Abstract
Progesterone is a steroid hormone that plays an important role in the breast. Progesterone exerts its action through binding to progesterone receptor (PR), a transcription factor. Deregulation of the progesterone signaling pathway is implicated in the formation, development, and progression of breast cancer. Next-generation selective progesterone receptor modulators (SPRMs) have potent antiprogestin activity and are selective for PR, reducing the off-target effects on other nuclear receptors. To date, there is limited information on how the newer generation of SPRMs, specifically telapristone acetate (TPA), affect PR function at the molecular level. In this study, T47D breast cancer cells were used to investigate the molecular mechanism by which TPA antagonizes PR action. Global profiling of the PR cistrome and interactome was done with chromatin immunoprecipitation sequencing (ChIP-seq) and rapid immunoprecipitation mass spectrometry. Validation studies were done on key genes and interactions. Our results demonstrate that treatment with the progestin (R5020) alone resulted in robust PR recruitment to the chromatin, and addition of TPA reduced PR recruitment globally. TPA significantly changed coregulator recruitment to PR compared with R5020. Upon conservative analysis, three proteins (TRPS1, LASP1, and AP1G1) were identified in the R5020+TPA-treated group. Silencing TRPS1 with small interfering RNA increased PR occupancy to the known PR regulatory regions and attenuated the inhibition of gene expression after TPA treatment. TRPS1 silencing alleviated the inhibition of proliferation by TPA. In conclusion, TPA decreases PR occupancy on chromatin and recruits coregulators such as TRPS1 to the PR complex, thereby regulating PR target gene expression and associated cellular responses.
Breast cancer is the most common type of cancer and the second leading cause of cancer death among women in the United States. The National Cancer Institute estimates there will be 252,710 new cases in 2017 in the United States (1). The majority of breast cancers are estrogen receptor (ER) positive, and ~65% are ER and progesterone receptor (PR) positive. Progesterone (P4) and progestin (synthetic progesterone) exposure is increasingly recognized as a risk factor for breast cancer. The Million Women Study and Women’s Health Initiative trials showed that progestins such as medroxyprogesterone acetate (MPA) used in combination with estrogen in hormone replacement therapy are associated with increased risk for breast cancer when compared with estrogen alone (2, 3). However, other studies support P4 as having antiproliferative effects in breast cancer cells (4, 5). Mohammed et al. (6) showed that P4 inhibits estrogen-mediated growth of ER+ and PR+ xenografts, breast tumor implants, and cell lines. All together these studies suggest that the role of progestins in breast cancer is complex and context dependent. The actions are determined by tissue-specific effects, timing and delivery of progestins, source of hormones, and crosstalk with other nuclear receptors.
Deregulation of the P4 signaling pathway is implicated in the formation, development, and progression of breast cancer (7–9). P4 and progestins exert their action through binding to PR, a transcription factor that belongs to the nuclear receptor family. Unliganded PR is located in the cytoplasm bound to a multiprotein chaperone complex. Upon ligand-binding, PR dissociates from the chaperone proteins, dimerizes, translocates to the nucleus, and binds specific sites on the chromatin. Classically, PR binds to its cognate sequence, the progesterone response element (PRE), located in the promoter or enhancer regions of its target genes. In addition, DNA-bound PR interacts with transcriptional coactivators and corepressors to regulate gene expression (10, 11). The variability of PR actions is mediated by complex interactions between PR with DNA and coregulatory proteins, and it is also cell and context dependent (10, 12, 13).
Limited progress has been made in the development of PR targeting therapies for breast cancer. P4 antagonists such as mifepristone (RU486) and onapristone (ZK98299) showed tumor inhibitory effects in animal models (14, 15) and tumor remission in patients with breast cancer (16). However, clinical trials were stopped because of liver toxicity, caused by binding and inhibiting other nuclear receptors, such as glucocorticoid receptor (GR) (12, 17). Nevertheless, recent data suggest that blocking PR signaling may be effective for the prevention and treatment of breast cancer. Selective progesterone receptor modulators (SPRMs) have been used for various gynecological applications, such as contraception, emergency contraception, treatment of endometriosis, and uterine leiomyoma (18). Next-generation SPRMs such as telapristone acetate (TPA, CDB-4124) have potent antiprogestin activity and are selective for PR, reducing the off-target effects on other nuclear receptors (19, 20). Recent studies showed that TPA can suppress the development of precancerous lesions and mammary tumors in rats (21). Furthermore, TPA inhibited cell proliferation and induced apoptosis in 1-methyl-1-nitrosourea–induced mammary tumors (22). In the breast cancer cell line T47D, TPA suppressed G1-S phase transition by inhibiting CDK2 and CDK4 expression (21). In addition, Singhal et al. (23) showed that TPA in combination with tamoxifen promoted tumor regression in T47D tumor xenografts. Previously, we demonstrated in human breast cancer cells that TPA decreased cell proliferation and reduced cell cycle progression and inhibited G2/M-associated genes (24). We also identified a panel of 16 genes associated with proliferation in response to the progestin R5020 that were inhibited by TPA treatment (24). In addition, we showed that TPA inhibited tumor formation in mouse mammary glands, opposed the hyperplastic effects of progestin MPA, and decreased tumor burden in rats (25).
Given the complex and at times opposing effects of PR on breast cancer cells, this study aimed at investigating the molecular mechanisms by which TPA antagonizes PR action in breast cancer cells by using global and unbiased approaches.
Materials and Methods
Cell culture and reagents
T47D human breast cancer cells were obtained from ATCC (Manassas, VA; catalog no. HTB-133; RRID: CVCL_0553) (26). MCF7 cells were obtained from Sigma-Aldrich (ECACC; St. Louis, MO; catalog no. 86012803; RRID: CVCL_0031) (27). The cell lines were authenticated by the Northwestern University DNA sequencing core. These cells were maintained in RPMI-1640 (Life Technologies, Carlsbad, CA) media supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA), 1% sodium pyruvate (Life Technologies), and 100 U/mL penicillin/streptomycin (Life Technologies). MCF7 cells were maintained in DMEM (Caisson Laboratories) media supplemented with 10% FBS (Atlanta Biologicals), 1% sodium pyruvate (Life Technologies), and 100 U/mL penicillin/streptomycin. Before all experiments, cells were cultured for 24 hours in phenol-free RPMI 1640 (Thermo Fisher Scientific, Waltham, MA) supplemented with 5% charcoal-stripped FBS and 1% sodium pyruvate and 100 U/mL penicillin/streptomycin and maintained in the same manner for the duration of the experiment. Cell lines were determined to be free from mycoplasma contamination with the Myco-Alert Mycoplasma Detection Kit (Lonza Inc., Basel, Switzerland). Promegestone (R5020) was obtained from PerkinElmer (Santa Clara, CA), mifepristone (RU486) was purchased from Sigma-Aldrich, onapristone (ZK89299) was provided by Dr. Asgi Fazleabas (Michigan State University), and TPA (CDB4124) was provided by Repros Therapeutics (The Woodlands, TX). All chemicals were dissolved in cell culture–grade ethanol and stored at −20°C.
Small interfering RNA transfection
T47D cells were transfected with small interfering RNA (siRNA) with Lipofectamine RNAiMax (Life Technologies) according to the manufacturer’s protocol. ON-TARGETplus SmartPool of siRNA to TRPS1 (catalog no. L-009644-00-0020) and nontargeting control (catalog no. D-001810-10-20) were purchased from GE Dharmacon (Lafayette, CO).
RNA extraction and real-time PCR
RNA was isolated with the RNeasy Plus minikit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. First strand cDNA synthesis was then performed with 2 μg of RNA and M-MLV reverse transcription (Life Technologies). Primer sequences were obtained from previously published data (24), and other primer sequences are listed in an online repository (28). Fold change values were calculated with the comparative Ct method, with GAPDH as housekeeping gene.
