Abstract
Parkinson's disease is typically treated with oral dopamine replacement therapies. However, long-term use is complicated by motor fluctuations from intermittent stimulation of dopamine receptors and off-target effects. ProSavin, a lentiviral vector based gene therapy that delivers local and continuous dopamine, was previously shown to be well tolerated in a Phase I/II first-in-human study, with significant improvements in motor behavior from baseline at 1 year. Here, patients with Parkinson's disease from the open-label trial were followed up in the long term to assess the safety and efficacy of ProSavin after bilateral injection into the putamen. Fifteen patients who were previously treated with ProSavin have been followed for up to 5 years, with some having been seen for 8 years. Eight patients received deep brain stimulation at different time points, and their subsequent assessments continued to assess safety. Ninety-six drug-related adverse events were reported (87 mild, 6 moderate, 3 severe) of which more than half occurred in the first year. The most common drug-related events were dyskinesias (33 events, 11 patients) and on–off phenomena (22 events, 11 patients). A significant improvement in the defined “off” Unified Parkinson's Disease Rating Scale part III motor scores, compared to baseline, was seen at 2 years (mean score 29 · 2 vs. 38 · 4, n = 14, p < 0.05) and at 4 years in 8/15 patients. ProSavin continued to be safe and well tolerated in patients with Parkinson's disease. Moderate improvements in motor behavior over baseline continued to be reported in the majority of patients who could still be evaluated up to 5 years of follow-up.
Keywords: : gene therapy, lentiviral vector, Parkinson's disease, dopamine
Introduction
The core motor features of Parkinson's disease are associated with a loss of dopaminergic neurons in the substantia nigra pars compacta and their projection to the posterior striatum where dopamine is released.1,2 Current Parkinson's disease treatments focus on the acute restoration of dopaminergic activity in the striatum through daily oral administration of the dopamine precursor L-Dopa, and/or dopaminergic agonists.3 Although such treatments provide good control of many of the motor features in the initial stages of the disease, L-Dopa therapy becomes less reliable with disease progression as a range of motor and non-motor complications emerge, such as on–off phenomena, peak-dose dyskinesias, and a range of neuropsychiatric and cognitive problems.4,5 A therapeutic approach that restores long-term continuous dopaminergic function, restricted to the dopamine-depleted striatum, may provide an effective and durable treatment while minimizing off-target effects.6
Gene therapy approaches for Parkinson's disease using adeno-associated virus vectors have been shown to be well tolerated in early-phase clinical trials of mid- to late-stage patients.7–12 The therapeutic rationale for these studies was based on: (i) neuroprotection of nigral dopaminergic neurons and their projections through delivery of genes encoding a neurotrophic factor,10,13 (ii) enhanced conversion of L-Dopa to dopamine by gene transfer of the enzyme aromatic amino acid decarboxylase (AADC),7–9 or (iii) modulation of basal ganglia outputs by delivery of glutamic acid decarboxylase (GAD) to the subthalamic nucleus.11,12 Although Phase I studies reported encouraging efficacy data, follow-up studies showed no or modest difference in motor improvements compared to placebo-control patients.12,13
The 1 year results were previously reported of the open-label part of a Phase I/II clinical trial evaluating the safety and efficacy of ProSavin—an equine infectious anemia virus (EIAV)-based gene therapy approach aimed at local and continuous dopamine replacement to the motor striatum of Parkinson's disease patients.14 The ProSavin vector encodes the three dopamine biosynthetic enzymes—tyrosine hydroxylase (TH), AADC, and GTP-cyclohydrolase 1 (CH1)—and has been shown to convert transduced non-dopaminergic striatal neurons into dopamine-producing cells.15
This clinical study showed that ProSavin was well tolerated, with promising indications of efficacy.14 Here, the long-term follow-up of these patients is reported, including assessments of safety and efficacy in 13 patients up to at least 5 years, and in some cases to 8 years, post treatment with ProSavin.
Methods
Study design
A detailed description of the Phase I/II study has been previously published.14 All patients were subsequently enrolled into this open-label follow-up study to investigate the long-term safety and efficacy of ProSavin for up to 10 years post treatment. The study protocols were approved by the ethics committee of each participating institution and complied with the Declaration of Helsinki, current Good Clinical Practice guidelines, and local laws and regulations. The Phase I/II study and ongoing open-label follow-up are registered with the ClinicalTrials.gov registry (NTC00627588 and NCT01856439; EudraCT numbers: 2007-001109-26 and 2009-017253-35).
Participants
As previously described, 15 patients with idiopathic Parkinson's disease, as defined by the diagnostic criteria from the core assessment program for surgical interventional therapies (CAPSIT [1999]), received ProSavin in one of four dose cohorts.14 Entry criteria included: age between 48 and 65 years, disease duration of at least 5 years, Hoehn and Yahr stage 3 or 4 in the off state, Unified Parkinson's Disease Rating Scale (UPDRS) part III (off) scores between 20 and 60, motor complications associated with L-Dopa therapy, a stable medication regimen for at least 4 weeks prior to surgery, and ≥50% improvement in the UPDRS part III score between the off and on states in response to an acute L-Dopa challenge (Table 1).
