Abstract
Long noncoding RNAs (lncRNAs) exhibit highly lineage-specific expression and act through diverse mechanisms to exert control over a wide range of cellular processes. lncRNAs can function as potent modulators of innate immune responses through control of transcriptional and posttranscriptional regulation of mRNA expression and processing. Recent studies have demonstrated that lncRNAs participate in the regulation of antiviral responses and autoimmune disease. Despite their emerging role as immune mediators, the mechanisms that govern lncRNA expression and function have only begun to be characterized. In this study, we explore the role of lncRNAs in human plasmacytoid dendritic cells (pDCs), which are critical sentinel sensors of viral infection and contribute to the development of autoimmune disease. Using genome-wide sequencing approaches, we dissect the contributions of Toll-like receptor 7 (TLR7) and type I interferon (IFN-I) in the regulation of coding and noncoding RNA expression in CAL-1 pDCs treated with R848 or IFNβ. Functional enrichment analysis reveals both the unique and synergistic roles of TLR7 and IFN-I signaling in the orchestration of pDC function. These observations were consistent with primary cell immune responses elicited by detection of viral infection. We identified and characterized the conditional TLR7- and IFN-I-dependent regulation of 588 lncRNAs. Dysregulation of these lncRNAs could significantly alter pDC maturation, IFN-I and inflammatory cytokine production, antigen presentation, costimulation or tolerance cues, turnover, or localization, all consequential events during viral infection or IFN-I-driven autoimmune diseases such as systemic lupus erythematosus. These findings demonstrate the differential responsiveness of lncRNAs to unique immune stimuli, uncover regulatory mechanisms of lncRNA expression, and reveal a novel and tractable platform for the study of lncRNA expression and function.
Keywords: : interferon, Toll-like receptor, plasmacytoid dendritic cell, long noncoding RNA, innate immunity, RNA sequencing
Introduction
Detection of microbial infection by the innate immune system relies critically upon pattern recognition receptor (PRR) activation by conserved microbial motifs (Takeuchi and Akira 2010). Pathogen-derived nucleic acids engage with Toll-like receptors (TLRs) or RIG-I-like receptors to initiate rapid production of type I interferons (IFN-I), which act locally and distally on cells through the IFN-I receptor (IFNAR) (Thompson and others 2011). IFN-I triggers intracellular signaling cascades resulting in the transcriptional induction of hundreds of IFN-stimulated genes (ISGs), which confer protective antiviral activities and limit the spread of viral infections (Sadler and Williams 2008; Swiecki and Colonna 2011; Hoffmann and others 2015). IFN-I also acts upon cells of the immune system to activate innate and adaptive immune responses to control infection (Gonzalez-Navajas and others 2012). Similarly, aberrant detection of host-derived nucleic acids by TLRs stimulates release of IFN-I, which drives inflammation and promotes self-antigen-specific autoreactivity in many systemic autoimmune diseases and interferonopathies (Crowl and others 2017). Such aberrant IFN-I signaling is implicated in the pathogenesis of systemic lupus erythematosus (SLE) (Elkon and Wiedeman 2012), where an elevated ISG signature in patient blood correlates with disease severity (Baechler and others 2003; Kirou and others 2005). Further evidence of the role of IFN-I in facilitating the initiation of autoreactivity is also provided by the observation that IFNAR-deficient mice are protected from the development of disease in adjuvant-induced and genetic SLE models (Nacionales and others 2007; Agrawal and others 2009).
Plasmacytoid dendritic cells (pDCs) have been extensively implicated in the pathogenesis of SLE (Rowland and others 2014; Sisirak and others 2014) as a major source of IFN-I, generating up to 1,000 times more than any other cell type (Cella and others 1999; Siegal and others 1999). Acting as phagocytic surveillance sentinels, pDCs are specialized in the detection of nucleic acids and production of IFN-I through high constitutive expression of the endosomal PRRs, TLR7 and TLR9, which engage single-stranded RNA or unmethylated CpG DNA, respectively (Reizis and others 2011). IFN-I produced by pDC is key in initiating protective resistance to infection in peripheral tissues and modulating the activity of other immune cells, as studies in murine models of pDC depletion have shown (Swiecki and Colonna 2010). As such, pDCs bridge the intersection of innate and adaptive immunity, and therefore are strategic targets for the manipulation of autoimmune responses (Swiecki and Colonna 2015). IFN-I is also essential to the function of pDCs, as autocrine/paracrine IFN-I signaling in activated pDCs results in amplification of subsequent IFN-I, inflammatory cytokine production, and modulation of pDC effector functions such as expression of costimulatory molecules and chemokine receptors (Asselin-Paturel and others 2005; Gautier and others 2005; Prakash and others 2005; Siren and others 2005; Liao and others 2010; Lorenzi and others 2011; Oh and others 2011; Bao and Liu 2013; Kim and others 2014; Pantel and others 2014).
Long noncoding RNAs (lncRNAs) (noncoding RNAs >200 nucleotides in length) have emerged as potent regulators of varied cellular processes through diverse mechanisms (Cech and Steitz 2014). lncRNAs are derived from pseudogenes, alternatively transcribed or spliced coding genes, antisense transcription, introns, or standalone intergenic regions (Griffiths-Jones 2007; ENCODE Project Consortium 2012; Derrien and others 2012; Djebali and others 2012). Many lncRNAs share features of coding genes such as promoter-controlled expression, exon splicing, and polyadenylation (Cabili and others 2011; Hung and others 2011). The functional activities of lncRNAs are largely linked to their subcellular localization. In the nucleus, lncRNAs direct epigenetic and transcriptional regulation (Khalil and others 2009; Bonasio and Shiekhattar 2014). In the cytosol, lncRNAs are key participants in posttranscriptional mRNA regulation (Yoon and others 2013), where lncRNA sequence complementarity, conserved RNA binding protein (RBP) motifs, and labile secondary and tertiary structures can influence their selective dynamic associations (Cook and others 2015). In the absence of a requirement to maintain codon integrity, lncRNAs display a high degree of evolutionary permissiveness to mutation, resulting in highly species-specific signatures, with <10% of lncRNA loci conserved between human and mouse (Johnsson and others 2014). While sequence conservation among lncRNA loci across species is low, an increasing appreciation for conservation in lncRNA structural elements is emerging (Nitsche and Stadler 2017). Mutations that disrupt sequence specificity and RBP motifs, or perturb secondary/tertiary structure may alter lncRNA function, and thereby dysregulate processes that define disease outcomes. Indeed, genome-wide association studies demonstrate that the majority of disease-associated variants fall outside protein-coding regions (Ricano-Ponce and Wijmenga 2013; Welter and others 2014), suggesting the functional importance of lncRNAs at the host–pathogen interface and in disease pathogenesis (Kapusta and Feschotte 2014).
lncRNAs are highly lineage specific (Kim and others 2015; Gloss and Dinger 2016), even more so than coding or small noncoding RNA signatures. Such features position them as critical regulators of cell subset differentiation and cell type-specific activities; however, the exact mechanisms through which lncRNAs modulate pDC development and function are unknown. While gene expression profiling in human pDCs has defined cell type-specific coding genes (Waddell and others 2010; Miller and others 2012), a comprehensive description of the long noncoding transcriptional landscape in pDCs has been challenging due to limited numbers of cells and low levels of lncRNA expression. To gain a better understanding of the noncoding transcriptional landscape in activated pDCs, we undertook comprehensive transcriptome profiling in a tractable human pDC line following TLR7 engagement or IFN-I signaling. We identified novel subsets of lncRNA species that are differentially regulated by these innate immune stimuli, neighboring genes that are co-regulated with lncRNAs, and transcriptional regulators likely to coordinate lncRNA expression. We compared these transcriptome profiles with those of activated primary pDCs, demonstrating that these data provide a solid platform for the study of lncRNA expression and their roles in pDC development and effector functions. Further characterization of lncRNAs described in this study will identify new biomarkers and therapeutic targets for disease.
Materials and Methods
Cell culture and reagents
CAL-1 pDCs (a kind gift from Takahiro Maeda) were cultured as previously reported (Steinhagen and others 2013). Cells were rested for 6 h in 0.1% fetal bovine serum (FBS) CAL-1 media before stimulations with 1,000 U/mL recombinant human IFN-β (PBL Interferon Source), 1 μg/mL R848 (InvivoGen) with or without 1 μg/mL recombinant vaccinia virus B18R protein (Thermo Fisher Scientific), or CpG-B (InvivoGen). Stimulated cells were incubated at 37°C for the indicated times; 20 × 106 frozen MUTZ3 progenitor cells were resuspended in 40 mL 20% minimum essential media (MEM)-alpha containing ribonucleosides/deoxyribonucleosides, 20% FBS, and 10% conditioned medium from bladder carcinoma cell line 5638 and cultured at 37°C for 4 days. Differentiation to immature conventional dendritic cell (cDC) was induced by culture at 0.25 × 106/mL MEM-alpha 20% FBS without 10% bladder line media, with the addition of 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (PeproTech), 10 ng/mL interleukin (IL)-4 (Shenandoah Biotechnology), and 2.5 ng/mL tumor necrosis factor-α for 7 days, replacing half medium after 3 days. Cells were then stimulated with 100 ng/mL lipopolysaccharide (LPS; Invivogen) for 12 h. THP-1 monocyte-derived dendritic cells (moDC) were derived and stimulated as previously described (Lim and others 2016).
Primary cell analyses
Primary monocytes and monocyte-derived DCs were obtained, cultured, and stimulated with LPS or Pam2CSK4 as previously described (Lim and others 2016).
Subcellular fractionation
CAL-1 cells were cultured and stimulated as described above for 0, 3, and 12 h. RNA from nuclear and cytoplasmic cellular fractions was isolated from cell lysates using the Ambion PARIS™ system (Thermo Fisher Scientific) as per the manufacturer's instructions.
siRNA knockdowns
CAL-1 pDC were nucleofected with 1 μM siRNAs at 1.2 × 106 cells/well in supplemented SF media from the SF Cell Line 4D-Nucleofector X Kit (Lonza) using an Amaxa nucleofector with 96-well plate strips (Lonza) on program DN-100 as per the manufacturer's instructions. siRNAs were obtained from Integrated DNA Technologies (IDT) targeting lncRNAs using the following sequences: lnc-DC 5′-GAGTTATCTTAAGGATCAT-3′, lnc-1133 5′-CUUGCAGGAAGGAUGGAUUCUCC (CA)-3′, and lnc-ROR 5′-GGAGAGGAAGCCTGAGAGT-3′. Proprietary triplicate siRNA mixes were obtained from Dharmacon targeting lncRNAs: lnc-515 SMARTpool: Lincode LINC00515 siRNA R-189581-00-0005 and lnc-SIPA1L1-2 SMARTpool: Lincode Loc145474 siRNA R189240-00-0005. Nucleofected cells were rested and stimulated with R848 as described above for the indicated times, with RNA collection at 18 h postnucleofection.