Western blot and coimmunoprecipitation
Nuclear and cytoplasmic extracts were prepared with the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Membranes were probed with the following antibodies: PR H-190X (Santa Cruz Biotechnology, Paso Robles, CA; catalog no. sc-7208; RRID: AB_2164331) (29), PR F-4X (Santa Cruz Biotechnology; catalog no. sc-166169; RRID: AB_2166687) (30), TRPS1 (R and D Systems; catalog no. AF4838; RRID: AB_2208891) (31) and β-actin (Sigma-Aldrich; catalog no. A1978; RRID: AB_476692) (32). Immunoprecipitation experiments were performed with 200 μg of nuclear extracts.
Cell proliferation
T47D cells were plated at an initial density of 5 × 103 cells per well in a 96-well plate in media as described above. The cells were treated with vehicle (VC) control (ethanol), R5020, TPA, or R5020+TPA for 24 hours. Cell proliferation was assessed with the Cell Proliferation ELISA bromodeoxyuridine (BrdU) colorimetric kit (Sigma-Aldrich) according to the manufacturer’s protocol, with a 4-hour incubation time for BrdU incorporation.
Chromatin immunoprecipitation
T47D cells were treated with VC (ethanol), R5020, TPA, or R5020+TPA for 30 minutes. Chromatin immunoprecipitation (ChIP) was performed with the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling, Danvers, MA) according to the manufacturer’s instructions. Rabbit IgG (Cell Signaling), p300 (Santa Cruz Biotechnology; catalog no. sc-585; RRID: AB_2231120) (33), and PR (Santa Cruz Biotechnology; catalog no. sc-7208; RRID: AB_2164331) (29) antibodies were used for ChIP. Immunoprecipitated DNA was extracted with the QIAquick PCR purification kit (Qiagen), and DNA was subjected to real-time PCR analysis. Primer sequences were obtained from a previously published study (34). Enrichment was calculated as a percentage of total input DNA. All experiments were performed in triplicate (35).
ChIP sequencing
ChIP sequencing (ChIP-seq) experiments were performed as previously described (35, 36). All experiments were performed in triplicate, and 10 μg of PR (#SC-7208X) antibody was used. Libraries were prepared with Kapa Biosystems Hyper Prep kit (#KK8500) according to the manufacturer’s protocol. 1 ng of DNA was used, and 12-cycle library amplification was performed. Amplified libraries were checked on a Bioanalyzer (Agilent Technologies) and were sequenced on the Illumina NextSeq500 with 75-bp single-end reads. ChIP-seq analysis was performed as previously described (35). Easeq interactive ChIP analysis software (http://easeq.net) was used for visualization (37). The ChIP-seq data have been deposited on the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo/) and can be accessed through Gene Expression Omnibus accession number GSE113607.
Rapid immunoprecipitation mass spectrometry experiments
Rapid immunoprecipitation mass spectrometry (RIME) was performed as previously described (35, 38). T47D cells were grown in phenol-free RPMI media and treated with VC (ethanol), R5020, TPA, or R5020+TPA for 30 minutes. ChIP was performed with the SimpleChIP Enzymatic Chromatin IP Kit (Cell Signaling) according to the manufacturer’s instructions. Three replicates were performed in each of the treatment groups.
CRISPR-Cas9 knockout for PR
In six-well plates 1 × 105 T47D cells were cultured in RPMI media supplemented with 10% FBS, 1% sodium pyruvate to achieve 80% to 90% confluency on the day of transfection. Cells were cotransfected with the PR CRISPR/Cas9 KO plasmid (SC-400198; Santa Cruz) and the PR HDR plasmid (SC-400198-HDR; Santa Cruz) with a Neon electroporation transfection system (Thermo Fisher Scientific) according to the manufacturer’s protocol. Cells were then seeded into 96-well plates as single cells via flow cytometry. Single cell–derived colonies were harvested and genomic DNA was extracted with the Qiagen DNeasy blood and tissue kit (Qiagen). The PR gene was sequenced to confirm loss of sequences from the genomic DNA. Protein levels of PR were measured with immunofluorescent staining and Western blotting to verify PR knockout (KO).
Immunofluorescence
T47D scrambled control (sr-c) cells and CRIPSR PR-KO cells were grown on coverslips in a 12-well plate in complete RPMI medium. Cells were washed, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 5% bovine serum albumin in PBS. Cells were then incubated with anti-PR (Santa Cruz Biotechnology; catalog no. sc-7208; RRID: AB_2164331) (29) followed by anti-rabbit secondary antibody Alexa Fluor 488 (Thermo Fisher Scientific) and mounted with ProLong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific). Images were taken with a Leica DM5000 B microscope.
Statistical analysis
All statistical analysis was performed in Graphpad Prism version 7.0c (Graphpad Software, La Jolla, CA). All data represent the mean ± SEM of a minimum of three independent experiments, and data were considered statistically significant if the P was < 0.05. Statistical analysis was performed as described in the figure legends.
Results
TPA alters genomewide recruitment of PR
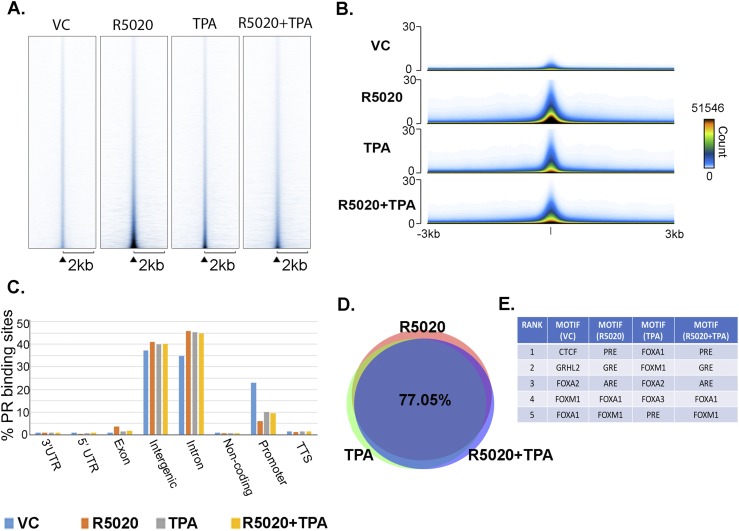
To obtain a global analysis of the effect of TPA on the PR cistrome, PR ChIP-seq was performed. T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 30 minutes, and ChIP-seq was performed in triplicate and normalized to input. The heat map and signal track generated by Easeq (37), as shown in Fig. 1A and 1B, were used to quantitatively demonstrate differences in occupancy throughout the genome. These maps are a collection of peak scores at all sites of occupancy. A heat map of the data set signals (peak score) are shown in Fig. 1A. The y-axis represents the individual positions of the occupancy regions, and the x-axis shows a horizontal window of ±2 kb. Shown in Fig. 1B are the track signals for all data sets. The y-axis shows the superimposed signal of all regions in a horizontal window of ±3 kb. Treatment with R5020 alone resulted in robust PR recruitment to the chromatin compared with VC-treated cells. TPA treatment alone showed an increase in PR occupancy compared with VC-treated cells, indicating the ability of TPA to direct PR to chromatin. However, in the presence of R5020, TPA treatment resulted in reduced PR occupancy compared with R5020-treated cells (Fig. 1A and 1B). These data show that the presence of TPA globally reduces R5020-driven PR recruitment to the chromatin, but alone it can recruit PR to chromatin, albeit at significantly lower levels. Similar to what has been previously reported in T47D cells (39), the majority of PR binding sites were located within introns and intergenic regions (Fig. 1C). In all the data sets, except for the VC-treated group, ~45% of PR binding regions were located in the intronic regions, 40% in intergenic regions, and 10% in promoter regions (Fig. 1C). Furthermore, ~8171, ~19,865, ~19,838, and ~19,892 PR binding regions were identified in VC, R5020, TPA, and R5020+TPA data sets, respectively. When we compared R5020 and R5020+TPA data sets, 83% (18,113) of binding regions overlapped. R5020 and TPA data sets had 82% (17,888) of overlapping regions, and TPA and R5020+TPA data sets had 86.8% (18,466) of overlapping PR regions (28). Comparison of all three data sets, R5020, TPA, and R5020+TPA, showed an overlap of 77.05% of PR binding regions (Fig. 1D). These data indicate that the presence of TPA, whether alone or with R5020, does not alter where PR is recruited on the chromatin. HOMER motif analysis indicated similar motifs enriched between R5020 and R5020+TPA data sets, identifying PRE as the most significantly enriched motif. Interestingly, although the top five enriched motifs were similar in the TPA-only treatment group, FOXA1 was the most significantly enriched motif (Fig. 1E). Collectively, the ChIP-seq data show that R5020 and TPA recruit PR to similar sites on the chromatin and that the presence of TPA globally reduces PR occupancy on the chromatin, without changing PR location on the genome.
