Abstract
Transcription factors of the AP-1/ATF family, including c-Fos, c-Jun, and ATF-2, play an important role in the regulation of cell proliferation and differentiation, and changes in their levels and/or activities may contribute to oncogenesis. We analyzed the alterations of AP-1/ATF transcription factors upon immortalization and transformation in a panel of cell lines derived from rat embryo fibroblast (REF) cells. The tumorigenic E1A + cHa-ras cells are characterized by high and constitutive DNA binding activities of AP-1, in contrast to nontransformed cells and the E1A cells. The expression of c-fos and c-jun genes was affected differently by the oncogenic transformation. By using antibodies to c-Jun and c-Fos proteins in electrophoretic mobility shift assays (EMSA), we showed that E1A + ras-ras transformants did not contain c-Fos under any condition of cell cultivation and growth factor stimulation, whereas c-Jun was constitutively upregulated. In the absence of c-fos gene expression, c-Fos protein appears to be replaced by proteins of Fos family (Fra-1) and ATF family (ATF-2 and ATFa). To determine the possible mechanisms of c-fos downregulation in E1A + cHa-ras transformants we have obtained populations of geneticin-resistant clones containing integrated reporter construct -711Fos-CAT and its mutants in serum-responsive element (SRE) and cAMP-responsive element (CRE). Data obtained show that the mutations within the SRE lead to a manifold activation of fos-CAT expression. This allows to suggest that c-fos downregulation in E1A + cHa-ras transformants is provided by a negative control mediated through the SRE regulatory region. The profound differences in regulation and composition of transcription factors of the AP-1 family probably play a pivotal role in the transformation of REF cells by E1A and cHa-ras oncogenes.
Keywords: E1A and cHa-ras oncogenes, fos and jun expression, AP-1 transcription factors
STIMULATION of quiescent normal cells to proliferation by growth factors initiates their transition from phase G0 to G1 of the cell cycle and induces the transcription of a large number of so-called immediate-early genes and genes involved in signal transduction (13,24). The first group includes the proto-oncogenes c-jun and c-fos, the products of which are members of the AP-1 transcription factors family. The AP-1 factors play an important role in the regulation of cell proliferation and differentiation in response to various stimuli. Transcription factors of this family constitute Fos/Jun heterodimers, Jun/Jun homodimers, or Jun heterodimers with members of the ATF/CREB family (i.e., Jun/ATF-2, Jun/ATF-3, and Jun/ATFa) (1,6,7,19,21,26). Depending on the composition of the dimers, these proteins are capable of binding to specific subsets of AP-1-regulatory elements and respond to different stimuli (21,26,40). The 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-responsive element (TRE) of the collagenase gene promoter (coll-TRE) is bound preferably by Jun/Fos and Jun/Jun dimers, and is activated mainly by mitogenic stimuli. Similar AP-1-responsive elements have been identified in the promoters of many genes activated upon the stimulation of cells by growth factors or TPA (3,30). Therefore, the Fos and Jun proteins possibly play an important role in the control of cell proliferation and transformation (9,27,48,49). The TREs of the c-jun gene itself (junl-TKE and jun2-TRE) are preferentially targeted by Jun/ATF-2 dimers, and can be activated by UV light and DNA-damaging agents, such as MMS (19,46,47). The ATF-containing complexes may thus be involved primarily in various stress responses. It is possible that perturbations in the levels of the different protein complexes and/or in the regulation of their activities by genetic mutation or by viruses may have profound consequences for the cell proliferation and differentiation control and thereby contribute to malignant cell transformation. This suggestion is supported by the finding that various viral and cellular nuclear onco-proteins, such as human adenoviruses E1A proteins, SV40 Large T, c-myc, and several cytoplasmic onco-proteins (cHa-ras, c-Raf, or polyoma middle T), indeed influence transcription of a number of cellular genes via AP-1-responsive DNA elements by modulating the composition and/or activities of AP-1 complexes (3,9,16,17,49). These oncoproteins belong to distinct subgroups: the nuclear oncoprotein (e.g., E1A), which can immortalize primary cells (5), and oncoproteins such as cHa-Ras, which can cooperate with the immortalizing proteins for full oncogenic transformation (35,39,48,50). Accordingly, distinct changes in the composition of AP-1 protein complexes or in the activities of their constituents may contribute to the immortalization process, and additional changes may be necessary for full transformation. In this study, we show that E1A + cHa-ras transformed cells are characterized by high and constitutive DNA binding activity of the AP-1 complex. In these cells, c-fos gene is downregulated and c-jun gene is upregulated. Moreover, significant changes of the AP-1 complex composition have been detected: c-Fos appears to be replaced by Fra-1 protein and factors of the ATF family (ATF-2, ATFa). The expression of fos-CAT mutants integrated in E1A + cHa-ras transformants allows to suggest that down-regulation of c-fos gene expression is likely to be mediated through the SRE regulatory region of c-fos gene promoter.
MATERIALS AND METHODS
Cell Lines
Rat embryo fibroblasts (REF) immortalized by stable transfection of the Ad5 E1 A oncogene or transformed by a combination of E1A + cHa-ras onco-genes have been described earlier (36). In contrast to the E1A-immortalized cells, E1A + cHa-ras cells display an increased saturation density and form colonies in soft agar. When injected into nude mice, E1A + cHa-ras cells give rise to tumors within a few weeks. E1A + E1B19kD cell lines have been established by cotransfection of primary REF cells with expression vectors encoding for Ad5 E1A and Ad5 E1B19kD (43). The REF cells (second passage) and the cell lines were grown in DMEM supplemented with 10% fetal calf serum (FCS; Gibco or Biolot). Cells were serum starved for 48 h in the presence of 0.5% FCS and stimulated by addition of 10% FCS, 12-O-tetradecanoyl-phorbol-13-acetate (TPA, 50 ng/ ml, Sigma), epidermal growth factor (EGF, 100 ng/ ml, Serva), dibutyryl cAMP (dbcAMP, 0.001 M, Sigma) for 1 h.
Nuclear Extracts
Nuclear extracts were prepared by using a protocol that has already been described (37). Briefly, 5 × 106 cells were resuspended in 1.5 ml of PBS solution and centrifuged, after which the pellet was resuspended in 800 nl of cold hypotonic solution (10 mM HEPES, pH 7.9, 10 mM KC1, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF) for 15 min. Subsequently, 50 μ1 of 10% NP-40 was added and the mixture was vigorously shaken. Sedimented nuclei were gently shaken in a solution consisting of 20 mM HEPES, pH 7.9, 0.42 M NaCl, 1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, and other protease inhibitors for 15 min at 0°C. Subsequently, the extract was cleared by centrifugation. The nuclear extracts were stored at 70°C in 10-fil aliquots. Protein concentration was determined according to Bradford’s method (12).
Oligonucleotides
The oligonucleotides used in this work are as follows. The TRE from the promoter of the human collagenase I gene, 5′AGCATGAGTCAGCC-3′ (coll-TRE); a mutated TRE derived from the same promoter, 5′-AGCTGGAGTCAGCC-3′; one of the TREs of the c-jun gene, 5′-AGCTAGCATTACCT CATCCC-3′ (jun2-TRE). The oligonucleotides were labeled with [32P]dNTPs with the Klenow fragment of E. coli DNA-polymerase I or phosphorylated by polynucleotide kinase with [γ−32P]ATP.
Electrophoretic Mobility Band Shift Assay (EMSA)
The incubation reaction mixture (10 μl) consisted of 10 mM HEPES, pH 7.9, 1 mM DTT, 1 mM EDTA, 8 mM MgCl2, 10% glycerol, 2 μg of nuclear extracts, 1 ng poly(dl-dC). The mixture was incubated for 20 min at 4°C followed by addition of labeled oligonucleotides (30,000 cpm/ng) for a 20-min period. Specific and nonspecific oligonucleotides were used in competition experiments at a 100-fold molar excess. DNA-protein complexes were separated by electrophoresis in a 5% polyacrylamide gel (30:1) in lx TBE buffer, pH 8.3. Gels were transferred to filter paper, dried, and exposed to X-ray film. EMSA experiments with specific antibodies (a supershift analysis) were carried out as follows: nuclear extracts were incubated in the presence of 2 M-l PBS, 2 μl nonimmune serum (MS), or specific antibodies for 2 h on ice, before addition of the labeled oligonucleotides. Antibodies used in these supershift experiments were purchased from Santa Cruz Biotechnologies: c-Fos (#sc-52x, #sc-413x), c-Jun (#sc-45x), ATF-2 (#sc-187x), JunD (#sc-74x), ATF-3 (#sc-188), Fra-1 (#sc-183x). Mouse monoclonal 3C12 antibody against full-length ATFa3 was a kind gift of B. Chatton (14). Rabbit polyclonal antibodies to FosB, (83-138 aa), Fra-1 (1–82 aa), and Fra-2 (200–252 aa) were kindly supplied by M. Yaniv (29).
