Abstract
Background. Recent policy changes require discussing the potential benefits and harms of lung cancer screening with low-dose computed tomography. This study explored how current and former smokers value potential benefits and harms after watching a patient decision aid, and their screening intentions. Methods. Current or former smokers (quit within 15 years) with no history of lung cancer watched the decision aid and responded to items assessing the value of potential benefits and harms in their decision making, and their screening intentions. Results. After viewing the decision aid, participants (n = 30; mean age 61.5 years, mean 30.4 pack-year history) were well-informed (mean 80.5% correct responses) and rated anticipated regret and finding cancer early as highly important in their decision (medians >9 out of 10), along with moderate but variable concerns about false positives, overdiagnosis, and radiation exposure (medians 7.0, 6.0, and 5.0, respectively). Most participants (90.0% to 96.7%) felt clear about how they personally valued the potential benefits and harms and prepared for decision making (mean 86.7 out of 100, SD = 21.3). After viewing the decision aid, most participants (90%) intended to discuss screening with their doctor. Limitations. The study is limited to current and former smokers enrolled in a tobacco treatment program, and it may not generalize to other patient populations. Conclusions. The majority of current and former smokers were strongly concerned about anticipated regret and finding cancer early, while concerns about radiation exposure, false positives, and overdiagnosis were variable. After viewing the decision aid, current and former smokers reported strong preparedness and intentions to talk with their doctor about lung cancer screening with low-dose computed tomography.
Keywords: decision support, decision making, lung neoplasms, tomography x-ray computed, smokers
Lung cancer is the second most diagnosed cancer in both men and women, and the leading cause of cancer-related death in the United States (estimated 222 500 new cases and 155 870 deaths).1 In 2011, the National Lung Screening Trial reported a 20% reduction in lung cancer deaths over an average follow-up period of 6.5 years among high-risk current and former smokers screened annually for 3 years with low-dose computed tomography (LDCT) compared to those screened with standard chest radiography.2 The Centers for Medicare & Medicaid Services (CMS) issued a national coverage determination providing reimbursement for annual lung cancer screening with LDCT for beneficiaries aged 55 to 77 years who have at least a 30 pack-year smoking history and currently smoke or have quit within the past 15 years, plus meet other eligibility criteria.3
The US Preventive Services Task Force (USPSTF) concluded that annual screening with LDCT can prevent lung cancer deaths based on a randomized trial that reported a 20% reduction in deaths over 6 years.4 However, the magnitude of benefit depends on each individual’s risk factors (e.g., pack-years, age, comorbidities), and there are potential harms of screening with LDCT.5 While LDCT involves less radiation exposure than a standard CT, it is still more radiation exposure than the standard chest X-ray and may lead to increased rates of breast, lung, or thyroid cancers in the future.5,6 LDCT also has a high false positive rate; 95% of positive results do not lead to a cancer diagnosis.5,6 Screening may lead to invasive follow-up testing, overdiagnosis, and overtreatment of cancers that may never have become harmful or fatal.5,7 These concepts may be new to patients and can be difficult to communicate, comprehend, and personalize.7
To facilitate informed decision making about lung cancer screening, CMS, the USPSTF, and the American Cancer Society and the American Thoracic Society recommend including a shared decision-making process for eligible, high-risk smokers.3,6,8–10 At minimum, these shared decision-making visits should include discussion of a patient’s knowledge of screening with LDCT, the potential benefits and harms, and clarification of their decision-making values, that is, the personal importance they place on each benefit and harm.5,11,12 As of February 2015, CMS requires a patient counseling and shared decision-making visit with a health care provider using one or more decision aids that address the benefits and harms of lung cancer screening with LDCT before a patient is referred for screening.3
Patient decision aids provide high-quality, up-to-date evidence, including a balanced presentation of the potential benefits and harms of screening or treatment.13,14 They also provide evidence-based deliberative guidance to help patients personalize the information, gain clarity about the benefit/harm trade-offs, and prepare to communicate with their doctor(s).13,15 The 2017 update of the Cochrane Collaboration systematic review of 89 randomized controlled trials of patient decision aids concluded that patient decision aids improve patients’ knowledge, accurate perceptions of risk, decisional conflict, clarity about their values, and preparation for decision making.16 It also reported a reduction in the number of patients who remain passive or undecided, and an increase in the proportion of patients who are satisfied with their patient-practitioner communication.16
Following the CMS requirement of the use of decision aids,3 evidence is needed regarding how patients who have viewed a patient decision aid value the potential benefits and harms of lung cancer screening with LDCT. As part of an ongoing series of studies of patient decision making for lung cancer screening,17–19 we report the effects of the use of a video-based decision aid about screening with LDCT to elicit the values that current and former smokers place on the potential benefits and harms, as well as their intentions toward screening with LDCT.