RNA isolation, reverse transcription, and quantification of gene expression
Total RNA was extracted by the NucleoSpin RNA extraction kit (Macherey-Nagel) and cDNA was reverse transcribed with the QuantiTect RT kit (Qiagen) according to the manufacturer's instructions. TaqMan qPCR was conducted using the ViiA7 qPCR system (Life Technologies) with the following probe assays obtained from IDT: lnc-DC Hs.PT.58.4925540, lnc-SIP1AL1-2 Hs.PT.58.24273269.g, lnc-ROR Hs.PT.58.40897944, lnc-515 Hs.PT.56a.24878664.g, lnc-578 Hs.PT.58.23294839, and lnc-1133 Hs.PT.58.40059900. Gene expression levels were normalized to HPRT or GAPDH as indicated. Data were analyzed using the QuantStudio real-time PCR software (Applied Biosystems).
RNA sequencing and bioinformatic analysis
CAL-1 cells (1 million cells/mL) were cultured in 0.1% FBS CAL-1 media in triplicate in a 12-well tissue culture plate for 6 h before stimulation with 1,000 U/mL recombinant human IFN-β (PBL Interferon Source) or 1 μg/mL R848 (Invitrogen), or 1 μg/mL R848 (Invitrogen) +1 μg/mL recombinant vaccinia virus B18R protein (Thermo Fisher Scientific) or mock stimulation. Stimulated cells were incubated for 12 h at 37°C and RNA was isolated as described above. RNA integrity was determined using the RNA 6000 Nano Kit with a 2100 bioanalyzer (Agilent Genomics) and quantified using the fluorometric Qubit™ RNA BR assay kit (Invitrogen). cDNA libraries were prepared with the TruSeq Stranded mRNA Library Prep Kit and sequenced on an Ilumina NextSeq 500 sequencer. Library preparation, QC, and sequencing were carried out by Seattle Genomics (www.seattlegenomics.com).
Both the genome sequence (fasta) and gene transfer files (gtf) for human were obtained using igenomes (https://support.illumina.com/sequencing/sequencing_software/igenome.html). Raw RNAseq data (Fastq files) were demultiplexed and checked for quality (FastQC; version 0.11.3). Ribosomal RNA was digitally removed using Bowtie2 (version 2.3.4). Host were mapped to the human genome (GRCh37) using STAR (version 2.5.3a) and converted into gene counts with HTSeq (version 0.6.1). Prior to statistical analysis, genes were filtered with a cutoff of a mean of 5 or greater read counts across all samples using R statistical programming language (version 3.4.3) and “edgeR” (version 3.20.9) using RStudio. Gene counts were normalized using “voom” and statistical analysis of differential expression was conducted using “limma” (version 3.34.8) in R. Fold changes were calculated for R848 over mock, R848 + B18R over mock, and IFN-β over mock with cutoffs for differentially expressed (DE) = >1.0 log2 fold, P < 0.01. Functional analysis of coding gene transcriptional responses was performed using Ingenuity Pathway Analysis.
Transcript annotation
Annotation of lncRNAs was done by filtering transcripts through “transcript biotype” designations and universal nomenclatures were derived from LNCipedia database (Volders and others 2015). Chromosome location, start and end positions, and strand orientation of the lncRNAs were retrieved using “biomaRt” (version 2.34.2) in R. Circos plots were generated using the RCircos (version 1.2.0) package in R.
Nearest neighbor analysis
Nearest neighbor genes for DE lncRNAs were identified with the seq2pathway package in R statistical programming language using RStudio. Functional analysis of nearest neighbor coding genes by gene ontology term enrichment was performed using Enrichr (Chen and others 2013).
Transcription factor enrichment analysis
Transcription factor (TF) enrichment was performed using the ENCODE Chip-seq dataset (wgEncodeRegTfbsClusteredV3.bed) obtained from UCSC (http://hgdownload.soe.ucsc.edu/goldenPath/hg19/encodeDCC/wgEncodeRegTfbsClustered/). The analysis was restricted to the proximal 500 bp upstream of lncRNAs. The TF binding site and lncRNA promoter overlaps were identified using the Bioconductor “GenomicRanges” (version 1.32.3) package in R statistical programming language (version 3.5.0) using RStudio.
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc.) was used for statistical analysis and plotting of reverse transcription–polymerase chain reaction (RT-PCR) data. Significance in gene expression differences is plotted as mean ± standard error of the mean and was determined using 2-tailed unpaired Student's t-test for stimulations versus mock or by one-way analysis of variance across multiple stimulations (P < 0.05, indicated by asterisk *). Pearson correlation analyses and plots were performed using the ggplot package in R statistical programming language using RStudio.
Data dissemination
The data generated in this study are available through the following accession identifiers on the NCBI GEO database (GSE117127). RNA sequencing data from primary monocytes treated with IFN-α for 6 h were retrieved from the NCBI-GEO database under accession number GSE72502 (Hung and others 2015). RNA sequencing data from influenza-infected primary pDC1/2/3 subset were retrieved from NCBI-GEO database under accession number GSE84204 (Alculumbre and others 2018).
Results
TLR7 and IFN-I signals drive broad transcriptional change in human pDCs
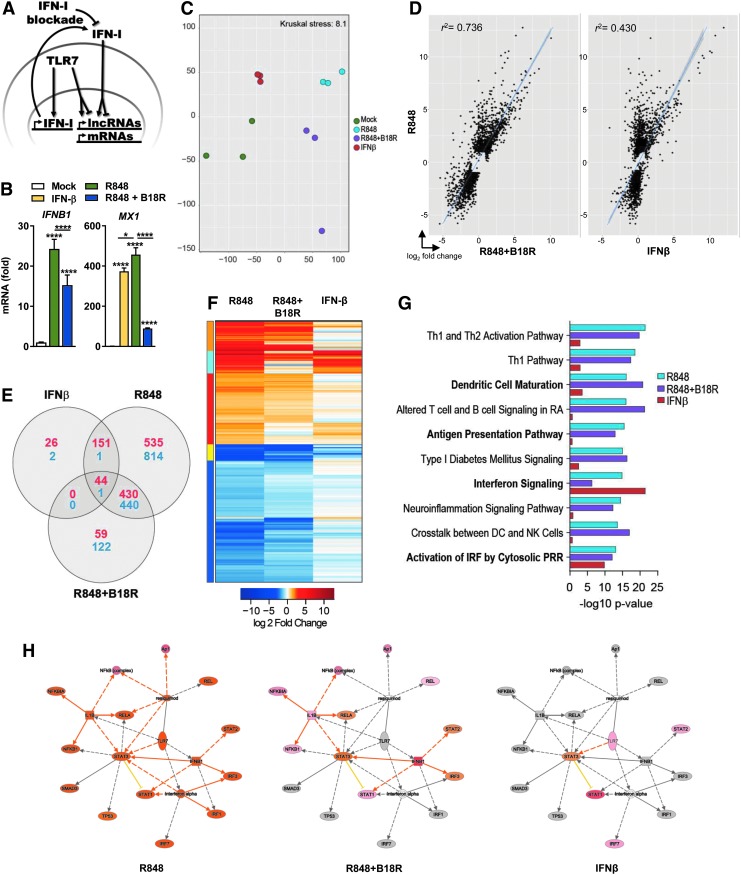
We utilized human CAL-1 cells, which phenotypically and functionally resemble primary peripheral pDCs (Maeda and others 2005; Steinhagen and others 2013), to understand the differences in the response to TLR7 and/or IFNAR ligation. CAL-1 cells were treated for 12 h with the TLR7 agonist R848 in the presence or absence of the pan-IFN-I inhibitory protein B18R (Colamonici and others 1995; Symons and others 1995), or treated with IFN-β, enabling dissection of the relative contributions of TLR and IFN-I signaling to gene expression (Fig. 1A). Treatment with R848 led to a significant induction of IFNB1 expression, which was partially decreased upon blockade of the IFNAR-mediated TLR amplification loop (R848 + B18R) (Fig. 1B left and Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/jir). Expression of the ISG, MX1, was induced by both R848 and IFN-β treatment and its expression was abrogated in the presence of B18R in R848-treated cells, demonstrating efficient IFN-I blockade (Fig. 1B right and Supplementary Fig. S1B, C). Genome-wide transcriptional profiling revealed substantial changes in gene expression in response to TLR ligation and IFN-I (Fig. 1C). Moreover, IFN-I blockade had a noticeable impact in the transcriptional signatures captured after R848 treatment (Fig. 1C). Statistical analysis of gene expression changes yielded 1,899 coding and 709 noncoding significantly differentially expressed genes (DEGs) in response to R848, R848 + B18R, or IFN-β. Within the detected signatures, we observed a high correlation between R848 and R848 + B18R treatment (r2 = 0.736) and moderate correlations between R848 or IFN-β treatments (r2 = 0.430), consistent with the relative exposures to IFN-I and TLR engagement in each set (Fig. 1D). Consistent with this observation, the majority of the transcripts induced after IFN-β treatment were also found to respond to R848 stimulation. However, following IFN-I blockade, the induction of many of these transcripts was abrogated (Fig. 1E). In addition, a contrast in fold change between R848 and R848 + B18R conditions evidences the impact of IFN-I feedback in amplifying TLR-induced gene expression (Fig. 1F). These data demonstrate that although TLR7 and IFN-I activate distinct signaling pathways, IFN-I production amplifies TLR7 signaling in a feed-forward loop, which enhances pDC responses to PRR ligands.
FIG. 1.
