Abstract
The World Health Organization HCV Guideline Development Group is considering a “treat all” recommendation for persons infected with hepatitis C virus (HCV). We reviewed the model-based evidence of cost-effectiveness and population health impacts comparing expanded treatment policies to more limited treatment access policies, focusing primarily on evaluations of all-oral directly acting antivirals published after 2012. Searching PubMed, we identified 2,917 unique titles. Sequentially reviewing titles and abstracts identified 226 potentially relevant articles for full-text review. Sixty-nine articles met all inclusion criteria—42 cost-effectiveness analyses and 30 models of population-health impacts, with 3 articles presenting both types of analysis. Cost-effectiveness studies for many countries concluded that expanding treatment to people with mild liver fibrosis, who inject drugs (PWID), or who are incarcerated is generally cost-effective compared to more restrictive treatment access policies at country-specific prices. For certain patient subpopulations in some countries—for example, elderly individuals without fibrosis—treatment is only cost-effective at lower prices. A frequent limitation is the omission of benefits and consequences of HCV transmission (i.e., treatment as prevention; risks of reinfection), which may underestimate or overestimate the cost-effectiveness of a “treat all” policy. Epidemiologic modeling studies project that through a combination of prevention, aggressive screening and diagnosis, and prompt treatment for all fibrosis stages, it may be possible to virtually eliminate HCV in many countries. Studies show that if resources are not available to diagnose and treat all HCV-infected individuals, treatment prioritization may be needed, with alternative prioritization strategies resulting in tradeoffs between reducing mortality or reducing incidence. Notably, because most new HCV infections are among PWID in many settings, HCV elimination requires unrestricted treatment access combined with injection transmission disruption strategies. The model-based evidence suggests that a properly constructed strategy that substantially expands HCV treatment could achieve cost-effective improvements in population health in many countries.
Keywords: hepatitis C, cost-effectiveness analysis, epidemic modeling, infectious disease control, patient prioritization, treatment expansion
The World Health Organization (WHO) estimated that 71 million people were living with chronic hepatitis C virus (HCV) infection worldwide in 2015.1 In 2016, the World Health Assembly endorsed the Global Health Sector Strategy for 2016–2021 that proposes to eliminate viral hepatitis as a public health threat by 2030 (90% reduction in incidence and 65% reduction in mortality).2 The WHO hepatitis strategy includes improving the data with which countries are developing evidence-based hepatitis response plans; advocacy for prevention including a safe transfusion blood supply, reducing the risk of transmission in health care settings, and harm reduction services for people who inject drugs (PWID); timely diagnosis and enhancing linkages to care after diagnosis; and expanded access to treatment. While the hepatitis strategy set a target of treatment coverage for HCV reaching 80% of eligible persons by 2030,2 it also recognized the need for HCV treatment prioritization due to high prices and limited health care infrastructure.3 It advised prioritizing people with advanced fibrosis and people at highest risk of transmitting HCV. Since 2016, the price of highly effective direct-acting antivirals (DAAs) has decreased substantially and several pan-genotypic DAAs have been approved reducing the need for expensive genotyping—greatly improving the feasibility of a “treat all” strategy and opening the question of the cost-effectiveness of such a strategy.
While many studies have evaluated the economic value of newer HCV treatments compared to older treatments, few assess a policy of treating all patients compared to more restrictive access policies. Analyses considering new versus old treatments generally find new treatments to be cost-effective, despite their high costs, for nearly all patient subgroups. Recent studies consider many countries: Canada,4,5 China,6,7 Egypt,8 Germany,9 Italy,10,11 Japan,12–15 Norway,16 Singapore,17,18 South Africa,19 Spain,20–22 Switzerland,23 Thailand,24 the United Kingdom,25–27 and the United States of America.28–32 Earlier studies of this type have been summarized in published reviews.33–35 The cost-effectiveness of DAAs compared to older regimens continues to improve with the availability of multiple new regimens and a concomitant decline in prices.
Our review aimed to synthesize model-based evidence on the cost-effectiveness and the population health impacts of a “treat all” policy for HCV. Specifically, the WHO Global Hepatitis Programme charged us with reviewing the model-based literature to identify 1) the cost-effectiveness of expanded access to HCV treatment compared to more restrictive treatment access policies; and 2) the population health impacts—for example, reductions in incidence, prevalence, and HCV-related morbidity and mortality—of expanded access to HCV treatment compared to more restrictive treatment access policies. To answer the first question, we focused on reports that assessed the cost-effectiveness, measured in incremental costs per quality-adjusted life-years (QALY)-gained, of offering treatment to all individuals chronically infected with HCV compared to providing treatment to only a subgroup of such individuals or delaying treatment conditional on an event (e.g., fibrosis progression or cessation of injection drug use). To answer the second question, we focused on analyses that compared projected HCV incidence, prevalence, and HCV mortality over the next 10, 15, or 20 years under different HCV treatment access policies.