Figure 1.
TPA globally reduces PR binding but does not alter location of PR binding. (A) T47D cells were treated with ethanol for the VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 30 minutes. Heat map of aligned reads of PR ChIP-seq data of each treatment were generated by Easeq. Easeq tool was aligned to hg19. The heat map is shown in a horizontal window of 2 kb. n = 3 independent biological replicates. The y-axis represents individual positions of the PR binding regions, and the x-axis represents a surrounding area that corresponds to 6000 bp of each peak’s region and was segmented into 200 bins. Regions were sorted according to decreasing peak score. (B) Easeq visualization of tracks of the peak intensity of PR ChIP-seq data of each treatment group. A bar showing the relationship between coloring and the overlaid signal can be seen on the right side of the plot. (C) VC, R5020, TPA, and R5020+TPA treatment group peaks were annotated, and the distribution of PR binding regions is displayed. (D) Venn diagram shows the overlap of PR binding sites between R5020, TPA, and R5020+TPA groups. (E) Enriched motifs and top five motifs are shown for each treatment.
Validation of the effect of TPA on PR recruitment
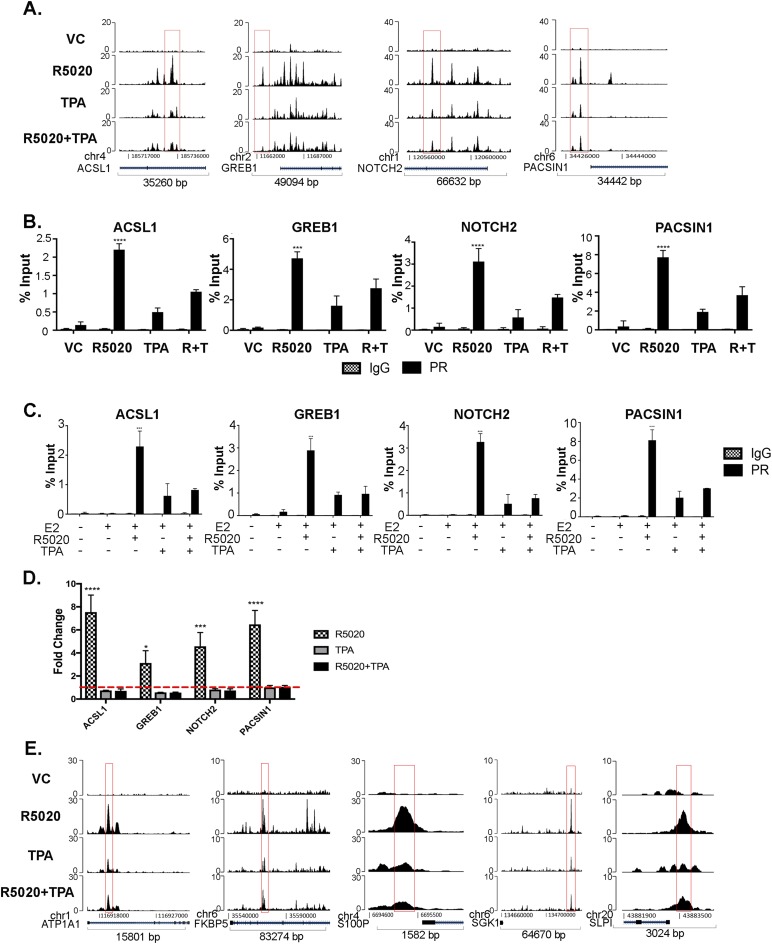
It was previously shown by Clarke and Graham (34) that PR was recruited to regulatory regions of ACSL1, NOTCH2, PACSIN1, and GREB1 genes. Recruitment of PR to these sites was visualized with Easeq tracks from our ChIP-seq data (Fig. 2A), and quantitative peak scores are shown in an online repository (28). In response to R5020, PR occupied these regulatory regions as previously reported. TPA alone also recruited PR to these sites but at lower levels. In response to R5020+TPA, a significant reduction of PR was observed compared with R5020 alone. There was no recruitment of PR in the VC-treated group at these sites. These sites were further validated by PR ChIP quantitative real-time PCR. T47D cells were treated with VC, R5020, TPA, and R5020+TPA for 30 minutes. As shown in Fig. 2B, PR occupancy was significantly increased upon R5020 treatment and decreased at these sites with the addition of TPA.
Figure 2.
TPA reduces recruitment of PR at specific PR target regions. (A) Easeq track visualization of PR recruitment to ACSL1, GREB1, NOTCH2, and PACSIN1 gene regions. ChIP-seq data were aligned to hg19. (B) T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, and R5020+TPA for 30 minutes and subjected to ChIP assay with anti-PR and IgG as control. PR binding regions identified by ChIP-seq were validated by direct PR ChIP by quantitative real-time PCR, by use of previously published primers specific to binding regions. The data are represented as percentage input, mean ± SEM from three independent experiments. ***P < 0.0005; ****P < 0.0001. (C) T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, and R5020+TPA in the presence of 1 nM E2 for 30 min and subjected to ChIP assay with anti-PR and IgG as control. The data are shown as percentage input, mean ± SEM from three independent experiments. ***P < 0.0005; ****P < 0.0001. (D) T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, and R5020+TPA for 24 hours. RNA was extracted and real-time PCR analysis was performed. Primer sequences are in an online repository (26). The data are represented as fold change of VC treatment, mean ± SEM from three independent experiments. *P < 0.05; ***P < 0.0005; ****P < 0.0001. (E) Easeq track visualization of PR recruitment near candidate genes that were significantly upregulated by R5020 treatment and blocked by TPA treatment. ChIP-seq data were aligned to hg19.
As a comparison with TPA, PR ChIP experiments were repeated in T47D cells with other SPRMs, RU486 (mifepristone), and ZK89299 (onapristone). As shown in an online repository (28), we observed similar results to that of TPA in that RU486 and ZK89299 treatment in the presence or absence of R5020 decreased PR occupancy compared with R5020 treatment alone. Given the intricate crosstalk between ER and PR (6, 8, 23, 39), we next tested the effect of estradiol (E2) on PR binding to chromatin. As shown in Fig. 2C, PR occupancy was not altered in the presence of 1 nM E2, at least in the timeframe of treatment tested and the specific sites examined. Next, expression of ACSL1, GREB1, NOTCH2, and PACSIN1 genes was assessed upon treatment with VC, R5020, TPA, or R5020+TPA for 24 hours. As predicted, R5020 significantly increased the expression of ACSL1, GREB1, NOTCH2, and PACSIN1 genes, and the addition of TPA treatment significantly decreased R5020-driven expression of these genes (Fig. 2D).
Previously, we identified seven genes (S100P, SLPI, FKBP5, MAFB, TRNP1, ATP1A1, and SGK1) that were significantly upregulated by R5020 and antagonized by TPA in T47D cells (24). We identified PRE sites in the neighboring regions of these genes by using IGVTools (IGV, Broad Institute). As shown in Fig. 2E, recruitment of PR to these sites increased upon R5020 treatment in five genes. The addition of TPA reduced PR occupancy at these sites, visualized by Easeq, and quantitative peak scores are shown in an online repository (28).
Next, regulatory regions of the previously reported P4-driven proliferation signature genes (24) were examined for PR occupancy. The ChIP-seq data showed modest PR recruitment to proximal regulatory regions of these genes with R5020 treatment, which was not affected upon addition of TPA, as visualized with Easeq (28). These data suggest that the decreased expression of the 16 P4-driven proliferation genes by TPA involves other distal regulatory elements or differential cofactor recruitment, which warrant additional investigation. Collectively, these results indicate that TPA reduces R5020-driven PR recruitment in specific regions but does not significantly alter PR recruitment in other regions, suggesting multiple mechanisms of action.