Table 1.
Baseline demographic data
| Patients | Cohort | Age (years) | Disease duration (years) | UPDRS motor score, off/on | Total UPDRS score, off/on | L-Dopa equivalent dose |
|---|---|---|---|---|---|---|
| L1 | 1 | 62 | 8 | 23/6 | 49/19 | 2,547 |
| L2 | 1 | 57 | 8 | 30/8 | 61/24 | 1,329 |
| L3 | 1 | 58 | 16 | 28/11 | 70/35 | 1,998 |
| M4 | 2a | 57 | 17 | 29/8 | 63/20 | 2,164 |
| M5 | 2a | 56 | 12 | 30/14 | 58/24 | 1,572 |
| M6 | 2a | 49 | 9 | 34/7 | 74/23 | 2,523 |
| M7 | 2b | 64 | 9 | 49/19 | 83/31 | 1,785 |
| M8 | 2b | 59 | 13 | 38/15 | 67/31 | 1,088 |
| M9 | 2b | 57 | 15 | 46/9 | 68/20 | 1,775 |
| H10 | 3 | 48 | 22 | 37/8 | 59/21 | 1,535 |
| H11 | 3 | 58 | 10 | 35/10 | 71/27 | 1,844 |
| H12 | 3 | 61 | 26 | 52/13 | 91/25 | 1,180 |
| H13 | 3 | 63 | 16 | 49/23 | 90/52 | 1,691 |
| H14 | 3 | 57 | 19 | 52/23 | 94/47 | 699 |
| H15 | 3 | 55 | 9 | 44/18 | 71/30 | 1,593 |
| Mean | NA | 57.4 | 13.9 | 38/13 | 71/29 | 1,688 |
| SD | NA | 4.47 | 5.47 | 9.61/5.70 | 13.17/9.74 | 505.03 |
Patients are listed in the order in which they received treatment.
UPDRS, Unified Parkinson's Disease Rating Scale; L, low (1.9 × 107 TU); M, mid (4.0 × 107 TU); H, high dose (1 × 108 TU); Off, off-medication state; On, on-medication state.
Procedure
All patients received ProSavin via bilateral injections into the striatum under general anesthesia. Three dose levels of ProSavin were assessed in four patient cohorts. Three patients were included at dose level 1 (low dose, 2 × 107 transducing units [TU]; cohort 1), six patients at dose level 2 (mid dose, 4 × 107 TU; cohorts 2a and 2b), and six patients at dose level 3 (high dose, 1 × 108 TU; cohort 3). A modified delivery method of administration was introduced for cohorts 2b and 3 to increase the rate of delivery and enhance the distribution of the vector. The modified delivery method included a change from 1 to 3 μL/min, with a reduction of diameter of the cannula from 25 to 28 gauge and from five needle tracks (each with five depots each) to three needle tracks (each with one depot; see Supplementary Data; Supplementary Data are available online at www.liebertpub.com/humc).
Outcomes
The primary endpoints of the Phase I/II study were the number and severity of adverse events (AEs) associated with ProSavin administration and motor responses as assessed using the UPDRS part III in the defined off state 6 months post vector administration. The UPDRS is a widely used assessment tool used in patients with Parkinson's disease. It is made up of six different sections. Part I evaluates mentation, behavior, and mood. Part II evaluates activities of daily life (ADLs), including speech, swallowing, handwriting, dressing, hygiene, falling, levels of salivation, turning in bed, walking, and cutting food. Part III is a clinician-scored itemized motor evaluation. Part IV evaluates the complications of dopaminergic therapy. Part V gives a Hoehn and Yahr staging of the severity of the Parkinson's disease. Part VI contains the Schwab and England ADL scale. Patients were thereafter evaluated at least every 6 months for 3 years and thereafter on an annual basis (which will be up to 10 years in the open-label follow-up). AEs were assessed at every visit, and all events were recorded, including those that were reported spontaneously or on general questioning and those observed directly by the investigators. Efficacy assessments, including the UPDRS parts I, II, and III (in the off and on states) and UPDRS part IV, were done at least every 6 months up to 3 years and yearly thereafter up to year 6, with a final assessment planned at year 10. Eight patients have received deep brain stimulation (DBS) at different time points post ProSavin injections, and subsequent assessments for these patients were not included in the UPDRS analysis. UPDRS off assessments were performed in the practically defined off state following overnight drug withdrawal. UPDRS on assessments were performed 1 h after a dose of L-Dopa that was tailored for each patient at baseline, with the same dose being used at each subsequent assessment. Individual doses of dopaminergic medication were kept constant throughout follow-up, unless alterations were required in response to AEs. Doses were assessed at every visit and expressed as L-Dopa equivalent daily dose (LEDD). Quality of life (using the Parkinson's Disease Questionnaire [PDQ-39]) was assessed at least every 6 months up to 3 years and yearly thereafter.
Statistical analysis
The UPDRS and PDQ-39 scores were analyzed by a Wilcoxon paired test at 24 months. Data management and statistical summaries were performed by Quanticate (UK) Ltd. (Hitchin, United Kingdom). Verbatim AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA).