Transcriptome sequencing in IFN-I- and TLR7-stimulated human pDCs. (A) Experimental schematic of TLR7 and IFN-I engagement in CAL-1 human pDC line used for transcriptome sequencing. (B) CAL-1 cells were stimulated with TLR7 agonist (R848) with or without blockade of type I IFN signaling by pan-IFN-I blocking protein (B18R) or treated with human IFN-β for 12 h and IFNB1, MX1, or mRNA quantified by RT-PCR normalized to GAPDH. *p < 0.05; ****p < 0.0001. (C) Multidimensional scaling of the whole genome transcriptome profiles from mock, IFN-β, R848, and R848 + B18R-treated CAL-1 pDCs. (D) Scatterplot depictions of correlation in the union of 2,610 DE genes in R848, R848 + B18R, and IFN-β stimulations. (E) Venn diagram depicting overlap across treatment conditions in the numbers of DE transcripts. Red indicates upregulated genes and blue indicates downregulated genes. (F) Heatmap showing unsupervised clustering of 2,610 gene DE over mock in any one condition. (G) Functional analysis of biologic pathways enriched within DE genes across each condition. (H) Ingenuity Pathway Analysis molecular network mapping of DE genes within each condition. IFN-1, type I interferon; TLR-7, Toll-like receptor 7; pDC, plasmacytoid dendritic cell; DE, differentially expressed; RT-PCR, reverse transcription–polymerase chain reaction.
Functional analysis of coding DEGs across these stimulatory conditions revealed enrichment of gene sets pertaining to IFN-I signaling, PRR activation, and dendritic cell (DC) functions (Fig. 1G). Accordingly, we observed enrichment of IFN signaling modules and strong induction of classical ISGs (Supplementary Table S1) by TLR7 and IFN-I stimulations, which were markedly diminished by IFN-I blockade during TLR7 activation. In contrast, enrichment of DEGs related to DC functions such as antigen presentation and lymphocyte engagement was modulated primarily by TLR engagement versus IFN-I (Fig. 1G). However, control of DEG expression regulated by interferon regulatory factors (IRFs) was similar downstream of both IFN-I and TLR stimulations, indicating redundancy in the pathways that control the activity of these factors (Fig. 1G). To better understand the complexity of TLR7-mediated responses, we implemented a regulatory network analysis, which demonstrated robust production of IFN-I (IFN-α and IFN-β), the activation of the signal transducer, and activator of transcription (STAT; STAT1; and STAT3), IRFs, NF-κB (RELA, NFKB1, and NFKBIA), and activator protein 1 (AP-1) (Fig. 1H, left). The strength of these activities was diminished in the absence of IFN-I signaling following TLR7 activation, but the diversity of mediators is preserved (Fig. 1H, middle). In contrast, responses to IFN-I were predicted to be primarily driven by STATs and IRFs, and there was a notable loss of NF-κB and AP1 activity (Fig. 1H, right). These data demonstrate that the stimulus-specific transcriptional response to TLR7 agonists and IFN-I is driven by unique TFs. In addition, the induction of IFN-I further promotes cellular activation, and cytokine production and maturation. Given the consistency of the observed gene expression patterns in CAL-1 cells with those observed in primary human and murine pDCs (Merad and others 2013; Heidkamp and others 2016), this cell line provides a viable platform for the investigation of immune-responsive noncoding RNAs.
TLR7 and IFN-I stimulations induce distinct lncRNA signatures
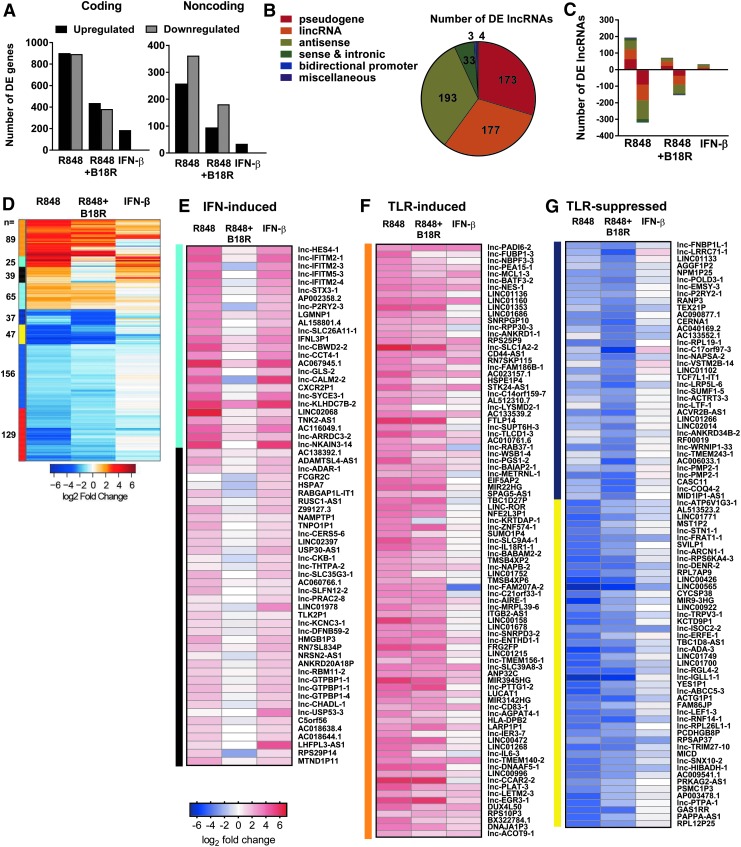
Further characterization of the DEGs modulated in these conditions revealed that roughly one-fourth are noncoding transcripts (Fig. 2A and Supplementary Fig. S2A). Notably, in contrast to nearly equivalent numbers of upregulated and downregulated DEGs among coding loci, noncoding loci display a marked trend toward more genes downregulated than upregulated following TLR7 activation, and IFN-I blockade enhances this phenomenon (Fig. 2A). Among the noncoding transcripts, the majority (n = 588) were identified as lncRNAs, with their distribution across lncRNA biotypes biased toward antisense transcripts (33%), standalone intergenic lincRNAs (30%), and pseudogenes (29%) (Fig. 2B). We did not capture a bias among lncRNA biotypes in the directionality of expression (Fig. 2C and Supplementary Fig. S2B).
FIG. 2.
TLR- and IFN-I-modulated lncRNAs in human pDCs. (A) Distribution of DE coding and noncoding transcripts after stimulation of CAL-1 cells. Distribution of lncRNA biotypes among (B) all lncRNAs DE within any one condition and (C) within each stimulation condition, plotted by directionality of fold change. (D) Heatmap of relative expression of 588 lncRNAs and unsupervised Euclidean distance clustering of transcripts. Annotated heatmaps were generated from modules representing unique immune responsiveness. These were defined as (E) IFN-I-induced lncRNAs (cyan and black clusters, n = 64), (F) TLR7-induced lncRNAs (orange cluster, n = 89), and (G) TLR-suppressed lncRNAs (dark blue and yellow clusters, n = 84). lncRNA, long noncoding RNA.
Unsupervised clustering of lncRNA relative expression yielded unique response modules, identifying lncRNAs under the regulation of either TLR7 or IFN-I (Fig. 2D and Supplementary Table S2). Consistent with the induction of coding ISGs by IFN-I, we identified 64 lncRNAs induced by exogenous IFN-β and whose induction is specifically dependent upon auto/paracrine IFN-I signaling in the context of TLR7 activation, as evidenced by the impact of IFN-I blockade (Fig. 2E). Conversely, we identified 89 lncRNAs which are generally indifferent to IFN-I signaling but are induced strictly by TLR engagement through mechanisms independent of IFN-I feedback (Fig. 2F). We identified a large set of lncRNAs (n = 369) whose expression is inhibited following TLR engagement (Fig. 2D and Supplementary Table S3). Of these downregulated lncRNAs, 84 were markedly downregulated by R848 treatment despite IFN-I exposure (Fig. 2G). In sum, these 588 DE lncRNAs comprise a novel definition of lncRNA sensitivity to TLR and IFN-I stimuli.