RIME identifies TRPS1 as a PR corepressor
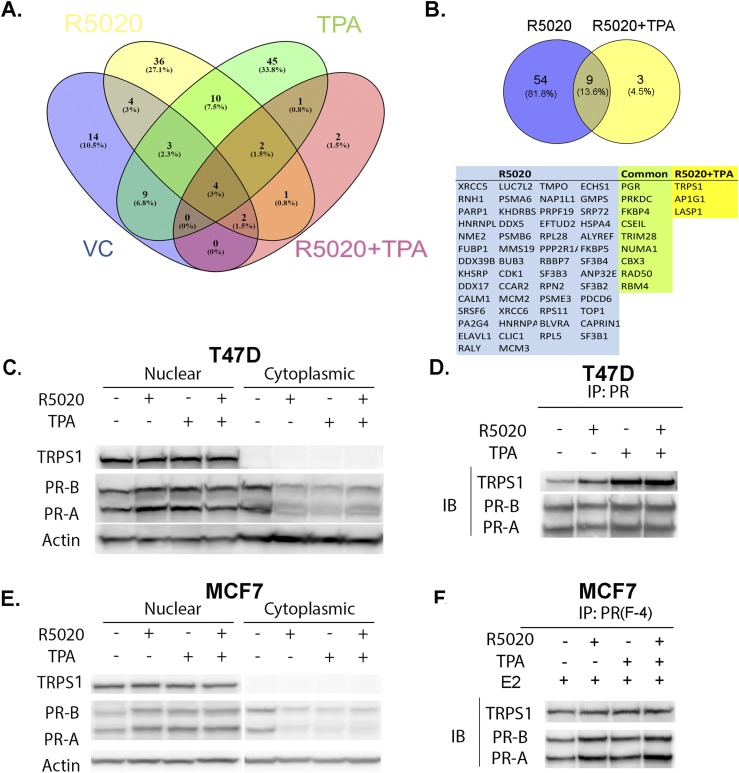
To identify the coregulators that are recruited to PR sites by TPA, ChIP mass spectrometry was performed. This method has been successfully used to identify cofactors of ER in breast cancer cells (38) and PR in endometrial cancer cells (35). A study by Edwards (10) showed that SPRMs that do not affect PR binding to DNA induce conformational changes in the AF-2 region to block the association of coactivators and promote corepressor interactions. Thus, we hypothesized that TPA influences cofactor binding to PR and modulates its transcriptional activity. T47D cells were treated with VC, R5020, TPA, and R5020+TPA for 30 minutes, followed by ChIP for PR, and interacting proteins were analyzed by mass spectrometry. Stringent conditions were set for identification of bona fide interacting proteins, in that proteins were detected in three independent replicates, excluded if proteins appeared in the IgG control group, and a cutoff of peptide spectrum match of at least twofold difference was used for comparing the IgG and PR groups. As a result, 37 (VC), 63 (R5020), 73 (TPA), and 12 (R5020+TPA) proteins were identified (28). PR was identified in all the data sets as one of the top-scoring proteins. A comparison between all four treatment groups is shown in Fig. 3A. When comparing R5020 and R5020+TPA groups, we identified three proteins (TRPS1, LASP1, and AP1G1) that were detected in the R5020+TPA group. In addition, TRPS1 was also present and identified in the TPA-only treatment group when compared with R5020-only treatment. Because TRPS1 has been shown to be a transcriptional corepressor and is overexpressed in the majority of human breast cancers, this protein was chosen for further analysis.
Figure 3.
ChIP mass spectrometry analysis identifies TRPS1 as a PR interacting protein. (A) Venn diagram shows the distribution and overlap of the identified proteins complexing with PR in the VC, R5020, TPA, and R5020+TPA treatment groups (IgG-bound proteins are excluded), n = 3 independent replicates. (B) Venn diagram shows unique and common proteins identified in R5020 or R5020+TPA treatment groups, and the list of the proteins is provided. (C) T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. Nuclear and cytoplasmic extracts were isolated and immunoblotted for TRPS1, PR, and actin. (D) T47D cells were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. Nuclear extracts were prepared and immunoprecipitated with PR and immunoblotted for TRPS1 and PR. (E) MCF7 cells were pretreated with E2 (1 nM) for 24 hours and treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. Nuclear and cytoplasmic extracts were isolated and immunoblotted for TRPS1, PR, and actin. (F) MCF7 cells were pretreated with E2 (1 nM) for 24 hours and treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. Nuclear extracts were prepared and immunoprecipitated with PR and immunoblotted for TRPS1 and PR. IB, immunoblot; IP, immunoprecipitation.
To confirm the RIME analysis and validate the interaction between PR and TRPS1, coimmunoprecipitation was performed after treatment with VC, R5020, TPA, or R5020+TPA for 24 hours. As shown in Fig. 3C, ligand-bound PR relocates to the nucleus. TRPS1 is also detected in the nuclear extracts only. The nuclear extracts were then immunoprecipitated with PR and immunoblotted with TRPS1. An increase in PR and TRPS1 complex with TPA and R5020+TPA compared with VC or R5020 treatment was observed (Fig. 3D), indicating a favorable interaction of TRPS1 with PR in the presence of TPA treatment. The experiment was repeated in the luminal breast cancer cell line MCF7. In comparison with T47D cells, MCF7 cells expressed less PR, and therefore MCF7 cells were pretreated with E2 for 24 hours to increase PR expression. As shown in Fig. 3E, similar to T47D cells, ligand-bound PR relocated to the nucleus and TRPS1 was detected in the nuclear extracts only. We observed that under these conditions, interaction between PR and TRPS1 occurred (Fig. 3F), but unlike in T47D cells, there was no enrichment in binding of these two factors when they were treated with TPA. In addition, coimmunoprecipitation experiments were performed in T47D cells treated with RU486 and ZK89299. As shown in an online repository (28), there was interaction between PR and TRPS1 upon treatment with RU486 and ZK89299; however, there were no obvious differences in the level of interaction between the different treatments. Overall, these data demonstrate that PR and TRPS1 interact in T47D and MCF7 cells, with TPA favoring this interaction in T47D cells.
TRPS1 influences PR action
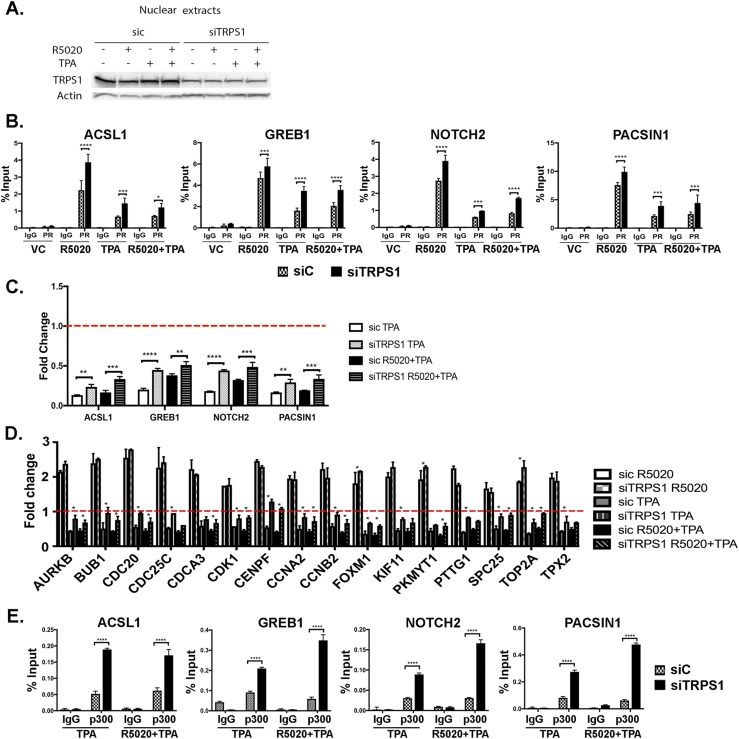
To determine the role of TRPS1 in regulating PR occupancy and transcriptional activity, TRPS1 was knocked down with siRNA in T47D cells followed by hormone treatments. Successful knockdown of TRPS1 was measured by Western blot (Fig. 4A) (28). Upon TRPS1 knockdown, PR occupancy on regulatory regions of ACSL1, GREB1, NOTCH2, and PACSIN1 was increased, as shown by PR ChIP (Fig. 4B), suggesting that TRPS1 hinders PR binding to these regulatory regions.