Results
Patients
All baseline patient characteristics have been previously described. With the exception of one patient (H14) who declined assessment of efficacy after the 12-month assessment (but continued safety assessments), all patients were assessed for efficacy for at least 2 years of follow-up (Fig. 1).
Figure 1.
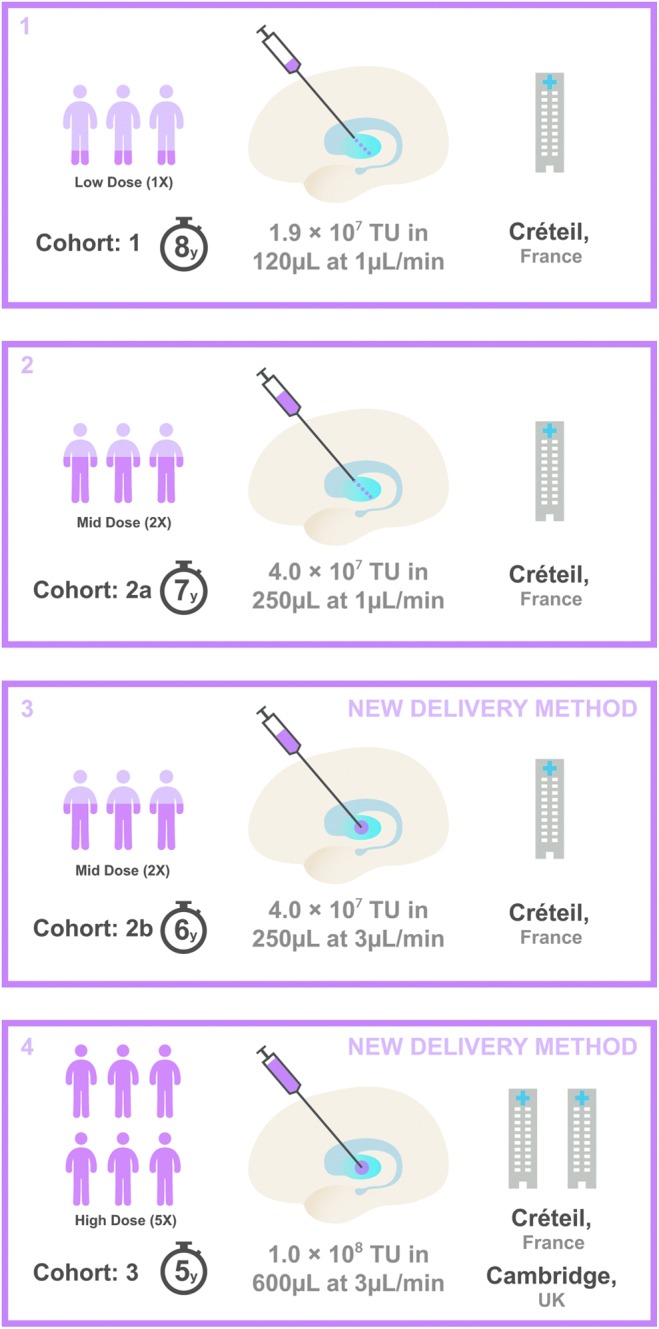
Trial profile. Box 1 represents cohort 1: n = 3 patients included at dose level 1 (low dose, 1.9 × 107 transducing units [TU]; 8 years of follow-up), which involved four needle tracks of five ProSavin deposits per track in each motor putamen. Box 2 represents cohort 2: n = 3 patients at dose level 2 (mid dose, 4.0 × 107 TU; 7 years of follow-up), which involved five needle tracks of five ProSavin deposits in each track placed within the motor putamen. Box 3 represents cohort 2b: n = 3 patients at dose level 2 (mid dose, 4.0 × 107 TU; 6 years of follow-up), with the new delivery method leading to three needle tracks with one deposit per track in each motor putamen. Box 4 represents cohort 3: n = 6 patients at dose level 3 (high dose, 1 × 108 TU; 5 years of follow-up), with the new delivery method of three needle tracks, one deposit per track, in each motor putamen. A modified delivery method of administration was introduced for cohorts 2b and 3 to increase the rate of delivery from 1 to 3 μL/min and a reduction of diameter of the cannula from 25 to 28 gauge.
Eight patients in total received bilateral DBS of the subthalamic nucleus after the 2- (M7, M8, H11), 3- (H14), 4- (H12), 5- (M4, H10), or 6-year (M6) assessment battery. Post-DBS assessments were not included in this analysis except for overall safety.
Safety
Across all time points of follow-up, treatment-emergent AEs were reported in every patient, with the majority (575/671) considered to be unrelated to ProSavin (Supplementary Table S1, S2, S3). Thirty serious AEs were reported across 12 patients, and all were considered unrelated or unlikely to be related to the study drug and the surgical procedure. Two deaths were reported: L1 after their year 6 assessment (peritonitis), and M7 after year 4 (cardio-respiratory arrest), with neither death considered related to ProSavin treatment or to their underlying Parkinson's disease.