Immune-regulatory pathways govern TLR- and IFN-I-modulated lncRNAs and co-expressed neighboring coding genes
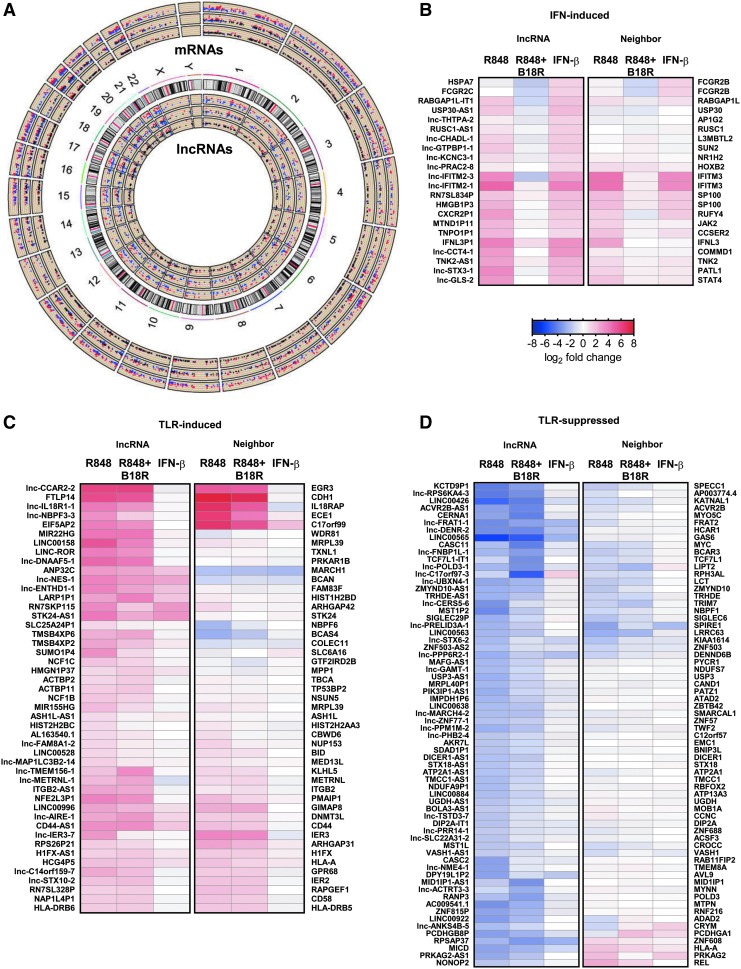
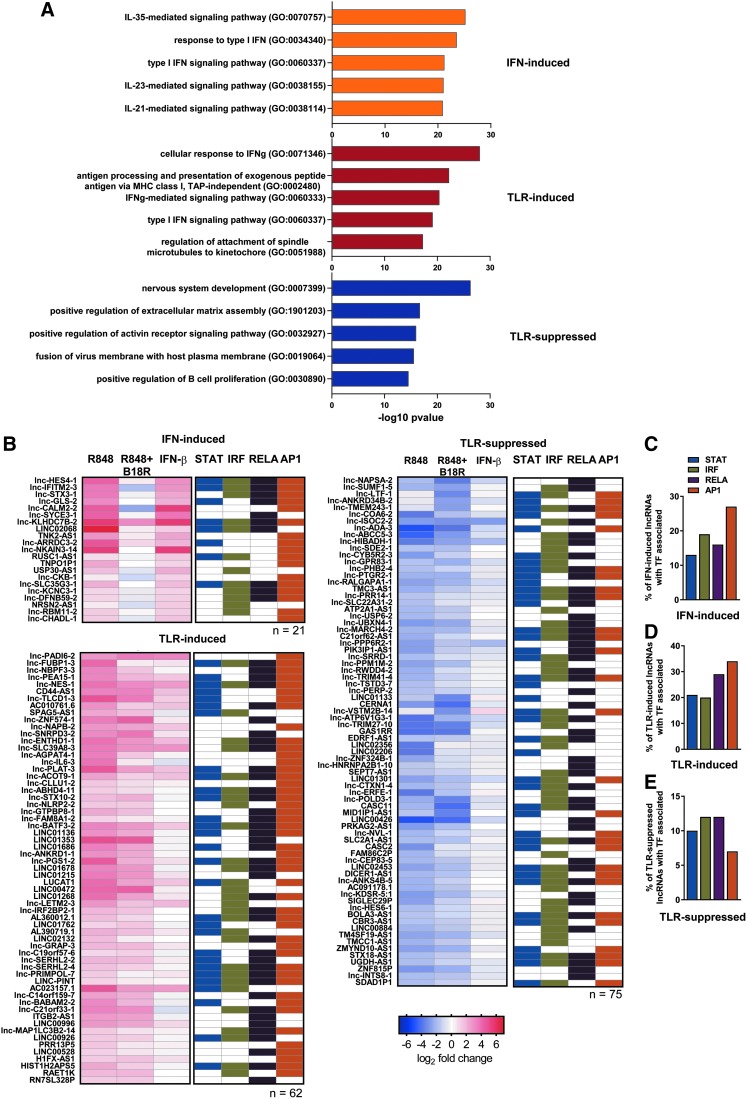
Global analyses of lncRNA and neighboring coding gene expression patterns have revealed modest associations in expression levels (Kutter and others 2012). Patterns of co-expression in IFN-I-induced lncRNAs and nearby coding genes have been identified in murine (Josset and others 2014) and human (Barriocanal and others 2014; Carnero and others 2014; Kambara and others 2014a, 2014b) epithelia and demonstrated for several lncRNA-neighboring ISG pairs (Carnero and others 2014). To better understand the distinct co-regulation of lncRNAs and neighboring genes by TLR7 and IFN-I signaling cascades in human pDCs, we undertook a global co-expression analysis. First, we identified broad genomic distribution across the DE coding genes and lncRNAs (Fig. 3A). Then, we identified the nearest coding neighbor across the previously identified TLR7- and IFN-I-regulated modules. Noticeable concordance in the directionality of gene expression was noted between the IFN-I-induced lncRNAs and their neighboring genes (Fig. 3B). Similar co-expression was observed among the TLR-induced (Fig. 3C) and TLR-suppressed (Fig. 3D) lncRNAs and their neighboring genes, with some examples of inverse directionality, suggesting it is likely that lncRNA expression is transcriptionally regulated by similar factors regulating coding gene expression. To explore the signaling and transcriptional influences responsible for lncRNA-neighbor gene co-expression, we performed functional analysis of the nearest neighbor coding genes. Functional enrichment analysis demonstrated that the coding genes neighboring IFN-induced lncRNAs are involved in the response to IFN-I and STAT activation (IL-35, IL-23, and IL-21 signaling pathways) (Fig. 4A; top). TLR-inducible lncRNAs were in close proximity with not only ISGs but also genes involved in antigen presentation (Fig. 4A; middle). Interestingly, TLR-suppressed lncRNAs were found near genes involved in hormonal regulation and membrane integrity pathways (Fig. 4A; bottom). Given the parallels in functional enrichment between these nearest neighbor coding genes (Fig. 4A) and those observed from the global signatures (Fig. 1G), these IFN-responsive and TLR-responsive lncRNAs are likely under the regulation of the previously identified master regulators of TLR7- and IFNAR-mediated signaling (Fig. 1H).
FIG. 3.
TLR-/IFN-modulated lncRNAs are co-expressed with neighboring coding genes. (A) Circos plot of genomic distribution of all DE mRNAs (outer tracks) and lncRNAs (inner tracks) in CAL-1 pDC treated with R848, R848 + B18R, and IFN-β, respectively. Red indicates upregulated genes and blue indicates downregulates genes. Black denotes expressions values <|1|. Each condition is represented in an individual track, where the outermost track corresponds to R848 treatment and the innermost track corresponds to IFN-β. Heatmaps of DE lncRNAs (left) mapped against log2 fold change in expression of their nearest neighbor genes (right) across each condition for (B) IFN-induced lncRNAs, (C) TLR-induced lncRNAs, and (D) TLR-suppressed lncRNAs.
FIG. 4.
Immune modulatory control of lncRNA expression changes. (A) Combined scores of GO term enrichment of nearest neighbor coding gene sets identified in Fig. 3B–D for IFN- and TLR-induced and TLR-suppressed lncRNAs whose nearest neighbor mapped to a coding gene. Heatmaps of expression change within each stimulatory condition of these (B) IFN- and TLR-induced and TLR-suppressed lncRNAs plotted against matrices of select immune-regulatory TFs, associated within 500 bp upstream of these lncRNA loci, as determined by ChIP-seq (via ENCODE). Color-filled cells indicate association by each TF. Quantification of the proportion of all IFN- or TLR-induced/suppressed lncRNAs that show enrichment by each TF as outlined in (B) for (C) IFN-induced lncRNAs, (D) TLR-induced lncRNAs, and (E) TLR-suppressed lncRNAs. GO, gene ontology; TF, transcription factor.
We leveraged the ENCODE ChIP-seq database to identify TF binding sites upstream of the transcriptional start site of the IFN- and TLR-responsive lncRNA loci. We found an appreciable enrichment of STAT, IRF, NF-κB, and AP-1 binding at sites proximal to a subset of lncRNAs (Fig. 4B). Overall, IFN-induced lncRNAs display enhanced representation of IRF binding sites and AP-1 binding sites (Fig. 4C), while TLR-induced lncRNAs display enhanced representation of NF-κB and AP-1 binding sites (Fig. 4D). In contrast, TLR-suppressed lncRNAs displayed less enrichment of binding sites for these TFs (Fig. 4E), suggesting that TLR activation likely induces additional negative regulators of gene expression. Collectively, these analyses demonstrate the role of TLR- and IFN-I-dependent transcriptional mediators in control of lncRNAs and their neighboring coding gene expression patterns in pDCs, and specifically implicate NF-κB and IRF proteins in their respective transcriptional control. These data suggest that many lncRNAs and their neighboring genes are coordinately regulated by similar canonical TLR- and IFN-I-signaling pathway mediators.
IFN-I- and TLR-modulated lncRNAs have diverse cytoplasmic functions
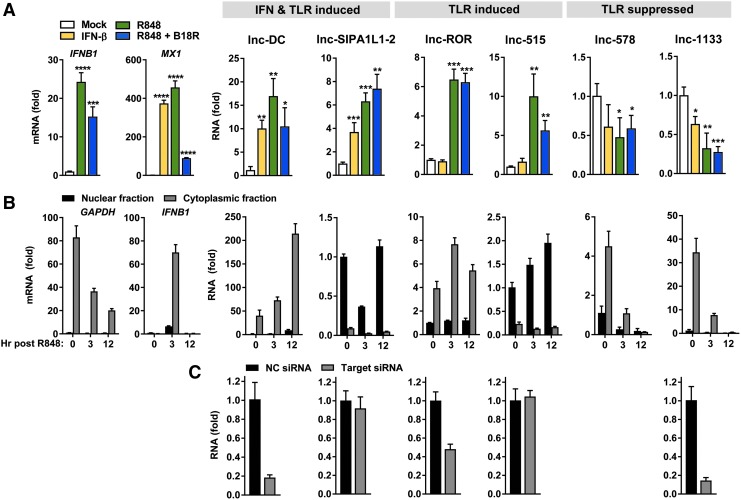
The limited abundance of primary pDCs is prohibitive for investigations of lncRNA function. To test the validity of CAL-1 as a model for exploration of functionally relevant lncRNAs, we screened these transcriptome profiles in conjunction with reports of known lncRNA functions to identify 6 lncRNAs representative of each TLR/IFN modulation group for further study: lnc-DC (WFDC21P, LOC645638), lnc-SIPA1L1-2 (LOC145474), lnc-ROR (LINC-ROR), lnc-515 (LINC00515), lnc-578 (LINC00578), and lnc-1133 (LINC01133) (Table 1). Validation of expression changes by RT-PCR in IFN-β and R848 +/− B18R treated CAL-1 cells revealed lnc-DC and lnc-SIPA1L1-2 displaying IFN-I and TLR inducibility, lnc-ROR and lnc-515 induced only by TLR stimulation, and lnc-1133 and lnc-578 showing significant downregulation following TLR engagement (Fig. 5A). Analysis of transcript modulation kinetics across 18 h following stimulation revealed variable patterns in the timing of inducibility/suppressibility in response to these stimuli, with evident effects of waves of autocrine/paracrine cytokine signaling as well as the influence of translated ISGs in boosting lncRNA levels (Supplementary Fig. S3A). We also tested the response of these lncRNAs to TLR9 agonism with CpG DNA, resulting in expression changes comparable to those observed in TLR7 stimulation (Supplementary Fig. S3B). Modulation in response to TLR2 and TLR4 agonists in myeloid cDC lineages showed similar patterns of change in abundance of these lncRNAs to that of TLR7 stimulation in pDCs, with the exceptions in lnc-ROR and lnc-578 (Supplementary Fig. S3C–E). We found similar patterns of expression in primary human myeloid lineages following TLR or IFN-I stimulation (Supplementary Fig. S3F, G).