Figure 4.
Knockdown of TRPS1 increases PR occupancy and increases the expression of PR-regulated genes. T47D cells were transfected with siRNA to either control (sic) or TRPS1. After transfection, cells were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. (A) Nuclear extracts were harvested and immunoblotted for TRPS1 and actin. (B) ChIP assay was done with anti-PR and IgG as control after 30 minutes of treatment. (C) RNA was extracted and real-time PCR analysis was performed. Primer sequences are in an online repository (26). The data are represented as fold change of VC treatment (dotted red line), mean ± SEM from three independent experiments. **P < 0.01; ***P < 0.0005; ****P < 0.0001. (D) RNA was extracted and real-time PCR analysis was performed. Primer sequences are from previously published data (24). The data are represented as fold change of VC treatment (dotted red line), mean ± SEM from three independent experiments. *P < 0.05. (E) After 30 minutes of hormone treatment, ChIP was done with antip300 or IgG as control. All data are represented as the mean ± SEM from three independent experiments. *****P < 0.05; **P < 0.001; ***P < 0.0005; ****P < 0.0001.
Next, the influence of TRPS1 on PR-mediated gene expression in the presence of TPA was assessed. TPA inhibited the R5020-mediated expression of ACSL1, NOTCH2, PACSIN1, and GREB1 genes in siCONTROL (siC) transfected cells, whereas knockdown of TRPS1 resulted in a rescue of this inhibition (Fig. 4C). Knockdown of TRPS1 did not affect PR-mediated gene expression in the R5020-only treatment (28). Expression of the 16 P4-driven proliferation signature genes increased upon TRPS1 knockdown with TPA compared with siC cells treated with TPA (Fig. 4D). These data show that TRPS1 plays an active role in TPA-mediated PR regulation of gene expression.
Given that p300 is a coactivator of PR that is recruited to functional regulatory regions (6), the effect of TRPS1 on p300 recruitment to PR was assessed. TRPS1 was knocked down by siRNA, and ChIP for p300 was performed in T47D cells treated with TPA alone or R5020+TPA. TRPS1 knockdown significantly increased p300 binding on the regulatory regions tested (Fig. 4E), suggesting that TRPS1 hinders coactivator or the transcriptional machinery binding at these PR regulatory sites upon TPA treatment. Additionally, the increased PR recruitment upon TRPS1 knockdown (Fig. 4B) could have promoted the increase in p300 recruitment (Fig. 4E).
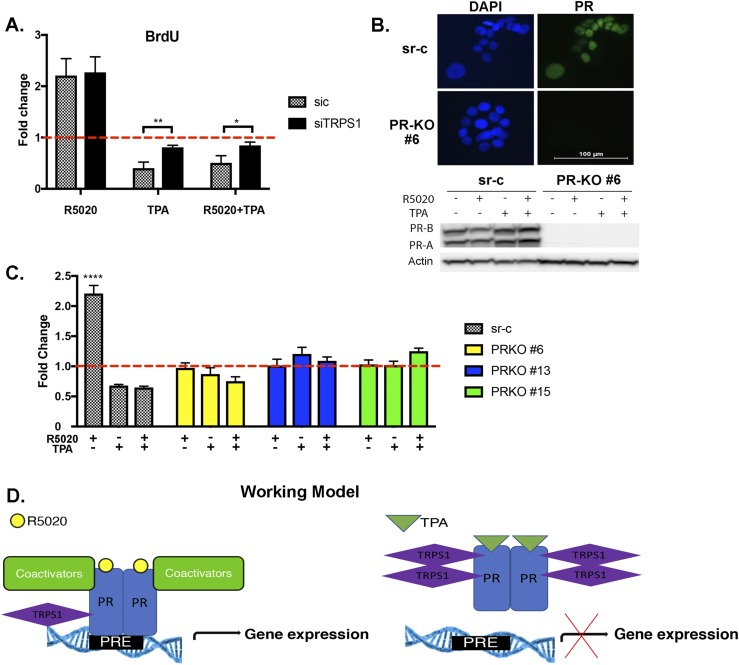
TRPS1 mediates TPA-dependent inhibition of cell proliferation
Gene ontology analysis of nearby genes of the PR-occupied sites revealed that cell cycle and proliferation pathways were enriched in R5020 and R5020+TPA groups. These data are in line with our previous gene expression analysis in T47D cells, which showed that blocking PR signaling by TPA resulted in decreased G2/M cells (24). To examine the role of TRPS1 in cell proliferation, a BrdU assay was performed in siC and siTRPS1 transfected T47D cells, treated with VC, R5020, TPA, or R5020+TPA for 24 hours. As expected, R5020 increased cell proliferation in control cells, and knockdown of TRPS1 did not affect this response (Fig. 5A). However, whereas TPA and R5020+TPA treatment decreased cell proliferation compared with R5020 treatment, knockdown of TRPS1 alleviated this decrease, resulting in an increase in cell proliferation (Fig. 5A). To examine whether the observed effect of TRPS1 on cell proliferation was dependent on PR, T47D CRISPR PR KO clones were generated and used. PR KO was confirmed by immunofluorescence and immunoblotting (Fig. 5B) (28). CRISPR sr-c and PR KO clones (#6, #13, and #15) were treated with VC, R5020, TPA, and R5020+TPA for 24 hours, and cell proliferation was assessed by BrdU assay. As shown in Fig. 5C, cell proliferation in PR KO clones did not change with any of the treatments. In fact, the increase in cell proliferation with R5020 did not occur, and TRPS1 did not increase proliferation either, demonstrating the central role of PR in TPA-mediated attenuation of proliferation.
Figure 5.
TRPS1 regulates cell proliferation, which is PR-dependent. (A) T47D cells were transfected with either control (siC) or TRPS1 siRNA. After transfection, cells were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours and subjected to BrdU assay. The data are represented as fold change of VC treatment (dotted red line), mean ± SEM from three independent experiments. *P < 0.05; **P < 0.001. (B) PR levels were measured in CRISPR sr-c and PR knockout (PR-KO) clone #6 with immunofluorescent staining of PR (green) and DAPI (blue) to visualize the nuclei. Immunoblotting was done to monitor levels of PR in the sr-c and PR-KO cells upon treatment with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours. Successful KO of PR is shown. (C) CRISPR sr-c and PR-KO clones #6, #13, and #15 were treated with VC, 10 nM R5020, 1 μM TPA, or R5020+TPA for 24 hours and subjected to BrdU assay to measure proliferation. The data are represented as fold change of sr-c VC treatment (dotted red line), mean ± SEM from three independent experiments. *****P < 0.0001. (D) Working model demonstrates a possible mechanism of TPA on PR action.