There were 96 drug-related AEs reported post treatment (Table 2), and the majority of these (n = 87; 91%) were mild and/or occurred in the first year of follow-up (n = 57; 61%). The most common ProSavin-related AEs, in both the first year post treatment and follow-up thereafter, were increased dyskinesias (33 AEs in 11 patients; 30 mild, 2 moderate, 1 severe) and on–off phenomena (22 AEs in 11 patients; all mild). Increased dyskinesias generally resolved with a reduction in the patients' oral dopaminergic medication. The safety profile was similar across all dose cohorts.
Table 2.
All drug-related adverse events
| Number of events | |||||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | Number of patients | |
| Total number of adverse events | 87 | 6 | 3 | 96 | 15 |
| Nervous system disorders | 64 | 3 | 1 | 68 | 15 |
| Dyskinesia | 30 | 2 | 1 | 33 | 11 |
| On and off phenomenon | 22 | 0 | 0 | 22 | 11 |
| Headache | 4 | 0 | 0 | 4 | 3 |
| Akinesia | 3 | 0 | 0 | 3 | 3 |
| Balance disorder | 1 | 0 | 0 | 1 | 1 |
| Brain edemaa | 1 | 0 | 0 | 1 | 1 |
| Dysarthria | 1 | 0 | 0 | 1 | 1 |
| Speech disorder | 1 | 0 | 0 | 1 | 1 |
| Tremor | 0 | 1 | 0 | 1 | 1 |
| Psychiatric disorders | 9 | 1 | 1 | 11 | 7 |
| Delusional perception | 3 | 0 | 0 | 3 | 3 |
| Anxiety | 1 | 1 | 0 | 2 | 2 |
| Hallucination | 2 | 0 | 0 | 2 | 2 |
| Abnormal dreams | 1 | 0 | 0 | 1 | 1 |
| Acute psychosis | 0 | 0 | 1 | 1 | 1 |
| Confused state | 1 | 0 | 0 | 1 | 1 |
| Hallucination, visual | 1 | 0 | 0 | 1 | 1 |
| Investigations | 7 | 0 | 0 | 7 | 5 |
| Nuclear magnetic resonance imaging brain abnormal | 3 | 0 | 0 | 3 | 3 |
| Nuclear magnetic resonance imaging abnormal | 2 | 0 | 0 | 2 | 2 |
| Weight decreased | 1 | 0 | 0 | 1 | 1 |
| Weight increased | 1 | 0 | 0 | 1 | 1 |
| Musculoskeletal and connective tissue disorders | 4 | 0 | 0 | 4 | 4 |
| Back pain | 1 | 0 | 0 | 1 | 1 |
| Musculoskeletal pain | 1 | 0 | 0 | 1 | 1 |
| Myalgia | 1 | 0 | 0 | 1 | 1 |
| Neck pain | 1 | 0 | 0 | 1 | 1 |
| Injury, poisoning, and procedural complications | 2 | 0 | 0 | 2 | 2 |
| Fall | 1 | 0 | 0 | 1 | 1 |
| Subdural hematomab | 1 | 0 | 0 | 1 | 1 |
| Blood and lymphatic system disorders | 0 | 1 | 0 | 1 | 1 |
| Anemia | 0 | 1 | 0 | 1 | 1 |
| Gastrointestinal disorders | 1 | 0 | 0 | 1 | 1 |
| Nausea | 1 | 0 | 0 | 1 | 1 |
| Renal and urinary disorders | 0 | 1 | 0 | 1 | 1 |
| Glomerulonephritis | 0 | 1 | 0 | 1 | 1 |
Along the injection site only.
Bleeding under burr hole.
The only immune responses seen were in cohort 3 where low-level antibody responses against the VSV-G envelope protein were detected in four of the six patients, and in three of these four patients, antibodies to p26 protein (part of the gag protein that makes up the viral particle) were observed.14
Efficacy
Similar to the previously reported data for the 6- and 12-month assessments, a significant reduction in mean UPDRS part III (off) motor scores compared to baseline scores was observed across the 14 patients evaluated after 2 years of follow-up (Table 3; 29.2 vs. 37.4, p < 0.05). Of the 14 patients, 10 showed sustained improvements from baseline at this time point. No statistically significant difference was seen between the differently dosed cohorts. Although four patients who received DBS were withdrawn from the efficacy analysis at follow-up after the 2- or 3-year assessments, 10/11 patients and 8/10 patients continued to demonstrate an improvement in UPDRS III (off) scores relative to baseline at the 3- or 4-year follow-up, respectively. The magnitude of improvement in these patients was generally similar to the effects observed at the 12- and 24-month time points. Of the patients who reached the 5- or 6-year follow-up, six out of nine patients and four out of six patients continued to have improved UPDRS III (off) scores relative to their baseline, which would also be consistent with the fact that they did not require further intervention with DBS.
Table 3.