RNA Analysis
Total cellular RNA was prepared as described (10, 36a). For Northern blots, total RNA (20–30 μg/lane) was denatured and electrophoresed on 1.5% agarose gels containing 2.2 M formaldehyde. The amount and quality of the RNA samples were checked by staining the gels with ethidium bromide (data not shown). Gels were capillary blotted onto nylon membranes (Gene Screen Plus, NEN) and baked for 2 h at 80°C. The DNA fragments used as probes are a 1.1 kb PstI fragment of the v-fos gene and a 1.0 kb PstI fragment of the c-jun gene. The DNA fragments were nick-translated with [32P]dNTPs. Hybridization was performed in rotating cylinders for 36 h at 68°C in a solution consisting of 3x SSC, lx Denhardt, 0.5% SDS, and denatured E. coli DNA (50 pg/ml). Membranes were washed, dried, and autoradiographed at -70°C in the presence of an intensifying screen (Curix MR 600).
Populations of Clones With Integrated Plasmid Constructs
E1A + cHa-ras cells were cotransfected with a selectable vector pSVneo conferring geneticin resistance and the following plasmids: fos-CAT (-711fos-CAT) or point mutants in SRE site [substitution G > A in positions of -319 and -304 nucleotides (11)]; a deletion mutant of CRE site (−71l/fosΔ−65/-52CAT) (22); jun-CAT (−1600/+170m/jun-CAT) and its mutants in jun1-TRE (−1600/+170Δ1) or jun2-TRE (−1600/+170Δ2) (44). The ratio of selectable/reporter plasmids was 1:5. Following the transfection, cells were grown for 2 days without antibiotic, then were plated on a selective medium containing 400 pg/ml of geneticin (G-418, Gibco). Selection was continued until the visible clones could be seen. All clones were pooled in a population containing the corresponding reporter construct. The populations of clones were used for obtaining cell extracts to monitor for the CAT activity. Cell extracts were normalized with respect to total protein measured by Bradford’s method (12).
Determination of CAT Activity
CAT activity was determined according to a method previously described (18) by using [14C]-chloramphenicol (Amersham) as an acceptor of ace-tyl groups. Acetylated products were separated by thin-layer chromatography on silicagel-covered plates (Merck) in a mixture of chloroform/methanol (95:5). Dried plates were exposed to Kodak X-ray films. All experiments were repeated not less than three times. Fold induction was calculated as the increase in CAT activity relative to that obtained with the control (wild-type) reporter plasmid. Values are given as the averages of the three independent experiments, and errors show standard deviations.
Western Blot Analysis
After removing medium, cells were rinsed with cold PBS. RIPA buffer (0.6 ml) was added to a 100-mm petri dish (RIPA buffer composition: PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitors including 1 mM PMSF, pepstatin A, leupeptin, aprotinin, trypsin inhibitor, 100 nM sodium orthovanadate). Cells were scrapped with a rubber scraper, transferred to a microfuge tube, vigorously vortexed, and centrifuged for 15 min at 12,000 rpm. Protein concentration in supernatant was measured by Bradford’s method (12). Proteins were separated by electrophoresis in 7.5–10% polyacrylamide gel and then transferred from the gel to Immobilon P membrane (Millipore). The membranes were incubated with primary antibodies: to c-Fos (sc-52), to c-Jun (sc-45), to JunD (sc-74) (Santa Cruz Biotech.). After washing, binding primary antibodies were detected with horseradish-conjugated secondary antibodies and revealed using the enhanced chemilumi-nescence method (ECL) following the manufacturer’s instructions (Amersham).
RESULTS
High and Constitutive DNA Binding Activity ofAP-1 Complexes in E1A + cHa-ras Transformed Cells
We analyzed DNA binding activities of the AP-1 complexes, their composition and regulation by growth factors in REF cells immortalized by E1A, or transformed by a combination of E1A and cHa-ras oncogenes. An electrophoretic mobility-shift analysis (EMSA) of AP-1 transcription factors in nuclear extracts of cells grown in the presence of 10% FCS was performed with the TRE of the human collagenase I gene promoter (coll-TRE) or one of the TREs of the c-jun gene (jun2-TKE) as probes. These oligonucleo-tides were chosen because they differ in their affinities for various AP-1 dimers (47). The jun2-TRE binds mainly Jun/ATF dimers, whereas the coll-TRE binds preferably Fos/Jun complexes (40,47). Slower and faster migrating I and II complexes can be resolved with the used oligonucleotide probes (20,46,47) (Fig. 1A). Competition experiments with cold nonmutated and a mutated oligonucleotide confirmed the specificities of the observed binding (results not shown). Two important differences between E1A + cHa-ras cells and REF and E1A cells jun2-TRE (i.e., complex I) was found to be low in untransformed REF and E1A-immortal-ized cells, but was drastically increased in E1A + cHa-ras transformed cells. The amount of the faster migrating complex II bound to the jun2-TRE was also increased in the E1A + cHa-ras transformed cells, albeit to a lesser extent. These results suggest that E1A + cHa-ras cells contain significantly higher levels of ATF-containing dimers (complex I). As for the coll-TRE bound complexes, the amount of complex II was clearly increased upon transformation. Taken together, these results suggest that E1A+ cHa-ras cells contain significantly higher levels of both dimer types (see also below).
FIG. 1.
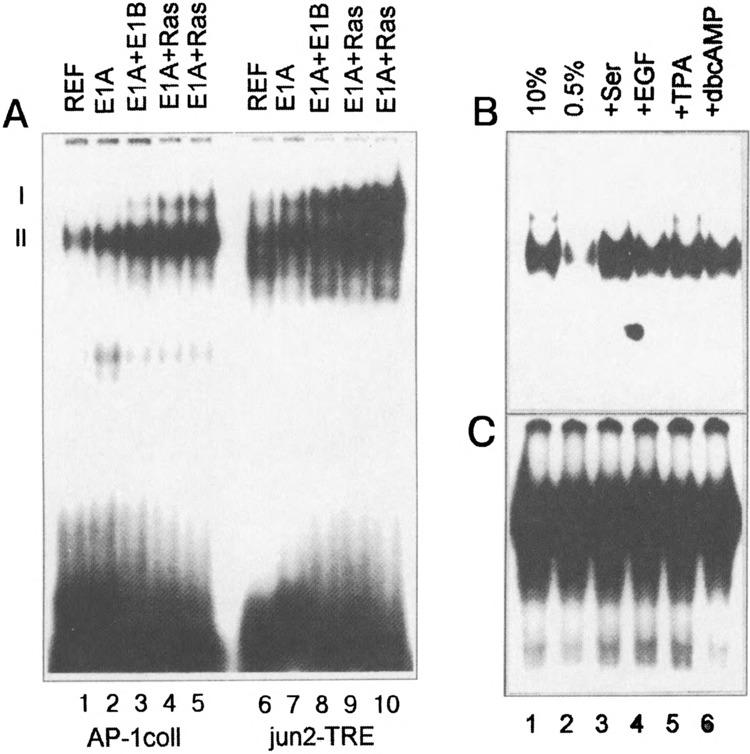
(A) Changes of AP-1 complexes I and II detected in nuclear extracts of REF, E1A-immortalized, E1A + E1B19kD, and E1A + cHa-ras cells with the coll-TRE or jun2-TRE probes in electrophoretic mobility shift assays (EMSA). Nuclear extracts were prepared from cells grown in medium supplemented with 10% FCS. The positions of the slower migrating complex (I) and the faster complex (II) have been indicated. (B, C) Regulation of AP-1 DNA binding activity in REF cells (B) and E1A + cHa-ras transformants (C). Cells were serum starved in the presence of 0.5% FCS for 48 h and stimulated by addition of 10% FCS, EGF, TPA, or dbcAMP for 1 h (see Materials and Methods). Nuclear extracts were isolated and used in EMSA. The labeled coll-TRE oligonucleotide was used as a probe.
