Abstract
Objective: To assess whether a self-care toolkit (SCT) provided to breast cancer patients undergoing surgery could mitigate distress and lessen symptoms associated with surgery.
Design: One hundred women with breast cancer, planning to undergo initial surgery, were randomly assigned to either one of two groups: treatment as usual (TAU; n = 49) or TAU with the addition of an SCT (n = 51). The SCT contained an MP3 player with audio-files of guided mind–body techniques (breathing, progressive muscle relaxation, meditation, guided imagery, and self-hypnosis) and acupressure antinausea wristbands. Anxiety, pain, nausea, sleep, fatigue, global health, and quality of life (QOL) were assessed using validated outcome measures. Two inflammatory blood markers (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) were measured serially. Data were collected at baseline (T1), immediately before surgery (T2), within 10 h postoperatively (T3), and ∼2 weeks postsurgery (T4).
Settings: Numerous studies have shown that psychological distress associated with a cancer diagnosis can affect pain perception and QOL.
Results: Between T1 and T4, there were significant between-group differences in Patient-Reported Outcomes Measurement Information System (PROMIS)-57 scores of Pain Interference, Fatigue, and Satisfaction with Social Roles, favoring the SCT group compared with TAU (p = 0.005, p = 0.023, and p = 0.021, respectively). There was a significant mean change in Defense and Veterans Pain Rating Scale (DVPRS) scores from T2 to T3, with the SCT group having significantly smaller increases in postoperative pain (p = 0.008) and in postoperative ESR (p = 0.0197) compared with the TAU group. Clinically significant reductions in anxiety occurred in the SCT group during the main intervention period.
Conclusion: These results suggest that using the SCT in the perioperative period decreased pain perceptions, fatigue, and inflammatory cytokine secretion.
Keywords: : self-care, mind–body skills, breast cancer, surgery, pain, anxiety
Introduction
More than one-third of women with breast cancer experience significant emotional distress, anxiety, and/or depression following diagnosis.1 Anticipating surgery for breast cancer can create negative cognitions and emotions, such as anxiety and fear.2 Higher preoperative distress is associated with poorer psychological outcomes following breast cancer surgery.3 Psychological distress may adversely impact pain perception, immune-mediated wound healing, and return to physical function.4 Consequently, it is important to investigate nonpharmacologic interventions that might help decrease preoperative emotional distress.
Psychological preparation before surgery
Preoperative psychological preparation lessens postoperative pain and negative affect across a range of clinical populations.5 A recent meta-analysis found strategies, including relaxation, hypnosis, procedural information, or describing sensations one might experience had statistically significant impact on negative affect, whereas relaxation and techniques to help contextualize emotions were more effective in alleviating postoperative pain.4
Mind and body techniques in cancer populations
Among the most commonly studied mind and body techniques are breathing exercises, mindfulness meditation, progressive muscle relaxation (PMR), guided imagery, and self-hypnosis. These physical and cognitive relaxation strategies induce muscle relaxation and instill mental and emotional calm.6 They are simple to learn, can be practiced virtually anywhere, are considered safe, and can be effectively used as self-management strategies.7
Strategies using a combination of mind and body techniques have safely and effectively decreased psychological distress in cancer populations.8 The combination of breathing exercises and PMR has been shown to decrease anxiety and emotional distress in diverse cancer populations.9–11 Guided imagery combined with breathing and PMR has improved anxiety and distress in persons with cancer as well.12,13 Practice of the standardized mindfulness-based stress reduction program has improved anxiety and mood symptoms in cancer populations,14,15 as have shorter versions of mindfulness meditation training.16–18 Brief hypnosis interventions have significantly reduced emotional distress related to invasive medical procedures19; pain, anxiety, and procedure length20; emotional upset and depressed mood; and increased relaxation compared with attention-control groups.21 In light of this evidence, the authors created a self-care toolkit (SCT) with mind–body audio-files to be used pre- and postoperatively to address perisurgical distress and pain.
The present study aimed primarily to determine whether an SCT with guided mind–body techniques (breathing, PMR, meditation, guided imagery, self-hypnosis suggestions) delivered pre- and postoperatively would be more effective in decreasing anxiety in women newly diagnosed with breast cancer than treatment as usual (TAU). The study also tested the effects of the SCT on secondary outcomes of pain, nausea, sleep, fatigue, global health, quality of life (QOL), and inflammatory blood markers. The authors hypothesized that when compared with a TAU control group, the SCT group would have improved (1) anxiety, pain intensity, pain interference, sleep, fatigue, global health status, QOL scores, and inflammatory blood markers (erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]) at longer term time points (T1 vs. T4); and (2) anxiety, pain, and nausea in the immediate pre- to postoperative time periods (T2 vs. T3).
Materials and Methods
SCT development and testing
The SCT contains an MP3 player with audio-files of mind–body skills (i.e., breathing, relaxation, meditation, guided imagery, and self-hypnosis suggestions) and tools (i.e., Sea-Band® acupressure wristbands and journal). It was designed to help individuals regulate their physiologic and emotional reactions to stressful situations, prevent symptoms associated with surgery and anesthesia (i.e., pain, anxiety, nausea, insomnia, and fatigue), and enhance postoperative recovery. The SCT components are described in Table 1.
Table 1.