UPDRS part III scores in on and off states for each patient, by time point—post DBS data excluded
| Patients | Baseline | 6 months | 12 months | 24 months | 36 months | 48 months | 60 months | 72 months |
|---|---|---|---|---|---|---|---|---|
| UPDRS part III off score (% change from baseline) | ||||||||
| L1 | 23 | 12 (−48%) | 13 (−43%) | 16 (−30%) | 20 (−13%) | 19 (−17%) | 29 (26%) | 28 (22%) |
| L2 | 30 | 26 (−13%) | 27 (−10%) | 30 (0%) | 35 (17%) | 32 (7%) | 31 (3%) | 26 (−13%) |
| L3 | 28 | 20 (−29%) | 19 (−32%) | 20 (−29%) | 21 (−25%) | 22 (−21%) | 26 (−7%) | 27 (−4%) |
| M4 | 29 | 21 (−28%) | 24 (−17%) | 30 (3%) | 26 (−10%) | 27 (−7%) | 34 (17%) | NA |
| M5 | 30 | 24 (−20%) | 26 (−13%) | 19 (−37%) | 24 (−20%) | 24 (−20%) | 29 (−3%) | 29 (−3%) |
| M6 | 34 | 16 (−53%) | 15 (−56%) | 18 (−47%) | 16 (−53%) | 15 (−56%) | 15 (−56%) | 24 (−29%) |
| M7 | 49 | 34 (−31%) | 39 (−20%) | 44 (−10%) | NA | NA | NA | NA |
| M8 | 38 | 24 (−37%) | 32 (−16%) | 39 (3%) | NA | NA | NA | NA |
| M9 | 46 | 18 (−61%) | 24 (−48%) | 22 (−52%) | 28 (−39%) | 26 (−43%) | 35 (−24%) | 46 (0%) |
| H10 | 37 | 26 (−30%) | 17 (−54%) | 21 (−43%) | 28 (−24%) | NA | 19 (−49%) | NA |
| H11 | 35 | 24 (−31%) | 27 (−23%) | 39 (11%) | NA | NA | NA | NA |
| H12 | 52 | 46 (−12%) | 39 (−25%) | 46 (−12%) | 41 (−21%) | 28 (−46%) | NA | NA |
| H13 | 49 | 29 (−41%) | 33 (−33%) | 30 (−39%) | 31 (−37%) | 37 (−24%) | 45 (−8%) | NA |
| H14 | 52 | 33 (−37%) | 38 (−27%) | NA | NA | NA | NA | NA |
| H15 | 44 | 32 (−27%) | 26 (−41%) | 35 (−20%) | 31 (−30%) | 47 (7%) | NA | NA |
| UPDRS part III on score (% change from baseline) | ||||||||
| L1 | 6 | 7 (17%) | 6 (0%) | 7 (17%) | 14 (133%) | 10 (67%) | 21 (250%) | 24 (300%) |
| L2 | 8 | 10 (25%) | 11 (38%) | 11 (38%) | 19 (138%) | 19 (138%) | 18 (125%) | 21 (163%) |
| L3 | 11 | 7 (−36%) | 7 (−36%) | 10 (−9%) | 15 (36%) | 12 (9%) | 19 (73%) | 21 (91%) |
| M4 | 8 | 7 (−13%) | 8 (0%) | 10 (25%) | 10 (25%) | 9 (13%) | 10 (25%) | NA |
| M5 | 14 | 14 (0%) | 14 (0%) | 13 (−7%) | 16 (14%) | 15 (7%) | 17 (21%) | 20 (43%) |
| M6 | 7 | 7 (0%) | 6 (−14%) | 9 (29%) | 7 (0%) | 6 (−14%) | 6 (−14%) | 5 (−29%) |
| M7 | 19 | 15 (−21%) | 15 (−21%) | 17 (−11%) | NA | NA | NA | NA |
| M8 | 15 | 13 (−13%) | 15 (0%) | 12 (−20%) | NA | NA | NA | NA |
| M9 | 9 | 5 (−44%) | 5 (−44%) | 8 (−11%) | 12 (33%) | 11 (22%) | 14 (56%) | 10 (11%) |
| H10 | 8 | 8 (0%) | 8 (0%) | 9 (13%) | 8 (0%) | NA | 8 (0%) | NA |
| H11 | 10 | 8 (−20%) | 9 (−10%) | 11 (10%) | NA | NA | NA | NA |
| H12 | 13 | 11 (−15%) | 9 (−31%) | 15 (15%) | 12 (−8%) | 12 (−8%) | NA | NA |
| H13 | 23 | 16 (−30%) | 21 (−9%) | 25 (9%) | 14 (−39%) | 20 (−13%) | 19 (−17%) | NA |
| H14 | 23 | 24 (4%) | 33 (43%) | NA | NA | NA | NA | NA |
| H15 | 18 | 20 (11%) | 15 (−17%) | 13 (−28%) | 10 (−44%) | 10 (−44%) | NA | NA |
No significant improvements in UPDRS part III (on) motor scores, compared to baseline, were observed for up to 2 years of follow-up (Table 3). All patients in the low-dose cohort, and three of the five mid-dose patients who could be evaluated, showed worsening UPDRS part III (on) scores compared to baseline at the 3-year follow-up. However, in the high-dose cohort, all of the patients who were evaluated from 3 years onwards showed equivalent or improved scores relative to baseline at every time point.
The previously reported significant reduction in mean total UPDRS (off) scores compared to baseline scores at 1 year was maintained at the 2-year follow-up time point in the 14 evaluable patients (Table 3; 69·6 vs. 58·3, p < 0.05).14 No significant improvement in mean total UPDRS (on) scores at 2 years versus baseline was observed (24.4 vs. 27.3; p = n.s.), and there were no sustained improvements observed in the majority of patients at longer follow-up times (Table 4).