Methods
Study Design
In this pre-/postintervention study, participants completed an online baseline questionnaire assessing their sociodemographic characteristics, smoking history, and knowledge of lung cancer screening with LDCT. Participants then watched the decision aid video and completed an online questionnaire assessing their post–decision aid knowledge, decision-making values, and screening intentions, as well as their overall sense of being well-informed, clear about their decision-making values, and prepared to make a decision.
The institutional review board at The University of Texas MD Anderson Cancer Center provided ethical review and approval of the study protocol. Informed consent was provided by all participants. The study is registered with www.clinicaltrials.gov (NCT02282969).
Participants and Setting
Eligible individuals were English-speaking men and women aged 55 to 80 years with no history of lung cancer, who were current smokers or had quit within the past 15 years. This study did not include a pack-year minimum as an eligibility criterion because one of the patient-requested features in the decision aid was an activity to help smokers learn to calculate their pack-year history and consider their eligibility. To obtain a more diverse sample of higher risk current and former smokers, individuals were recruited from the Tobacco Treatment Program at The University of Texas MD Anderson Cancer Center. Data collection occurred from March 29, 2016, to May 5, 2016.
Intervention
The patient decision aid video, “Lung Cancer Screening: Is It Right for Me?” was developed based on theories in cognitive psychology and decision making, including the integrative model of behavior.20,21 In accordance with the recommendations of the International Patient Decision Aid Standards Collaboration, the decision aid video meets 28 out of 32 relevant quality criteria.22
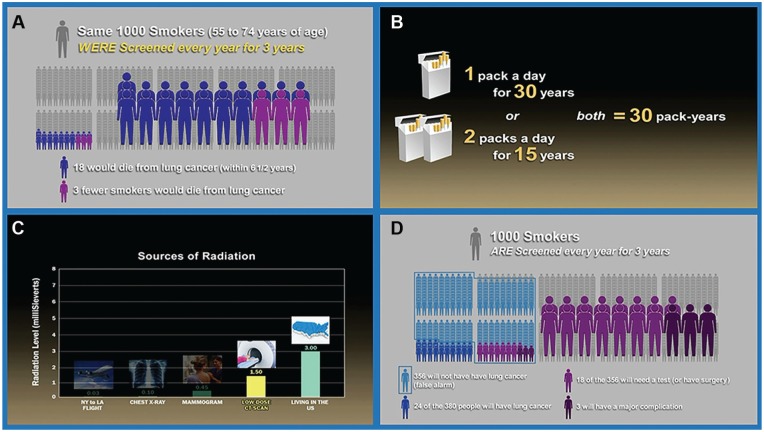
Figure 1 provides selected screenshots of the video. A narrator guides viewers through descriptions and images of the natural history of smoking and lung cancer, with an emphasis on the importance of quitting smoking. The USPSTF screening recommendation is described. The narrator explains that screening with LDCT is a personal decision, and describes the importance of considering the potential benefits and harms so that “you can have an informed discussion with your doctor about whether screening with low-dose computed tomography is right for you.”
Figure 1.
Selected screen shots from the patient decision aid video. (A) mortality benefit from annual screening with low-dose computed tomography, (B) pack-year calculation, (C) radiation exposure contextualized, (D) comparing benefits and harms of lung cancer screening with low-dose computed tomography.