Self-Care Toolkit Components
| Component | Description |
|---|---|
| Manual | Spiral-bound instruction manual with a section to be used as a journal |
| Sea-Band® acupressure wristband | FDA-approved medical device designed to apply pressure to the P6 or “Nei-Kuan,” acupuncture point to decrease nausea |
| MP3 player with headphones | Includes seven audio-files designed to teach individuals self-regulating skills to enhance relaxation and diminish anxiety, pain, fatigue, nausea, and insomnia |
| Track 1: Introduction (1:17 min) | Instructions about how to use the toolkit, disclaimer about use only when in safe relaxing environment |
| Track 2: Breathing exercise with introduction (4:56 min) | Diaphragmatic breathing exercise with instructions how to position the body, notice chest versus diaphragmatic breathing |
| Track 3: Breathing exercise without introduction (3:31 min) | Diaphragmatic breathing exercise without introduction |
| Track 4: Mindfulness meditation exercise (11:29 min) | Guided meditation to pay attention to the in-breath, the out-breath, sounds, chosen mantra |
| Track 5: PMR (17:53 min) | Guided exercise to sequentially tense and relax muscle groups from head, upper body, and midbody to lower body and extremities |
| Track 6: Guided imagery and preprocedure track (21:19 min) | Breathing and relaxation induction with safe place imagery; specific suggestions for deepening relaxation with environmental cues, confidence in healthcare team, restoring comfort, normal body function, healing, restful sleep, energy, and strength postoperatively |
| Track 7: Guided imagery and postprocedure track (19:32 min) | Imagery with healing island of serenity; specific suggestions for trusting body's inner wisdom, advancing diet while making healthy choices, being patient with the healing process, restful sleep, energy, and strength |
PMR, progressive muscle relaxation.
The content for the audio-files was created specifically for this toolkit by mind–body experts. The breathing, PMR, and meditation tracks were created by a mind–body subject matter expert (Katherine Smith) with extensive training and experience teaching experiential mind–body workshops and courses. The guided imagery and two procedural tracks were created by one of the investigators (D.B.), who is a nurse practitioner and mind–body researcher with certifications in hypnotherapy and Interactive Guided ImagerySM. The scripts for the audio-files were vetted externally with subject matter experts in mind–body medicine and revised based on their feedback. The audio-files were recorded at a professional recording studio. Background instrumental music was included only on the two guided imagery procedural tracks. Both researchers created the content for the SCT instructional manual.
The research team conducted an initial institutional review board (IRB)-approved product evaluation study in a cohort of twelve adults undergoing elective cosmetic surgery. Participants responded favorably, stating that the mind–body skills were easy to learn and helpful before surgery. Negative comments focused on physical attributes of the toolkit (e.g., difficulty navigating tracks on the MP3 player, uncomfortable earbuds, and bulky manual). The research team incorporated the suggested improvements and used the modified SCT in the present study.
Study design overview
This two-group, nonblinded, randomized controlled study was conducted in 100 women recently diagnosed with nonmetastatic breast cancer, for whom surgery was the initial treatment. Participants were randomized to either receive the SCT in addition to usual medical care or TAU only. Data were collected at baseline (T1), immediately before surgery (T2), within 10 h postoperatively (T3), and ∼2 weeks postsurgery (T4).
Setting
The study was approved to be conducted at two sites: Brooke Army Medical Center (BAMC) and Carl R. Darnall Army Medical Center (CRDAMC). Regulatory approvals were obtained from the Department of Clinical Investigation (DCI) and BAMC IRB, and by the Human Research Protection Office at U.S. Army Medical Research and Materiel Command. The protocol was registered on clinicaltrials.gov (ID: NCT02387320). All study staff completed relevant Collaborative Institutional Training Initiative (CITI) training before study initiation.
Participants
Eligible participants included women older than 18 years who were newly diagnosed with nonmetastatic breast cancer and for whom surgery (e.g., lumpectomy or mastectomy) would be their initial treatment. Exclusion criteria included women who received neoadjuvant treatment or individuals with severe hearing impairment who would be unable to listen to the audio-files.
Study procedures
Research staff recruited potential study participants from the BAMC and CRDAMC General Surgery Departments. During their multidisciplinary oncology appointment, women diagnosed with breast cancer received an IRB-approved study flyer and were invited to meet with a researcher to learn about the study. Women meeting eligibility criteria and voicing interest in participation attended a baseline appointment, during which they received a detailed explanation of the study and signed IRB-approved informed consent documents. Participants were randomized to either the SCT or TAU group using a random integer generator.22 Due to the nature of the intervention, blinding was not possible.
Interventions
SCT group participants received instruction about the toolkit's components and demonstration of the MP3 player and acupressure wristbands by the research assistant. Participants were asked to read the manual and listen to each of the seven audio-files at least once, but encouraged to use them as many times as desired during the 2-week preoperative and postoperative periods. In addition, they were asked to wear the acupressure wristbands during surgery and instructed to remove them if they experienced adverse reactions.
Women randomized to the TAU group received perioperative medical care as per their oncology team. They received the SCT on study completion since the mind–body skills and antinausea wristbands could be used during future cancer treatments.
Outcome measures
Self-reported survey data were collected using Wi-Fi-enabled tablets at four time points. Primary and secondary outcome measures are shown in Table 2. The primary outcome, anxiety, was measured by the NIH Patient-Reported Outcomes Measurement Information System (PROMIS-57) Anxiety subscale. Secondary outcomes for Pain Intensity, Pain Interference, Fatigue, and Sleep Disturbance were measured with the corresponding NIH PROMIS-57 subscales. PROMIS-57 also includes subscales for Depression, Physical Function, and Satisfaction with Social Roles, which were not expected to be affected, but were administered as part of the full measure. The PROMIS-57 scales have excellent internal consistency to show expected patterns of convergent and discriminant validity.23 Two questions from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), which have been tested extensively in cancer populations,24 independently measure self-assessment of global health and overall QOL over the prior week on a 7-point Likert scale from 1 (very poor) to 7 (excellent). ESR and CRP levels were obtained to determine if the SCT group had comparatively lower inflammatory biomarker levels. Due to diurnal variability of ESR and CRP, laboratory draws were generally done before 10:00 am at T1, T2, and T4.25
Table 2.
Primary and Secondary Outcome Measures
| Instrument | Constructs | Baseline (T1) | Preoperative (T2) | Postoperative (T3) | Two-week follow-up (T4) |
|---|---|---|---|---|---|
| Demographics Survey | Demographics, breast cancer stage, type of surgery, past use of integrative techniques | X | |||
| NIH PROMIS-57 | Anxiety,a Pain Intensity,b Pain Interference,b Fatigue,b Sleep Disturbance,b Satisfaction with Social Roles,c Physical Function,c Depressionc | X | X | X | |
| EORTC QLQ-C30b (two items) | Global health status, Quality of life | X | X | ||
| GA-VASa | Anxiety | X | X | ||
| Defense and Veterans Pain Rating Scaleb | Pain | X | X | ||
| Nausea VASb | Nausea | X | X | ||
| Inflammatory biomarkersb | ESR, CRP | X | X | X | |
| Satisfaction surveyd | Satisfaction, use, helpfulness of SCT | X | |||
| Qualitative Interviewd | Open-ended questions about SCT | X |
Primary outcomes.