Table 4.
Total UPDRS scores in on and off states for each patient, by time point—post DBS data excluded
| Patients | Baseline | 6 months | 12 months | 24 months | 36 months | 48 months | 60 months | 72 months |
|---|---|---|---|---|---|---|---|---|
| Total UPDRS off score | ||||||||
| L1 | 49 | 30 | 30 | 37 | 44 | 43 | 67 | 62 |
| L2 | 61 | 45 | 59 | 62 | 73 | 65 | 70 | 61 |
| L3 | 70 | 41 | 43 | 38 | 42 | 49 | 61 | 65 |
| M4 | 63 | 50 | 52 | 69 | 59 | 59 | 65 | NA |
| M5 | 58 | 54 | 53 | 30 | 47 | 40 | 60 | 54 |
| M6 | 74 | 54 | 44 | 58 | 53 | 49 | 53 | 60 |
| M7 | 83 | 67 | 69 | 84 | NA | NA | NA | NA |
| M8 | 67 | 46 | 58 | 67 | NA | NA | NA | NA |
| M9 | 68 | 44 | 54 | 51 | 56 | 58 | 71 | 89 |
| H10 | 59 | 54 | 49 | 51 | 66 | NA | 57 | NA |
| H11 | 71 | 58 | 65 | 79 | NA | NA | NA | NA |
| H12 | 91 | 84 | 73 | 86 | 82 | 71 | NA | NA |
| H13 | 90 | 47 | 53 | 49 | 57 | 50 | 74 | NA |
| H14 | 94 | 63 | 64 | NA | NA | NA | NA | NA |
| H15 | 71 | 44 | 39 | 55 | 53 | 71 | NA | NA |
| Total UPDRS on score | ||||||||
| L1 | 19 | 13 | 14 | 15 | 23 | 22 | 48 | 48 |
| L2 | 24 | 19 | 28 | 26 | 38 | 42 | 38 | 45 |
| L3 | 35 | 15 | 18 | 17 | 22 | 27 | 39 | 49 |
| M4 | 20 | 17 | 17 | 28 | 21 | 19 | 19 | NA |
| M5 | 24 | 24 | 22 | 19 | 28 | 20 | 31 | 35 |
| M6 | 23 | 25 | 22 | 26 | 25 | 17 | 31 | 22 |
| M7 | 31 | 29 | 26 | 39 | NA | NA | NA | NA |
| M8 | 31 | 27 | 29 | 22 | NA | NA | NA | NA |
| M9 | 20 | 18 | 16 | 20 | 24 | 30 | 33 | 32 |
| H10 | 21 | 18 | 21 | 20 | 22 | NA | 23 | NA |
| H11 | 27 | 22 | 25 | 26 | NA | NA | NA | NA |
| H12 | 25 | 24 | 21 | 29 | 34 | 33 | NA | NA |
| H13 | 52 | 27 | 34 | 35 | 25 | 30 | 36 | NA |
| H14 | 47 | 33 | 41 | NA | NA | NA | NA | NA |
| H15 | 30 | 25 | 22 | 20 | 20 | 19 | NA | NA |
Analysis of the UPDRS I, II (on and off), and IV scores demonstrated no significant differences from baseline scores in the 14 patients evaluated at 2 years (Supplementary Tables S4–S6). However, the majority of patients who could be evaluated showed improved or sustained scores from baseline up to the 4-year assessment. In the smaller numbers of patients who were followed up beyond 4 years, responses were generally similar or less favorable than baseline. Analysis of PDQ-39 scores demonstrated similar scores to baseline at the 2-year follow-up (Supplementary Table S7; 32.5 vs 32.1, p = n.s.). At later time points, around half of patients assessed showed a gradual decline in scores.
In long-term follow-up, the majority of patients continued to require a lower LEDD compared to baseline. In total, 10/14 patients and 8/12 patients benefited from a reduction in LEDD compared to baseline at 2 and 3 years of follow-up, respectively (Table 5). Of the patients followed up for 4, 5, and 6 years, 5/11 patients, 5/10 patients, and 4/6 patients received a lower LEDD than baseline, respectively.
Table 5.