Next, the narrator explains the procedures for screening with LDCT, and the video shows a patient being screened. The narrator guides viewers through the potential benefits and potential harms, and icon arrays are provided to visualize the risk. With extensive input from smokers and health care providers, the video contextualizes the risk of radiation exposure by comparison with other medical imaging and environmental exposures. It describes false positives using the term false alarms and depicts the false positive rate using an icon array. Overdiagnosis is also presented as cancers that would never become life threatening, and the video explains that some people may be treated for cancer that would never have harmed them.7
Following the didactic information, the decision aid video provides theory-based key messages to prompt current and former smokers to consider what matters most to them personally (i.e., to clarify their decision-making values). It closes by summarizing the potential benefits and harms side-by-side, and emphasizing again the importance of considering this personal decision with your doctor.
Measures
To assess current and former smokers’ decision-making values after viewing a LDCT patient decision aid, participants were asked to respond to five questions using visual analog scales.23
If you made the decision not to be screened and were later diagnosed with lung cancer, would you have regrets? (0 = Would have no regrets about my decision not to be screened; 10 = Would strongly regret my decision not to be screened)
How important to you is it to find lung cancer early? (0 = Not at all important; 10 = Extremely important)
How concerned are you about radiation exposure from lung cancer screening and potential follow-up testing? (0 = Not at all concerned; 10 = Extremely concerned)
How concerned are you that your scan may say you have cancer when you do not (in other words, a false alarm)? (0 = Not at all concerned; 10 = Extremely concerned)
How concerned are you about being treated for a lung cancer that never would have harmed you (in other words, overdiagnosis)? (0 = Not at all concerned; 10 = Extremely concerned)
Participants also rated their intention to 1) discuss screening at their next appointment and 2) complete screening within the next year, using visual analog scales (1 = Definitely will not; 3 = Not sure; 5 = Definitely will).
To assess participants’ objective information comprehension, participants completed the LCS-12, a brief measure of lung cancer screening knowledge.18 This scale includes 12 items with three response categories: True, False, I Don’t Know (1 = Correct; 0 = Incorrect or I Don’t Know).17
In addition, three measures assessed participants’ subjective ratings of their knowledge, values clarity, and preparation for decision making after viewing the decision aid video. Participants were asked to respond to the question, “How informed do you feel about lung cancer screening?” on a scale of 0 (Not at all informed) to 10 (Very well informed). Values clarity was measured using the question format of the values clarity subscale of the Decisional Conflict Scale,24,25 with three low literacy response items—Yes, Unsure, or No (scored 0, 2, 4, respectively):
Are you clear about which benefits matter most to you?
Are you clear about which risks and side effects matter most to you?
Are you clear about which is more important to you (the benefits or the risks and side effects)?
Finally, participants completed the Preparation for Decision Making scale, using 5-point visual analog scales (0 = Not at all; 4 = A great deal).26
Data Management and Analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at MD Anderson.27 Data were de-identified and analyzed using the Statistical Package for the Social Sciences (SPSS v. 23, IBM 2016). Descriptive statistics assessed the distributions of responses for all items.
To address the primary objective, participants’ responses to the decision-making values questions were tabulated and box plots were created to illustrate the medians and variance of participants’ concerns about finding cancer early, anticipated regret, radiation exposure, overdiagnosis, and false alarms. Tabulations also summed the number of participants who responded “Definitely Will” or “Probably Will” to the two questions about intentions to discuss screening and to be screened.
Knowledge scores were calculated using the published scoring algorithm and a paired t test, as reported previously.17,18 To assess participants’ subjective perceptions, means and standard deviations were calculated for their ratings of being well-informed and for their scores on the Preparation for Decision Making scale. To quantify the amount of participants’ who felt clear about their values, analyses summed the number of “Yes” responses to the three Decisional Conflict Scale values clarity subscale items. To compare participants’ values clarity with other published studies, analyses summed the three items, divided by three, and multiplied by 25 to yield a 0 to 100 subscale scale score.24
Role of Financial Support
This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-03385) and The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. All statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the PCORI, its Board of Governors, or Methodology Committee. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. Ashley Housten was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA057730 (PI: Shine Chang, PhD) and by a Cancer Center Support Grant CA016672.