Secondary outcomes.
Other NIH PROMIS-57 subscales.
Administered only to SCT group.
CRP, C-reactive protein; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Questionnaire Core 30; ESR, erythrocyte sedimentation rate; GA-VAS, General Anxiety-Visual Analog Scale; PROMIS, Patient-Reported Outcomes Measurement Information System; SCT, self-care toolkit; VAS, Visual Analog Scale.
Three single-item measures were administered pre- and postoperatively to determine whether the SCT had immediate effects on anxiety, pain, and nausea. The 10-cm General Anxiety-Visual Analog Scale (GA-VAS) correlates well with other anxiety measures, with minimally important difference (MID) estimates clustering between 10 and 15 mm.26 The Defense and Veterans Pain Rating Scale (DVPRS) is a self-reported graphic pain scale from 0 to 10 (0 = “no pain” to 10 = “as bad as it could be, nothing else matters”) with an internal consistency reliability of Cronbach's alpha of 0.902.27 A standard 10-cm Visual Analog Scale (VAS; 0–10 cm) measured nausea on a scale from 0 to 10 (0 = “no nausea” to 10 = “extreme nausea”).
Two assessments administered to the SCT group at follow-up (T4) were a satisfaction survey and a brief qualitative interview with open-ended questions.
Statistical methods
The sample size calculation was based on expected minimal detectable effect size for the primary outcome of change in anxiety over time using the PROMIS-57 anxiety subscale. Using a 0.05 significance level and 80% power, the required sample size was estimated at 45 participants per group to detect a 0.6 mean difference in effect size (Cohen's d). Assuming a 10% participant dropout or lost to follow-up rate, an enrollment of 50 participants per group was required.
Subjects were compared using an intent-to-treat model based on their randomization assignment. Categorical variables and frequency counts were analyzed using chi-square or Fisher's exact tests, whichever was most appropriate. Means and standard deviations (SDs) were used as summary statistics for continuous variables and analyzed using Student's t test, analysis of variance (ANOVA), and/or Wilcoxon's test. For data measured at two time points, the delta change in values was calculated to detect within-group differences in SCT and TAU using Wilcoxon's rank sum test or paired t test. For factors measured at more than two time points, a two-way repeated measures ANOVA (RM-ANOVA) was implemented with a Bonferroni corrected post hoc analysis to determine between-group differences at each time point. Significance for results was established when p-values were <0.05. All statistical analyses were performed using SPSS v22.0 (IBM Corp.).
Results
Participant flow
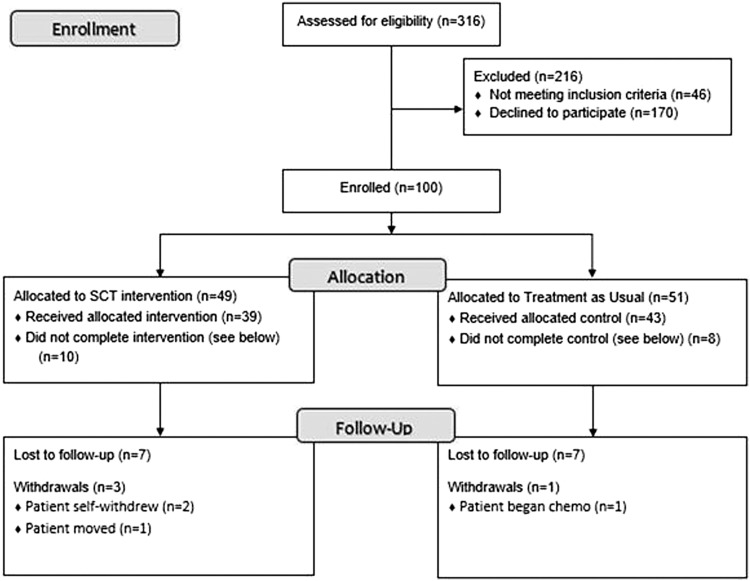
A total of 316 potential participants were screened for eligibility at BAMC and CRDAMC from April 2014 to June 2017. Of these, 216 were excluded for not meeting eligibility criteria (21%) or choosing not to participate (79%). Most declining participation cited lack of available personal time. One hundred participants were enrolled and randomly assigned to either one of two groups: SCT (n = 51) or TAU (n = 49) group. Four participants voluntarily withdrew from the study: two received treatment at another facility, one required neoadjuvant chemotherapy, and the fourth moved out of the area (Fig. 1). Fourteen participants were lost to follow-up and did not complete the 2-week follow-up measures. None of the study participants experienced any adverse effects.
FIG. 1.
Participant CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials. SCT, self-care toolkit.
Participant demographics
The majority of the women enrolled were Caucasian (59%), military dependents (68%), older than 45 years (87%), and with a Bachelor's degree (57%). Nearly half (42%) were diagnosed with Stage I breast cancer and the proportions of anticipated mastectomy and lumpectomy surgeries were not significantly different between study groups (p = 0.341). Thirty-four percent of participants were familiar with integrative modalities and 13% stated they had used them previously. No significant differences were detected between study groups at baseline for any demographic variables (Table 3).
Table 3.