Table 1.
Annotation, Genomic Loci, and Various Attributes of 6 Toll-Like Receptor/Interferon-modulated Long Noncoding RNAs Selected for Further Study in CAL-1 Plasmacytoid Dendritic Cell
| lncRNA | Symbol | Gene ID | Ensembl ID | Transcript ID | Location | Exons | Splice variants | bp | Type |
|---|---|---|---|---|---|---|---|---|---|
| Lnc-DC | WFDC21P | 645638 | ENSG00000261040 | NR_030732.1 | Chr 14: 71,487,861–71,489,714 forward strand | 3 | 8 | 447–630 | Pseudogene |
| Lnc-SIPA1L1-2 | LOC145474 | 145474 | N/A | NR_027046.1 | Chr 14: 71,487,861–71,489,714 forward strand | 1 | 1 | 1,854 | Misc ncRNA |
| Lnc-ROR | LINC-ROR | 100885779 | ENSG00000258609 | NR_048536.1 | Chr 18: 57,054,559–57,072,119 reverse strand | 4 | 1 | 2,630 | Intergenic (lincRNA) |
| Lnc-515 | LINC00515 | 282566 | ENSG00000260583 | NR_024092.1 | Chr 21: 25,582,770–25,583,326 reverse strand | 1 | 1 | 557 | Antisense |
| Lnc-578 | LINC00578 | 100505566 | ENSG00000228221 | NR_047568.1 | Chr 3: 177,441,921–177,752,704 forward strand | 4 | 1 | 1,222 | Intergenic (lincRNA) |
| Lnc-1133 | LINC01133 | 100505633 | ENSG00000224259 | NR_038849.1 | Chr 1: 159,961,218–159,984,750 forward strand | 3 | 3 | 1,113, 1,266, 1,996 | Intergenic (lincRNA) |
lncRNA, long noncoding RNA.
FIG. 5.
Analysis of select nuclear and cytoplasmic TLR-/IFN-modulated lncRNAs. (A) RT-PCR analysis of IFNB1, MX1, lnc-DC, lnc-SIP1AL1-2, lnc-ROR, lnc-515, lnc-578, and lnc-1133 transcript levels normalized to GAPDH in CAL-1 pDC following 12 h of mock, IFN-β (1,000 U/mL), or R848 (1 μg/mL) +/− B18R (1 μg/mL) treatments. (B) RT-PCR measurement of GAPDH, IFNB1, and TLR-/IFN-modulated lncRNAs as indicated in (A) in the nuclear and cytoplasmic extracts of CAL-1 stimulated with R848 for 0, 3, or 12 h. Transcript abundance is quantified relative to the lowest expressing sample in each set. (C) Transcript levels of lnc-DC, lnc-SIP1AL1-2, lnc-ROR, lnc-515, and lnc-1133 measured by RT-PCR normalized to GAPDH in CAL-1 cells nucleofected with 1 μM siRNA targeting each lncRNA (Target) or negative control (NC) siRNA for 18 h before harvest. Cells were stimulated with 1 μg/mL R848 for 12 h (lnc-DC, lnc-SIP1AL1-2, and lnc-515) or 3 h (lnc-ROR) before harvest or not stimulated (lnc-1133). (A) and (C) are representative of 3 or more independent experiments and (B) is representative of 2 independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
lncRNAs termed competing endogenous RNA (ceRNA) function as decoys, competing with mRNAs for occupation of RBPs and microRNAs (miRNAs), thereby relieving gene repression or stabilization by these factors (Tay and others 2014). These activities occur within the cytosol, where lncRNAs can also modulate mRNA decay by masking RBP or miRNA binding sites, structurally support mRNA stability, disrupt translational machinery, and control the activities of signaling mediators through occupation of protein interaction or modification sites (Jalali and others 2013; Forero and others 2017). Through their ability to regulate the repressive activities of miRNAs, RBPs, or other enzymes, cytoplasmic lncRNAs are critical determinants of posttranscriptional gene regulation and cellular signaling. Thus, we focused our analysis on lncRNAs within this cytoplasmic niche. We determined the subcellular localization of the 6 validated lncRNAs in CAL-1 pDC by fractionation of nuclear and cytoplasmic compartments, identifying 3 of 6 lncRNAs enriched within each compartment (Fig. 5B). Consistent with prior reports, lnc-DC (Wang and others 2014), lnc-ROR (Wang and others 2013), and lnc-1133 (Wu and others 2017) localize within the cytosol, a finding also evidenced by their sensitivity to RNAi (Fig. 5C). Our demonstration of the amenability of this culture system to RNAi manipulations and the consistency of lncRNA localization and modulation patterns in CAL-1 with reports of their behavior in other tissues and primary cells supports the validity of this model for subsequent lncRNA characterizations.
TLR7-mediated gene expression changes reflect those observed in primary pDCs
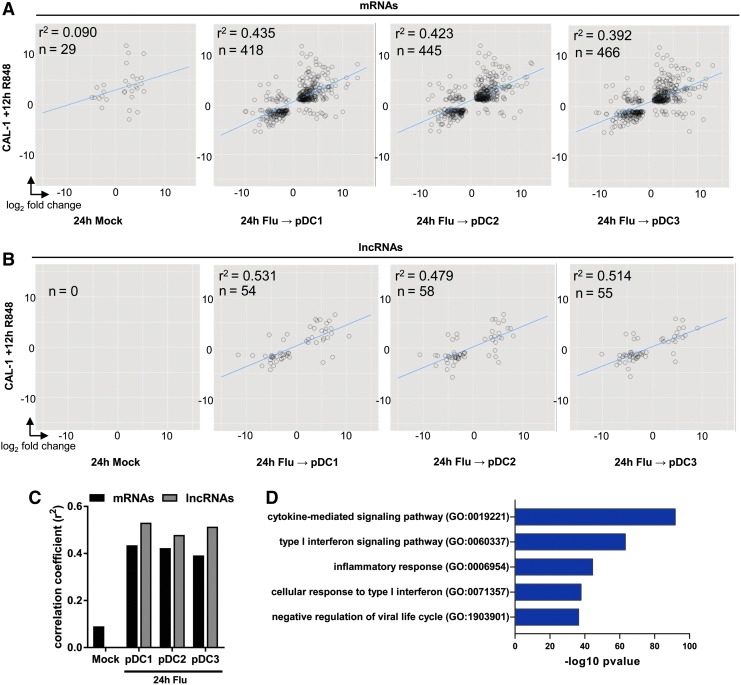
We have identified TLR7-responsive coding and noncoding RNAs, their regulators, and subcellular localization in in vitro pDC cultures. To evaluate whether these observations faithfully recapitulate the transcriptional changes captured in primary human pDCs, we compared CAL-1 gene signatures with those of ex vivo virus-infected pDCs (Alculumbre and others 2018). In vitro infection of pDCs with influenza virus is reported to activate TLR7 to induce IFN-I and proinflammatory cytokines (Cella and others 2000), and was shown to induce differentiation of ex vivo primary human pDCs into subsets that primarily produce IFN-I (“pDC1” which are PD-L1hi CD80lo), those with reduced IFN-I production, but elevated antigen presentation capacity (“pDC3,” which are PD-L1lo CD80hi), or an intermediate phenotype (“pDC2,” which are which are PD-L1hi CD80hi), as measured by flow cytometry (Alculumbre and others 2018). We compared the transcriptome of R848-stimulated CAL-1 with those of in vitro influenza-infected sort-purified primary pDC1, pDC2, and pDC3 cells. We observed a moderate correlation in coding DEG expression between TLR7-stimulated CAL-1 and virus-infected primary pDC subsets (Fig. 6A). More importantly, we found a high to moderate degree of correlation in the expression of DE lncRNAs between CAL-1 and pDC1, pDC2, and pDC3 subsets (Fig. 6B). Correlation of these DEG patterns between mRNAs and lncRNAs reflects concordance between TLR7 ligands and culture systems (Fig. 6C). Notably, we observe higher correlations between DE lncRNAs than mRNAs common to each dataset (Fig. 6C), consistent with the phenomenon of lncRNA signatures more tightly reflecting cellular lineage and activation states than coding gene signatures. Functional enrichment analysis of coding DEGs common to TLR7-stimulated CAL-1 and virus-infected primary pDCs revealed genes involved in inflammatory cytokine and IFN-I signaling, and antiviral responses (Fig. 6D). This analysis demonstrates that the CAL-1 cell line displays similarities with primary human pDCs in their responses to TLR7 agonists.
FIG. 6.
Transcriptomes of CAL-1 pDC correlate with in vitro infected primary pDCs. (A) Scatterplots and Pearson correlations of coding DEGs identified by RNA in CAL-1 pDC treated with R848 versus primary human pDCs cultured ex vivo for 24 h (mock) or infected with influenza virus for 24 h and sort-purified by flow cytometry according to expression of PD-L1 and CD80 into subsets that produce IFN-I (pDC1), present antigen (pDC3), or have an intermediate phenotype (pDC2). DEG values were calculated as R848-fold over mock in CAL-1, and in primary pDC mock as 24-h media over ex vivo, and pDC1/2/3 as 24-h infection over mock per pDC subset. (B) Scatterplots and Pearson correlations of lncRNA DEGs identified in R848-treated CAL-1 across mock and each influenza-infected pDC subset. (C) Bar plot of correlation coefficients listed in (A) and (B). (D) Combined scores of GO term enrichment within coding DEGs identified in (A). DEG, differentially expressed gene.
Discussion
lncRNAs have been implicated in essential processes in immune responses such as control over cytokine and antiviral gene expression, regulation of signal-mediating TFs, and control of PRR activation (Willingham and others 2005; Carpenter and others 2013; Gomez and others 2013; Rapicavoli and others 2013; Bidet and others 2014; Cui and others 2014; Heward and Lindsay 2014; Li and others 2014; Ouyang and others 2014; Turner and others 2014; Wang and others 2014; Chan and others 2015; Liu and others 2015; Atianand and others 2016, 2017; Castellanos-Rubio and others 2016; Hu and others 2016; Tong and others 2016; Jiang and others 2018). Studies of gene expression changes following viral infection have profiled marked changes in lncRNA signatures (Peng and others 2010; Yin and others 2013; Zhang and others 2013; Josset and others 2014; Ouyang and others 2014, 2016; Winterling and others 2014). Other studies have examined the contributions of antiviral cytokines or PRR stimuli as upstream regulators of lncRNA expression (Carpenter and others 2013; Dave and others 2013; Barriocanal and others 2014; Carnero and others 2014; Cui and others 2014; Kambara and others 2014a, 2014b; Ilott and others 2014). However, the mechanisms through which viral infection and inflammatory signals contribute to changes in lncRNA expression are poorly understood.