The AP-1 binding activity has also been analyzed with the coll-TRE as a probe in nuclear extracts of REF cells and E1A + cHa-ras transformants grown under various conditions (Fig. 1B, C). Serum-starved cells (0.5% FCS for 48 h) were stimulated for 1 h with 10% FCS, TPA, EGF, or dbcAMP. As shown in Fig. 1B, in serum-starved REF cells (lane 2) the AP-1 DNA binding activity was very low, but could be stimulated by addition of 10% FCS, EGF, or TPA. The inducibility of AP-1 DNA binding activity was also observed in E1A-immortalized cells (results not shown). In contrast to the REF and E1A cells, E1A + cHa-ras transformants reveal high levels of AP-1 complexes, which cannot be up- or downregulated by addition or removal of serum or by treatment with TPA, EGF, or dbcAMP (Fig. 1C).
The c-jun Gene Is Constitutively Expressed at High Levels, Whereas c-fos Is Downregulated and Cannot Be Induced in E1A + cHa-ras Cells
We have earlier analyzed by Northern blot hybridization the expression of c-fos and c-jun genes, whose products are the constituents of the AP-1 complexes (36a). Total RNA was prepared from REF cells, E1A-immortalized, and E1 A + cHa-ras transformed cells grown in the presence of 10% FCS or 0.5% FCS (serum starvation) and after stimulation of serum-starved cells with 10% FCS and TPA (Fig. 2). The results showed that the expression of c-fos and c-jun genes was found to be under stringent control in normal REF cells and that these genes could be activated by growth factors as expected from data in Fig. 1B. c-fos and c-jun genes were also found to be activated by serum in E1A-immortalized cells, although the noninduced levels of c-jun gene expression were somewhat higher than in REF cells (Fig. 2, middle). In E1A + cHa-ras transformed cells, which have very high level of AP-1 DNA binding activity (Fig. 1), the c-fos gene expression could not be detected under any conditions of cell cultivation, whereas c-jun was expressed at a high and constitutive level (Fig. 2, right). Thus, the expression of c-fos and c-jun genes was found to be affected to a different extent in E1A + cHa-ras transformed cells: c-fos was downregulated and could not be stimulated by any growth factor, whereas c-jun was constitutively upregulated.
FIG. 2.
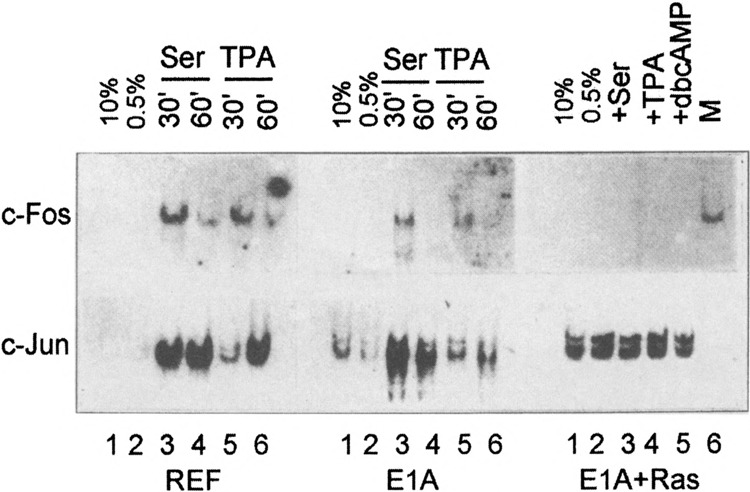
Northern blot hybridization of RNA isolated from REF cells, E1A-immortalized cells, and E1A + cHa-ras transformed cells with DNA probes specific for the c-fos and the c-jun genes (36a). REF and E1A cells were grown in DMEM supplemented with 0.5% FCS for 48 h (serum starvation) and subsequently were stimulated by addition of 10% FCS and TPA during 30 and 60 min. E1A + cHa-ras cells were stimulated with 10% FCS, TPA, and dbcAMP during 30 min. In the lane indicated with “marker,” a control RNA isolated from serum-stimulated E1A cells was hybridized with the c-fos probe.
Because the most significant increases in expression of immediately early genes, such as c-fos, can be detected upon serum stimulation of quiescent cells, we performed the EMSA after incubation of nuclear extracts prepared from serum-stimulated cells with c-Fos-specific antibodies. We used the concentrated antibodies (see Materials and Methods), which are able to form high molecular complexes with the transcription factors, giving rise to the supershifted bands upon electrophoresis (Fig. 3). It was found that c-Fos protein can indeed be detected in the serum-stimulated REF and E1A-immortalized cells (super-shifted complexes marked by SC). Under the same conditions, in nuclear extracts of E1A + cHa-ras or E1A + E1B19kD transformed cells the supershifted complexes were not detected (Fig. 3). A band marked by a star does represent the complex I described in Fig. 1A, and it migrates actually faster than the super-shifted Fos-specific complex detected in REF and E1 A cells. In agreement with these data, c-Fos proteins were also not detected by Western blot both in nonstimulated and stimulated E1A + cHa-ras cells (Fig. 4B, lanes 3 and 4), whereas in REF cells c-Fos proteins were readily seen after serum stimulation (Fig. 4B, lanes 1 and 2). In accordance with our Northern blot hybridization data, the amount of c-Jun protein was found to be increased in exponentially growing E1A + cHa-ras transformants (Fig. 4A, lane 4). Correspondingly, antibodies raised against c-Jun protein caused the formation of a supershifted band when incubated with nuclear extracts of not only serum-stimulated REF cells but also E1A + cHa-ras transformants growing in the presence of 10% FCS (Fig. 5A, lanes 3 and 7). Interestingly, the amount of JunD proteins measured by the immunoblot procedure was very similar in all investigated cell lines (Fig. 4A, top panel). But JunD-supershifting antibodies revealed detectable amount of JunD-containing AP-1 complexes both in nonstimulated and serum-stimulated REF and E1A cells (Fig. 5B), but barely in E1A + cHa-ras transformants (Fig. 5C, lanes 3 and 4 the supershifted complexes). Thus, though JunD protein is present in comparable amounts in REF and E1A + cHa-ras cells (Fig. 4A), the content of JunD-containing AP-1 complexes appears to be low in E1A + cHa-ras transformants compared with normal REF cells.
FIG. 3.
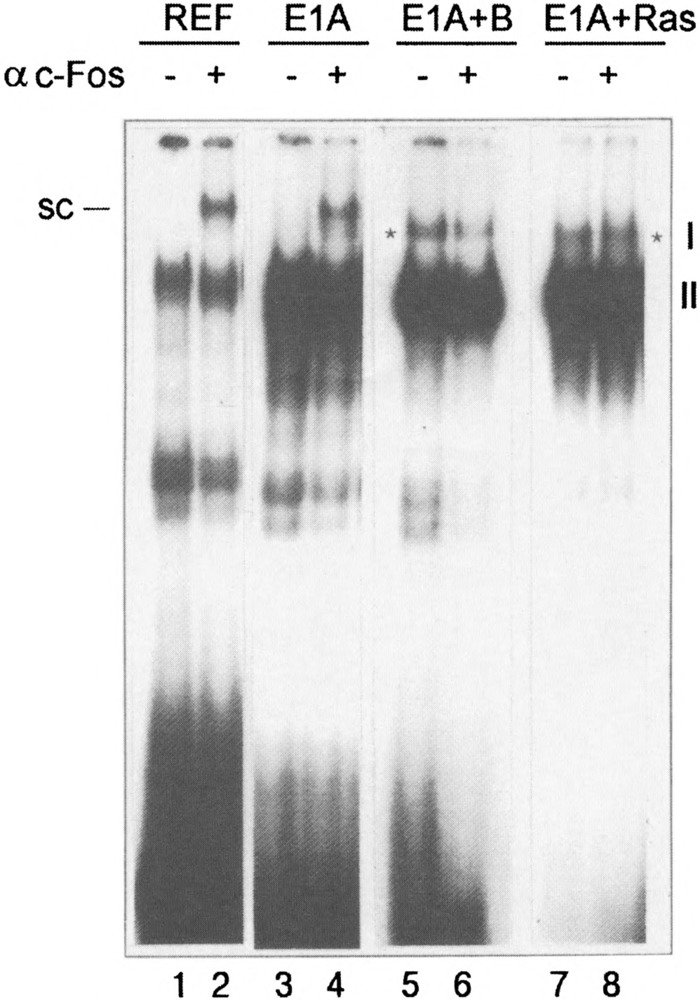
c-Fos protein is a component of AP-1 complex in nuclear extracts of serum-stimulated REF (lanes 1 and 2) and E1A-immortalized cells (lanes 3 and 4) but not in serum-stimulated E1 A + E1B19kD (lanes 5 and 6) and E1A + cHa-ras transformants (lanes 7 and 8). Cells were serum starved for 48 h (0.5% FCS), then subsequently stimulated by addition of 10% FCS for 1 h. Nuclear extracts (2 μg) were incubated with 2μ of PBS or with c-Fos-specific antibodies (Santa Cruz sc-52x). Subsequently, labeled coll-TRE was added followed by incubation for 20 min. SC indicates supershifted AP-1 complexes containing the c-Fos; asterisks indicate the position of complex I in relation to supershifted complex (SC).
