Abstract
Background: While overt thyroid disease is a well known risk factor for infertility, the potential consequences of mild thyroid dysfunction or thyroid autoimmunity remain unknown. Experimental studies suggest a considerable role for thyroid hormone in the physiological mechanisms of ovarian reserve, but translation of such findings to human studies remains rare. A potential role for thyroid function in female reproduction could be especially relevant when the cause of infertility remains unknown, such as in women with diminished ovarian reserve (DOR) or unexplained infertility. The aims of this study were to investigate the association of thyroid function and autoimmunity with markers of ovarian reserve day 3 follicle-stimulating hormone (FSH) concentrations and antral follicle count (AFC), and to investigate whether thyroid function or autoimmunity may have different effects in women with DOR or unexplained infertility.
Methods: Thyrotropin, free thyroxine, thyroxine, free triiodothyronine (fT3), triiodothyronine, thyroid peroxidase antibodies (TPOAbs), and thyroglobulin antibodies (TgAbs), as well as AFC and the day 3 FSH concentration, were measured among women seeking fertility treatment at the Massachusetts General Hospital Fertility Center. Multiple linear or mixed regression models were used to study the association of thyroid function or autoimmunity with AFC or day 3 FSH.
Results: In the total study population (436 women, 530 AFC measurements), there was no association of thyroid function or TPOAb positivity with AFC. However, TgAb positivity was associated with a higher AFC (mean difference = 3.4 [95% confidence interval (CI) 1.8–5.1], p < 0.001). In women with DOR or unexplained infertility, lower fT3 and TPOAb positivity were associated with a lower AFC (fT3: continuous nonlinear association, p = 0.009; TPOAb positivity: −2.3 follicles [confidence interval −3.8 to −0.5], p = 0.01), while TgAb positivity was not associated with AFC. Neither thyroid function nor thyroid antibody positivity was associated with the day 3 FSH concentration.
Conclusions: This study found that lower fT3 and TPOAb positivity are associated with a lower AFC in women with DOR or unexplained infertility. Future studies are required to replicate these findings and further elucidate the role of TgAbs and underlying mechanisms through which thyroid function and autoimmunity is associated with ovarian reserve.
Keywords: : thyroid, TPO antibody, antral follicle count
Introduction
Thyroid hormone regulates metabolism in practically all tissues in the human body. Adequate thyroid hormone availability is important for normal female reproduction. This is reflected by the consequences of overt hypothyroidism, which can present with a blunting of luteinizing hormone (LH) pulsatility, hyperprolactinemia, menstrual and ovulation disturbances, and reduced overall fertility that can be reversed by re-establishing a euthyroid state (1). In contrast to overt thyroid disease, mild forms of thyroid dysfunction and/or thyroid autoimmunity often remain clinically unidentified because patients do not present with typical hypothyroidism symptoms. However, some studies indicate that even mild forms of thyroid dysfunction or thyroid autoimmunity may adversely affect female reproduction (1–3).
The effects of mild thyroid dysfunction or thyroid autoimmunity on female fertility have been studied predominantly in infertile women, including those who undergo assisted reproductive technology (ART). Various studies show that the prevalence of mild hypothyroidism or thyroid autoimmunity is higher in infertile women (1–3). Clinical studies investigating the effects of thyroid function or autoimmunity in an ART setting have focused on assessing outcomes such as the estradiol trigger peak, oocyte retrieval, maturation or fertilization, embryo implantation, clinical pregnancy, and/or live birth (1,4–7). While the results of these studies differ considerably and remain inconclusive, experimental studies suggest that thyroid hormone has effects on ovarian reserve, an important determinant of downstream ART outcomes (8).
Thyroid hormone receptors are abundantly present throughout the human female reproductive tract, including in granulosa cells and oocytes (9–13). Preclinical studies suggest that thyroid hormone variation already within the normal range regulates the stimulatory effects of follicle-stimulating hormone (FSH) on follicular growth and apoptosis suppression (14,15). On the other hand, high thyroid hormone concentrations may reduce granulosa cell aromatase activity and impair pre-antral follicle development (12,16). Animal studies show that thyroid hormone regulates ovarian function, potentially in part via its effects on nitric oxide synthase, and that low thyroid hormone availability leads to a decreased number of antral follicles (1,17–19). Furthermore, both hypothyroid and hyperthyroid immature mice have a reduced number of primordial, growing, and antral follicles (17,18). However, efforts to translate these findings to human studies, for example by investigating potential dose-dependent effects of thyrotropin (TSH), free thyroxine (fT4), free triiodothyronine (fT3), or thyroid autoimmunity on outcomes reflective of ovarian reserve, remain sparse (20–23).
The current study therefore aimed to investigate the association of the full spectrum of clinical thyroid function measurements with measures of ovarian reserve such as day 3 FSH concentration and antral follicle count (AFC). It was hypothesized that thyroid hormone concentrations would have a positive, dose-dependent effect on AFC and a negative, dose-dependent effect on day 3 FSH concentrations. Furthermore, it was hypothesized that the effects of thyroid function or autoimmunity would be more prominent in women with an infertility diagnosis of diminished ovarian reserve (DOR) or unexplained infertility, given that no underlying pathophysiological mechanisms can be defined for these diagnoses.