Given the vast potential for discovery of novel mechanisms of immune response control by lncRNAs, we sought to describe the noncoding transcriptome of human pDCs and develop a model system, whereby functional analyses of lncRNA regulatory processes can be achieved. Collectively, these in vitro studies define the coding and noncoding transcriptome signatures of TLR7- and IFN-I-stimulated human pDCs. We isolated the impact of IFN-I feedback following TLR activation and identify coding and noncoding gene sets sensitive to this influence. Our analysis describes the biotype and genomic distribution of TLR- and IFN-I-modulated lncRNAs, and identifies regional co-expression patterns among DE lncRNAs and nearby coding genes, and their potential transcriptional regulators. This investigation also delineates the concordance in DEG patterns of the CAL-1 line with primary human pDCs. Our characterization of the lncRNA landscape in human pDCs adds a critical element to the growing body of annotation of tissue- and stimulation-specific lncRNA expression profiles and lays a foundation for further study of their functions in immune responses.
Poor conservation of lncRNAs across species and accelerated evolution at these loci warrant comprehensive documentation of expression profiles in human cells (Nitsche and Stadler 2017). High lineage specificity and stimulation-specific expression changes add a further layer of analysis required to fully catalogue human noncoding genomic output. lncRNA expression profiles and individual functional roles have yet to be fully elucidated for the breadth of innate immune stimuli and human cellular subsets. Although coding ISGs have been extensively annotated, noncoding loci were omitted from such studies and very few descriptions of IFN-stimulated lncRNAs exist. Functional examples of lncRNAs induced by TLR activation or IFN-I illustrate the capacity of these molecular players to potently regulate this critical pathway in innate antiviral immunity (Atianand and others 2017). In this study, we present the transcriptome signatures of TLR7- and IFN-I-stimulated human pDCs and identify lncRNAs whose expression is modulated by these stimuli.
In this analysis, we have defined patterns of lncRNA regulation by innate immune TLR activation and IFN-I signaling, subsetting cohorts of transcripts differentially induced or suppressed by TLR or IFN-I signaling. We characterized the genomic distribution of TLR- and IFN-I-modulated lncRNAs and performed an analysis of parallel and inverse expression patterns in their neighboring genes. This analysis revealed co-expression between lncRNAs and neighboring TLR- or IFN-inducible coding genes. This phenomenon may be a product of regulation of these neighboring loci by similar TFs, and our analysis of enrichment of several factors known to control coding gene expression following these stimuli supports this hypothesis. Specifically, we found that IFN- and TLR-induced lncRNA groups are notably enriched for IRF and NF-κB associations, respectively. These analyses suggest that canonical pathways dictating IFN-I- and TLR-driven coding transcriptional changes extend to noncoding loci as well. Co-expression patterns between lncRNAs and coding neighbors are a naturally intuitive phenomenon for lncRNAs derived from alternative splice forms, antisense or intronic transcription of coding genes, as well as those derived from pseudogenes with preserved promoters that parallel their neighboring ancestral ortholog. However, for many of these transcripts, co-expression with a neighboring coding gene may be not only coincidental but also causal, as in the case of antisense lncRNA cis transcriptional control. In addition, co-expressed pseudogenes may contain sequence similarity to their coding orthologues, thereby conferring ceRNA functions that enable relief of repressive elements targeting the coding gene. Thus, the roles of these lncRNA-coding gene neighbor pairs are intriguing points of study and are of particular relevance within contexts of robust transcriptional induction and protein production, such as those of TLR and IFN-I signals. Further study of lncRNAs identified in this analysis, whose expression positively or inversely correlates with that of their coding neighbor, is warranted.
Nuclear lncRNAs within this gene set may act in cis to regulate transcription of their neighboring coding genes (Villegas and Zaphiropoulos 2015). Cytoplasmic lncRNAs co-expressed with a neighboring coding gene may function as ceRNAs for the negative regulatory factors targeting the neighbor gene, thereby facilitating enhanced protein production (Salmena and others 2011). This is especially relevant in the case of pseudogene lncRNAs, which contain high sequence similarity to their coding orthologs (An and others 2017). We validated expression changes of 6 lncRNAs representative of TLR- and IFN-I-modulated signatures and determined their subcellular localization, identifying 3 cytoplasmic regulators whose activities may modulate pDC function. We discovered that expression of lnc-DC, a cytoplasmic noncoding pseudogene specific to DCs and reported to promote STAT3 activity through antagonism of SHP1–STAT3 interaction (Wang and others 2014), is induced following TLR7 and IFN-I stimulation in pDCs and cDCs. Our findings suggest that DCs experience increased sensitivity to STAT3-mediated signals through elevated expression of lnc-DC in the hours subsequent to TLR activation. However, whether this signal-amplifying role might extend to other STAT proteins has not been explored, nor has the contribution of lnc-DC to pDC function been defined. Notably, lnc-DC polymorphisms associate with SLE incidence and levels are diminished in SLE patient blood relative to healthy controls (Li and others 2017), despite elevated IFN-I signaling and pDC activation in this context, indicating possible negative regulatory feedback in chronic exposure states in vivo. We found that a well-described cytoplasmic ceRNA, lnc-ROR, is also induced upon TLR activation in CAL-1 cells. In epithelial tissues, this transcript sponges miR-145 from targets critical to control of pluripotency (Pan and others 2016), suggesting that in pDCs, lnc-ROR may exert ceRNA functions that relieve repression of mRNAs essential to pDC activation. Among the many lncRNAs downregulated following TLR7 stimulation, we identified lnc-1133, a cytoplasmic ceRNA, which has been reported to sponge miRNAs from targets critical to tumorigenesis and proliferation in several cancers (Zhang and others 2015, 2017; Kong and others 2016; Wu and others 2017). We show that in pDC and myeloid lineages, lnc-1133 undergoes rapid and sustained downregulation following TLR stimulation. We did not observe an impact on cell proliferation or viability upon its knockdown (data not shown), thus lnc-1133 may exert other lineage-specific functions in this cell type. Further study of this transcript in hematopoietic lineages and TLR-activated epithelial tissues is warranted to determine the full range of its functions across tissue types.
Restraint of inflammatory responses following TLR engagement is regulated by diverse means, including signaling by anti-inflammatory cytokines as well as transcriptional and posttranscriptional repression, and is critical in limiting tissue damage (Murray and Smale 2012). Several lncRNAs have been shown to function as negative regulators of inflammation (Willingham and others 2005; Yoon and others 2012; Rapicavoli and others 2013; Cui and others 2014; Ouyang and others 2014; Liu and others 2015; Atianand and others 2016; Castellanos-Rubio and others 2016; Jiang and others 2018), including IFN-I production (Kambara and others 2014b; Valadkhan and Gunawardane 2016). In our data set, we observe a trend of substantially more noncoding genes being downregulated than upregulated following TLR stimulation compared to an equivalent distribution of coding DEG directionality. This phenomenon appears in our study to be a broad feature of lncRNAs and is unrestricted to certain lncRNA biotypes. We hypothesize that many of these downregulated transcripts act as negative regulators of cellular processes that are augmented following pDC TLR activation. Relief of the repression enforced by these lncRNAs by their downregulation after TLR activation would facilitate a burst of the cellular activities they restrain. Notably, downregulation of many lncRNAs has been observed in many cancers (Cancer Genome Atlas Research Network and others 2013; Wu and others 2016; Terashima and others 2017; Yang and others 2017), a phenomenon that is often a product of chronic inflammation, as well as following administration of probiotic dietary supplements (Nunez-Acuna and others 2017). While our findings indicate IRF- and NF-κB-driven control of IFN-I- and TLR-inducible lncRNAs, many factors such as miRNA-mediated decay or transcriptional repression may influence the downregulation of lncRNAs following TLR activation. Further study of the factors controlling lncRNA downregulation is warranted and may yield insight into potential transcript functional mechanisms. Many of the downregulated lncRNAs identified in this study may function as a critical brake on inflammatory processes; thus, dysfunction in their upstream regulators might have deleterious consequences due to uncontrolled inflammation. In addition, the TLR7- and IFN-I-induced lncRNAs we have identified could also act as negative regulators of other repressors, as in the case of ceRNAs sponging miRNAs away from their target mRNAs, thereby facilitating increased mRNA stability and translation of inflammatory proteins. We hypothesize that many of the IFN-/TLR-induced and TLR-suppressed lncRNAs identified in this study thus act as critical regulators of the cytokine production, antigen presentation, and migratory activity that ensue once pDCs become activated.
Such regulatory activities have been described for lncRNAs in other pathways such as pluripotency, but in the context of innate immune PRR activation, these roles may contribute to the rapid and robust cytokine production that ensues following ligand engagement. This is particularly relevant to the function of pDCs, whose primary role is production of IFNs and inflammatory cytokines following PRR activation. It has been reported that individual DCs vary greatly in their cytokine production in response to a common stimulus (Shalek and others 2014; Alculumbre and others 2018), and that in pDCs, such individual variability in IFN-I production is independent of the amplifying IFN-I feedback loop (Bauer and others 2016). Differences in the propensity of individual cells to produce inflammatory cytokines could be dictated by the status of regulatory lncRNAs such as those identified in this study, which may be acting as potent repressors of cytokines in otherwise precocious producers. If the activity of such lncRNAs is dysregulated due to polymorphism or other anomalous influences, exaggerated cytokine production in response to infectious or innocuous stimuli may yield the inflammatory foundation upon which the development of autoimmune pathogenesis depends. Further genetic and functional investigations of the lncRNAs identified in this study are thus warranted to identify their regulatory roles in IFN-I and inflammatory cytokine production, or other cellular activities that may contribute to antiviral responses or aberrant pDC activity in systemic autoinflammatory disease.