Participant Demographics
| SCT n (%) | TAU n (%) | p | |
|---|---|---|---|
| Age (years) | |||
| 31–35 | 1 (2) | 0 (0) | 0.476 |
| 36–45 | 5 (11) | 6 (13) | |
| >45 | 42 (87) | 39 (87) | |
| Race | |||
| Caucasian/white | 31 (66) | 23 (51) | 0.148 |
| African American | 7 (12) | 13 (27) | |
| Hispanic/Latino | 9 (19) | 5 (11) | |
| Asian/Pacific Islander | 1 (2) | 3 (7) | |
| Highest education level | |||
| No high school diploma | 0 (0) | 0 (0) | 0.113 |
| High school diploma | 6 (12) | 4 (9) | |
| Vocational school | 1 (2) | 2 (4) | |
| Some college | 9 (19) | 9 (20) | |
| Associates | 9 (19) | 4 (9) | |
| Bachelors | 12 (26) | 14 (31) | |
| Masters | 10 (21) | 7 (16) | |
| PhD | 0 (0) | 5 (11) | |
| Military status | |||
| Active duty | 5 (11) | 2 (4) | 0.58 |
| Reservist | 1 (2) | 0 (0) | |
| National Guard | 0 (0) | 0 (0) | |
| Retired | 10 (21) | 9 (20) | |
| Dependent | 30 (64) | 33 (73) | |
| Civil service | 1 (2) | 1 (2) | |
| Pay grade | |||
| E1–E4 | 1 (2) | 0 (0) | 0.106 |
| E5–E6 | 1 (2) | 0 (0) | |
| E7–E9 | 1 (2) | 2 (4) | |
| W1–W5 | 2 (4) | 0 (0) | |
| 01–03 | 0 (0) | 0 (0) | |
| 04–06 | 5 (11) | 1 (2) | |
| Other | 37 (79) | 42 (93) | |
| Marital status | |||
| Married, together | 34 (72) | 36 (80) | 0.097 |
| Married, separate | 1 (2) | 2 (4) | |
| Separated | 0 (0) | 1 (2) | |
| Divorced | 1 (2) | 3 (7) | |
| Widowed | 0 (0) | 3 (7) | |
| In relationship | 2 (4) | 0 (0) | |
| Single | 3 (6) | 0 (0) | |
| Years with spouse | 6.0 ± 1.7 | 6.2 ± 1.7 | 0.574 |
| Cancer stage | |||
| Stage 0 | 19 (40) | 15 (33) | 0.56 |
| Stage I | 20 (42) | 19 (4) | |
| Stage II | 8 (17) | 10 (22) | |
| Stage III | 0 (0) | 1 (2) | |
| Surgery type | |||
| Lumpectomy | 24 (52) | 19 (48) | 0.341 |
| Mastectomy | 19 (42) | 26 (58) | |
| Breast | |||
| Right breast | 20 (42) | 22 (49) | 0.353 |
| Left breast | 22 (47) | 15 (33) | |
| Both | 5 (11) | 8 (18) | |
| Familiar with integrative modalities | |||
| Yes | 19 (40) | 12 (27) | 0.161 |
| No | 28 (60) | 33 (73) | |
| Past use of integrative modalities | |||
| Yes | 6 (12) | 6 (13) | 0.936 |
| No | 41 (87) | 39 (87) | |
SCT, self-care toolkit; TAU, treatment as usual.
SCT use and satisfaction
Most participants in the SCT group completed the satisfaction survey (n = 36; 71%) and provided feedback about the toolkit. All respondents endorsed using the SCT and the MP3 player; however, there were variations in use of the individual components (Table 4).
Table 4.
Reported Use of Self-Care Toolkit Components
| Frequency of use | ||||
|---|---|---|---|---|
| SCT Component | Used Component (%) | Found Helpful (%) | Low Usersa(%) | High Usersb(%) |
| Overall toolkit | 100 | 97 | — | — |
| Manual | 74 | 93 | 85 | 15 |
| Journal | 40 | 71 | 61 | 39 |
| Wristbands | 66 | 67 | 64 | 37 |
| MP3 player | 100 | 97 | 33 | 67 |
| Track 1: Introduction | 80 | 89 | 96 | 4 |
| Track 2: Breathing with instructions | 89 | 100 | 73 | 27 |
| Track 3: Breathing without instructions | 91 | 97 | 41 | 59 |
| Track 4: Meditation | 94 | 80 | 47 | 53 |
| Track 5: PMR | 91 | 87 | 48 | 52 |
| Track 6: Guided imagery with preoperative suggestions | 86 | 83 | 73 | 27 |
| Track 7: Guided imagery with postoperative suggestions | 83 | 93 | 76 | 24 |
Participants who used component only once or several times over the study.
Participants who used component many times, daily, or >1 time a day.
SCT, self-care toolkit; PMR, progressive muscle relaxation.
The most commonly used mind–body audio-files were the meditation (94%), PMR (91%), and breathing (91%) tracks. Participants also reported listening to the guided imagery with preoperative (86%) and postoperative (83%) suggestions. The least used component was the journal section of the manual (40%). About two-thirds reported using the antinausea wristbands and a similar proportion found them helpful. The components rated most helpful were the breathing track with instructions (100%), guided imagery with postoperative suggestions (93%), and PMR (87%) tracks. Overall, 94% of SCT participants were very satisfied or satisfied with the toolkit and 74% rated the quality as very good or good (Table 4). Analyses to examine correlations between frequency of SCT use and outcomes showed no significant relationships; however, the small subgroup sample sizes were underpowered to show effects (data not shown).
Symptom changes over time
Baseline (T1) to preoperative (T2) time interactions
Baseline values were higher in the SCT group when compared with the TAU group for PROMIS-57 subscales of Anxiety (p = 0.0045), Pain Intensity, Pain Interference, Fatigue (p = 0.0147), and Sleep Disturbance. The mean change in the primary outcome, PROMIS Anxiety, from T1 to T2 in the SCT group was −5.08 (SD 5.61) and in the TAU group −1.70 (SD 10.96); however, this was not statistically significant (p = 0.098). For all other PROMIS-57 subscales, there were no significant between-group mean differences from T1 to T2 (Table 5).
Table 5.