FIG. 4.
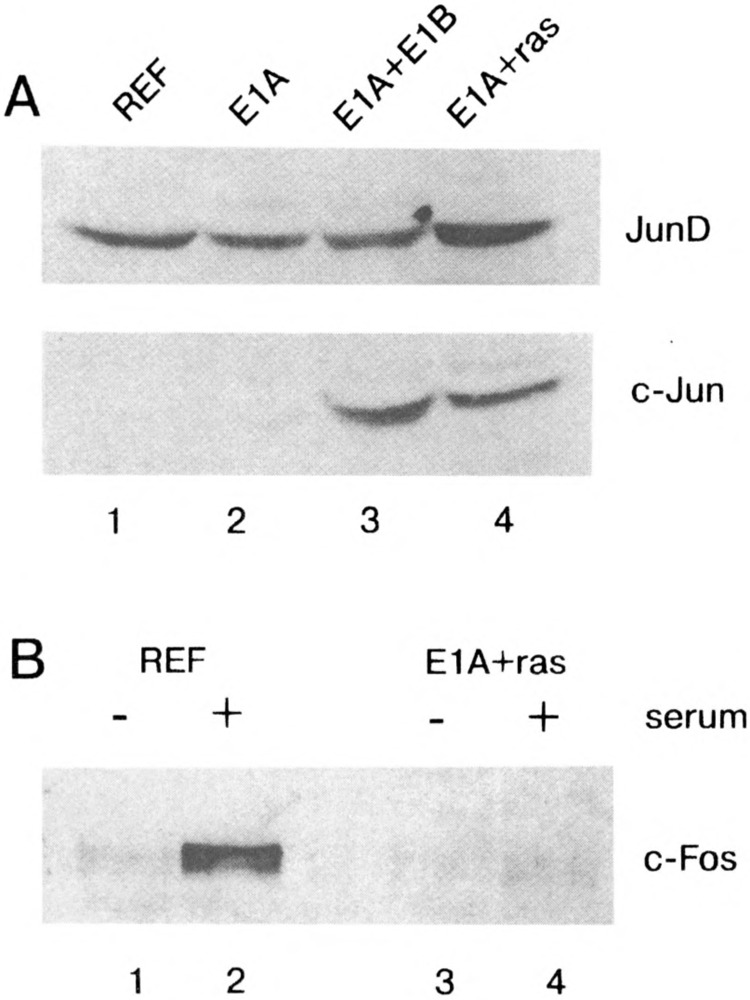
(A) Western blot analysis of total proteins from REF, E1A, E1A + E1B19kD, and E1A + cHa-ras cells probed with antibodies to JunD and c-Jun proteins. Cells were grown in the presence of 10% FCS and lysed with a full RIPA buffer (see Materials and Methods). Proteins were separated by electrophoresis in 10% polyacrylamide gel and stained by antibodies specific to JunD and c-Jun proteins (Santa Cruz sc-74 and sc-45). (B) c-Fos protein is not serum stimulated in E1A + cHa-ras transformants. REF (lanes 1, 2) and E1A + cHa-ras (lanes 3, 4) cells were serum starved (0.5% FCS for 48 h, lanes 1 and 3), then serum stimulated with 10% FCS for 1 h (lanes 2 and 4). Proteins were separated by electrophoresis and visualized with antibodies specific to c-Fos protein (Santa Cruz sc-52).
FIG. 5.
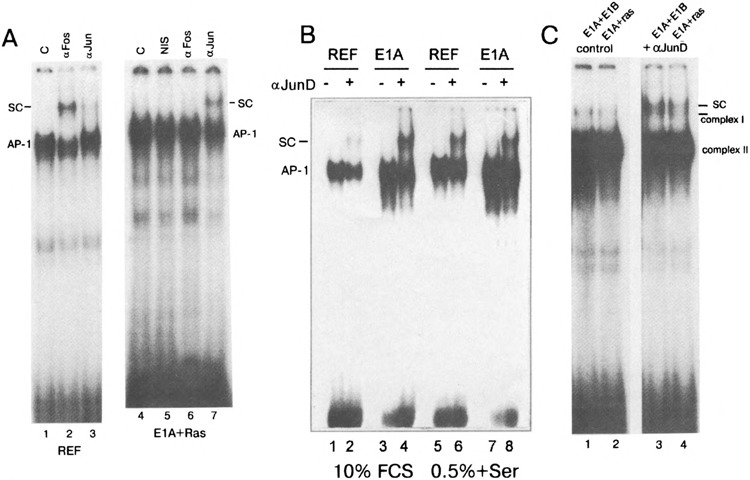
(A) c-Jun proteins are present in AP-1 complex of exponentially growing E1A + cHa-ras cells. Nuclear extracts of serum-stimulated REF cells (lanes W3) and exponentially growing E1A + cHa-ras cells (lanes 4–7) were incubated with antibodies to c-Jun protein (Santa Cruz #sc-45x, lanes 3 and 7) and c-Fos protein (Santa Cruz sc-52x, lanes 2 and 6). NIS: nonimmune serum; SC: supershifted AP-1 complexes formed with the specific antibodies. (B, C) JunD proteins are detected in AP-1 complex of nuclear extracts from normal and transformed cells by the EMSA supershift analysis. (B) REF and E1A cells were grown in the presence of 10% FCS (lanes 1-*) or serum starved (0.5% FCS for 24 h) and then serum stimulated with 10% FCS for 1 h (lanes 5–7). Nuclear extracts were incubated with PBS (lanes 1, 3, 5, 7) or with JunD-specific antibodies (lanes 2, 4, 6, 8, Santa Cruz sc-74x), and complexes were separated in 5% gel. SC: AP-1 complexes super-shifted by JunD-specific antibody. (C) Nuclear extracts from E1A + E1B19kD cells (lanes 1 and 3) and E1A + cHa-ras cells (lanes 2 and 4) were incubated with PBS (control, lanes 1 and 2) or with JunD-specific antibodies (lanes 3 and 4). Two horizontal lines are given to discriminate mobilities of supershifted AP-1 complexes (SC) and the complex I that migrates faster. The labeled coll-TRE oligonucleotide was used as a probe.
c-Fos Appears to be Replaced by Fra-1 Protein in E1A + cHa-ras Transformants
Because c-Fos protein was found to be absent in E1A + cHa-ras transformants, we also checked other proteins of the Fos family by incubating the nuclear extracts with antibodies to FosB, Fra-1, and Fra-2 transcription factors. Data presented in Fig. 6A show that the FosB protein expression appears to follow by the same mode as for c-Fos: exponentially growing REF cells (10% FCS, Fig. 6, lane 2)) do not practically reveal FosB in AP-1 complex, whereas serum stimulation leads to FosB accumulation (Fig. 6A, lane 5). However, AP-1 complexes from serum-stimulated E1A + cHa-ras transformants did not contain FosB protein (Fig. 6, lane 11). The Fra-2 protein can be detected in unstimulated REF cells and in less amount in stimulated REF and E1A cells (Fig. 6, lanes 3, 6, and 9), but not in AP-1 complex of E1A + cHa-ras transformants (no supershifted complexes, no reduction of the AP-1 complex intensity, Fig. 6, lane 12). Quite different data were obtained with antibodies specific to Fra-1 protein (Fig. 6B, C). If unstimulated (Fig. 6B) and stimulated (Fig. 6C) REF and E1A cells contain little but detectable quantity of the factor, the amount of Fra-1 in E1A + cHa-ras transformants was found to be significantly increased (Fig. 6B, C, supershifted complexes). Thus, in the absence of c-fos gene expression, c-Fos protein appears to be replaced by Fra-1, and Fra-1 becomes a predominant component of the Fos family presented in the AP-1 complex of E1A + cHa-ras cells.
FIG. 6.