Our dissection of the contribution of IFN-I to gene expression changes in the context of TLR7 activation uniquely identifies coding and noncoding genes whose upregulation in activated human pDCs critically depends on IFN-I feedback. This phenomenon is reported to be due to the amplification of interferon response factor 7 levels by IFN-I feedback (Prakash and others 2005), which subsequently drives elevated TLR7-dependent gene induction, and our data support this hypothesis (Supplementary Fig. S1E). Disparate activity of these IFN-I-dependent genes may underlie the mechanisms responsible for the effects of IFN-I antagonism therapy in SLE patients (Kirou and Gkrouzman 2013), and the protection from disease pathogenesis provided by IFNAR1−/− or blockade in murine SLE models. Thus, explorations of functional relevance and polymorphic risk factors within these coding and noncoding genes may reveal potential causal mechanisms in SLE and other IFN-I-driven autoreactivities and interferonopathies.
Our description of TLR-/IFN-modulated lncRNAs provides a foundation for identification of novel functional mechanisms and extensive interrogation of potential players in disease. The paucity of pDCs in peripheral blood and the generally low expression levels of lncRNAs are major limitations to functional lncRNA analysis in primary pDCs ex vivo. Through comparison with influenza-infected primary human pDC, we have established that lncRNA signatures in the CAL-1 pDC line resemble primary pDCs and defined their core common elements, demonstrating that this is a suitable model amenable to the biochemical manipulations required for subsequent interrogations of lncRNA function in pDCs. As noncoding loci are sites of evolutionary pressure at host–pathogen interface, we posit that the lncRNA transcripts identified in this study may play vital roles in host defense to viral infection and, when aberrantly expressed or dysfunctional, in autoimmune disease. Further interrogations of the mechanisms utilized by these lncRNAs to regulate pDC function are thus warranted in the in vitro system we have established. Given demonstrated effectiveness of RNA-based interventions in disease (Lieberman 2018), this characterization of the lncRNA landscape during the innate immune response reveals potential novel targets for treatment of viral infection and IFN-driven autoimmunity. We foresee the capacity of lncRNAs defined in this study to act through diverse mechanisms to modulate DC activities, wielding outcome-defining influence during infection and autoimmune disease.
Supplementary Material
Acknowledgments
This work was supported, in part, by R21AI137956 (R.S.), TL1 TR000422 (R.C.J.), and T32 HL007312 (A.F.). We thank the members of R.S. laboratory for helpful discussions.
Author Disclosure Statement
No competing financial interests exist.
References
- Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, Neas B, Mathian A, Koss MN, Stohl W, Kovats S, Jacob CO. 2009. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol 183(9):6021–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alculumbre SG, Saint-Andre V, Di Domizio J, Vargas P, Sirven P, Bost P, Maurin M, Maiuri P, Wery M, Roman MS, Savey L, Touzot M, Terrier B, Saadoun D, Conrad C, Gilliet M, Morillon A, Soumelis V. 2018. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat Immunol 19(1):63–75 [DOI] [PubMed] [Google Scholar]
- An Y, Furber KL, Ji S. 2017. Pseudogenes regulate parental gene expression via ceRNA network. J Cell Mol Med 21(1):185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med 201(7):1157–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atianand MK, Caffrey DR, Fitzgerald KA. 2017. Immunobiology of long noncoding RNAs. Annu Rev Immunol 35:177–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD, Blin J, Braun JE, Gandhi P, Moore MJ, Chang HY, Lodish HF, Caffrey DR, Fitzgerald KA. 2016. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell 165(7):1672–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100(5):2610–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Liu YJ. 2013. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 4(1):40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal M, Carnero E, Segura V, Fortes P. 2014. Long non-coding RNA BST2/BISPR is induced by IFN and regulates the expression of the antiviral factor tetherin. Front Immunol 5:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Dress RJ, Schulze A, Dresing P, Ali S, Deenen R, Alferink J, Scheu S. 2016. Cutting edge: IFN-beta expression in the spleen is restricted to a subpopulation of plasmacytoid dendritic cells exhibiting a specific immune modulatory transcriptome signature. J Immunol 196(11):4447–4451 [DOI] [PubMed] [Google Scholar]
- Bidet K, Dadlani D, Garcia-Blanco MA. 2014. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog 10(7):e1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R, Shiekhattar R. 2014. Regulation of transcription by long noncoding RNAs. Annu Rev Genet 48:433–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL. 2011. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 25(18):1915–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. 2013. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 45(10):1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero E, Barriocanal M, Segura V, Guruceaga E, Prior C, Borner K, Grimm D, Fortes P. 2014. Type I interferon regulates the expression of long non-coding RNAs. Front Immunol 5:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, O'Neill LA, Moore MJ, Caffrey DR, Fitzgerald KA. 2013. A long noncoding RNA mediates both activation and repression of immune response genes. Science 341(6147):789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, Luo X, Bhagat G, Green PH, Schneider R, Kiledjian M, Bilbao JR, Ghosh S. 2016. A long noncoding RNA associated with susceptibility to celiac disease. Science 352(6281):91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR, Steitz JA. 2014. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157(1):77–94 [DOI] [PubMed] [Google Scholar]
- Cella M, Facchetti F, Lanzavecchia A, Colonna M. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol 1(4):305–310 [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5(8):919–923 [DOI] [PubMed] [Google Scholar]
- Chan J, Atianand M, Jiang Z, Carpenter S, Aiello D, Elling R, Fitzgerald KA, Caffrey DR. 2015. Cutting edge: a natural antisense transcript, AS-IL1alpha, controls inducible transcription of the proinflammatory cytokine IL-1alpha. J Immunol 195(4):1359–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma'ayan A. 2013. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. 1995. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem 270(27):15974–15978 [DOI] [PubMed] [Google Scholar]
- Cook KB, Hughes TR, Morris QD. 2015. High-throughput characterization of protein-RNA interactions. Brief Funct Genomics 14(1):74–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl JT, Gray EE, Pestal K, Volkman HE, Stetson DB. 2017. Intracellular nucleic acid detection in autoimmunity. Annu Rev Immunol 35:313–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Xie N, Tan Z, Banerjee S, Thannickal VJ, Abraham E, Liu G. 2014. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol 44(7):2085–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RK, Dinger ME, Andrew M, Askarian-Amiri M, Hume DA, Kellie S. 2013. Regulated expression of PTPRJ/CD148 and an antisense long noncoding RNA in macrophages by proinflammatory stimuli. PLoS One 8(6):e68306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, Lagarde J, Veeravalli L, Ruan X, Ruan Y, Lassmann T, Carninci P, Brown JB, Lipovich L, Gonzalez JM, Thomas M, Davis CA, Shiekhattar R, Gingeras TR, Hubbard TJ, Notredame C, Harrow J, Guigo R. 2012. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22(9):1775–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. 2012. Landscape of transcription in human cells. Nature 489(7414):101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon KB, Wiedeman A. 2012. Type I IFN system in the development and manifestations of SLE. Curr Opin Rheumatol 24(5):499–505 [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414):57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero A, So L, Savan R. 2017. Re-evaluating strategies to define the immunoregulatory roles of miRNAs. Trends Immunol 38(8):558–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201(9):1435–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss BS, Dinger ME. 2016. The specificity of long noncoding RNA expression. Biochim Biophys Acta 1859(1):16–22 [DOI] [PubMed] [Google Scholar]
- Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K. 2013. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 152(4):743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Navajas JM, Lee J, David M, Raz E. 2012. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12(2):125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S. 2007. Annotating noncoding RNA genes. Annu Rev Genomics Hum Genet 8:279–298 [DOI] [PubMed] [Google Scholar]
- Heidkamp GF, Sander J, Lehmann CHK, Heger L, Eissing N, Baranska A, Luhr JJ, Hoffmann A, Reimer KC, Lux A, Soder S, Hartmann A, Zenk J, Ulas T, McGovern N, Alexiou C, Spriewald B, Mackensen A, Schuler G, Schauf B, Forster A, Repp R, Fasching PA, Purbojo A, Cesnjevar R, Ullrich E, Ginhoux F, Schlitzer A, Nimmerjahn F, Schultze JL, Dudziak D. 2016. Human lymphoid organ dendritic cell identity is predominantly dictated by ontogeny, not tissue microenvironment. Sci Immunol 1(6):pii: [DOI] [PubMed] [Google Scholar]
- Heward JA, Lindsay MA. 2014. Long non-coding RNAs in the regulation of the immune response. Trends Immunol 35(9):408–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HH, Schneider WM, Rice CM. 2015. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol 36(3):124–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Gong AY, Wang Y, Ma S, Chen X, Chen J, Su CJ, Shibata A, Strauss-Soukup JK, Drescher KM, Chen XM. 2016. LincRNA-Cox2 promotes late inflammatory gene transcription in macrophages through modulating SWI/SNF-mediated chromatin remodeling. J Immunol 196(6):2799–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, Graham RR, Ortmann W, Criswell LA, Yeo GW, Behrens TW. 2015. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science 350(6259):455–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Wang Y, Kong B, Langerod A, Borresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY. 2011. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43(7):621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, Donnelly LE, Sims D, Lindsay MA. 2014. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun 5:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali S, Bhartiya D, Lalwani MK, Sivasubbu S, Scaria V. 2013. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One 8(2):e53823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang S, Yang Z, Lin H, Zhu J, Liu L, Wang W, Liu S, Liu W, Ma Y, Zhang L, Cao X. 2018. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell 173(4):906.e13–919.e13 [DOI] [PubMed] [Google Scholar]
- Johnsson P, Lipovich L, Grander D, Morris KV. 2014. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta 1840(3):1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josset L, Tchitchek N, Gralinski LE, Ferris MT, Eisfeld AJ, Green RR, Thomas MJ, Tisoncik-Go J, Schroth GP, Kawaoka Y, Manuel de Villena FP, Baric RS, Heise MT, Peng X, Katze MG. 2014. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol 11(7):875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Gunawardane L, Zebrowski E, Kostadinova L, Jobava R, Krokowski D, Hatzoglou M, Anthony DD, Valadkhan S. 2014a. Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Front Immunol 5:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Niazi F, Kostadinova L, Moonka DK, Siegel CT, Post AB, Carnero E, Barriocanal M, Fortes P, Anthony DD, Valadkhan S. 2014b. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res 42(16):10668–10680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta A, Feschotte C. 2014. Volatile evolution of long noncoding RNA repertoires: mechanisms and biological implications. Trends Genet 30(10):439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL. 2009. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 106(28):11667–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Marinov GK, Pepke S, Singer ZS, He P, Williams B, Schroth GP, Elowitz MB, Wold BJ. 2015. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell 16(1):88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kaiser V, Beier E, Bechheim M, Guenthner-Biller M, Ablasser A, Berger M, Endres S, Hartmann G, Hornung V. 2014. Self-priming determines high type I IFN production by plasmacytoid dendritic cells. Eur J Immunol 44(3):807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou KA, Gkrouzman E. 2013. Anti-interferon alpha treatment in SLE. Clin Immunol 148(3):303–312 [DOI] [PubMed] [Google Scholar]
- Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. 2005. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 52(5):1491–1503 [DOI] [PubMed] [Google Scholar]
- Kong J, Sun W, Li C, Wan L, Wang S, Wu Y, Xu E, Zhang H, Lai M. 2016. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett 380(2):476–484 [DOI] [PubMed] [Google Scholar]
- Kutter C, Watt S, Stefflova K, Wilson MD, Goncalves A, Ponting CP, Odom DT, Marques AC. 2012. Rapid turnover of long noncoding RNAs and the evolution of gene expression. PLoS Genet 8(7):e1002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wu GC, Zhang TP, Yang XK, Chen SS, Li LJ, Xu SZ, Lv TT, Leng RX, Pan HF, Ye DQ. 2017. Association of long noncoding RNAs expression levels and their gene polymorphisms with systemic lupus erythematosus. Sci Rep 7(1):15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, Head SR, Burns JC, Rana TM. 2014. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A 111(3):1002–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao AP, Salajegheh M, Morehouse C, Nazareno R, Jubin RG, Jallal B, Yao Y, Greenberg SA. 2010. Human plasmacytoid dendritic cell accumulation amplifies their type 1 interferon production. Clin Immunol 136(1):130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. 2018. Tapping the RNA world for therapeutics. Nat Struct Mol Biol 25(5):357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Hong M, Savan R. 2016. Human IL-22 binding protein isoforms act as a rheostat for IL-22 signaling. Sci Signal 9(447):ra95. [DOI] [PubMed] [Google Scholar]
- Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, Zeng M, Song E. 2015. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 27(3):370–381 [DOI] [PubMed] [Google Scholar]
- Lorenzi S, Mattei F, Sistigu A, Bracci L, Spadaro F, Sanchez M, Spada M, Belardelli F, Gabriele L, Schiavoni G. 2011. Type I IFNs control antigen retention and survival of CD8alpha(+) dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 186(9):5142–5150 [DOI] [PubMed] [Google Scholar]
- Maeda T, Murata K, Fukushima T, Sugahara K, Tsuruda K, Anami M, Onimaru Y, Tsukasaki K, Tomonaga M, Moriuchi R, Hasegawa H, Yamada Y, Kamihira S. 2005. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol 81(2):148–154 [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. 2013. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31:563–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M, Immunological Genome C. 2012. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol 13(9):888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Smale ST. 2012. Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nat Immunol 13(10):916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. 2007. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum 56(11):3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche A, Stadler PF. 2017. Evolutionary clues in lncRNAs. Wiley Interdiscip Rev RNA 8(1) [Epub ahead of print]; DOI: 10.1002/wrna.1376 [DOI] [PubMed] [Google Scholar]
- Nunez-Acuna G, Detree C, Gallardo-Escarate C, Goncalves AT. 2017. Functional diets modulate lncRNA-coding RNAs and gene interactions in the intestine of rainbow trout Oncorhynchus mykiss. Mar Biotechnol (NY) 19(3):287–300 [DOI] [PubMed] [Google Scholar]
- Oh JZ, Kurche JS, Burchill MA, Kedl RM. 2011. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 118(11):3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Hu J, Chen JL. 2016. lncRNAs regulate the innate immune response to viral infection. Wiley Interdiscip Rev RNA 7(1):129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang J, Zhu X, Chen Y, Wei H, Chen Q, Chi X, Qi B, Zhang L, Zhao Y, Gao GF, Wang G, Chen JL. 2014. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe 16(5):616–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. 2016. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem 40(1–2):219–229 [DOI] [PubMed] [Google Scholar]
- Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, Longhi MP. 2014. Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol 12(1):e1001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gralinski L, Armour CD, Ferris MT, Thomas MJ, Proll S, Bradel-Tretheway BG, Korth MJ, Castle JC, Biery MC, Bouzek HK, Haynor DR, Frieman MB, Heise M, Raymond CK, Baric RS, Katze MG. 2010. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. MBio 1(5):e00206-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Smith E, Lee CK, Levy DE. 2005. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J Biol Chem 280(19):18651–18657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapicavoli NA, Qu K, Zhang J, Mikhail M, Laberge RM, Chang HY. 2013. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2:e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. 2011. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol 11(8):558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricano-Ponce I, Wijmenga C. 2013. Mapping of immune-mediated disease genes. Annu Rev Genomics Hum Genet 14:325–353 [DOI] [PubMed] [Google Scholar]
- Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M. 2014. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med 211(10):1977–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8(7):559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. 2011. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell 146(3):353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Shuga J, Trombetta JJ, Gennert D, Lu D, Chen P, Gertner RS, Gaublomme JT, Yosef N, Schwartz S, Fowler B, Weaver S, Wang J, Wang X, Ding R, Raychowdhury R, Friedman N, Hacohen N, Park H, May AP, Regev A. 2014. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 510(7505):363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284(5421):1835–1837 [DOI] [PubMed] [Google Scholar]
- Siren J, Pirhonen J, Julkunen I, Matikainen S. 2005. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol 174(4):1932–1937 [DOI] [PubMed] [Google Scholar]
- Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D'Agati V, Elkon KB, Reizis B. 2014. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med 211(10):1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen F, McFarland AP, Rodriguez LG, Tewary P, Jarret A, Savan R, Klinman DM. 2013. IRF-5 and NF-kappaB p50 co-regulate IFN-beta and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells. Eur J Immunol 43(7):1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. 2010. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev 234(1):142–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. 2011. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol 1(6):463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15(8):471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JA, Alcami A, Smith GL. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81(4):551–560 [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140(6):805–820 [DOI] [PubMed] [Google Scholar]
- Tay Y, Rinn J, Pandolfi PP. 2014. The multilayered complexity of ceRNA crosstalk and competition. Nature 505(7483):344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima M, Tange S, Ishimura A, Suzuki T. 2017. MEG3 long noncoding RNA contributes to the epigenetic regulation of epithelial-mesenchymal transition in lung cancer cell lines. J Biol Chem 292(1):82–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. 2011. Pattern recognition receptors and the innate immune response to viral infection. Viruses 3(6):920–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Gong AY, Zhang XT, Lin C, Ma S, Chen J, Hu G, Chen XM. 2016. LincRNA-Cox2 modulates TNF-alpha-induced transcription of Il12b gene in intestinal epithelial cells through regulation of Mi-2/NuRD-mediated epigenetic histone modifications. FASEB J 30(3):1187–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Galloway A, Vigorito E. 2014. Noncoding RNA and its associated proteins as regulatory elements of the immune system. Nat Immunol 15(6):484–491 [DOI] [PubMed] [Google Scholar]
- Valadkhan S, Gunawardane LS. 2016. lncRNA-mediated regulation of the interferon response. Virus Res 212:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas VE, Zaphiropoulos PG. 2015. Neighboring gene regulation by antisense long non-coding RNAs. Int J Mol Sci 16(2):3251–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders PJ, Verheggen K, Menschaert G, Vandepoele K, Martens L, Vandesompele J, Mestdagh P. 2015. An update on LNCipedia: a database for annotated human lncRNA sequences. Nucleic Acids Res 43(Database issue):D174–D180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell SJ, Popper SJ, Rubins KH, Griffiths MJ, Brown PO, Levin M, Relman DA. 2010. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS One 5(3):e9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, Jiang Z, Xu J, Liu Q, Cao X. 2014. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344(6181):310–313 [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. 2013. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell 25(1):69–80 [DOI] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. 2014. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res 42(Database issue):D1001–D1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG. 2005. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309(5740):1570–1573 [DOI] [PubMed] [Google Scholar]
- Winterling C, Koch M, Koeppel M, Garcia-Alcalde F, Karlas A, Meyer TF. 2014. Evidence for a crucial role of a host non-coding RNA in influenza A virus replication. RNA Biol 11(1):66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wang F, Li HF, Hu YP, Jiang L, Zhang F, Li ML, Wang XA, Jin YP, Zhang YJ, Lu W, Wu WG, Shu YJ, Weng H, Cao Y, Bao RF, Liang HB, Wang Z, Zhang YC, Gong W, Zheng L, Sun SH, Liu YB. 2017. lncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep 18(10):1837–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lyu H, Liu H, Shi X, Song Y, Liu B. 2016. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci Rep 6:31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen L, Gu J, Zhang H, Yuan J, Lian Q, Lv G, Wang S, Wu Y, Yang YT, Wang D, Liu Y, Tang J, Luo G, Li Y, Hu L, Sun X, Wang D, Guo M, Xi Q, Xi J, Wang H, Zhang MQ, Lu ZJ. 2017. Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nat Commun 8:14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Guan D, Fan Q, Su J, Zheng W, Ma W, Ke C. 2013. lncRNA expression signatures in response to enterovirus 71 infection. Biochem Biophys Res Commun 430(2):629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Gorospe M. 2013. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol 425(19):3723–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M. 2012. LincRNA-p21 suppresses target mRNA translation. Mol Cell 47(4):648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu N, Chen X. 2015. A novel long noncoding RNA LINC01133 is upregulated in lung squamous cell cancer and predicts survival. Tumour Biol 36(10):7465–7471 [DOI] [PubMed] [Google Scholar]
- Zhang JH, Li AY, Wei N. 2017. Downregulation of long non-coding RNA LINC01133 is predictive of poor prognosis in colorectal cancer patients. Eur Rev Med Pharmacol Sci 21(9):2103–2107 [PubMed] [Google Scholar]
- Zhang Q, Chen CY, Yedavalli VS, Jeang KT. 2013. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. MBio 4(1):e00596-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.