Methods
Design and population
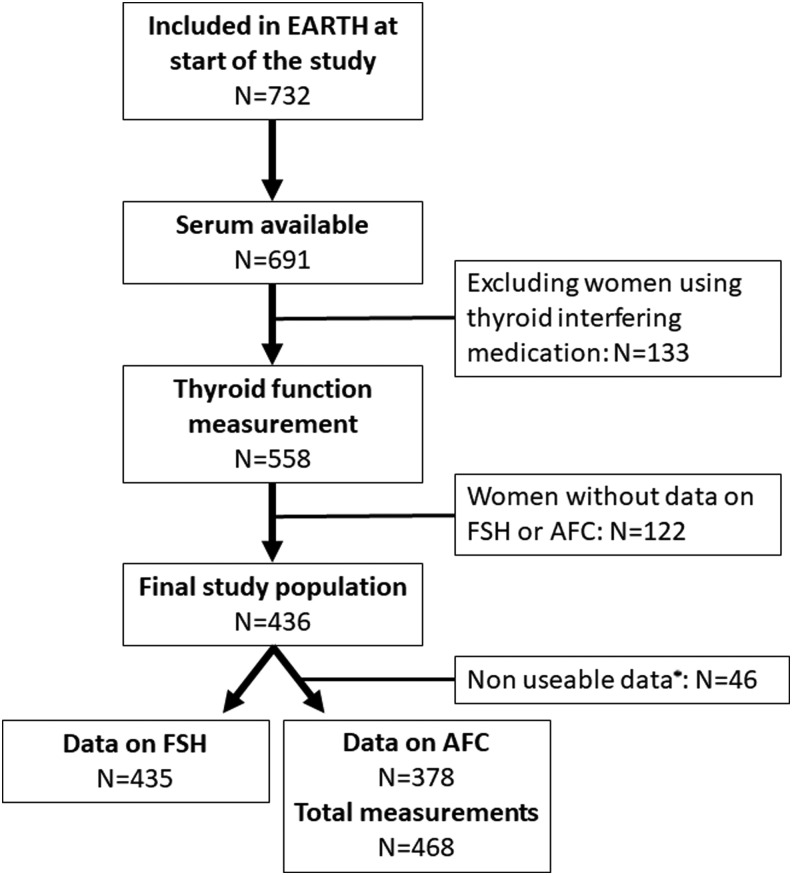
This study was embedded within the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2004 to evaluate environmental and dietary determinants of fertility (24). Eligible women were those who attended the Massachusetts General Hospital (MGH) Fertility Center and were between 18 and 45 years old. Of the 732 women included in the EARTH study by November 8, 2016, 691 (94.4%) had a stored serum sample collected at their entry visit. After exclusion of women who were using thyroid-interfering medication at entry (n = 133; predominantly levothyroxine, methimazole, propylthiouracil, amiodarone, antipsychotics, anticonvulsants, or high-dose steroids), thyroid function and antibodies were measured in the remaining 558 women. All women with data on day 3 FSH (n = 435) and/or AFC (n = 378) were included in the current study (Fig. 1). The study was approved by the Human Studies Institutional Review Boards of both the MGH and Harvard T.H. Chan School of Public Health. Participants signed an informed consent prior to study entry and after the study procedures were explained by trained research study staff.
FIG. 1.
Flow chart for final study population selection. *Excluded due to multicystic ovaries, difficult to visualize, or absent ovary.
Serum measurements
Serum measurements were performed in stored serum samples obtained at the entry visit of the study. This sample does not necessarily reflect the start of the clinical fertility trajectory for all women, since eligible women were recruited from the MGH during any stage of their clinical infertility trajectory. Serum TSH, fT4, T4, fT3, T3, and thyroid peroxidase antibodies (TPOAbs) were measured with commercial kits on a Modular Analytics E170, and serum thyroglobulin antibodies (TgAbs) were measured using the Cobas e601 analyzer (both Roche Diagnostics, Mannheim, Germany) at the Department of Clinical Chemistry, Máxima Medical Center (Veldhoven, The Netherlands). Inter- and intra-assay coefficients of variation were 2.1%, 3.5%, 3.8%, 3.8%, and 7.7% for TSH, fT4, T4, fT3, and T3, respectively. TPOAb and TgAb concentrations were considered positive if >35 IU/mL or >115 IU/L (manufacturer cutoffs), respectively, and the coefficients of variation were 12.4% and 7.1% for TPOAbs at 33 or 100 IU/L, respectively, and 10.9% and 8.6% for TgAbs at 76 and 218 IU/L, respectively. Serum concentrations of menstrual cycle day 3 FSH were measured using an automated electrochemiluminescence immunoassay at the MGH Core Laboratory, as previously described (25).
Clinical outcomes
Ovarian AFC and measurement of FSH concentrations in serum were assessed on day 3 of an unstimulated menstrual cycle. AFC was defined as the sum of antral follicles in both ovaries as measured by transvaginal ultrasound in the early follicular phase by a trained MGH reproductive endocrinologist. Women with multicystic ovaries, with an AFC >20 for the left or right side separately, or with ovaries difficult to visualize were excluded from analyses (n = 46; Fig. 1). AFC and FSH measurements were prospectively collected, and additional measurements that preceded study inclusion were obtained from electronical medical records when available (days between serum collection and AFC examination: median −5 [95% range −328 to 669]; or day 3 FSH measurement: median −42 [95% range: −280 to 68]). An infertility diagnosis was assigned by the treating physician according to the definition of the Society for Assisted Reproductive Technology.
Covariates
Demographic and clinical information at study entry, such as age at enrollment and race, were obtained from a baseline questionnaire, and parity was abstracted from the electronic medical records by trained study staff. Height and weight were measured at enrollment by the study nurse, and body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared (kg/m2).
Statistical analyses
The association of thyroid function measurements or thyroid antibody positivity with AFC was studied using two different approaches: (i) by analyzing all available AFC measurements, including multiple measurements for some women (full analysis) using generalized linear mixed effects regression models with a Poisson distribution, a log link function, and a random intercept for each individual; and (ii) by analyzing the measurement of AFC that was obtained in the closest proximity to the entry visit using generalized linear regression models with a Poisson distribution and a log link function. The association of thyroid function measurements or thyroid antibody positivity with day 3 FSH was studied using linear regression models. Nonlinearity was assessed using restricted cubic splines with three or four knots. The study investigated if the associations of thyroid function measurements or thyroid antibody positivity with AFC or day 3 FSH was influenced by the time between outcome assessment and the entry visit by additionally adjusting for, and testing for effect modification by, the number of days between both time points. Covariates and potential confounders were added to the model based on biological plausibility, a 10% change of the effect estimates, and residual variability of the model. All models were adjusted for age, BMI, smoking, ethnicity, and infertility diagnosis. A preanalytical power calculation was not considered, and multiple comparisons were not adjusted for, given the exploratory nature of this study, the low number of tested hypotheses, and the context of previous literature. All analyses were performed using R v3.3.2 (packages rms, lme4, and sjPlot).