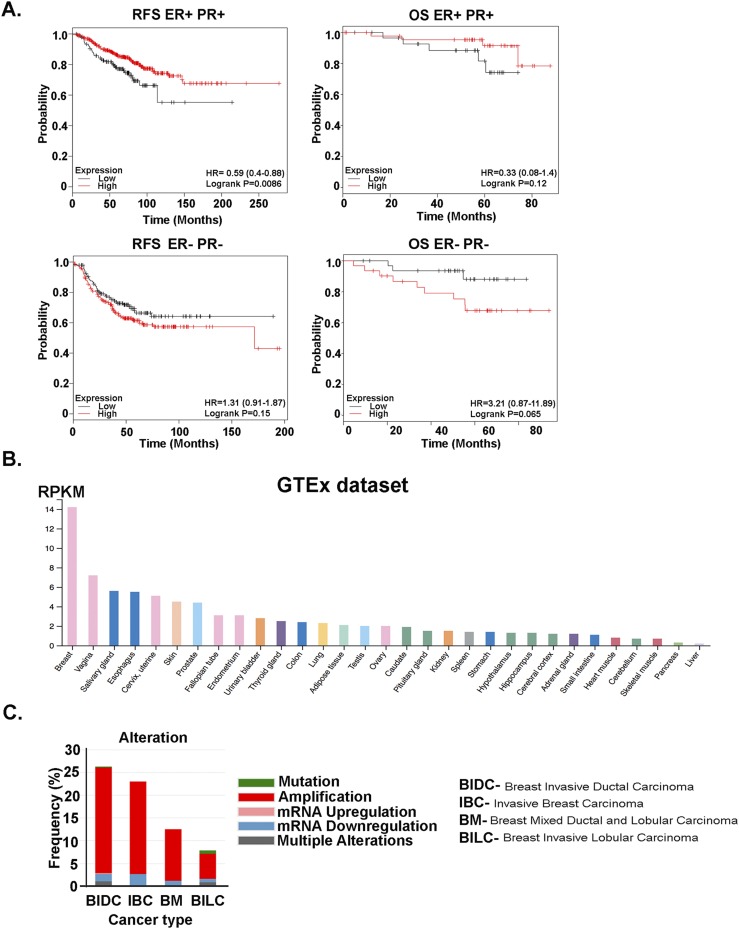
TRPS1 alterations are common in breast cancer
Given that TRPS1 has been shown to be overexpressed in majority of human breast cancers (40, 41), we explored the relevance of TRPS1 in breast cancer survival. We used an online survival analysis tool (42) and assessed breast cancer survival that correlated with TRPS1 mRNA expression. Specifically, the cohorts were divided into two groups according to the median (or upper/lower quartile) expression of the particular gene. Bonferroni multiple testing correction was applied for generating the P value. Using this database (kmplot.com), patients with breast cancer with high TRPS1 mRNA levels had increased overall survival (OS) and relapse-free survival (RFS) compared with patients with low TRPS1 (Fig. 6A). This increased OS and RFS was observed only in ER- and PR-positive breast cancer but not in ER- and PR-negative cancers (Fig. 6A). Examination of the Human Protein Atlas (proteinatlas.org) revealed that TRPS1 mRNA expression was highly expressed in breast tissue compared with all the other organs and tissues (Fig. 6B) (43). In addition, the Breast Invasive Carcinoma TCGA data (44) on cbioportal.org were examined for TRPS1 genetic alterations. As shown in Fig. 6C, TRPS1 amplification is the most common type of alteration, with some rare mutations in different types of breast cancer.
Figure 6.
TRPS1 alterations are common in breast cancer. (A) Kaplan-Meier plots show TRPS1 mRNA levels associated with RFS and OS in patients with ER+ PR+ (top plots) and ER− PR− (bottom plots) breast cancer. (B) Tissue-specific mRNA expression of TRPS1 from the Human Protein Atlas is shown. RNA-seq data are reported as median reads per kilobase per million mapped reads (RPKM), generated by Genotype-Tissue Expression (GTEx) project. (C) Alterations of the TRPS1 gene in the breast cancer TCGA data set are shown.
Discussion
Our study demonstrates that TPA globally decreases recruitment of PR to the chromatin and recruits corepressor TRPS1 to the PR complex, where it is functionally linked with the transcriptional output of the PR complex, as depicted in our working model (Fig. 5D). We observed that TRPS1 is a critical regulator of PR in TPA-treated breast cancer cells and regulates PR target gene expression in this comprehensive analysis of the global effects of TPA on the PR cistrome. TPA alone was able to recruit PR to the same sites as the agonist R5020, albeit to significantly lower levels. Edwards et al. (45) reported that type I PR antagonists such as onapristone (ZK 98299) prevent PR from relocating to the nucleus and prevent PR binding to the chromatin. On the contrary, type II PR antagonists such as mifepristone (RU486) promote receptor dissociation from chaperone proteins such as HSP90, dimerization, and the interaction with DNA, and its ability to inhibit PR action is downstream of DNA binding. The same group showed that when PR is bound to mixed ligands (agonist and antagonist), PR forms heterodimers, thereby reducing the binding affinity to DNA (46). In our studies, the SPRMs TPA, mifepristone, and onapristone inhibited the R5020-mediated occupancy of PR on chromatin (Fig. 2B) (28). The mechanisms by which the SPRMs inhibit this binding may differ. Similar to what has been reported (45), onapristone did not change the cytoplasmic and nuclear content of PR, whereas RU486 and TPA favored nuclear PR (Fig. 3C) (28). The decrease in PR occupancy with the SPRMs combined with R5020 could be a result of the inhibition of translocation to the nucleus, mixed agonist/antagonist heterodimers of PR reducing binding to DNA, or a general change in conformation of PR that would affect residence time on the chromatin or promote weaker binding to sites on chromatin.
Because agonists (progestins) and antagonists (RU486) induce distinct conformational changes in PR structure (47–49), it would be of interest to determine how TPA changes conformation of PR. Studies have characterized the binding of another SPRM, ulipristal acetate (UPA), to PR, as well as other receptors including androgen receptor, GR, and mineralocorticoid receptor. Using the crystal structure of the ligand-binding domain (LBD) of PR, Petit-Topin et al. (50), found the C11 aryl group of UPA forms stabilizing contacts to the LBD of PR that gives it the high potency as a PR antagonist. UPA exhibits less potency with androgen receptor, GR, and mineralocorticoid receptor because of the weak or ineffective binding contacts of UPA with the LBD of these other nuclear receptors, providing increased specificity of UPA to PR (51). In a study by Colucci and Ortlund (52), mifepristone (RU486) can bind to and interfere at the coregulator binding site of the P4-bound ancestral 3-ketosteroid receptor. To date, there are no published studies describing TPA and the nature of its interactions with PR.
In addition, we did not detect PR binding sites (PRE) and PR occupancy in the proximal promoter of the 16 P4-driven proliferation genes. It is possible that the PR site that regulates the transcription of these signature genes may be distal to the gene. Clarke and Graham (34) showed that PR effects were mediated over a longer distance. In fact, only about 20% of PR binding regions were associated with active transcriptional regulation (34). In addition, Ballaré et al. (39) showed that in 68% of upregulated and 35% of downregulated PR target genes, there was at least one PR site within 10 kb upstream and 5 kb downstream of the transcription start site.
Compared with VC treatment, TPA alone was able to recruit PR to the same sites as the agonist R5020, albeit to significantly lower levels, yet TPA did not induce PR target gene expression. There may be a threshold of PR occupancy that is necessary for PR to regulate target gene expression. Alternatively, coregulators play an important role in regulating PR action. We identified PR interacting proteins by ChIP mass spectrometry in all treatment groups. There were 65 unique proteins identified in the R5020-only data set (28) compared with TPA-only data set. It is possible that the absence of important coactivators to regulate transcription is the reason for the decrease in gene expression in the presence of TPA. Alternatively, the presence of important corepressors could inhibit transcription. TRPS1 was chosen for further investigation because of its role as a transcriptional corepressor and its expression in normal breast tissue and breast cancer (52, 53). This protein was detected in our screen only in the presence of TPA. To our knowledge, TRPS1 has not been shown to interact with and regulate PR transcriptional function.
TRPS1 was first discovered as a gene associated with a rare genetic disorder, trichorhinophalangeal syndrome I. TRPS1 is an atypical member of the GATA family transcription factors and has been shown to regulate differentiation and development of bone, cartilage, and hair follicles (54). TRPS1 has been implicated in hormone-dependent cancers, such as prostate cancer (55), endometrial cancer (56), and breast cancer (52, 53, 57, 58). Several other reports have shown that TRPS1 inhibits EMT during breast cancer development (57–59). Though not considered a classic coregulator of steroid hormone receptors, other GATA family members have been shown to regulate nuclear receptor action, with the most recognized interaction in breast cancer being GATA-3 and ER (34, 60, 61). Su et al. (62) showed that TRPS1 can interact with various proteins to either inhibit or promote cancer, depending on the context. Finally, other studies in breast cancer indicated that TRPS1 could be a prognostic marker in early stage breast cancer because high expression of TRPS1 is associated with increased OS (52, 58, 59). Our data agree with these studies in that higher mRNA expression of TRPS1 is associated with increased OS and RFS (Fig. 6A). This association was significant only in patients with ER+ and PR+ breast cancer.