Methods
We searched the peer-reviewed medical literature for research articles evaluating expanded-access strategies compared to more restrictive policies, focusing on recent model-based evaluations of all-oral directly acting antivirals that considered impacts on HCV epidemiology (e.g., incidence, prevalence), population health outcomes (e.g., total cases of hepatocellular carcinoma [HCC] or HCV-related mortality), or economic value. We searched PubMed using combinations of free text and MeSH search terms, shown in Table 1. We then scanned the references of review articles for additional studies. We also searched for relevant gray literature to identify additional sources.
Table 1.
Search Term Combinations and the Number of Results They Returned (Search Performed on June 24, 2017)
| Searches: Search Term Combinations | # of Resultsa |
|---|---|
| (“Hepatitis C”[Mesh] OR “Hepatitis C, Chronic”[Mesh]) AND (“Disease Transmission, Infectious”[Mesh] OR “Computer Simulation”[Mesh] OR “Markov Chains”[Mesh] OR “Models, Theoretical”[Mesh] OR “Models, Biological”[Mesh]) AND (“Disease Eradication”[Mesh] OR “Communicable Disease Control”[Mesh]) | 259 |
| (“Hepatitis C”[Mesh] OR “Hepatitis C, Chronic”[Mesh]) AND (“Disease Transmission, Infectious”[Mesh] OR “Computer Simulation”[Mesh] OR “Markov Chains”[Mesh] OR “Models, Theoretical”[Mesh] OR “Models, Biological”[Mesh]) AND (“Cost-Benefit Analysis”[Mesh] OR “Decision Support Techniques”[Mesh] OR “Policy Making”[Mesh]) | 216 |
| (“Hepatitis C” OR “HCV”) AND (“Markov” OR “dynamic transmission” OR “decision science” OR “decision-analytic” OR “decision analytic” OR “simulation” OR “agent-based” or “agent based” OR “simulation” OR “microsimulation” OR “differential equation” OR “network”) | 1,904 |
| (“Hepatitis C” OR “HCV”) AND (“cost-effectiveness”) | 633 |
| (“Hepatitis C” OR “HCV”) AND (“cost-utility”) | 49 |
| (“Hepatitis C” OR “HCV”) AND (“cost-benefit” OR “benefit-cost”) | 604 |
| (“Hepatitis C” OR “HCV”) AND (“policy analysis” OR “economic analysis” OR “economic evaluation”) | 80 |
| (“Hepatitis C” OR “HCV”) AND (“incremental cost effectiveness ratio” OR “incremental cost-effectiveness ratio” OR “ICER” OR “QALY”) | 222 |
| (“Hepatitis C” OR “HCV”) AND (“elimination” OR “eradication” OR “disease control” OR “epidemic control” OR “herd immunity” OR “herd effects” OR “indirect benefits” OR “incidence” OR “prevalence” OR “population benefit”) AND (“model-based” OR “model based”) | 25 |
| Identified under PubMed feature “Titles with your search terms” in addition to those identified via the main search (for all search terms above) | 362 |
Total number of unique articles returned by all searches was less than the sum of the numbers returned by each search because some were duplicates.
We screened the titles and applied exclusion criteria conservatively (i.e., when uncertain about an article’s relevance, we included it in the next round of evaluation; Table 2). We reviewed abstracts, if available, and retrieved full text when an abstract was unavailable to evaluate relevance. We retrieved the full text and reviewed articles for analyses related to our specific questions of interest.
Table 2.
Exclusion Criteria
| • Referred to other diseases as primary topic (HIV, hemophilia, etc.) • Letters to the editor, editorials, or other short commentaries • Microbiology/biochemistry studies • Within cell or within host modeling • Descriptive observational, natural history, or epidemiological studies • Direct cost estimates only • Elicitation of quality of life weights • Studies dealing with health worker infection prevention • Studies related to screening donor blood supply • Actual focus was not on curative treatment of HCV but on management of health consequences related to HCV (i.e., timing of liver transplant, screening for liver cancer in patients with HCV) • Methodological articles with highly stylized examples that might refer to HCV tangentially • Study focus was the timing of treatment before or after liver transplant |
HCV, hepatitis C virus.