Results
Thirty-one individuals were recruited from the Tobacco Treatment Program and one withdrew when a family member became ill. The majority of participants were current smokers (20, 67%) aged 55 to 70 years (mean = 61.5 years) with a 4.6 to 90 pack-year history (mean 30.4 pack-years).17 Fifteen participants (50%) were female, and 11 (36.7%) were non-White, had a college degree, and/or had private insurance.17
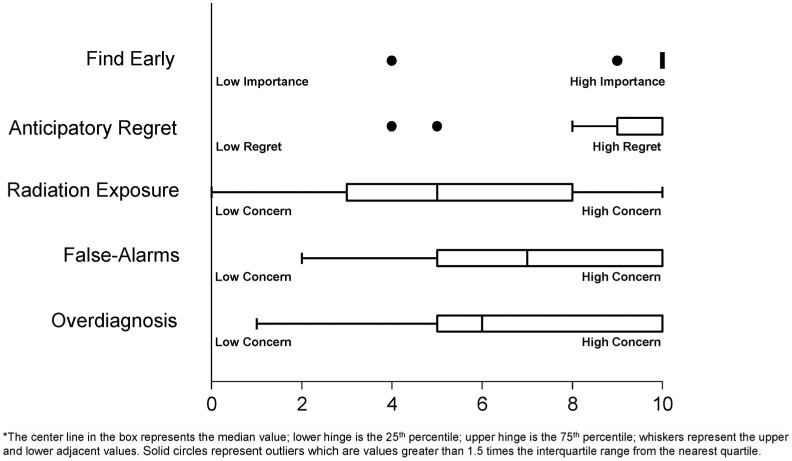
Figure 2 illustrates the median, interquartile range (IQR), and outliers of participants’ decision-making values (i.e., their ratings of the importance of each factor for their personal decision). Twenty-seven participants responded that finding lung cancer early was extremely important to their decision making (median 10.0, IQR 0, min. 4, max. 10). Twenty-two of the 30 participants reported very high anticipated regret (10 on a scale of 0 to 10) if they were diagnosed with lung cancer after choosing to forego screening (median 10.0, IQR 1, min. 4, max. 10). Participants reported moderate and variable concerns about false positive results (median 7.0, IQR 5, min. 2, max. 10), overdiagnosis (median 6.0, IQR 5, min. 1, max. 10), and radiation exposure (median 5.0, IQR 6, min. 0, max. 10).
Figure 2.
Current and former smokers’ ratings of the value (i.e., importance) of potential risks of lung cancer screening with low-dose computed tomography; medians, interquartile ranges, and outliers. The box represents the interquartile range (IQR), with the center line in the box representing the median value; whiskers representing the data points within 1.5 IQR on the upper and lower ends; and solid dots representing outliers that are values outside 1.5 IQR. For Find Early, responses are clustered at 10, represented by the bolded bar.
Table 1 shows that after watching the decision aid video, the majority of participants reported feeling well-informed (mean 8.7 out of 10, SD = 1.6). As reported previously, watching the decision aid video improved patients’ mean knowledge scores on the 12-item measure from 5.7 (47.3% correct) pre–decision aid to 9.6 (80.3% correct) post–decision aid, and the 3.9 mean difference (SD = 2.9, 95% confidence interval 2.9–5.0) was significant (t = 7.6, P < 0.001).17 The largest increases in correct responses were seen in questions assessing individuals’ awareness of the overall benefit of CT scanning, where lung cancer ranks as a cancer-related cause of death in the United States, potential for false positives, and likelihood of receiving a lung cancer diagnosis given an abnormal CT scan result.17
Table 1.
Current and Former Smokers’ Feelings of Being Well-Informed, Clear About Their Decision-Making Values, Preparation, and Intentions for Lung Cancer Screening (N = 30)
| Measure | Response |
|---|---|
| Knowledge, 0–10, mean (SD) | |
| How informed do you feel about lung cancer screening? | 8.7 (1.6) |
| Decisional Conflict Values Clarity Subscale, 0-100, mean (SD) | 3.9 (10.4) |
| Are you clear about: n (%) | |
| Which benefits of lung cancer screening matter most to you? | 29 (96.7%) |
| Which risks and side effects of lung cancer screening matter most to you? | 28 (93.3%) |
| Which is more important to you (the benefits or the risks and side effects of lung cancer screening)? | 27 (90%) |
| Preparation for Decision Making Scale, 0–100, mean (SD) | 81.7 (21.3) |
| Intentions, n (%) | |
| To discuss screening at next appointment | 27 (90%) |
| To be screened within the coming year | 19 (63.3%) |
Participants reported feeling clear about which risks and benefits were important to them (90% to 96.7% positive responses). Their mean score on the Decisional Conflict Values Clarity Subscale was 3.9 (where 0 = feels extremely certain and 100 = feels extremely uncertain about the best choice).