Results
The final study population comprised 436 women, of whom 378 and 435 had data on AFC and/or day 3 FSH, respectively (Fig. 1). Descriptive characteristics of the study population are shown in Table 1. There was no difference in mean age, mean BMI, or infertility diagnosis between women with or without data on thyroid function (p = 0.41, p = 0.79, and p = 0.19, respectively; data not shown). The study population had a median age of 35 years (95% range 27–42 years), and mainly consisted of Caucasian (83%) women who did not smoke (73%). Women with DOR had a higher median day 3 FSH (11.2 vs. 6.1–6.9) and a lower median AFC (8 vs. 13–22) than women with other infertility diagnosis (data not shown). Of all women in the study population, 5.6% had a TSH above the reference range (TSH >4.0 mIU/L; 21/378), while TPOAb positivity and TgAb positivity were detected in 10.6% and 9.2%, respectively (overlap n = 20; 4.6%).
Table 1.
Baseline Characteristics of 436 Women in the EARTH Study
| Characteristic | |
|---|---|
| TSH (mU/l) | 1.90 (0.68–4.70)a |
| fT4 (pmol/l) | 15.2 (11.5–19.6)a |
| T4 (nmol/l) | 95.8 (66.0–141.3)a |
| fT3 (pmol/l) | 4.7 (3.7–6.1)a |
| T3 (nmol/l) | 1.78 (1.27–2.819)a |
| TPOAb positive | 46 (10.6)b |
| TgAb positive | 40 (9.2)b |
| Antral follicle count | 12 (4–29)a |
| Day 3 FSH concentration | 6.7 (3.5–14.8)a |
| Age | 35 (27–42)a |
| BMI | 23.4 (18.6–36.4)a |
| Ethnicities | |
| Caucasian | 361 (82.8)b |
| Black | 17 (3.9)b |
| Asian | 40 (9.2)b |
| Other | 18 (4.1)b |
| Ever smoker | 118 (27.1)b |
| Baseline reproductive characteristics | |
| Previous IUI | 155 (35.6)b |
| Previous IVF | 72 (16.5)b |
| Initial infertility diagnosis | |
| Diminished ovarian reserve | 39 (8.9)b |
| Ovulation disorders | 49 (11.2)b |
| Endometriosis/tubal/uterine | 56 (12.8)b |
| Male factor | 119 (27.3)b |
| Unexplained | 173 (39.7)b |
| Initial treatment protocol | |
| Antagonist | 26 (6)b |
| Flare* | 25 (5.7)b |
| Luteal phase agonist** | 385 (88.3)b |
Data shown are median (95% range).
Data shown are n (%).
Follicular-phase GnRH-agonist/flare protocol.
Luteal-phase GnRH-agonist protocol.
EARTH, Environment and Reproductive Health; TSH, thyrotropin; fT4, free thyroxine; fT3, free triiodothyronine; TPOAb, thyroid peroxidase antibodies; TgAb, thyroglobulin antibodies; BMI, body mass index; IUI, intrauterine insemination; IVF, in vitro fertilization.
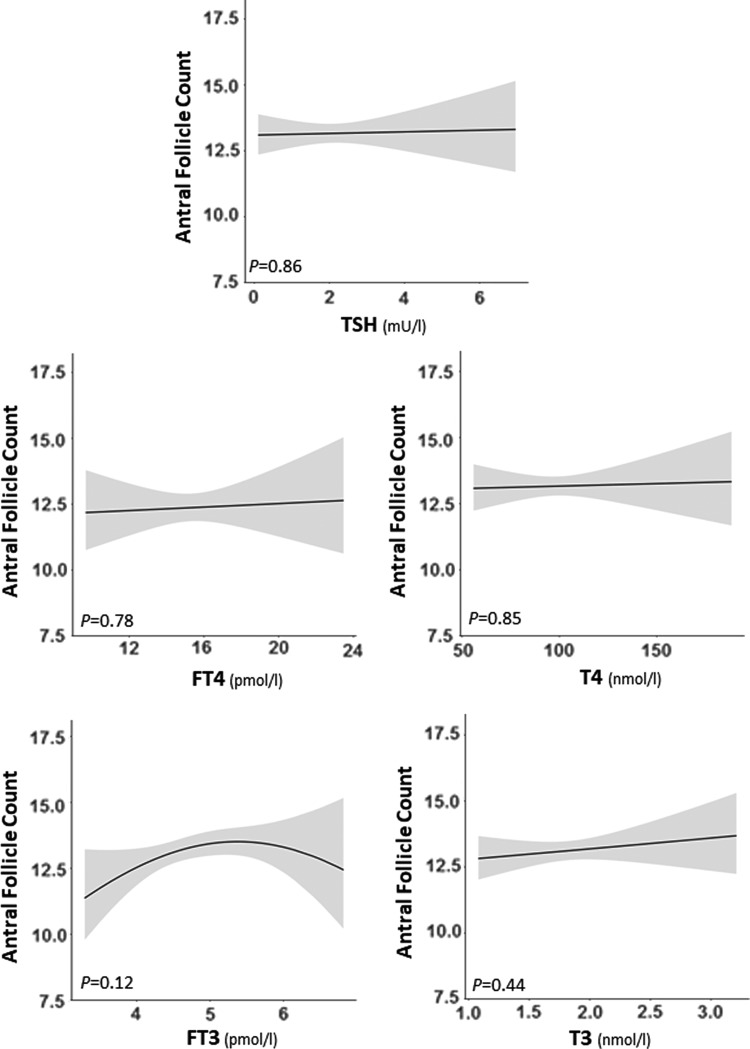
