Masullo C., Logroscino G., Solfrizzi V., and Pilotto A. (2012). Apolipoprotein E genotypes and neuropsychiatric symptoms and syndromes in late-onset Alzheimer's disease. Ageing Res. Rev. 11, 87–103 [DOI] [PubMed] [Google Scholar]
- 81.Hodgkinson A., Gillett L., and Simpson G.K. (2009). Does Apolipoprotein E play a role in otucome after severe traumatic brain injury? Brain Impair. 10, 162–168 [Google Scholar]