Participants also felt prepared for making a decision with their doctor (mean 86.7 out of 100, SD = 21.3), with highest scores reported for the decision aid video helping them recognize “that a decision needs to be made” (mean 4.2, SD = 0.9), knowing “that the decision depends on you” (mean 4.5, SD = 0.7), and thinking “about the pros and cons of each option” (mean 4.3, SD = 0.8).
After watching the decision aid video, 27 (90%) participants intended to discuss lung cancer screening at their next doctor’s appointment. Twelve participants (40%) would meet the CMS pack-year eligibility criterion, and 19 (63%) indicated that they were interested in being screened within the next year.
Discussion
Overall, use of a patient decision aid video resulted in current and former smokers placing high value on anticipated regret if they chose not to be screened and later were diagnosed with lung cancer, and finding cancer early as well as moderate but variable concerns about radiation exposure, false positives, and overdiagnosis. The majority of participants felt prepared for, and remained enthusiastic about, discussing lung cancer screening with LDCT at their next doctor’s visit.
These results add to the literature by providing an example of using a patient decision aid to elicit and report current and former smokers’ decision-making values about LDCT. Several qualitative studies have identified barriers to lung cancer screening—lack of awareness, costs, concerns about the procedures/exposures, anxiety, uncertainty, and misunderstanding of the meaning of the results—and recommended patient-focused educational and shared decision-making tools.28–32 However, some of these studies occurred prior to the CMS policy change and/or did not inform smokers about the potential risks and benefits of LDCT. Since the CMS policy change, a number of patient decision aids about LDCT have been developed and show positive effects on individuals’ awareness, knowledge, risk perceptions, and decision making.19,32,33
Notably, these results differ slightly from a study comparing direct invitation to lung cancer screening (including mailing Veterans who met the risk guidelines a brochure decision aid describing LDCT) with usual care.33 In that study, Veterans placed the highest value on “personal risk of lung cancer” and “fear of getting cancer.” Lower value was observed for “risks of lung cancer screening”; however, Veterans exposed to the decision aid brochure did rate potential harms higher than Veterans in the usual care group.33 Our study expands these findings by assessing the value of the types of potential harms (overdiagnosis, anticipated regret, etc.), after confirming participants’ knowledge of the risks and benefits.
While the sample size is not large enough to assess potential associations, the high and moderate importance ratings may reflect increased understanding and awareness of the potential risks of screening. Similar studies in other cancer screening contexts have noted that increased knowledge and awareness of the potential risks of screening resulted in patients reporting high importance for those factors, and continued intentions to be screened.13,34,35 The integrative model of behavior posits that, when risk is present, supporting patients in feeling clear about their values and being well-informed and prepared to discuss their preferences with their clinician(s) may improve intention and action.36–38
While the effects of increased knowledge and values on intentions toward cancer screening vary by cancer type, a recent meta-analysis found anticipated regret to be a strong predictor of intentions to engage in a health behavior, especially anticipated inaction regret.39 Schapira and colleagues noted the challenges in helping patients consider the unknowns of their current health state (ambiguity) and potential future health outcomes (uncertainty).40 Shared decision-making interventions (patient decision aids, decision coaching consultations, etc.) may confirm that patients are knowledgeable about the risks and benefits and hold realistic expectations when considering lung cancer screening with LDCT.
Limitations
This study was limited to current and former smokers participating in a tobacco treatment program at a large cancer center. While many may not have been actively considering this decision, all participants were seeking smoking cessation services and represent a target population for raising screening awareness. Accordingly, a higher percentage of participants expressed intentions to discuss screening compared to actually being screened within the next year. The difference in intention for discussion and intention for screening may reflect increased awareness of the characteristics of smokers for whom screening with LDCT is or is not recommended. Additional testing is needed to explore the use of the decision aid with individuals in routine clinical care.