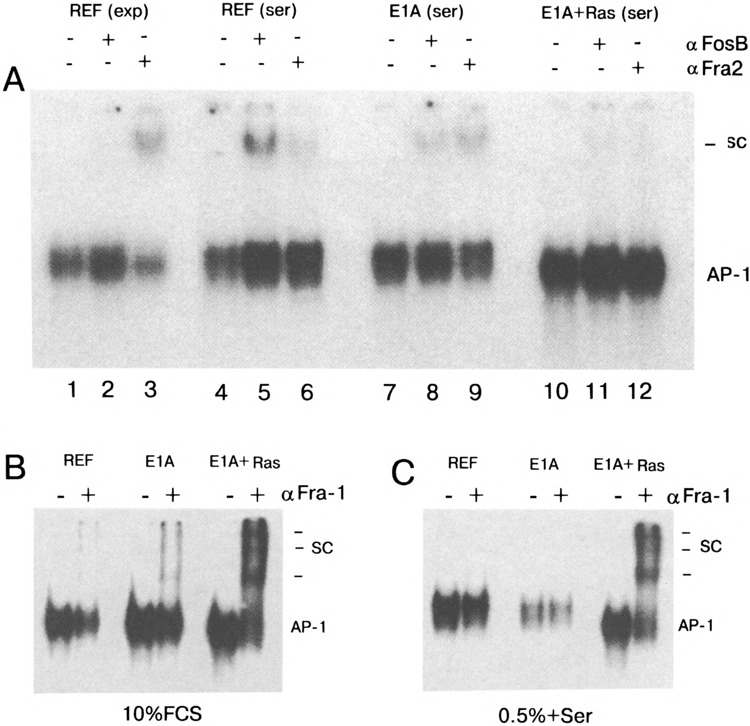
(A) FosB and Fra-2 proteins in AP-1 complexes of normal and transformed cells. Nuclear extracts have been isolated from exponentially growing REF cells (10% FCS, lanes 1–3) or REF cells stimulated by 10% FCS for 1 h (lanes 4–6), from serum-stimulated E1A cells (lanes 7–9), and serum-stimulated E1A + cHa-ras cells (lanes 10 and 12). Nuclear extracts were incubated with antibodies to FosB and Fra-2 proteins (29). The labeled coll-TRE oligonucleotide was used as a probe. SC: high molecular complexes (supershifts) formed in the presence of the specific antibodies. (B, C) Fra-1 protein is accumulated in nuclear extracts of E1A + cHa-ras transformed cells. Nuclear extracts have been obtained from exponentially growing (B) and serum-stimulated (C) REF, E1A, and E1A + cHa-ras cells. In both panels, lanes (-) without addition of Fra-1-specific antibodies, lanes (+) added Fra-1 specific antibodies (Santa Cruz sc-183x). The labeled coll-TRE oligonucleotide was used as a probe. Horizontal lines indicate the positions of supershifted complexes (SC).
E1A + cHa-ras Transformants Contain High Levels of ATF-Containing Dimers
To study whether AP-1 complex of E1A + cHa-ras transformants does contain, except of Fra-1 and Jun, the proteins of the ATF family, we used antibodies specific for ATF-2, ATF-3, and ATFa factors and the labeled jun2-TRE as a probe in supershift EMSA experiments. Data presented in Fig. 7 show that E1A + cHa-ra.s cells do contain significant amount of ATF-2 and ATFa factors, which are the components of complex I. One can see the supershifted complexes concomitant with the reduction of the complex I intensity (Fig. 7A, B). No supershifted complexes could be detected with ATF-3-specific antibodies, although ATF-3 factor could be readily detected with the same antibodies in control extracts (HER cells transformed by E1 region of Ad5) (19) (results not shown). Thus, the composition of AP-1 complexes of E1A + cHa-ras cells is changed from c-Fos/c-Jun and Jun/Jun dimers to those composed of Fra-l/ATF/Jun.
FIG. 7.
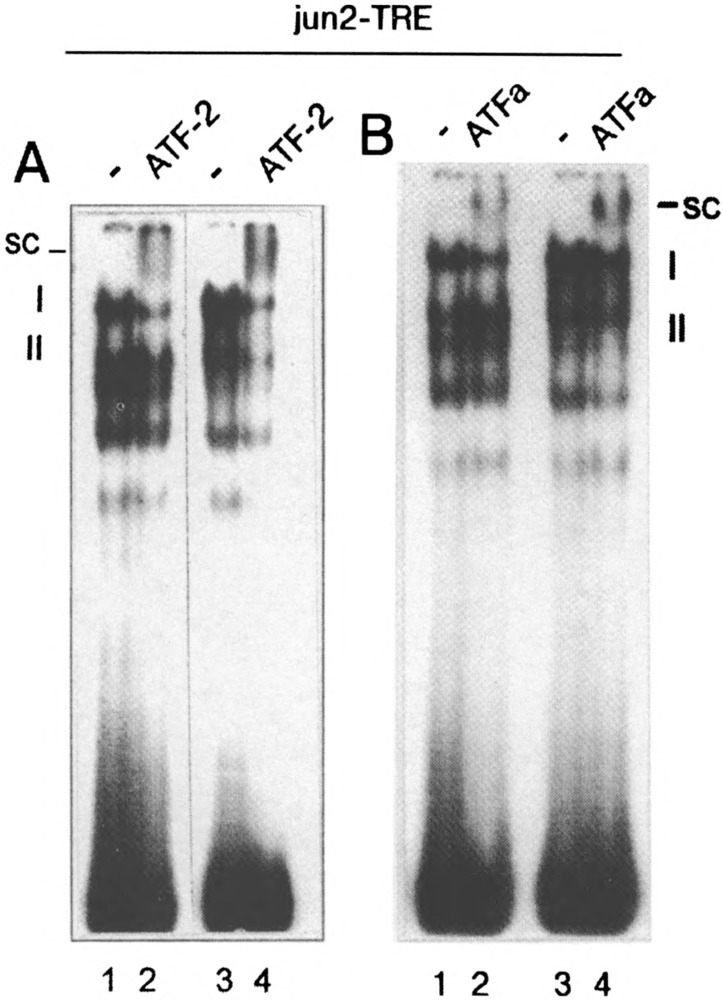
Detection of ATF-containing complexes with the jun2-TRE probe in supershift EMSA experiment. (A) ATF-2-containing AP-1 complexes in nuclear extracts of E1A + E1B19kD (lanes 1 and 2) and E1A + cHa-ras (lanes 3 and 4) cells were detected with thev"im2-TRE-labeled probe and ATF-2-specific antibodies (lanes 2 and 4). (B) ATFa-containing AP-1 complexes were detected in nuclear extracts of E1A + E1B19kD cells (lanes 1 and 2), and E1A + cHa-ras cells (lanes 3 and 4) with ATFa-specific antibodies (14) (lanes 2 and 4) and jun2-TRE-labeled probe. I and II indicate the AP-1 complexes, and SC indicates the supershifted complexes.
Negative Regulation of c-fos Gene Promoter in E1A + cHa-ras Cells Is Likely to Be Mediated Through the SRE Site
To determine the possible mechanisms of c-fos downregulation we stably integrated a plasmid construct containing a fragment of c-fos gene promoter (-711/+45) linked to a reporter bacterial CAT gene (−711fos-CAT). To study the effects produced by mutations in the c-fos promoter, we used several mutant constructs: point mutants in serum response element (SRE) (G>A transitions at positions −319 at the TCF binding site and −304 nt at the SRF binding site), a deletion mutant of cAMP-responsive element (CRE) (−711fosΔ-65/−52CAT). The used mutations inhibited the serum-stimulated fos-CAT activity differently, in particular, in the TCF binding site to 80−90%, in the SRF binding site to 60% (11), and in the CRE site up to 70-80% (22). All plasmids were introduced into E1A + cHa-ras cells by cotransfect-ing with a selectable vector pSVneo conferring genet-icin resistance to antibiotic G-418. The formed genet-icin-resistant clones were pooled and used to monitor the levels of CAT activity. The CAT assay given in Fig. 8 represents one of three independent experiments with each mutant construct. The averages and standard deviations are given below in the text. As expected, the activity of wild-type -711fos-CAT construct was very low in E1A + cHa-ras cells that was in clear agreement with the lack of c-fos gene expression in these cells (see Fig. 2). A deletion of the CRE site (ACRE) only slightly affected the activity of -711fos-CAT (1.55 ± 0.4-fold activation). The fact that the CRE mutation did not lead to any significant increase in expression suggests that the c-fos promoter repression was not based on this element in E1A + cHa-ras transformants. On the other hand, both mutations within the SRE site (at -319 and -304 nt positions) caused a several fold increase of fos-CAT activity (mTCF: 13.9 ±0.8-and mSRF: 33.9 ± 0.1-fold activation, respectively) (Fig. 8A). Because these mutations affect the TCF and SRF binding to the SRE (45,52), this allows to suggest that negative regulation of c-fos promoter in E1A + cHa-ras cells is mediated through the SRE-SRF/TCF ternary complex. To prove that c-fos promoter mutants behave properly in other cell lines, we transiently transfected the plasmids into REF and NIH 3T3 cells. The cells were grown in 10% FCS (c-fos is repressed) or serum starved in 0.5% FCS and then stimulated with 10% FCS for 1-3 h (c-fos is induced). Data presented in Fig. 8B show that under conditions of c-fos downreg-ulation the mutations affecting TCF and SRF binding caused elevated fos-CAT expression (Fig. 8B, lanes 2 and 3). In contrast, the mutations inhibited serum-stimulated c-fos promoter expression (Fig. 8B, lanes 5 and 6).