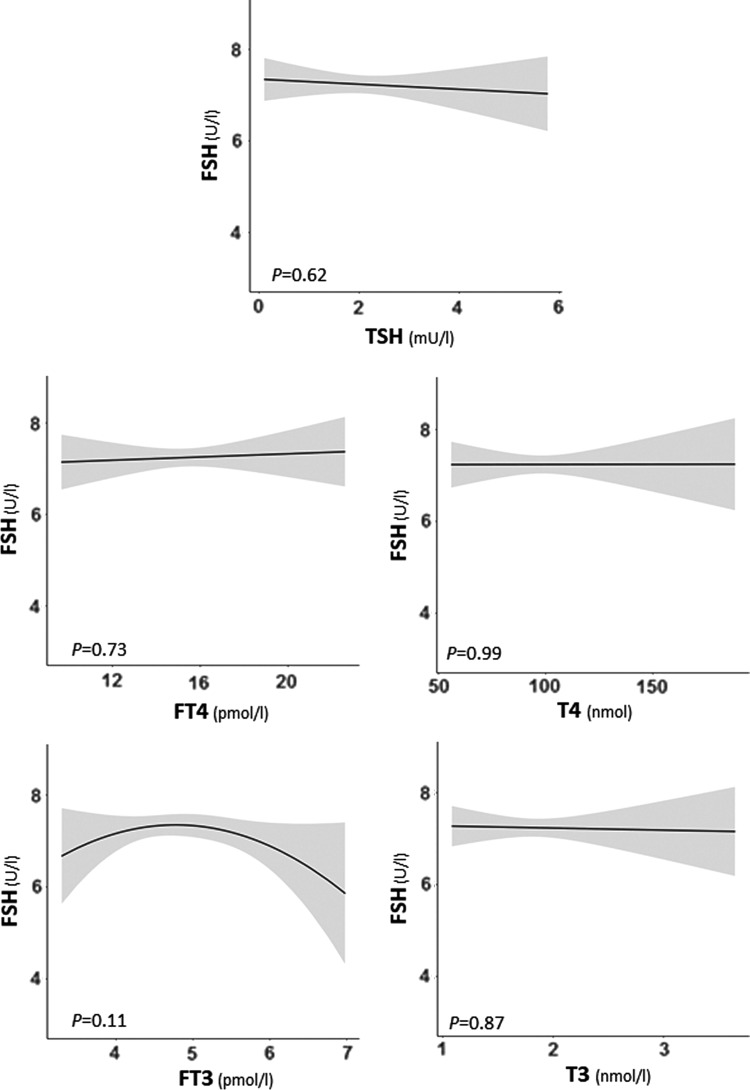
There was no association of TSH, fT4, T4, fT3, or T3 with AFC as analyzed across all available measurements (full analysis; p ≥ 0.29 for all analyses; Fig. 2) or when analyzed as the closest AFC measurement (p ≥ 0.12 for all analyses; Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy). Similarly, there was no association of TSH, fT4, T4, fT3, or T3 with day 3 FSH (p ≥ 0.11 for all analyses; Fig. 3).
FIG. 2.
Plots show the association of thyrotropin (TSH), free thyroxine (fT4), or free triiodothyronine (fT3) with the antral follicle counts as the predicted mean count (black lines) with confidence interval (gray area). All analyses were adjusted for age, body mass index (BMI), smoking, ethnicity, and infertility cause.
FIG. 3.
Plots show the association of TSH, fT4, or fT3 with the follicle-stimulating hormone (FSH) concentration measured at the third day of the menstrual cycle as the predicted mean count (black lines) with confidence interval (gray area). All analyses were adjusted for age, BMI, smoking, ethnicity, and infertility cause.
In the total study population, there was no association of TPOAb positivity with either AFC or day 3 FSH (Table 2). TgAb positivity was associated with a higher mean AFC in both the full analysis and the closest measurement analysis (mean difference = 2.9 [95% confidence interval (CI) 0.8–5.2], p = 0.006; and mean difference = 3.4 [CI 1.8–5.1], p < 0.001, respectively; Table 2).
Table 2.
Association of Thyroid Antibodies with AFC and Day 3 FSH Concentration
| AFC | AFC | |||||
|---|---|---|---|---|---|---|
| Overall | DOR or unexplained only | |||||
| Mean difference | [95% CI] | p | Mean difference | [95% CI] | p | |
| TPOAb positivity | ||||||
| Full trajectory | 0.1 | [−1.6 to 2.1] | 0.93 | −2.4 | [−4.2 to −0.3] | 0.04 |
| Closest measurement | 0.9 | [−0.5 to 2.4] | 0.24 | −2.3 | [−3.8 to −0.5] | 0.01 |
| TgAb positivity | ||||||
| Full trajectory | 2.9 | [0.8–5.2] | 0.006 | −0.8 | [−3.2 to 2.2] | 0.55 |
| Closest measurement | 3.4 | [1.8–5.1] | <0.001 | 0.2 | [−1.8 to 2.6] | 0.83 |
| FSH concentration | FSH concentration | |||||
| TPOAb positivity | 0.47 | [−0.55 to 1.49] | 0.37 | −0.52 | [−1.60 to 0.56] | 0.35 |
| TgAb positivity | −0.08 | [−1.26 to 1.10] | 0.90 | −0.19 | [−1.35 to 1.03] | 0.76 |
AFC, antral follicle count; DOR, diminished ovarian reserve; CI, confidence interval; FSH, follicle-stimulating hormone.