The sample size of this study also limits exploration of several downstream factors that influence decision making and utilization. Larger trials are needed to evaluate effects of patient decision support on utilization of LDCT and subgroup effects due to sociodemographic characteristics and lung cancer risk levels. Longitudinal studies are also needed to assess the decision aid’s effect on patients’ retained knowledge and stability of values when new concepts (e.g., overdiagnosis) and policies become more familiar, and whether these factors influence rates of initial or repeat testing.
Conclusion
A decision aid for lung cancer screening with LDCT may help current and former smokers clarify their values about the benefits and harms of screening, and assist clinicians in diagnosing potentially dominating drivers of screening decisions, such as anticipated regret. Decision aids for LDCT may help smokers contextualize less-familiar risks, such as radiation exposure, and ensure that they are clear, well-informed, and prepared to make decisions about lung cancer screening with LDCT. Decision support tools may also be useful for identifying gaps in information comprehension or mismatches between patients’ values and treatment preferences. While variance was observed in patients’ values (i.e., importance ratings) regarding radiation exposure, overdiagnosis, and false positives, enthusiasm for finding lung cancer early and screening with LDCT remained strong.
Acknowledgments
The authors would like to thank the Tobacco Treatment Program at The University of Texas MD Anderson Cancer Center for assistance with recruitment and acknowledge Gary Michael Deyter for his support as a scientific writer.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support for this study was provided in part by a grant from the Patient-Centered Outcomes Research Institute (Award CER-1306-03385) and by The Shared Decision Making Collaborative though an award by The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
The statements presented in this work are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Presented at the 38th Annual North American Meeting of the Society for Medical Decision Making in Vancouver, Canada, October 23-26, 2016
Authorship Contributions: Conceptualization: RJV; Formal analysis: ASH, APH, AJH, VFR; Investigation: APH; Writing—original draft: APH, ASH, AJH; Writing—review and editing: APH, ASH, AJH, VFR, LML, VBL, RJV; Visualization: AJH, APH, ASH; Project administration: APH, VBL; Funding acquisition: RJV.
ORCID iD: Robert J. Volk  https://orcid.org/0000-0001-8811-5854
https://orcid.org/0000-0001-8811-5854
References
- 1. American Cancer Society. Cancer facts & figures 2016 [cited March 21, 2017]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html
- 2. Patz EF, Jr, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17(5):590–9. doi: 10.1016/s1470-2045(15)00621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Medicare and Medicaid Services. Decision Memo for Screening for Lung Cancer With Low Dose Computed Tomography (LDCT) (CAG-00439N). Baltimore, MD: Centers for Medicare and Medicaid Services; 2015. [Google Scholar]
- 4. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moyer VA. Screening for lung cancer: US preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–8. doi: 10.7326/m13-2771. [DOI] [PubMed] [Google Scholar]
- 6. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159(6):411–20. doi:10.7326/0003-4819-159-6-201309170- 00690. [DOI] [PubMed] [Google Scholar]
- 7. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 8. Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society policy statement. Chest. 2015;147(2):295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–8. doi:10.1016/j .jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman AS. Teaching diverse orthopaedic patient populations about deliberative decision making skills: testing a design strategy for online patients’ decision aids (PhD thesis). Dartmouth College, Hanover, NH; 2011. [Google Scholar]