Between-Group Differences in Patient-Reported Outcomes Measurement Information System-57 Subscales
| Baseline (T1) | Preoperative (T2) | Follow-up (T4) | Preoperative to baseline (T2 vs. T1) | Follow-up to baseline (T4 vs. T1) | |||
|---|---|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean, difference (SD) | pa | Mean difference (SD)a | pa |
| Anxiety | |||||||
| SCT | 59.91 (7.91) | 54.67 (7.92) | 51.43 (9.54) | −5.08 (5.61) | 0.098 | −8.96 (8.45) | 0.099 |
| TAU | 55.22 (9.38) | 53.97 (9.34) | 48.67 (8.40) | −1.7 (10.96) | −5.69 (8.63) | ||
| Pain Intensity | |||||||
| SCT | 2.34 (2.29) | 2.26 (2.54) | 3.02 (2.19) | −0.17 (2.05) | 0.267 | 0.32 (1.72) | 0.095 |
| TAU | 1.82 (2.42) | 1.35 (2.23) | 2.78 (2.16) | −0.72 (2.13) | 1.08 (2.14) | ||
| Pain Interference | |||||||
| SCT | 50.25 (9.11) | 48.59 (9.64) | 54.67 (8.15) | −2.68 (7.23) | 0.522 | 2.89 (8.92) | 0.005* |
| TAU | 46.49 (8.97) | 45.70 (8.79) | 56.20 (10.45) | −1.58 (7.53) | 9.93 (11.92) | ||
| Fatigue | |||||||
| SCT | 51.25 (10.02) | 49.66 (9.56) | 51.76 (8.15) | −1.03 (7.01) | 0.938 | 0.02 (7.64) | 0.023* |
| TAU | 46.30 (10.91) | 44.84 (10.33) | 50.70 (9.74) | −1.16 (7.84) | 4.13 (7.82) | ||
| Sleep Disturbance | |||||||
| SCT | 52.65 (3.69) | 52.94 (3.97) | 52.70 (3.51) | 0.36 (3.66) | 0.625 | −0.29 (4.10) | 0.351 |
| TAU | 51.54 (3.96) | 52.44 (4.23) | 52.25 (3.32) | 0.79 (3.90) | 0.59 (4.00) | ||
| Satisfaction with Social Roles and Activities | |||||||
| SCT | 51.05 (10.14) | 53.02 (11.50) | 49.08 (10.96) | 2.76 (8.89) | 0.518 | −1.53 (9.79) | 0.021* |
| TAU | 52.99 (10.79) | 55.06 (10.84) | 45.52 (11.10) | 1.43 (8.67) | −7.38 (11.79) | ||
| Physical Function | |||||||
| SCT | 27.84 (7.46) | 27.00 (6.64) | 34.63 (6.38) | −0.74 (3.22) | 0.416 | 6.54 (6.77) | 0.232 |
| TAU | 25.58 (6.78) | 24.60 (6.63) | 34.20 (6.91) | −0.04 (4.03) | 8.73 (8.99) | ||
| Depression | |||||||
| SCT | 49.25 (8.75) | 45.38 (7.18) | 46.20 (7.57) | −3.08 (7.12) | 0.313 | −3.23 (7.17) | 0.819 |
| TAU | 47.11 (7.89) | 46.54 (8.22) | 44.19 (6.37) | −0.99 (10.29) | −2.85 (6.99) | ||
t Test on delta change.
t-test, p < 0.05.
SCT, self-care toolkit; SD, standard deviation; TAU, treatment as usual.
Baseline (T1) to follow-up (T4) time interactions
Several significant between-group mean differences were observed when examining the time interaction between T1 and T4 (Table 5). The mean change in Pain Interference was significant (p = 0.005) with a larger increase in Pain Interference in the TAU group (mean difference: 9.93 [TAU] vs. 2.89 [SCT]). While Fatigue was significantly higher among SCT participants at baseline, both groups were equivalent at the 2-week follow-up. The mean change in Fatigue from T1 to T4 was also significantly greater in the TAU group (4.13 [TAU] vs. 0.02 [SCT], p = 0.023). Satisfaction with Social Roles and Activities significantly decreased in the TAU group and plateaued in the SCT group (p = 0.021). There were nonsignificant differences in Anxiety (p = 0.099), Pain Intensity (p = 0.095), or Sleep Disturbance (p = 0.351) over this time period.
Group by time interactions
There were significant group-by-time interaction effects for Pain Interference (p = 0.0152), Fatigue (p = 0.0288), and Social Roles and Activities (p = 0.0130) in RM-ANOVA. There were no significant interaction effects in PROMIS subscales for Anxiety, Pain Intensity, or Sleep Disturbance. The results of the QOL Survey (QLQ-C30) showed no difference between the two study groups (data not shown).
Preoperative (T2) to postoperative (T3)
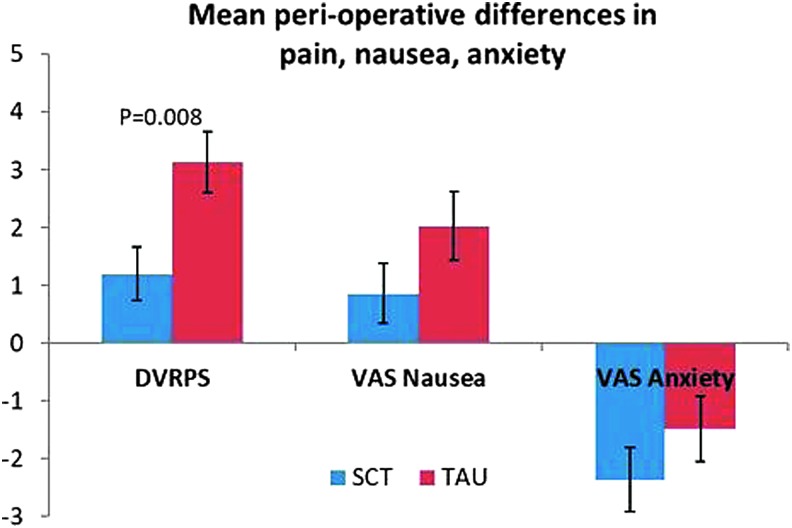
There was a significant difference in mean change in DVPRS scores from T2 to T3, with the SCT group having a lower increase in postoperative pain (mean difference: 1.2 [SCT] vs. 3.14 [TAU], p = 0.008). VAS nausea scores increased less for the SCT group. VAS anxiety decreased for both groups, more so in the SCT group (Table 6).