FIG. 8.
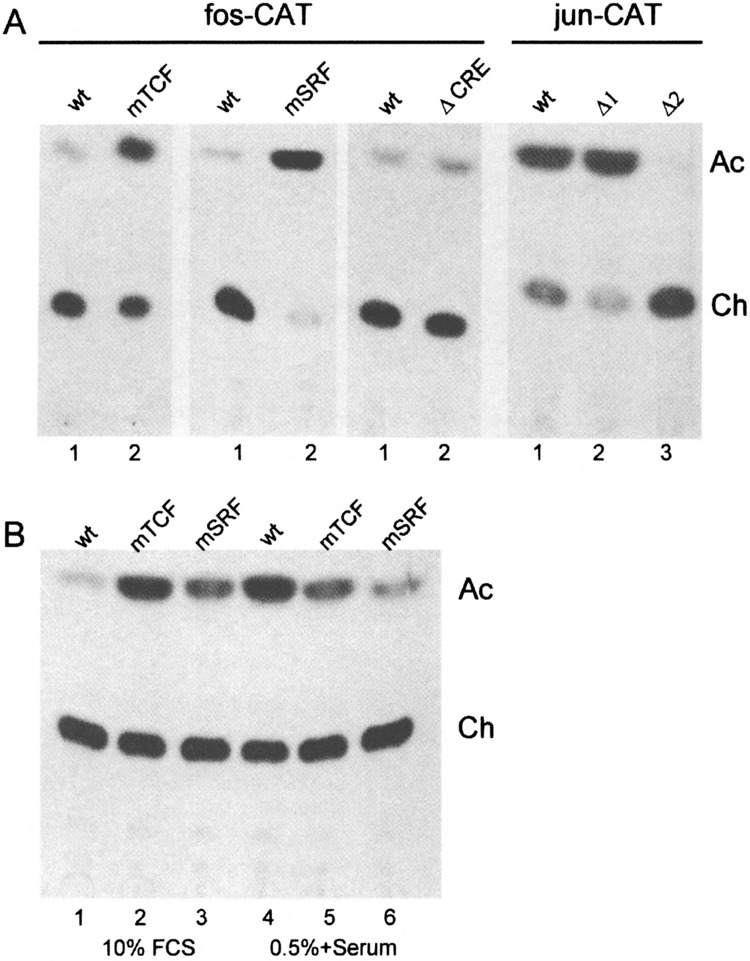
(A) Chloramphenicol acetyltransferase activity of wild-type fos-CAT and jun-CAT constructs and their mutants stably integrated into E1A + cHa-ras cells. Cell lysates were prepared from populations of clones containing integrated wild-type constructs or mutant derivatives. The levels of CAT activity were measured for wild-type fos-CAT (wt), a mutant in the TCF binding site (mTCF), a mutant in the SRF binding site (mSRF), a mutant in the CREB binding site (ACRE). The right panel of CAT assays is for wild-type jun-CAT construct (wt) and two mutants: Δ1 and Δ2 lacking the functional jun1-TRE and jun2-TRE, respectively (45). Ch and Ac: nonacetylated and acetylated forms of [14C]chloramphenicol. (B) Mutants affecting TCF and SRF binding cause overexpression of fos-CAT construct in REF cells grown in 10% FCS (c-fos is repressed). Cells were transiently transfected with wild-type fos-CAT (wt) or its mutants at the TCF (mTCF) and SRF (mSRF) binding. At 16 h after transfection, cells were either grown in 10% FCS (lanes 1–3) or serum starved in 0.5% FCS and then stimulated with 10% FCS for 1–3 h (lanes 4–6).
To confirm a functional significance of Jun/ATF heterodimers that are in abundance in nuclear extracts of E1A + cHa-ras cells, we stably integrated by similar method the the CAT constructs driven by c-jun gene promoter: wild-type -1600/+740/Kn-CAT, mutants in jun1-TRE (−1600/+740A1) and in jun2-TRE (−1600/+740A2) (44). As expected, activity of wild-type jun-CAT was very high in E1A + cHa-ras cells; the results obtained for jun1-TRE mutation (Al) showed that this AP-1 site contributed little to c-jun expression because there can be even seen slight activation (1.14 ±0.02). On the other hand, jun2-TRE mutation (A2) practically turned the c-jun promoter off (Fig. 8A). Thus, the jun2-TRE element is functionally significant for the expression of c-jun promoter in E1A + cHa-ras cells, and Jun/ATF heterodimers that fail to bind to the mutant jun2-TRE site are not capable of effectively using another AP-1 element, jun1-TRE, to facilitate the transcription of c-jun promoter in these cells.
DISCUSSION
Changes in the levels and/or activities of AP-1/ ATF factors by genetic mutation or by the action of viral proteins may contribute to oncogenesis. In this article, we show that REF cells transformed by Ad5 E1A and cHa-ras oncogenes are characterized by high and constitutive DNA binding activity of AP-1 complexes when various AP-1 elements have been used as probes in EMSA: coll-TRE and jun2-TRE. Also, we observed significant changes in the composition of AP-1 complexes: c-fos was no longer expressed or stimulated by serum in E1A + cHa-ras transformants, whereas some other members of Fos family (FosB, Fra-2) contributed very little, if at all, to the AP-1 complex in these cells. In contrast, Fra-1 factor, another member of the Fos family, was found to be accumulated in the AP-1 complex of E1A + cHa-ras transformants. It appears that c-Fos/c-Jun heterodimers, which are specific for the G0 > G1 transition of the cell cycle, are replaced by the complexes containing Fra-1 factors. Because Fra-1 protein was found to be accumulated in NIH 3T3 cells transformed by a single cHa-ras oncogene (33), one may suggest that the E1A oncogene does contribute little to elevation of the Fra-1 content of E1A + cHa-ras cells (see also Fig. 6B, C).
The above-mentioned shift in the AP-1 composition may have profound effects on the regulation of gene expression. The c-Jun/c-Fos and c-Jun/ATF complexes have distinct DNA binding specifities and influence on different sets of target genes. In addition, the c-Jun/c-Fos and the c-Jun/ATF complexes are differentially affected by various stimuli. The first mentioned complexes are stimulated mainly by mito-genic agents, whereas the latter are predominantly activated by stress- and DNA-damaging agents. Moreover, these complexes are affected differently by E1A. Whereas E1 A oncogene represses transcription mediated by c-Jun/c-Fos complexes, possibly by interference with their DNA binding activities, and by squelching the transcriptional coactivator p300, E1A stimulates transcription via c-Jun/ATF heterodimers (2,4,20). Taking into account that the E1A + cHa-ras cells contain high and constitutive levels of Jun/ATF complexes and no c-Fos-containing complexes under any of the tested growth conditions, one can expect that especially genes containing regulatory elements resembling the jun1-TRE and the jun2-TRE will be activated. Which target genes are important for transformation by E1A + cHa-ras largely remain to be determined; however, it seems highly probable that high and constitutive expression of the c-jun gene (and probably fra-1) may contribute to transformation by E1A + cHa-ras oncogenes. Consistently, fi-broblasts from c-jun gene knock-out mice cannot be transformed by the cHa-ras oncogene, whereas ec-topic expression of c-jun leads to partial transformation of NIH 3T3 cells (27).