It remains to be determined how TPA promotes interaction of PR with TRPS1. The conformational change of PR by TPA could favor interaction with TRPS1. It is unknown whether interaction between these two proteins is direct or if it is part of a larger complex. TRPS1 is known to be a transcriptional corepressor by binding to GATA sites, but it also has a conserved C-terminal region that is responsible for its repressive actions (40). In our studies, it was possible that TRPS1 independently occupied GATA sites near PR sites, but preliminary ChIP data suggested that TRPS1 occupancy at PR sites depended on PR occupancy (data not shown). Furthermore, a scan of the TRPS1 protein did not show classic nuclear receptor binding motifs for coactivators (LXXLL) or corepressors (IXXXI/L). Additional research is needed to address these questions. In another RIME that was performed in the study by Mohammed et al. (6), TRPS1 was detected in P4- or R5020-treated T47D and MCF7 cells. Our RIME also detected TRPS1 in R5020-treated cells but only in one of the three replicates performed. Additionally, our coimmunoprecipitation validation studies showed interaction between TRPS1 and PR- in R5020-treated cells. Differences in experimental design, including the presence of E2 and a longer time of hormone treatment in their study, could have influenced the levels of TRPS1 detected by mass spectrometry. Interestingly, in the Mohammed et al. (6) study, P4 inhibited estrogen-driven growth of ER+ breast cancer cell line xenografts and primary ER1 primary breast tumor explants. Whether the corepressor function of TRPS1 mediates the inhibitory effect of P4 in the presence of estrogen is an interesting possibility. A recent study by Serandour et al. (63) showed that TRPS1 regulated ER binding and histone acetylation at enhancer regions in MCF7 cells, and thus there could be a dynamic crosstalk between ER and PR and TRPS1 and would be worth investigation.
Given the prominent role TRPS1 plays in influencing the PR cistrome in response to TPA, expression of PR target genes, and cell proliferation and its association with OS, it could serve as a predictive biomarker of SPRM response in PR-positive breast cancer. This possibility remains to be tested. By understanding the detailed molecular actions of TPA and other SPRMs, we can tailor the use of SPRMs for the prevention and treatment of human breast cancer.
Acknowledgments
We acknowledge the Northwestern Proteomics Core Facility and Northwestern Next Generation Sequencing Core Facility, and in particular Dr. Matthew Schipma, for the ChIP-seq analysis. We also thank Dr. Brandon Parker for his assistance in the generation of the PR CRISPR knockout cells and the members of the Kim laboratory, especially Zhenxiao Lu, for technical assistance and insightful discussions.
Financial Support: This work is supported by National Institutes of Health/National Cancer Institute Grant R01CA192124 (to S.A.K. and J.J.K.).
Author Contributions: Conception and design: B.D., S.A.K., S.E.C., O.L., and J.J.K. Development of methods: B.D. and J.J.K. Data acquisition: B.D. and A.R.M. Analysis and interpretation of data: B.D. and J.J.K. Writing and review of manuscript: B.D., A.R.M., S.A.K., S.E.C., O.L., and J.J.K. All coauthors read and approved the final draft of this manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BrdU
bromodeoxyuridine
- ChIP
chromatin immunoprecipitation
- ChIP-seq
chromatin immunoprecipitation sequencing
- DAPI
4′,6-diamidino-2-phenylindole
- E2
estradiol
- ER
estrogen receptor
- FBS
fetal bovine serum
- GR
glucocorticoid receptor
- KO
knockout
- LBD
ligand-binding domain
- MPA
medroxyprogesterone acetate
- OS
overall survival
- PR
progesterone receptor
- P4
progesterone
- PRE
progesterone response element
- RIME
rapid immunoprecipitation mass spectrometry
- RFS
relapse-free survival
- siC
siCONTROL
- siRNA
small interfering RNA
- SPRM
selective progesterone receptor modulator
- sr-c
scrambled control
- TPA
telapristone acetate
- UPA
ulipristal acetate
- VC
vehicle
References
- 1. National Cancer Institute SEER stat fact sheets: breast cancer. 2017. Available at: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 21 March 2018.
- 2. Beral V; Million Women Study Collaborators . Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. [DOI] [PubMed] [Google Scholar]
- 3. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 4. Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11(11):1593–1607. [DOI] [PubMed] [Google Scholar]
- 5. Clarke RB, Howell A, Anderson E. Estrogen sensitivity of normal human breast tissue in vivo and implanted into athymic nude mice: analysis of the relationship between estrogen-induced proliferation and progesterone receptor expression. Breast Cancer Res Treat. 1997;45(2):121–133. [DOI] [PubMed] [Google Scholar]
- 6. Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, Menon S, Hadfield J, Pugh M, Raj GV, Brown GD, D’Santos C, Robinson JL, Silva G, Launchbury R, Perou CM, Stingl J, Caldas C, Tilley WD, Carroll JS. Progesterone receptor modulates ERα action in breast cancer [published correction appears in Nature. 2015;526(7571):144] Nature. 2015;523(7560):313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. [DOI] [PubMed] [Google Scholar]
- 8. Hilton HN, Clarke CL, Graham JD. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol. 2018;466:2–14 [DOI] [PubMed] [Google Scholar]
- 9. Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, Lane DS, Johnson KC, Wactawski-Wende J, Chen C, Qi L, Yasmeen S, Newcomb PA, Prentice RL. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105(8):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5(3):307–324. [DOI] [PubMed] [Google Scholar]
- 11. Beato M. Gene regulation by steroid hormones. Cell. 1989;56(3):335–344. [DOI] [PubMed] [Google Scholar]
- 12. Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13(6):385–396. [DOI] [PubMed] [Google Scholar]
- 14. Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314(5804):1467–1470. [DOI] [PubMed] [Google Scholar]
- 15. Michna H, Schneider MR, Nishino Y, el Etreby MF. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: mechanistic studies. Breast Cancer Res Treat. 1989;14(3):275–288. [DOI] [PubMed] [Google Scholar]
- 16. Robertson JF, Willsher PC, Winterbottom L, Blamey RW, Thorpe S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur J Cancer. 1999;35(2):214–218. [DOI] [PubMed] [Google Scholar]
- 17. Spitz IM. Progesterone receptor antagonists and selective progesterone receptor modulators (SPRMs). Semin Reprod Med. 2005;23(1):3–7. [DOI] [PubMed] [Google Scholar]
- 18. Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11(3):293–307. [DOI] [PubMed] [Google Scholar]
- 19. Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188(1–2):111–123. [DOI] [PubMed] [Google Scholar]
- 20. Attardi BJ, Burgenson J, Hild SA, Reel JR. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. 2004;88(3):277–288. [DOI] [PubMed] [Google Scholar]
- 21. Wiehle R, Lantvit D, Yamada T, Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila). 2011;4(3):414–424. [DOI] [PubMed] [Google Scholar]
- 22. Wiehle RD, Christov K, Mehta R. Anti-progestins suppress the growth of established tumors induced by 7,12-dimethylbenz(a)anthracene: comparison between RU486 and a new 21-substituted-19-nor-progestin. Oncol Rep. 2007;18(1):167–174. [PubMed] [Google Scholar]
- 23. Singhal H, Greene ME, Tarulli G, Zarnke AL, Bourgo RJ, Laine M, Chang YF, Ma S, Dembo AG, Raj GV, Hickey TE, Tilley WD, Greene GL. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;2(6):e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clare SE, Gupta A, Choi M, Ranjan M, Lee O, Wang J, Ivancic DZ, Kim JJ, Khan SA. Progesterone receptor blockade in human breast cancer cells decreases cell cycle progression through G2/M by repressing G2/M genes. BMC Cancer. 2016;16(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee O, Choi MR, Christov K, Ivancic D, Khan SA. Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett. 2016;376(2):310–317. [DOI] [PubMed] [Google Scholar]
- 26. RRID:CVCL_0553.
- 27. RRID:CVCL_0031.