- 12. Hoffman AS, Llewellyn-Thomas HA, Tosteson AN, et al. Launching a virtual decision lab: development and field-testing of a web-based patient decision support research platform. BMC Med Inform Decis Mak. 2014;14:112. doi: 10.1186/s12911-014-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. doi:10.1002/ 14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 14. Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl. 2):S1. doi:10.1186/1472-6947- 13-s2-s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stacey D, Kryworuchko J, Belkora J, et al. Coaching and guidance with patient decision aids: a review of theoretical and empirical evidence. BMC Med Inform Decis Mak. 2013;13(Suppl. 2):S11. doi: 10.1186/1472-6947-13-s2-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Housten AJ, Lowenstein LM, Leal VB, Volk RJ. Responsiveness of a brief measure of lung cancer screening knowledge. J Cancer Educ. Epub Dec 2016. doi: 10.1007/s13187-016-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowenstein LM, Richards VF, Leal VB, et al. A brief measure of smokers’ knowledge of lung cancer screening with low-dose computed tomography. Prev Med Rep. 2016;4:351–6. doi: 10.1016/j.pmedr.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Volk RJ, Linder SK, Leal VB, et al. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Prev Med. 2014;62:60–3. doi: 10.1016/j.ypmed.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llewellyn-Thomas HA. Patients’ health-care decision making: a framework for descriptive and experimental investigations. Med Decis Making. 1995;15(2):101–6. [DOI] [PubMed] [Google Scholar]
- 21. O’Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267–79. [DOI] [PubMed] [Google Scholar]
- 22. Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Connor AM. User Manual: Values Scale. Ottawa, Canada: Ottawa Health Research Institute, 2004. [Google Scholar]
- 24. O’Connor A. Users Manual: Decisional Conflict Scale. Ottawa, Canada: Ottawa Health Research Institute, 1993. [revised 2010]. [Google Scholar]
- 25. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 26. Graham IOCA. User Manual: Preparation for Decision Making Scale. Ottawa, Canada: Ottawa Health Research Institute, 1995. [revised 2010]. [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi:10.1016/j.jbi .2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gressard L, DeGroff AS, Richards TB, et al. A qualitative analysis of smokers’ perceptions about lung cancer screening. BMC Public Health. 2017;17(1):589. doi:10.1186/ s12889-017-4496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mishra SI, Sussman AL, Murrietta AM. Patient perspectives on low-dose computed tomography for lung cancer screening, New Mexico, 2014. Prev Chronic Dis. 2016;13:E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530–7. doi: 10.1001/jamainternmed.2015.3558. [DOI] [PubMed] [Google Scholar]
- 31. Poghosyan H,Kennedy Sheldon L Cooley ME.. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35(6):446–75. doi: 10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- 32. Lau YK, Caverly TJ, Cao P, et al. Evaluation of a personalized, web-based decision aid for lung cancer screening. Am J Prev Med. 2015;49(6):e125–9. doi:10.1016/j.amepre .2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lillie SE, Fu SS, Fabbrini AE, et al. What factors do patients consider most important in making lung cancer screening decisions? Findings from a demonstration project conducted in the Veterans Health Administration. Lung Cancer. 2017;104:38–44. doi: 10.1016/j.lungcan.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 34. Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. Am J Prev Med. 2016;51(5):779–91. doi: 10.1016/j.amepre.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trikalinos TA, Wieland LS, Adam GP, Zgodic A, Ntzani E. Decision Aids for Cancer Screening and Treatment. Rockville (MD): Agency for Healthcare Research and Quality; 2014 2014. [PubMed] [Google Scholar]
- 36. Fishbein M, Hennessy M, Yzer M, Douglas J. Can we explain why some people do and some people do not act on their intentions? Psychol Health Med. 2003;8(1):3–18. doi: 10.1080/1354850021000059223. [DOI] [PubMed] [Google Scholar]
- 37. Frosch DL, Legare F, Fishbein M, Elwyn G. Adjuncts or adversaries to shared decision-making? Applying the Integrative Model of behavior to the role and design of decision support interventions in healthcare interactions. Implement Sci. 2009;4:73. doi: 10.1186/1748-5908-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman AS, Lowenstein LM, Kamath GR, et al. An entertainment-education colorectal cancer screening decision aid for African American patients: a randomized controlled trial. Cancer. 2017;123(8):1401–8. doi:10.1002/cncr .30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brewer NT, DeFrank JT, Gilkey MB. Anticipated regret and health behavior: a meta-analysis. Health Psychol. 2016;35(11):1264–75. doi: 10.1037/hea0000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schapira MM, Aggarwal C, Akers S, et al. How patients view lung cancer screening. The role of uncertainty in medical decision making. Ann Am Thorac Soc. 2016;13(11):1969–76. doi: 10.1513/AnnalsATS.201604-290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]