L-Dopa equivalent dose (LEDD)—post DBS data excluded
| Patients | Baseline | 6 months | 12 months | 24 months | 36 months | 48 months | 60 months | 72 months |
|---|---|---|---|---|---|---|---|---|
| L1 | 2,547 | 2,257 | 2,382 | 2,507 | 2,507 | 3,102 | 986 | 1,461 |
| L2 | 1,329 | 1,319 | 1,103 | 1,019 | 1,582 | 1,582 | 1,530 | 1,680 |
| L3 | 1,998 | 1,448 | 750 | 1,149 | 1,282 | 999 | 875 | 1,575 |
| M4 | 2,164 | 2,164 | 1,025 | 1,229 | 1,487 | 2,015 | 2,414 | NA |
| M5 | 1,572 | 1,572 | 1,572 | 1,238 | 1,238 | 1,052 | 998 | 1,098 |
| M6 | 2,523 | 2,257 | 2,257 | 1,548 | 1,615 | 1,615 | 1,848 | 1,958 |
| M7 | 1,785 | 1,936 | 2,036 | 2,305 | NA | NA | NA | NA |
| M8 | 1,088 | 1,088 | 1,088 | 1,160 | NA | NA | NA | NA |
| M9 | 1,775 | 1,525 | 1,400 | 1,400 | 1,725 | 1,850 | 1,800 | 2,582 |
| H10 | 1,535 | 1,160 | 1,360 | 1,660 | 1,810 | 2,314 | 3,002 | NA |
| H11 | 1,844 | 1,549 | 1,615 | 2,148 | NA | NA | NA | NA |
| H12 | 1,180 | 1,030 | 1,130 | 1,055 | 1,165 | 2,253 | NA | NA |
| H13 | 1,691 | 1,391 | 1,391 | 1,391 | 1,391 | 1,125 | 1,125 | NA |
| H14 | 699 | 633 | 699 | NA | 699 | NA | NA | NA |
| H15 | 1,593 | 1,530 | 1,530 | 1,573 | 1,928 | 2,229 | 2,344 | NA |
Discussion
The results of a Phase I/II clinical trial were previously reported describing the first-in-human use of a lentiviral-based gene therapy vector. In this study, ProSavin, an EIAV-derived lentiviral vector, was shown to have a favorable safety profile and encouraging efficacy signals following injection into the motor striatum of 15 patients with Parkinson's disease.14
The data reported here describes follow-up, for up to 8 years, of patients from the Phase I/II study and provides important additional information on the long-term safety and potential efficacy of this therapeutic approach. The safety data are generally consistent with those previously reported,14 with no drug- or procedure-related serious AEs during follow-up. The majority of drug-related AEs were on-medication dyskinesias adequately managed by lowering their L-Dopa medication, and encouragingly, the majority of drug-related AEs occurred within the first 12 months after treatment.
Eight patients received treatment with DBS, as they developed worsening off periods alternating with L-Dopa dyskinesias as a result of disease progression. It should be noted that no safety issue was observed with lead implantation or high frequency stimulation of the subthalamic nucleus in any of these cases (S. Palfi, pers. commun.). There was a very similar safety profile before and after DBS (compare Table 2 and Supplementary Table S1).
In terms of efficacy, the significant improvement in motor function previously reported up to 12 months was maintained at 2 years across the 14 patients that were assessed at this time point (with one patient withdrawing consent for off assessments after 12 months of follow-up). Although statistical analyses were not performed beyond this time point, as several patients were withdrawn from the efficacy part of the study by virtue of having had DBS, the majority of patients who remained in the open-label follow-up continued to show improvements in their UPDRS (III) off scores at all time points. These efficacy findings are consistent with the fact that the lentiviral vector genome is integrated into the host-cell genome and transgene expression is maintained long term,16 as has recently been shown following administration of an EIAV lentiviral vector into the retina.17 Nonetheless, Parkinson's disease is a progressive disease. So, it is encouraging that the data indicate that the levels of dopamine achieved through this gene therapy approach may be sufficient to sustain a positive motor behavioral effect for several years.
Considering only the patients who were evaluated at the longer-term follow-up time points, it is interesting to note that patients M6, M9, H12, and H13, who were diagnosed with Parkinson's disease between 9 and 26 years prior to ProSavin treatment,14 showed particularly encouraging improvements from baseline in their UPDRS III (off) scores, with changes of 12–24 points at the 4-year assessment. Patient M6 was also evaluated at 6 years, and continued to show an approximately 30% improvement in both UPDRS III on and off scores, with a sustained reduction in LEDD, and a 17-unit improvement in PDQ-39 scores from baseline.
Although these data provide strong evidence to support an efficacy benefit for ProSavin in Parkinson's disease patients, the overall magnitude of effects are within the range reported for placebo study arms, albeit over shorter time frames, in other clinical trials for PD using surgical interventions.8,13 The duration of such placebo effects have not been well studied. However, given that the improvements in motor scores were sustained for 6 years in some patients and confined to their “off” state assessments, combined with the fact that Parkinson's disease is a progressive neurodegenerative disease with an expected three- to four-point increase in the UPDRS part III (off) motor score per year,18,19 the likelihood that the findings reported are attributable to the study treatment is strong. Nonetheless, interpretation of these findings must still be viewed with caution until a larger comparator study has been undertaken with placebo treatments, especially given the extent of patient attrition over the follow-up period in this study. An attrition that is not unexpected, given that the study recruited patients at relatively advanced stages of disease.
Given the small sample populations, it is difficult to assess dose response. It was previously reported that there were indications of positive responses in patients receiving the highest dose of ProSavin. These patients had the most consistent LEDD reduction post intervention, the highest mean improvement in UPDRS III (off) motor scores at 1 year, and a significant change from baseline in 11C-raclopride binding potential.14 At 2 years of follow-up, a similar mean improvement from baseline in UPDRS III (off) scores was observed in the mid- and high-dose groups, which was higher than in the low-dose group. Again, interpretation of these observations must be made with caution due to the small patient numbers and the changes seen in the UPDRS III scores.