Thyroid function and TPOAb positivity in women with either DOR or unexplained infertility
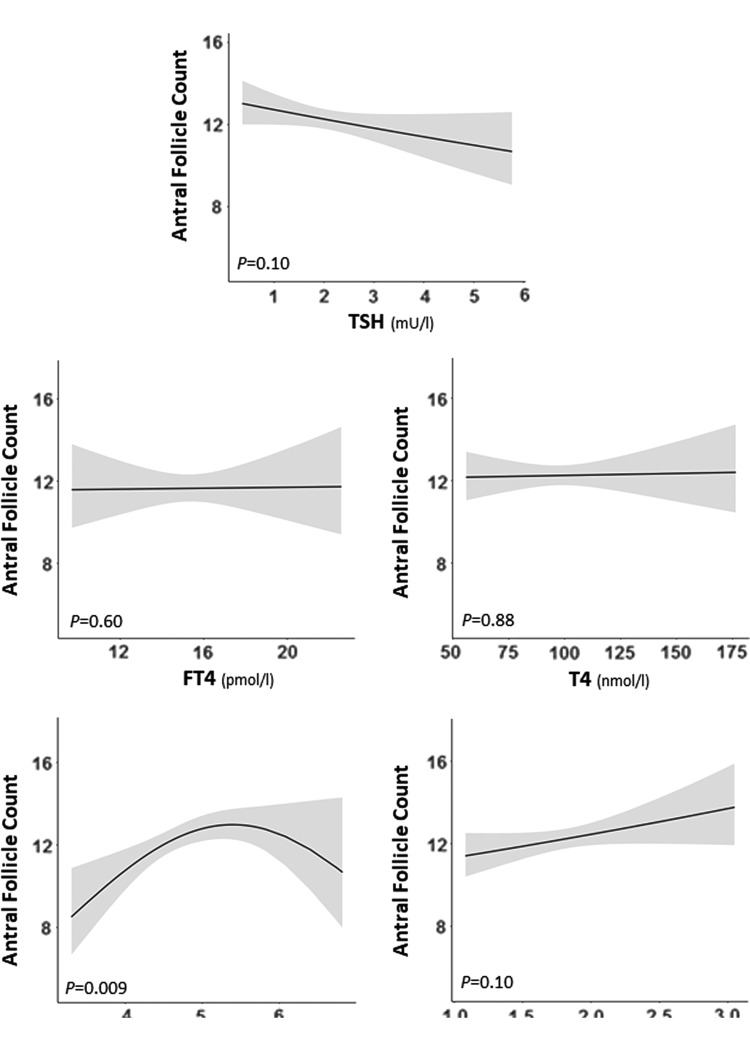
Since thyroid dysfunction or thyroid autoimmunity could contribute to unexplained infertility (1), a sensitivity analysis was performed in those women diagnosed with either DOR or unexplained infertility. In this subset, lower fT3 was associated with a lower AFC (Fig. 4 and Supplementary Fig. S2). There was no association of TSH, fT4, T4, or T3 with AFC in women with DOR or unexplained infertility (Fig. 4 and Supplementary Fig. S2). Furthermore, in women with either DOR or unexplained infertility, TPOAb positivity was associated with lower mean AFC in both the full analysis and the closest measurement analysis (mean difference = −2.3 [CI −3.8 to −0.5], p = 0.04; and mean difference = −2.3 [CI −3.8 to −0.3], p = 0.04, respectively; Table 2).
FIG. 4.
Plots show the association of TSH, FT4, or fT3 with the antral follicle count as the predicted mean count (black lines) with confidence interval (gray area). All analyses were adjusted for age, BMI, smoking, ethnicity, and infertility cause.
TgAb positivity in women with either DOR or unexplained infertility
In contrast, among women with either DOR or unexplained infertility, TgAb positivity was not associated with AFC (Table 2). This result is opposite to results from analyses performed in the whole population (Table 2), suggesting that the association of TgAb positivity with AFC differs between women depending on infertility diagnoses. Therefore, a post hoc sensitivity analysis was performed, stratifying TPOAb and TgAb positivity analyses for each infertility diagnosis subgroup for future references (Supplementary Table S1). Although statistical power was lacking, subgroup analyses indicated that predominantly for male factor infertility, TPOAb positivity and TgAb positivity were associated with a higher AFC (Supplementary Table S1). Furthermore, for infertility based on ovulation disorders, the results suggested that TPOAb positivity was associated with a lower AFC, while TgAb positivity was associated with a higher AFC, but these results did not reach statistical significance (Supplementary Table S1).
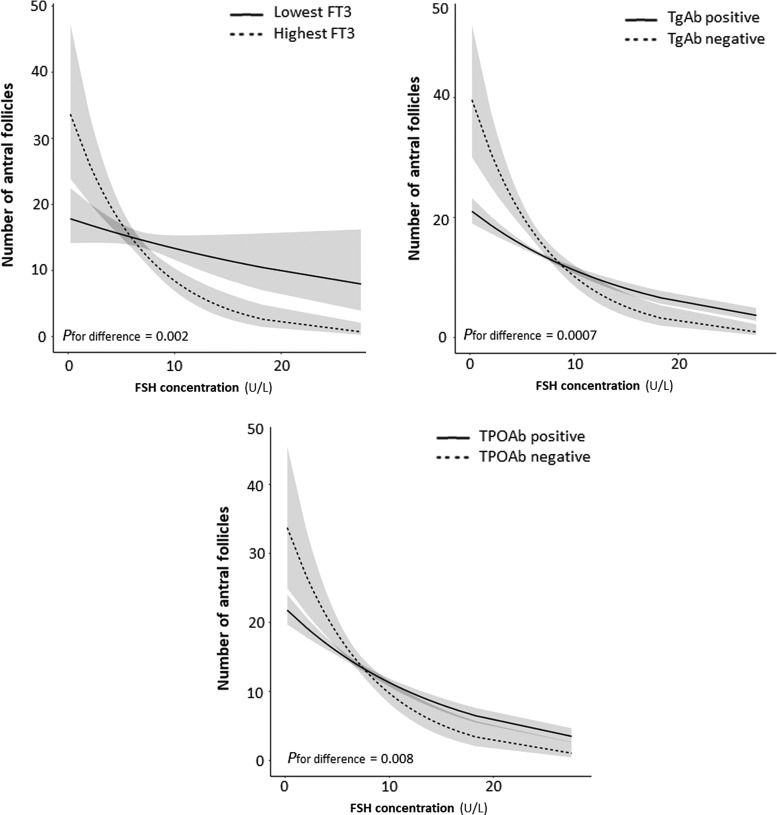
Thyroid function and autoimmunity modifies the association of FSH with AFC
Next, we investigated whether thyroid function or antibodies affect the relationship between FSH and AFC. As shown in Figure 5, the association of day 3 FSH with AFC attenuated considerably with lower fT3 concentrations (p = 0.002), TPOAb positivity (p = 0.008), and TgAb positivity (p = 0.0007). The association of day 3 FSH with AFC did not differ according to TSH (in thyroid antibody negative women), fT4, total T4, or total T3 (p = 0.21, 0.60, 0.33, and 0.80, respectively).