Table 6.
Mean Perioperative Change in Defense and Veterans Pain Rating Scale and Visual Analog Scale Variable Scores
| Group | Mean change (T3–T2) | SD | SE mean | p | |
|---|---|---|---|---|---|
| DVRPS Pain | SCT (n = 35) | 1.200 | 2.774 | 0.469 | 0.008* |
| TAU (n = 35) | 3.143 | 3.126 | 0.528 | ||
| VAS Anxiety | SCT (n = 33) | −2.364 | 3.151 | 0.548 | 0.269 |
| TAU (n = 35) | −1.486 | 3.346 | 0.566 | ||
| VAS Nausea | SCT (n = 35) | 0.857 | 3.060 | 0.517 | 0.141 |
| TAU (n = 35) | 2.029 | 3.510 | 0.593 |
Wilcoxon p < 0.05.
DVRPS, Defense and Veterans Pain Rating Scale; SD, standard deviation; SE, standard error; VAS, Visual Analog Scale.
While these between-group differences were not statistically significant for either nausea or anxiety (p = 0.141 and p = 0.269, respectively), the overall trend suggests a decrease in both variables from pre- to postoperative in the SCT group (Fig. 2).
FIG. 2.
Mean differences in pain, nausea, and anxiety from preoperative (T2) to postoperative (T3). Data displayed as mean ± SEM. Wilcoxon p-value = 0.008. DVRPS, Defense and Veterans Pain Rating Scale; SCT, self-care toolkit; SEM, standard error of the mean; TAU, treatment as usual; VAS, Visual Analog Scale.
Laboratory results
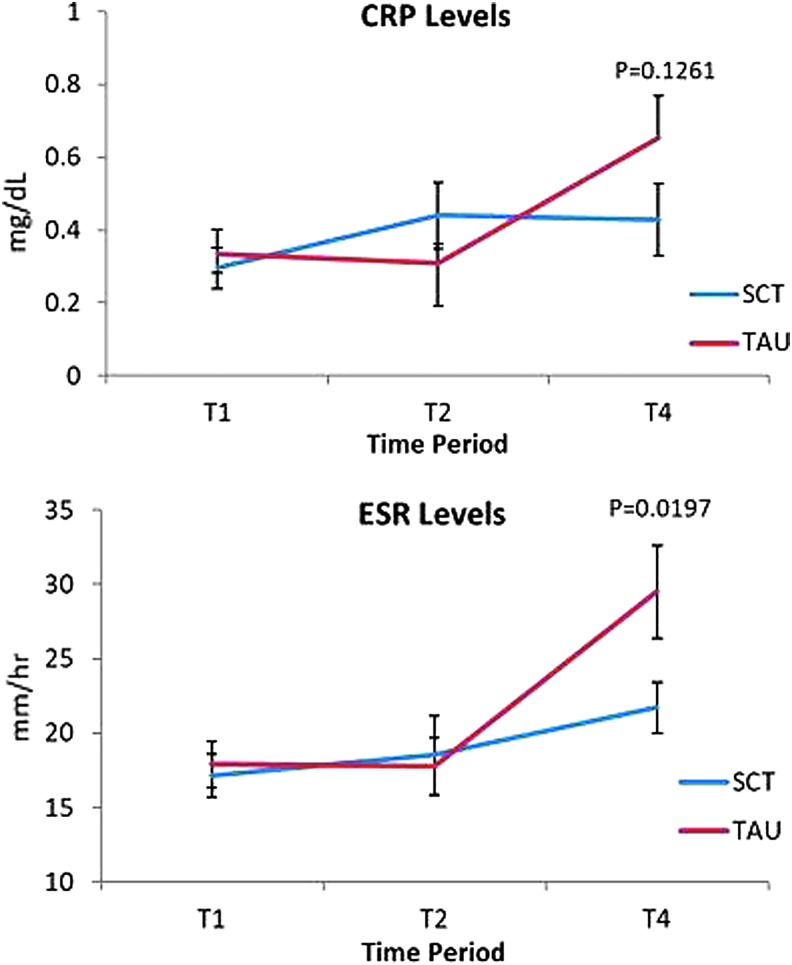
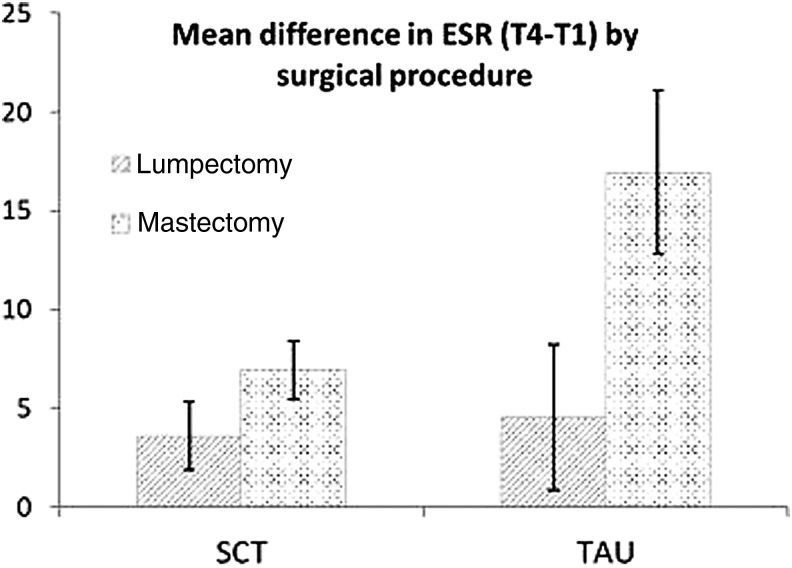
While both CRP and ESR increased from T2 to T4 in the TAU group, the SCT group's values did not show a similarly spiked increase (Fig. 3); however, only the ESR group by time interaction was significant at T4 (p = 0.0197). ESR subgroup analysis showed statistically significant between-group differences from T1 to T4 favoring the SCT group in the rate of increase in ESR among mastectomy, but not lumpectomy, patients (p = 0.023) (Fig. 4).