In conclusion, the new data demonstrate the long-term safety and promising efficacy profile of ProSavin in Parkinson's disease patients for up to 8 years of follow-up. These are the longest follow-up assessments reported in any Parkinson's disease gene therapy study. Although the results are encouraging, the data suggest that the optimal level of dopamine replacement may not have been achieved, since patients continued to require oral L-Dopa therapy to obtain maximal benefit, and some of the more severely affected patients required DBS 2–6 years following ProSavin administration. Further dose escalation using ProSavin would be challenging due to limitations on vector titers using current production processes and the volume of vector that can be safely administered into the human striatum. Therefore, a new vector (OXB-102) has been recently developed in which the configuration of three dopamine biosynthesis enzymes was further optimized to increase the capacity for dopamine production significantly compared to ProSavin.20 This vector is under preclinical development and, pending regulatory approval, will be assessed in a new Phase I/II study to determine the appropriate dose before a larger placebo-controlled Phase IIb clinical trial is undertaken.
Supplementary Material
Acknowledgments
We would like to thank the current and former staff at Henri Mondor Hospital, CEA, Oxford BioMedica and John van Geest Centre for Brain Repair, and Addenbrooke's Hospital. In addition, we would like to thank the members of the data-monitoring committee and of course the 15 patients for their commitment to participate in this study.
Oxford BioMedica (UK) Ltd. funded the study and was the study sponsor, with responsibility for the trial design, manufacture, and supply of the drug, data collection, clinical monitoring, and pharmacovigilance. Additional funding was provided by ARSC Foundation, and the NIHR funded Biomedical Research Centre award to Addenbrooke's Hospital and the University of Cambridge. R.A.B. is an NIHR Senior Investigator.
Author Disclosure
G.S.R., K.A.M., and N.J.T. are employees, or former employees, of Oxford BioMedica, which funded this study. They and their families have ownership interests in the company. S.P., R.A.B., P.B., and K.H. are consultants, or former consultants, of Oxford BioMedica. No competing financial interests exist for the remaining authors.
References
- 1.Schapira AH. Etiology and pathogenesis of Parkinson disease. Neurol Clin 2009;27:583–603 [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol 2006;5:525–535 [DOI] [PubMed] [Google Scholar]
- 3.Prashanth LK, Fox S, Meissner WG. L-dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol 2011;98:31–54 [DOI] [PubMed] [Google Scholar]
- 4.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16:448–458 [DOI] [PubMed] [Google Scholar]
- 5.Wu K, Politis M, Piccini P. Parkinson disease and impulse control disorders: a review of clinical features, pathophysiology and management. Postgrad Med J 2009;85:590–596 [DOI] [PubMed] [Google Scholar]
- 6.Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 2006;5:677–687 [DOI] [PubMed] [Google Scholar]
- 7.Muramatsu S, Fujimoto K, Kato S, et al. . A Phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol Ther 2010;18:1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christine CW, Starr PA, Larson PS, et al. . Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 2009;73:1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberling JL, Jagust WJ, Christine CW, et al. . Results from a Phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008;70:1980–1983 [DOI] [PubMed] [Google Scholar]
- 10.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. . Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, Phase I trial. Lancet Neurol 2008;7:400–408 [DOI] [PubMed] [Google Scholar]
- 11.Kaplitt MG, Feigin A, Tang C, et al. . Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, Phase I trial. Lancet 2007;369:2097–2105 [DOI] [PubMed] [Google Scholar]
- 12.LeWitt PA, Rezai AR, Leehy MA, et al. . AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 2011;10:309–319 [DOI] [PubMed] [Google Scholar]
- 13.Marks WJ, Jr, Bartus RT, Siff ert J, et al. . Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010;9:1164–1172 [DOI] [PubMed] [Google Scholar]
- 14.Palfi S, Gurruchaga JM, Ralph GS, et al. . Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, Phase 1/2 trial. Lancet 2014; 383: 1138–1146 [DOI] [PubMed] [Google Scholar]
- 15.Azzouz M, Martin-Rendon E, Barber RD, et al. . Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci 2002;22:10302–10312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarraya B, Boulet S, Ralph GS, et al. . Dopamine gene therapy for Parkinson's disease in a nonhuman primate without associated dyskinesia. Sci Transl Med 2009;1:2ra4. [DOI] [PubMed] [Google Scholar]
- 17.Campochiaro PA, Lauer AK, Sohn EH, et al. . Lentiviral vector Gene transfer of Endostatin/angiostatin for Macular degeneration (GEM) study. Hum Gene Ther 2017;28:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggers C, Pedrosa DJ, Kahraman D, et al. . Parkinson subtypes progress differently in clinical course and imaging pattern. PLoS One 2012;7:e46813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olanow CW, Goetz CG, Kordower JH, et al. . A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003;54:403–414 [DOI] [PubMed] [Google Scholar]
- 20.Stewart HJ, Ralph GS, Fong-Wong L, et al. . Optimizing transgene configuration and protein fusions to maximize dopamine production for the gene therapy of Parkinson's disease. Hum Gene Ther Clin Dev 2016;27:100–110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