FIG. 5.
Plots show the association of day 3 FSH with the antral follicle count as the predicted mean count (black lines) with confidence interval (gray area), as modeled using the whole data set for women within the lowest 10% of fT3, TgAb-positive or TPOAb-positive women (all shown by continuous lines), or the highest 10% of fT3, TgAb-negative, or TPOAb-positive women (dotted lines). All analyses were adjusted for age, BMI, smoking, ethnicity, and infertility cause.
All analyses on TPOAbs or TgAbs remained similar after additional adjustment for thyroid function measurements or when analyzed continuously (data not shown). The time between AFC or day 3 FSH assessment and thyroid function measurement did not influence the results.
Discussion
Thyroid hormone plays a role in ovarian function, and both mild thyroid dysfunction and thyroid autoimmunity have been associated with female infertility and suboptimal ART outcomes (1,4–7). The current study found that thyroid function or TPOAb positivity was not associated with AFC, while TgAb positivity was associated with a higher AFC, when infertility diagnosis was not taken into consideration. However, when analyses were stratified by infertility diagnosis, an association of lower fT3 and TPOAb positivity with lower AFC wes identified among women with either DOR or unexplained infertility. While there was no association of either thyroid function and/or autoimmunity with day 3 FSH, a lower fT3 as well as TPOAb and TgAb positivity were associated with an attenuation of the association between day 3 FSH and AFC. This indicates that in women with low fT3 or thyroid autoimmunity, even more so than in a general population, day 3 FSH is a less reliable marker for ovarian reserve.
These results may suggest a possible role for either thyroid dysfunction, low T3 availability, or autoimmunity in the pathophysiology of DOR and unexplained infertility. To the best of the authors' knowledge, this study is the first to demonstrate that low fT3 is associated with a lower AFC in women with DOR or unexplained infertility. T3 is the bioactive form of thyroid hormone, and roughly 80% of serum T3 is produced intracellularly via deiodination of T4 by deiodinase type I and II in peripheral tissues. Given that >99.5% of serum T3 is bound to hormone-binding proteins and thus is not biologically available, the consideration that T3 but not fT3 is affected by changes in binding proteins could explain the discrepant results. Alternatively, differences in the assay sensitivity between T3 and fT3 could have affected the results. The association of low fT3 with lower AFC and lack of association for TSH or fT4 may suggest that the ovary is particularly dependent on T3. This is in line with studies showing that both monocarboxylate transporter 8, a T3 and T4 transporter, and deiodinase type II are expressed in the human ovary (26,27). Furthermore, animal studies show that the ovary can concentrate iodine and produce local T3 through outer ring deiodination (28) and that local T3 has a time- and dose-dependent effect on local estrogen availability (12). Non-thyroidal illness syndrome, which in its mildest form is characterized by an isolated decrease in serum T3 levels, could confound the association of fT3 with AFC. Alternatively, other factors could contribute to selectively lower T3 concentrations such as fasting, stress, and depression. Future studies are necessary to replicate these results and further elucidate the role of serum (f)T3 availability for ovarian reserve.
Although the association of thyroid autoimmunity with ART, ovulation induction, or intrauterine insemination outcomes has been widely examined (4,5), studies that assess ovarian reserve are sparse. The present results showing that TPOAb positivity was not associated with AFC or day 3 FSH in the total population of women undergoing ART are in line with other studies that investigated this or a similar association (20–23,29). In line with a recent study (29), the current stratified analyses showed that TPOAb positivity was associated with a lower AFC in women with DOR or unexplained infertility. A lower AFC in women with TPOAb positivity could be explained by various underlying mechanisms. First, TPOAb positivity could be associated with differences in AFC via a decrease in thyroid function, which could for example result in changes in early follicular recruitment. However, this potential effect pathway seems unlikely based on the fact that the association of TPOAb positivity with AFC remained unchanged after additional correction for thyroid function (including fT3). Second, TPOAb positivity could reflect a higher general susceptibility to autoimmunity. As such, co-occurrence of thyroid autoimmunity with other autoimmune processes that could influence ovarian reserve such as, for example, anti-ovarian antibodies or in the setting of early-phase autoimmune polyglandular syndrome I and II could cause a spurious association between thyroid autoimmunity and AFC (30–33). Third, serum concentrations of both TPOAbs and TgAbs are positively correlated with the respective concentrations in follicular fluid, and antigen cross-reactivity has been described between zone pellucida antibodies and thyroid antibodies (34,35). This could suggest that thyroid antibodies can have a direct impact on ovarian tissue.
Unexpectedly, the current study found that among women with an infertility diagnosis other than DOR or unexplained infertility, TgAb positivity was associated with a higher AFC, but there was no association of TgAb positivity with AFC in the prespecified sensitivity analysis in women with DOR or unexplained infertility. Further exploratory stratified analyses indicated that the association of TPOAb or TgAb positivity with AFC differs considerably based on the infertility diagnoses. This warrants further adequately powered studies with detailed phenotyping of underlying infertility causes, for example also focusing on iatrogenic or genetic causes of low ovarian reserve (23). It is speculated that a possible underlying cause for a higher AFC in women with any form of thyroid autoimmunity could be the co-occurrence of isolated features and/or a subclinical form of polycystic ovary syndrome (PCOS). Various studies suggest a role for autoimmunity in the pathogenesis of PCOS, and women with PCOS have an up to a fivefold higher risk of chronic lymphocytic thyroiditis (36–38). Moreover, one study showed that already in young girls, thyroid autoimmunity was associated with considerable differences in various clinical and biochemical characteristics fitting with a PCOS phenotype (39).