Concerning the role of another immediate-early gene, c-fos, in oncogenic transformation, the situation is more complex. In fibroblast cells the formation of transformed foci by various oncogenes was found to be independent of c-fos gene expression (c-fos +/+ vs. c-fos -/- cells), suggesting that active c-fos is not obligatory, at least for initial steps of transformation (25), but c-fos is required for malignant progression of keratinocytes (41). On the other hand, rat fibro-blast cell line 208F can be morphologically transformed by inducible continuous expression of a single c-fos gene (34). These authors showed that the c-fos expression was not sufficient, however, to stimulate cell cycle progression, implying that other genes should be switched to start cell proliferation. Other experiments suggest that the noninducibility or attenuation of c-fos expression upon transformation by Ha-Ras may be a more general phenomenon (32,51).
For example, in rat 3Y1 cells transformed by cyto-plasmic oncogenes (v-src, v-sis, and v-raf) serum inducibility of several immediate early genes, including c-fos, was shown to be attenuated (51). In mouse NIH 3T3 cells, introduction of cHa-ras also results in loss of the c-fos inducibility (37). Apparently, downregu-lation and noninducibility of c-fos upon oncogenic transformation is not limited to the REF cell system and may contribute to a shift in balance of transcription factors and thereby may contribute to oncogenic transformation. It has been suggested that ATFa and c-Fos might have functional antagonistic property (14). The c-Jun heterodimers with ATF-2 and c-Fos can regulate positively and negatively for the uroki-nase gene enhancer, respectively (15). In the E1A + cHa-ras cells, the absence of c-Jun/c-Fos complexes and constitutively high levels of Jun/ATF may thus even further increase activation of certain genes via ATF-responsive elements.
As for Fra-1 expression in E1A + cHa-ras trans-formants, its expression can be mediated through an AP-1 site located in the intron I of the gene (8) due to high levels of Jun proteins in the transformants. Fra-l/Jun complexes, in turn, can modulate the c-fos transcription via the AP-1 site located downstream of the SRE in c-fos promoter. This suggestion is supported by the results evidencing that ectopic expression of fra-1 and c-jun genes is able to suppress c-fos response on serum stimulation (33); moreover, NIH 3T3 clones overexpressing both c-Jun and Fra-1 proteins display enhanced transformed properties (33). The experiments with c-fos promoter mutants presented in this study showed that negative regulation of c-fos promoter in E1A + cHa-ras cells was mediated through the SRE site. The SRE element of c-fos promoter is a target for negative factors in exponentially growing normal and minimally transformed cells, and in vivo titration by exogenous SRE can relieve the c-fos repression (28,38). It remains to determine whether the c-fos repression in E1A + cHa-ras transformants is mediated by a yet unidentified factor or the repression is provided by a modification of preexisting factors on a level of phosphorylation/de-phosphorylation of ternary complex SRE/SRF/TCF (23,52). Indeed, the SRE mutations used in the present work are located in those parts of the SRE, which are responsible for interaction with TCF and SRF factors. Therefore, one may suggest that in E1A + cHa-ras transformants the complex SRF-TCF permanently bound to the SRE (23) functions as a repressor. The repression can be provided, at least partly, by dephosphorylation of the TCF factor. Indeed, treatment of E1A + cHa-ras population bearing integrated wild-type fos-CAT construct with okadaic acid, an inhibitor of protein phosphatases, particularly PP2A (42), led to twofold activation of the fos-CAT expression. This might imply that fos promoter repression was reversible and was dependent on the phosphorylation/dephosphorylation equilibrium. We have noticed also that irradiation of wild-type fos-CAT-bearing E1A + cHa-ras population by UV light, which is known to activate the c-fos transcription due to phosphorylation of TCF factor (3), stimulated of fos-CAT expression (unpublished).
Antibodies to c-Fos, FosB, and Fra-2 proteins did not reveal these factors in the AP-1 complex of E1A + cHa-ras transformants, in contrast to Fra-1 factor, which was found to be significantly accumulated. A sequence analysis of promoters of c-fos, fosB, and fra-1 genes shows that only fra-1 gene promoter seems not to have the SRE (31). If c-fos (and fosB) repression is indeed mediated through the SRE regions of these genes, the absence of the SRE in promoter of fra-1 gene can give an explanation of why fra-1 gene is not downregulated in E1A + cHa-ras transformants.
In summary, it appears likely that E1A proteins alter the c-jun gene expression and the trans-activating capacities of ATF-containing factors, whereas E1A + cHa-ras promotes the maintenance of high levels of factors of the AP-1 family after transformation, which cannot be regulated by mitogenic stimuli. In addition, the shift from c-Jun/c-Fos towards constitutive levels of Fra-1/Jun/ATF-containing complexes may have profound consequences for the transcriptional regulation of relevant target genes.
ACKNOWLEDGMENTS
We thank A. K. Savel’ev, Yu. K. Kurash, H. van Ormondt, and A. Zantema for help in this work. The authors thank A. G. Jochemsen for the kind gift of CMV-21kDa plasmid, and M. Yaniv and B. Chatton for generously providing us with antibodies to proteins Fra-1, Fra-2, FosB, and ATFa. We are also grateful the H. van Dam, P. Herrlich, H. Rahmsdorf, and M. Bonfanti for valuable supply of fos-CAT and jun-CAT constructs and their mutants. This work was supported by grants from the Russian Foundation for Basic Research (98-04-49896 and 97-04-50199), the State Program “National Priorities in Medicine and Public Health” (21/97), and INTAS (94-4409).
REFERENCES
- 1. Abdel-Hafiz H. A.; Chen C. Y.; Marcell T.; Kroll D. J.; Hoeffler J. P. Structural determinants outside of the leucine zipper influence the interaction of CREB and ATF-2: Interaction of CREB with ATF-2 blocks Ela-ATF-2 complex formation. Oncogene 8:1161–1174; 1993. [PubMed] [Google Scholar]
- 2. Abraham S. E.; Lobo S.; Yaciuk P.; Wang H. G.; Moran E. p300, and p300-associated proteins, are components of TATA-binding (TBP) complexes. Oncogene 8:1639–1647; 1993. [PubMed] [Google Scholar]
- 3. Angel P.; Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129–157; 1991. [DOI] [PubMed] [Google Scholar]
- 4. Arany Z.; Newsome D.; Oldread E.; Livingston D. M.; Eckner R. A family of transcriptional adaptor proteins targeted by the ElA oncoprotein. Nature 374:81–84; 1995. [DOI] [PubMed] [Google Scholar]
- 5. Bayley S. T.; Mymryk J. S. Adenovirus E1A proteins and transformation (review). Int. J. Oncol. 5:425–444; 1984. [DOI] [PubMed] [Google Scholar]
- 6. Benbrook D. M.; Jones N. C. Heterodimer formation between CREB and jun proteins. Oncogene 5:295–302; 1990. [PubMed] [Google Scholar]
- 7. Benbrook D. M.; Jones N. C. Different binding specificities and transactivation of variant CRE’s by CREB complexes. Nucleic Acids Res. 22:1463–1469; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergers G.; Granninger P.; Brasselmann S.; Wrighton C.; Busslinger M. Transcription activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the I intron. Mol. Cell. Biol. 15:3747–3758; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Binetruy B.; Smeal T.; Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature 351:122–127; 1991. [DOI] [PubMed] [Google Scholar]
- 10. Birnboim S. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 16:1487–1497; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonfanti M.; Collela G.; Broggini M.; D’lncalci M. Effect of G:C > A :T transition, potentially arising from 06-guanine alkylation, in the transcription response of c-fos serum response element. Anticancer Res. 17: 2019–2024; 1997. [PubMed] [Google Scholar]
- 12. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 73:248–254; 1976. [DOI] [PubMed] [Google Scholar]
- 13. Bravo R. Growth factors, differentiation, and cytokines. Berlin: Springer Verlag Press; 1990:324–343. [Google Scholar]
- 14. Chatton B.; Bocco J. L.; Goetz J.; Gaire M.; Lutz Y.; Kedinger C. Jun and Fos heterodimerize with ATFa, a member of the ATF/CREB family and modulate its transcriptional activity. Oncogene 9:375–385; 1994. [PubMed] [Google Scholar]