FIG. 3.
Mean ESR and CRP levels over time. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SCT, self-care toolkit; TAU, treatment as usual.
FIG. 4.
Mean difference in ESR T4 versus T1 by surgical procedure. Data displayed as mean ± SEM. Wilcoxon p-values by surgery type: mastectomy, p = 0.023; lumpectomy, p = 0.8339; Wilcoxon p-values by randomization arm: SCT, p = 0.456; TAU, p = 0.008. ESR, erythrocyte sedimentation rate; SCT, self-care toolkit; TAU, treatment as usual.
Discussion
The primary hypothesis was that anxiety would decrease in the SCT group during the main intervention period from baseline to preoperative. While anxiety decreased a mean 5.08 T-scale points in the SCT and 1.7 in TAU groups, these between-group differences did not reach statistical significance. Nonetheless, this decrease in anxiety in the SCT group represents a clinically meaningful change, since a MID range of 3.0–4.5 in the PROMIS-Cancer Anxiety subscale has been found in persons with advanced cancers.28 While the average decrease in anxiety was even greater for the SCT group from baseline to follow-up (mean, 8.96), anxiety also decreased substantially during this time period for the TAU group as well (mean, 5.69), possibly explained by a lessening of preoperative anxiety once surgery had occurred. Both the SCT and TAU groups had substantially elevated anxiety at baseline (mean, 59.9 and 55.2, respectively), well above the 49.6 reference range for anxiety in newly diagnosed breast cancer patients.29 This heightened anxiety at baseline is likely due to the short interval between when participants learned of their treatment plan and the consenting research appointment.
Another unexpected finding is that the SCT group had significantly higher fatigue compared with the TAU group at baseline. The authors did not find any significant baseline differences between groups on demographic variables (e.g., type of surgery and cancer stage), nor did they collect data that might account for these baseline differences (e.g., pre-existing psychological conditions, prognosis, genetic risk factors, and postoperative treatment plan). Consequently, the authors are unable to definitively attribute the cause of these baseline between-group differences. One possible explanation is that since the IRB required an opt-in section of the consent to participate in qualitative interviews only for the SCT group, there may have been concern at baseline among SCT participants about the burden of additional research-related requirements.
The significant mean increase in Pain Interference from baseline to follow-up was 9.93 T-scale points in the TAU and 2.89 in the SCT group, reflecting Pain Interference in the TAU group that exceeded the MID range of 4.0–6.0.28 This statistically and clinically significant difference between groups suggests that the SCT group did not experience Pain Interference to the same extent as the TAU did. Fatigue was higher at baseline and stayed relatively constant at T2 and T4 in the SCT group, whereas it worsened 4.13 T-scale points in the TAU group within the clinically relevant MID range of 3.0–5.0.28 Although the authors cannot definitely attribute the cause, the pre- and postoperative tracks contained posthypnotic suggestions for comfort, ease, vitality, and vigor increasing each day as healing occurs.
Inflammatory conditions such as cancer, surgery, and tissue infarction commonly lead to changes in serum levels of acute-phase reactants (APRs) such as ESR and CRP.30 Recent studies show linkages between inflammation and psychological stress and that relaxation techniques can reduce physiologic and genetic markers of inflammation.25,31–34 In this study, the authors used ESR and CRP to investigate whether the SCT influenced the degree and duration of postoperative inflammation. These results showed a statistically significant between-group difference in the 2-week postsurgical ESR and a simultaneous, nonsignificant decrease in CRP (Fig. 3). Since both ESR and CRP showed similar trends in the postsurgical state, the SCT intervention may have had a direct or indirect effect on APR and proinflammatory cytokine secretion. The blunted rise in postoperative ESR in the SCT group may imply an improvement in wound healing associated with decreased proinflammatory cytokine levels.35,36 An unanticipated finding was that mastectomy patients in the SCT group had significantly lower postsurgical ESR compared with TAU mastectomy patients. It would be expected that mastectomy patients would have a greater increase in APRs since mastectomy is a more invasive surgery than lumpectomy.
Challenges and limitations
Several challenges were encountered during the course of this study. Following lengthy administrative processes for military IRB approval, the authors added a second site at CRDAMC to increase enrollment; however, many of their breast cancer patients were referred to other facilities. Another challenge was initiating the intervention at time of diagnosis. Although this seemed an ideal time to introduce a potentially helpful intervention for emotional distress, it was not a priority for many, explaining the high percentage of screened patients who declined to participate. Finally, during this 3-year study, the MP3 player intervention used was outpaced by technologic advances in mobile applications.
Conclusion
The mind–body skills used in this study, specifically meditation, guided imagery and self-hypnosis, have been shown to relieve distress and pain related to cancer and surgery. The authors created a customized SCT with guided meditations to teach individuals how to recognize and self-manage physical and emotional reactions to stressful events from cancer diagnosis and surgical treatment. They found significant differences in Pain Interference, Fatigue, and Satisfaction with Social Roles in the SCT group compared with TAU from baseline to follow-up. In the immediate perioperative period, the SCT group had a smaller increase in postoperative pain as measured by the DVPRS compared with the TAU group. There was also a significantly greater difference in ESR, but not CRP, favoring the SCT group at follow-up. These results suggest individuals may derive benefits from these practices without accompanying harm. These techniques may provide a healthy method of coping and increased resilience throughout cancer treatment.