In an effort to identify an underlying reason for the discrepancy in the present results showing association of thyroid function and autoimmunity with AFC but not with FSH, it was found that low fT3 as well as thyroid antibody positivity was associated with an abnormal ratio of FSH to AFC. In women with a low FSH concentration, those with low fT3 or thyroid antibody positivity had a considerably lower AFC (estimated 15–20 follicles fewer). From a biological point of view, this discrepancy may indicate that low fT3 and thyroid antibodies interfere with processes that regulate follicle growth and development through intracellular pathways that do not affect the hypothalamic–pituitary–gonadal axis, specifically serum concentrations of estrogen or inhibin. These results are in line with experimental studies that show that thyroid hormone enhances the stimulatory effects of FSH on follicular growth and apoptosis suppression, potentially via regulating nitric oxide synthase (1,14,15,17–19. Interestingly, the analyses on fT3 also indicate that for higher FSH concentrations, women with low fT3 have a higher AFC than women with a high fT3. This could suggest that the effects of fT3 on follicle growth and development have a U-shaped curve, depending on the concentration on FSH. This concept fits with the described effects of T3 on granulosa cell aromatase activity and pre-antral follicle development (12,16). Future studies focusing on follicular fluid and the downstream effects of thyroid hormone on follicle growth and development are required to elucidate these mechanisms further.
It was possible to do a prospective study on the association of thyroid function and autoimmunity with ovarian reserve using a broad panel of thyroid function tests and detailed data on the characteristics and clinical workup of the study participants. Although the statistical power of the current study allowed for the detection of clinically meaningful effect estimates and an adequate subgroup analysis in the 47% of women with DOR or unexplained infertility, a potential limitation of this study is that statistical power did not allow for further meaningful subgroup analyses. It was identified that the association of thyroid function or autoimmunity with AFC was considerably modified by the underlying infertility diagnosis, but some subgroups were too small to quantify differences in effect estimates adequately.
Another potential limitation is the fact that TSH screening is routinely performed during the infertility workup in women who were eligible to participate in this study. Although the results of this study are therefore only generalizable to women who were not considered eligible for thyroid treatment on the basis of an initial TSH screening, this could indicate that already mild alterations in thyroid function and/or milder forms of thyroid autoimmunity can affect ovarian reserve. In addition, this study included predominantly white women, which may hamper the generalizability of these results to other races/ethnicities.
Finally, another potential limitation is that the serum sampling was not performed at the same time as the AFC or FSH measurements for all study participants. However, the results did not systematically differ between the trajectory and closest measurement analysis, and confounding or effect modification was not identified according to the time difference between the measurements. Nonetheless, the absence of differences according to the time between measurements could suggest that the results are more likely to reflect a long-term effect of thyroid hormone or thyroid autoimmunity. As such, different results could be identified in future studies investigating more rapid changes in thyroid function or early-onset thyroid autoimmunity.
In conclusion, this study demonstrates that a lower fT3 and TPOAb positivity are associated with a lower AFC in women with DOR or unexplained infertility. It also shows that TgAb positivity is associated with a higher AFC in a population of infertile women when infertility diagnosis is not taken into consideration. Considerable differences were identified in the effects of thyroid hormone or thyroid autoimmunity on AFC according to the infertility diagnosis. This indicates that the different underlying pathophysiological mechanisms involved can modify the role of thyroid hormone or thyroid autoimmunity. Future studies are required to replicate the results of this exploratory study and to investigate further the role of the underlying infertility diagnosis in the association of thyroid hormone or thyroid autoimmunity with female reproduction outcomes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge all study participants and members of the EARTH study team, specifically the Harvard T.H. Chan School of Public Health research nurses, Jennifer B. Ford and Myra G. Keller, research staff, Ramace Dadd and Patricia Morey, and physicians and staff at Massachusetts General Hospital fertility center. This work was supported by financial support from the Royal Netherlands Academy of Arts and Sciences ter Meulen fonds, Stichting de Drie Lichten and the National Institute of Environmental Health Sciences (NIEHS) Grant (R01 009718).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Krassas GE, Poppe K, Glinoer D. 2010. Thyroid function and human reproductive health. Endocr Rev 31:702–755 [DOI] [PubMed] [Google Scholar]
- 2.Vaquero E, Lazzarin N, Caserta D, Valensise H, Baldi M, Moscarini M, Arduini D. 2006. Diagnostic evaluation of women experiencing repeated in vitro fertilization failure. Eur J Obstet Gynecol Reprod Biol 125:79–84 [DOI] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive M 2015. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 104:545–553 [DOI] [PubMed] [Google Scholar]
- 4.Busnelli A, Paffoni A, Fedele L, Somigliana E. 2016. The impact of thyroid autoimmunity on IVF/ICSI outcome: a systematic review and meta-analysis. Hum Reprod Update 22:793–794 [DOI] [PubMed] [Google Scholar]
- 5.Unuane D, Velkeniers B, Bravenboer B, Drakopoulos P, Tournaye H, Parra J, De Brucker M. 2017. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod 32:915–922 [DOI] [PubMed] [Google Scholar]
- 6.Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. 2013. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: systematic review and meta-analysis of RCTs. Hum Reprod Update 19:251–258 [DOI] [PubMed] [Google Scholar]
- 7.Karmon AE, Batsis M, Chavarro JE, Souter I. 2015. Preconceptional thyroid-stimulating hormone levels and outcomes of intrauterine insemination among euthyroid infertile women. Fertil Steril 103:258–263.e1 [DOI] [PubMed] [Google Scholar]