- 28.Davaadelger B, Murphy AR, Clare SE, Lee O, Khan SA, Kim JJ. Data From: Mechanism of telapristone acetate (CDB4124) on progesterone receptor action in breast cancer cells. Figshare 2018. Deposited 27 August 2018. 10.6084/m9.figshare.7015382.v1. [DOI] [PMC free article] [PubMed]
- 29. RRID:AB_2164331.
- 30. RRID:AB_2166687.
- 31. RRID:AB_2208891.
- 32. RRID:AB_476692.
- 33. RRID:AB_2231120.
- 34. Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS One. 2012;7(4):e35859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee II, Maniar K, Lydon JP, Kim JJ. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene. 2016;35(39):5191–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods. 2009;48(3):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lerdrup M, Johansen JV, Agrawal-Singh S, Hansen K. An interactive environment for agile analysis and visualization of ChIP-sequencing data. Nat Struct Mol Biol. 2016;23(4):349–357. [DOI] [PubMed] [Google Scholar]
- 38. Mohammed H, Taylor C, Brown GD, Papachristou EK, Carroll JS, D’Santos CS. Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of chromatin complexes. Nat Protoc. 2016;11(2):316–326. [DOI] [PubMed] [Google Scholar]
- 39. Ballaré C, Castellano G, Gaveglia L, Althammer S, González-Vallinas J, Eyras E, Le Dily F, Zaurin R, Soronellas D, Vicent GP, Beato M. Nucleosome-driven transcription factor binding and gene regulation. Mol Cell. 2013;49(1):67–79. [DOI] [PubMed] [Google Scholar]
- 40. Malik TH, Shoichet SA, Latham P, Kroll TG, Peters LL, Shivdasani RA. Transcriptional repression and developmental functions of the atypical vertebrate GATA protein TRPS1. EMBO J. 2001;20(7):1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin HY, Zeng D, Liang YK, Wei XL, Chen CF. GATA3 and TRPS1 are distinct biomarkers and prognostic factors in breast cancer: database mining for GATA family members in malignancies. Oncotarget. 2017;8(21):34750–34761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. [DOI] [PubMed] [Google Scholar]
- 43.The Human Protein Atlas. 2018. Available at: https://www.proteinatlas.org. Accessed 2 April 2018.
- 44. Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Perou CM; TCGA Research Network . Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards DP, Altmann M, DeMarzo A, Zhang Y, Weigel NL, Beck CA. Progesterone receptor and the mechanism of action of progesterone antagonists. J Steroid Biochem Mol Biol. 1995;53(1-6):449–458. [DOI] [PubMed] [Google Scholar]
- 46. Leonhardt SA, Altmann M, Edwards DP. Agonist and antagonists induce homodimerization and mixed ligand heterodimerization of human progesterone receptors in vivo by a mammalian two-hybrid assay. Mol Endocrinol. 1998;12(12):1914–1930. [DOI] [PubMed] [Google Scholar]
- 47. Spitz IM, Bardin CW. Mifepristone (RU 486)--a modulator of progestin and glucocorticoid action. N Engl J Med. 1993;329(6):404–412. [DOI] [PubMed] [Google Scholar]
- 48. Vegeto E, Allan GF, Schrader WT, Tsai MJ, McDonnell DP, O’Malley BW. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992;69(4):703–713. [DOI] [PubMed] [Google Scholar]
- 49. Weigel NL, Beck CA, Estes PA, Prendergast P, Altmann M, Christensen K, Edwards DP. Ligands induce conformational changes in the carboxyl-terminus of progesterone receptors which are detected by a site-directed antipeptide monoclonal antibody. Mol Endocrinol. 1992;6(10):1585–1597. [DOI] [PubMed] [Google Scholar]
- 50. Petit-Topin I, Fay M, Resche-Rigon M, Ulmann A, Gainer E, Rafestin-Oblin ME, Fagart J. Molecular determinants of the recognition of ulipristal acetate by oxo-steroid receptors. J Steroid Biochem Mol Biol. 2014;144(Pt B):427–435. [DOI] [PubMed] [Google Scholar]
- 51. Colucci JK, Ortlund EA. X-ray crystal structure of the ancestral 3-ketosteroid receptor-progesterone-mifepristone complex shows mifepristone bound at the coactivator binding interface. PLoS One. 2013;8(11):e80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Radvanyi L, Singh-Sandhu D, Gallichan S, Lovitt C, Pedyczak A, Mallo G, Gish K, Kwok K, Hanna W, Zubovits J, Armes J, Venter D, Hakimi J, Shortreed J, Donovan M, Parrington M, Dunn P, Oomen R, Tartaglia J, Berinstein NL. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci USA. 2005;102(31):11005–11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen JQ, Litton J, Xiao L, Zhang HZ, Warneke CL, Wu Y, Shen X, Wu S, Sahin A, Katz R, Bondy M, Hortobagyi G, Berinstein NL, Murray JL, Radvanyi L. Quantitative immunohistochemical analysis and prognostic significance of TRPS-1, a new GATA transcription factor family member, in breast cancer. Horm Cancer. 2010;1(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Malik TH, Von Stechow D, Bronson RT, Shivdasani RA. Deletion of the GATA domain of TRPS1 causes an absence of facial hair and provides new insights into the bone disorder in inherited tricho-rhino-phalangeal syndromes. Mol Cell Biol. 2002;22(24):8592–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chang GT, Steenbeek M, Schippers E, Blok LJ, van Weerden WM, van Alewijk DC, Eussen BH, van Steenbrugge GJ, Brinkmann AO. Characterization of a zinc-finger protein and its association with apoptosis in prostate cancer cells. J Natl Cancer Inst. 2000;92(17):1414–1421. [DOI] [PubMed] [Google Scholar]
- 56. Liang H, Cheung LW, Li J, Ju Z, Yu S, Stemke-Hale K, Dogruluk T, Lu Y, Liu X, Gu C, Guo W, Scherer SE, Carter H, Westin SN, Dyer MD, Verhaak RG, Zhang F, Karchin R, Liu CG, Lu KH, Broaddus RR, Scott KL, Hennessy BT, Mills GB. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012;22(11):2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu J, Su P, Jiao M, Bai X, Qi M, Liu H, Wu Z, Sun J, Zhou G, Han B. TRPS1 suppresses breast cancer epithelial-mesenchymal transition program as a negative regulator of SUZ12. Transl Oncol. 2018;11(2):416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen JQ, Bao Y, Litton J, Xiao L, Zhang HZ, Warneke CL, Wu Y, Shen X, Wu S, Katz RL, Sahin A, Bondy M, Murray JL, Radvanyi L. Expression and relevance of TRPS-1: a new GATA transcription factor in breast cancer. Horm Cancer. 2011;2(2):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen JQ, Bao Y, Lee J, Murray JL, Litton JK, Xiao L, Zhou R, Wu Y, Shen XY, Zhang H, Sahin AA, Katz RL, Bondy ML, Berinstein NL, Hortobagyi GN, Radvanyi LG. Prognostic value of the trichorhinophalangeal syndrome-1 (TRPS-1), a GATA family transcription factor, in early-stage breast cancer. Ann Oncol. 2013;24(10):2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee JY, Park YJ, Oh N, Kwack KB, Park KS. A transcriptional complex composed of ER(α), GATA3, FOXA1 and ELL3 regulates IL-20 expression in breast cancer cells. Oncotarget. 2017;8(26):42752–42760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carroll JS. Mechanisms of oestrogen receptor (ER) gene regulation in breast cancer. Eur J Endocrinol. 2016;175(1):R41–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Su P, Hu J, Zhang H, Jia M, Li W, Jing X, Zhou G. Association of TRPS1 gene with different EMT markers in ERα-positive and ERα-negative breast cancer. Diagn Pathol. 2014;9(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Serandour AA, Mohammed H, Miremadi A, Mulder KW, Carroll JS. TRPS1 regulates oestrogen receptor binding and histone acetylation at enhancers [published online ahead of print 12 June 2018]. Oncogene. doi: 10.1038/s41388-018-0312-2. [DOI] [PMC free article] [PubMed]