- 15. De Cesare D.; Vallone D.; Carraciolo A.; Sassone-Corsi P.; Nerlov C.; Verde P. Heterodimerization of c-Jun with ATF-2 and c-Fos is required for positive and negative regulation of the human urokinase enhancer. Oncogene 11:365–376; 1995. [PubMed] [Google Scholar]
- 16. Deng T.; Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature 371:171–175; 1994. [DOI] [PubMed] [Google Scholar]
- 17. Derijard B.; Hibi M.; Wu I. H.; Barrett T.; Su D.; Dery T.; Karin M.; Davis R. J. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025–1037; 1994. [DOI] [PubMed] [Google Scholar]
- 18. Gorman C. M.; Moffat L. F.; Howard B. H. Recombinant genomes which express chloramphenicol acetyl-transferase in mammalian cells. Mol. Cell. Biol. 2: 1944–1051; 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hagmeyer B. M.; Duyndam M. C. A.; Angel P.; de Groot R. P.; Verlaan M.; Elfferich P.; van der Eb A. J.; Zantema A. Altered AP-l/ATF complexes in adenovirus-El-transformed cells due to ElA-depen-dent induction of ATF-3. Oncogene 12:1025–1032; 1996. [PubMed] [Google Scholar]
- 20. Hagmeyer B. M.; Konig H.; Herr I.; Offringa R.; Zantema A.; van der Eb A.; Herrlich P.; Angel P. Adenovirus El A negatively and positively modulates transcription of AP-1 dependent genes by dimer-specific regulation of the DNA-binding and transactivation activities of jun. EMBO J. 12:3559–3572; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hai T.; Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88: 3720–3724; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartig E.; Loncarevic I. F.; Buscher M.; Herrlich P.; Rahmsdorf H. J. A new cAMP response element in the transcribed region of the human c-fos gene. Nucleic Acids Res. 19:4153–4159; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrera R. E.; Shaw P. E.; Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature 340:68–70; 1989. [DOI] [PubMed] [Google Scholar]
- 24. Hill C. S.; Treisman R. Transcriptional regulation by extracellular signals: Mechanisms and specificity. Cell 80:194–211; 1995. [DOI] [PubMed] [Google Scholar]
- 25. Hu E.; Mueller E.; Oliviero S.; Papaioannou V. E.; Johnson R.; Spiegelman B. M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 13:3094–3103; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivashkiv L. B.; Lion H. C.; Kara C. J.; Lamph W. W.; Verma I. M.; Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoyl phorbol-13-acetate response element. Mol. Cell. Biol. 10:1609–1621; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson R.; Spiegelman B.; Hanahan D.; Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol. Cell. Biol. 16:4504–4511; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konig H.; Ponta H.; Rahmsdorf H.; Buscher H.; Schonthal A.; Rahmsdorf H.-J.; Herrlich P. Autoregulation of c-fos: The dyad symmetry element as the major target of repression. EMBO J. 9:2559–2566; 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lallemand D.; Spyrou G.; Yaniv M.; Pfarr C. M. Variations in Jun and Fos protein expression and AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene 14:819–830; 1997. [DOI] [PubMed] [Google Scholar]
- 30. Lamph W. W.; Wamsley P.; Sassone-Corsi P.; Verma I. M. Induction of protooncogene JUN/AP-1 by serum and TPA. Nature 334:629–631; 1988. [DOI] [PubMed] [Google Scholar]
- 31. Lazo P. S.; Dorfman K.; Noguchi T.; Mattei M.-G.; Bravo R. Structure and mapping of the fosB gene. FosB downregulates the activity of the fosB promoter. Nucleic Acids Res. 20:343–350; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin A. H.; Groppi V. E.; Gorman R. R. Platelet-derived growth factor does not induce c-fos in NIH3T3 cells expressing the EJ-ras oncogene. Mol. Cell. Biol. 8:5052–5055; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mechta F.; Lallemand D.; Pfarr C. M.; Yaniv M. Transformation by Ras modifies AP-1 composition and activity. Oncogene 14:837–847; 1997. [DOI] [PubMed] [Google Scholar]
- 34. Miao G. G.; Curran T. Cell transformation by c-fos requires an extended period of expression and is independent of the cell cycle. Mol. Cell. Biol. 14:4295–4310; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peeper D. S.; Zantema A. Adenovirus ElA proteins transform cells by sequestering regulatory proteins. Mol. Biol. Rep. 17:197–207; 1993. [DOI] [PubMed] [Google Scholar]
- 36. Pospelova T. V.; Medvedev A. V.; Svetlikova S. B.; Pospelov V. A. Characteristics of the transformed phenotype and the expression of CAT-plasmids in rat embryo fibroblasts transformed with ElA + Ha-Ras oncogenes. Cytology 32:148–155; 1990. [PubMed] [Google Scholar]
- 36a. Pospelova T. V.; Kukushkin A. N.; Medvedev A. V.; Savel’ev A. K.; Pospelov V. A. Activity and composition of transcription factor AP-1 in rat embryo fibroblasts transformed with oncogenes ElA and Ha-ras. Mol. Biol. (Mosk.). 30:662–672; 1996. [PubMed] [Google Scholar]
- 37. Pospelov V. A.; Pospelova T. V.; Julien J.-P. AP-1 and Krox-24 transcription factors activate the neurofilament light gene promoter in P19 embryonal teratocarcinoma cells. Cell Growth Differ. 5:187–196; 1994. [PubMed] [Google Scholar]
- 38. Pospelova T. V.; Volkov I. V.; Kukushkin A. N.; Svetlikova S. B.; Pospelov V. A. Serum-response element SRE is a possible target for negative control of protooncogene c-fos promoter. Proc. Acad. Sci. USSR 315:1003–1007; 1990. [PubMed] [Google Scholar]
- 39. Ruley H. E. Adenovirus early region IA enables viral and cellular transforming genes to transform primary cells in culture. Nature 304:602–606; 1983. [DOI] [PubMed] [Google Scholar]
- 40. Ryseck R.-P.; Bravo R. C-Jun, JunB, and JunD differ in their binding affinities to AP-1 and CRE consensus sequences. Oncogene 6:533–542; 1991. [PubMed] [Google Scholar]
- 41. Saez E.; Rutberg S. E.; Mueller E.; Oppenheim H.; Smoluk J.; Yuspa S. H.; Spiegelman B. M. c-Fos is required for malignant progression of skin tumors. Cell 82:721–732; 1995. [DOI] [PubMed] [Google Scholar]
- 42. Schonthal A.; Tsukitani Y.; Feramisco J. R. Transcriptional and post-transcriptional regulation of c-fos expression by the tumor promoter okadaic acid. Oncogene 6:423–430; 1991. [PubMed] [Google Scholar]
- 43. Steegenga W. T.; van Laar T.; Shvarts A.; Terleth C.; van der Eb A. J.; Jochemsen A. G. Distinct modulation of p53 activity in transcription and cell-cycle regulation by the large (54 kDa) and small (21 kDa) adenovirus E1B proteins. Virology 212:543–554; 1995. [DOI] [PubMed] [Google Scholar]
- 44. Stein B.; Angel P.; van Dam H.; Ponte H.; Herrlich P.; van der Eb A.; Rahmsdorf H. L. Ultraviolet-radiation induced c-jun gene transcription: Two AP-1 like binding sites mediate the response. Photochem. Photo-biol. 55:409–415; 1992. [DOI] [PubMed] [Google Scholar]
- 45. Treisman R.; Marais R.; Wynne K. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 11:4631–4640; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Dam H.; Duyndam M.; Rottier R.; Borsch A.; de Vries-Smith L.; Herrlich P.; Zantema A.; Angel P.; van der Eb A. Heterodimer formation of c-Jun and ATF-2 is responsible for induction of c-Jun by the 243 amino acid adenovirus ElA protein. EMBO J. 12:479–487; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Dam H.; Wilhelm D.; Herr I.; Steffen A.; Herrlich P.; Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 14: 1798–1811; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandel L.; Montreau N.; Vial. E.; Pfarr C.M.; Bine-truy B.; Castelazzi M. Stepwise transformation of rat embryo fibroblasts: c-jun, junB, or junD can cooperate with Ras for focus formation, but a c-jun-containing heterodimer is required for immortalization. Oncogene 12:1881–1888; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wasylyk C.; Imler J. C.; Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEAl. EMBO J. 7:2475–2483; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wyllie A. H.; Rose K. A.; Morris R. C.; Steel C. M.; Foster E.; Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: Growth, metastasis and endogenous oncogene expression. Br. J. Cancer 56:251–259; 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu C.-L.; Prochovnik E. V.; Imperiale M. J.; Jove R. Attenuation of serum inducibility of immediately early genes by oncoproteins in tyrosine kinase signaling pathways. Mol. Cell. Biol. 13:2011–2019; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zinck R.; Hipskind R. A.; Pingoud V.; Nordheim A. C-fos transcriptional activation and repression correlated temporally with the phosphorylation status of TCF. EMBO J. 12:2377–2387; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