- 8.Bonilla-Musoles F, Castillo JC, Caballero O, Perez-Panades J, Bonilla F, Jr, Dolz M, Osborne N. 2012. Predicting ovarian reserve and reproductive outcome using antimullerian hormone (AMH) and antral follicle count (AFC) in patients with previous assisted reproduction technique (ART) failure. Clin Exp Obstet Gynecol 39:13–18 [PubMed] [Google Scholar]
- 9.Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, Hovatta O, Skjoldebrand-Sparre L. 2009. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online 18:337–347 [DOI] [PubMed] [Google Scholar]
- 10.Maruo T, Matsuo H, Mochizuki M. 1991. Thyroid hormone as a biological amplifier of differentiated trophoblast function in early pregnancy. Acta Endocrinol (Copenh) 125:58–66 [DOI] [PubMed] [Google Scholar]
- 11.Maruo T, Katayama K, Barnea ER, Mochizuki M. 1992. A role for thyroid hormone in the induction of ovulation and corpus luteum function. Horm Res 37:12–18 [DOI] [PubMed] [Google Scholar]
- 12.Cecconi S, Rucci N, Scaldaferri ML, Masciulli MP, Rossi G, Moretti C, D'Armiento M, Ulisse S. 1999. Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology 140:1783–1788 [DOI] [PubMed] [Google Scholar]
- 13.Wakim AN, Polizotto SL, Buffo MJ, Marrero MA, Burholt DR. 1993. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil Steril 59:1187–1190 [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Xia G, Tsang BK. 2011. Interactions of thyroid hormone and FSH in the regulation of rat granulosa cell apoptosis. Front Biosci (Elite Ed) 3:1401–1413 [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Wang X, Wang Z, Niu W, Zhu B, Xia G. 2013. Effect of different culture systems and 3, 5, 3′-triiodothyronine/follicle-stimulating hormone on preantral follicle development in mice. PLoS One 8:e61947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cecconi S, Rossi G, Coticchio G, Macchiarelli G, Borini A, Canipari R. 2004. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertil Steril 81:919–924 [DOI] [PubMed] [Google Scholar]
- 17.Fedail JS, Zheng K, Wei Q, Kong L, Shi F. 2014. Roles of thyroid hormones in follicular development in the ovary of neonatal and immature rats. Endocrine 46:594–604 [DOI] [PubMed] [Google Scholar]
- 18.Chan WY, Ng TB. 1995. Effect of hypothyroidism induced by propylthiouracil and thiourea on male and female reproductive systems of neonatal mice. J Exp Zool 273:160–169 [DOI] [PubMed] [Google Scholar]
- 19.Dijkstra G, de Rooij DG, de Jong FH, van den Hurk R. 1996. Effect of hypothyroidism on ovarian follicular development, granulosa cell proliferation and peripheral hormone levels in the prepubertal rat. Eur J Endocrinol 134:649–654 [DOI] [PubMed] [Google Scholar]
- 20.Sakar MN, Unal A, Atay AE, Zebitay AG, Verit FF, Demir S, Turfan M, Omer B. 2016. Is there an effect of thyroid autoimmunity on the outcomes of assisted reproduction? J Obstet Gynaecol 36:213–217 [DOI] [PubMed] [Google Scholar]
- 21.Zhong YP, Ying Y, Wu HT, Zhou CQ, Xu YW, Wang Q, Li J, Shen XT, Li J. 2012. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci 9:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revelli A, Casano S, Piane LD, Grassi G, Gennarelli G, Guidetti D, Massobrio M. 2009. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol 7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. 2015. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum Reprod 30:1690–1696 [DOI] [PubMed] [Google Scholar]
- 24.Messerlian C, Williams P, Ford JB, Chavarro J, Mínguez-Alarcón L, Dadd R, Braun J, Gaskins AJ, Meeker JD, James-Todd T, Chiu Y-H, Nassan FL, Souter I, Petrozza J, Keller M, Toth T, Calafat AM, Hauser R. 2018. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum Reprod Open 2018. February 20 [Epub ahead of print]; DOI: 10.1093/hropen/hoy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. 2010. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 33:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. 2011. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril 95:230–237, 237.e1–2 [DOI] [PubMed] [Google Scholar]
- 27.Rae MT, Gubbay O, Kostogiannou A, Price D, Critchley HO, Hillier SG. 2007. Thyroid hormone signaling in human ovarian surface epithelial cells. J Clin Endocrinol Metab 92:322–327 [DOI] [PubMed] [Google Scholar]
- 28.Slebodzinski AB. 2005. Ovarian iodide uptake and triiodothyronine generation in follicular fluid. The enigma of the thyroid ovary interaction. Domest Anim Endocrinol 29:97–103 [DOI] [PubMed] [Google Scholar]
- 29.Chen CW, Huang YL, Tzeng CR, Huang RL, Chen CH. 2017. Idiopathic low ovarian reserve is associated with more frequent positive thyroid peroxidase antibodies. Thyroid 27:1194–1200 [DOI] [PubMed] [Google Scholar]
- 30.Edassery SL, Shatavi SV, Kunkel JP, Hauer C, Brucker C, Penumatsa K, Yu Y, Dias JA, Luborsky JL. 2010. Autoantigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil Steril 94:2636–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luborsky J, Llanes B, Davies S, Binor Z, Radwanska E, Pong R. 1999. Ovarian autoimmunity: greater frequency of autoantibodies in premature menopause and unexplained infertility than in the general population. Clin Immunol 90:368–374 [DOI] [PubMed] [Google Scholar]
- 32.Cervera R, Balasch J. 2008. Bidirectional effects on autoimmunity and reproduction. Hum Reprod Update 14:359–366 [DOI] [PubMed] [Google Scholar]
- 33.Hoek A, Schoemaker J, Drexhage HA. 1997. Premature ovarian failure and ovarian autoimmunity. Endocr Rev 18:107–134 [DOI] [PubMed] [Google Scholar]
- 34.Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, Cela V, Genazzani AR, Artini PG. 2011. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol 66:108–114 [DOI] [PubMed] [Google Scholar]
- 35.Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. 2005. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Reprod Immunol 66:53–67 [DOI] [PubMed] [Google Scholar]
- 36.Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gartner R. 2004. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol 150:363–369 [DOI] [PubMed] [Google Scholar]
- 37.van Gelderen CJ, Gomes dos Santos ML. 1993. Polycystic ovarian syndrome. Evidence for an autoimmune mechanism in some cases. J Reprod Med 38:381–386 [PubMed] [Google Scholar]
- 38.Hefler-Frischmuth K, Walch K, Huebl W, Baumuehlner K, Tempfer C, Hefler L. 2010. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil Steril 93:2291–2294 [DOI] [PubMed] [Google Scholar]
- 39.Ganie MA, Marwaha RK, Aggarwal R, Singh S. 2010. High prevalence of polycystic ovary syndrome characteristics in girls with euthyroid chronic lymphocytic thyroiditis: a case-control study. Eur J Endocrinol 162:1117–1122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.