Acknowledgments
The authors acknowledge the following individuals for their contributions: Ms. Katherine Smith (codeveloper of SCT), Dr. Salvatore Libretto (protocol writing, study Principal Investigator), Dr. Karin Nicholson (onsite Principal Investigator), Dr. Bonnie Sakallaris and Ms. Sita Ananth (SCT project initiation), Mrs. Sandra Jarzombek (recruitment, data collection), Ms. Bianca Rodriguez (recruitment, data collection), Ms. Susan Ferrise (recruitment, data collection), Mrs. Edna Figuordias (recruitment, data collection), Ms. Ames Davis (recruitment, data collection), Ms. Ashley Price (protocol writing, project management), Dr. Shawnn Nichols (Associate Investigator), Dr. Peter Learn (Associate Investigator), and Dr. Christy Chai (Associate Investigator). This project was sponsored by the U.S. Army Medical Research and Materiel Command under Award Number W81XWH-11-1-0538. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of BAMC, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Air Force, the Department of the Army or the Department of Defense or the U.S. Government.
Author Disclosure Statement
The authors have no conflict of interest to report.
References
- 1.Vin-Raviv N, Akinyemiju TF, Galea S, Bovbjerg DH. Depression and anxiety disorders among hospitalized women with breast cancer. PLoS One 2015;10:e0129169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer 2004;90:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyranou BM, Puntillo EK, Dunn ML, et al. Predictors of initial levels and trajectories of anxiety in women before and for 6 months after breast cancer surgery. Cancer Nurs 2014;37:406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell R, Scott NW, Manyande A, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev 2016;5:CD008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston M, Vögele C. Benefits of psychological preparation for surgery: A meta-analysis. Ann Behav Med 1993;15:245–256 [Google Scholar]
- 6.Michie S, Johnston M, Francis J, et al. From theory to intervention: Mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660–680 [Google Scholar]
- 7.Crawford C, Wallerstedt DB, Khorsan R, et al. A systematic review of biopsychosocial training programs for the self-management of emotional stress: Potential applications for the military. Evid Based Complement Alternat Med 2013;2013:747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr 2014;50:346–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CW, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: Results of a psychoeducational randomized controlled trial. J Pain Symptom Manage 2011;41:347–357 [DOI] [PubMed] [Google Scholar]
- 10.Fawzy NW. A psychoeducational nursing intervention to enhance coping and affective state in newly diagnosed malignant melanoma patients. Cancer Nurs 1995;18:427–438 [PubMed] [Google Scholar]
- 11.Bridge LR, Benson P, Pietroni PC, Priest RG. Relaxation and imagery in the treatment of breast cancer. BMJ 1988;297:1169–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decker TW, Cline-Elsen J, Gallagher M. Relaxation therapy as an adjunct in radiation oncology. J Clin Psychiatry 1992;48:388–393 [DOI] [PubMed] [Google Scholar]
- 13.Nunes DF, Rodriguez AL, da Silva Hoffmann F, et al. Relaxation and guided imagery program in patients with breast cancer undergoing radiotherapy is not associated with neuroimmunomodulatory effects. J Psychosom Res 2007;63:647–655 [DOI] [PubMed] [Google Scholar]
- 14.Kabat-Zinn J, Santorelli S. Mindfulness-Based Stress Reduction Professional Training Resource Manual. Worchester, MA: Center for Mindfulness in Medicine Health Care and Society, 1999 [Google Scholar]
- 15.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 2010;78:169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med 2000;62:613–622 [DOI] [PubMed] [Google Scholar]
- 17.Lengacher CA, Johnson‐Mallard V, Post‐White J, et al. Randomized controlled trial of mindfulness‐based stress reduction (MBSR) for survivors of breast cancer. Psychooncology 2009;18:1261–1272 [DOI] [PubMed] [Google Scholar]
- 18.Branstrom R, Kvillemo P, Brandberg Y, Moskowitz JT. Self-report mindfulness as a mediator of psychological well-being in a stress reduction intervention for cancer patients—A randomized study. Ann Behav Med 2010;39:151–161 [DOI] [PubMed] [Google Scholar]
- 19.Schnur JB, Kafer I, Marcus C, Montgomery GH. Hypnosis to manage distress related to medical procedures: A meta-analysis. Contemp Hypn 2008;25:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnur JB, Bovbjerg DH, David D, et al. Hypnosis decreases presurgical distress in excisional breast biopsy patients. Anesth Analg 2008;106:440–444, table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomised trial. Lancet 2000;355:1486–1490 [DOI] [PubMed] [Google Scholar]
- 22.Alimi D, Rubino C, Pichard-Leandri E, et al. Analgesic effect of auricular acupuncture for cancer pain: A randomized, blinded, controlled trial. J Clin Oncol 2003;21:4120–4126 [DOI] [PubMed] [Google Scholar]
- 23.Lanting S, Saffer B, Koehle M, Iverson G. Reliability and validity of the PROMIS-57 health outcome measures. Paper presented at: Annual Convention of the Canadian Psychological Association, 2013, Quebec City, Quebec
- 24.Aaronson N, Ahmedzai S, Bergman B, Bullinger M. The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376 [DOI] [PubMed] [Google Scholar]
- 25.Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav Immun 2013;27:38–41 [DOI] [PubMed] [Google Scholar]
- 26.Williams VS, Morlock RJ, Feltner D. Psychometric evaluation of a Visual Analog Scale for the assessment of anxiety. Health Qual Life Outcomes 2010;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckenmaier CC, 3rd, Galloway KT, Polomano RC, et al. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med 2013;14:110–123 [DOI] [PubMed] [Google Scholar]
- 28.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol 2011;64:507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen RE, Potosky AL, Moinpour CM, et al. United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35:1913–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454 [DOI] [PubMed] [Google Scholar]
- 31.Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med 2003;65:571–581 [DOI] [PubMed] [Google Scholar]
- 32.Kaliman P, Alvarez-Lopez MJ, Cosin-Tomas M, et al. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 2014;40:96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain Behav Immun 2013;27:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkranz MA, Davidson RJ, Maccoon DG, et al. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 2013;27:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyapati L, Wang HL. The role of stress in periodontal disease and wound healing. Periodontol 2000 2007;44:195–210 [DOI] [PubMed] [Google Scholar]
- 36.Serra MB, Barroso WA, da Silva NN, et al. From inflammation to current and alternative therapies involved in wound healing. Int J Inflam 2017;2017:3406215. [DOI] [PMC free article] [PubMed] [Google Scholar]