We focused our evidence synthesis on full-text, peer-reviewed, original reports published after 2012 since highly effective, directly acting antivirals, especially those that were all-oral and interferon-free, became available after this date. We also reviewed the full text of selected titles published in 2012 or earlier if the potential for specific relevance to the review’s questions had been identified at the abstract stage or if they included analyses of low- and middle-income country settings where the number of overall publications was lower. Many studies included in our analysis did not focus primarily on expanded treatment access relative to more restricted access, but did present this comparison in sensitivity analyses.
Presentation and Discussion of Published Articles
Results of Search Strategy
Our search strategy produced a list of 2,917 unique titles after removing duplicates. Title review followed by abstract review reduced the number of titles to 339 abstracts—original research and review articles—to retrieve for full-text review. After searching the gray literature and the references of reviews, we compiled a list of 348 original research and review articles: 51 published in 2017; 55 published in 2016; 59 published in 2015; 37 published in 2014; 24 published in 2013; and 122 published between 1994 and 2012. Many of the full texts did not meet the inclusion/exclusion criteria, did not include the opportunity to delay treatment for patients with earlier-stage fibrosis, or did not present sufficient detail in the reporting of their sensitivity analysis to be included.
Review of the full texts identified 31 articles reporting the cost-effectiveness of expanded access to HCV treatment compared to more restrictive treatment access policies as their main or a secondary analysis; 4 cost-effectiveness analyses presenting results on highly stratified patient cohorts providing information on specifically which cohort’s treatment expansion is or is not cost-effective; and 7 additional articles reporting on the cost-effectiveness of newer HCV treatment alternatives compared to older treatments or no treatment in low- and middle-income countries. We also identified 30 articles that compared population health outcomes of various HCV treatment expansion strategies (three of which also included cost-effectiveness analyses).
Cost-Effectiveness of Expanded Access to HCV Treatment
General Population
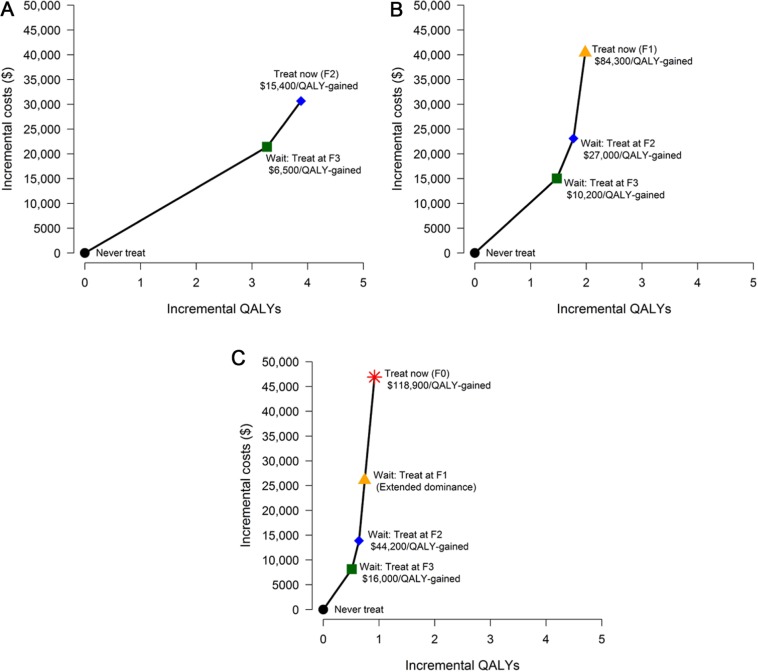
Cost-effectiveness analyses of treatment expansion to milder fibrosis stages compared to more restricted access to treatment exist for Canada,36 Egypt,37,38 France,39,40 Germany,41 Italy,42 the Republic of Korea,43 the United Kingdom,44 and the United States.45–57 In their own conclusions, these studies find that expanding treatment more broadly in the general population is cost-effective, though may add substantial shorter-term costs to pay for treatment (Table 3). Few studies specifically consider access for patients with each successively milder stage of liver disease; for those that do,45,48 delaying treatment for individuals with the mildest fibrosis (metavir score F0) until progression to F1 fibrosis is identified as the optimal strategy at current prices (e.g., Figure 1).
Table 3.
Cost-Effectiveness of Treating All Patients Versus Delaying Treatment to Later Fibrosis Stage or to Later Diagnosis and Fibrosis Stage: General Population
| Analysis |
Model Features |
Population |
LMIC | Newest Drug(s) | Comparator(s) | ICER ($/QALY Gained) | Currency (Year) | Notes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Genotype | Fibrosis Stratified | Delay Considered | Model Typea | Transmission | Reinfection | PWID-Focus | Incarcerated | Highly Stratified | ||||||
| Chahal, 201645 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | SOF-LDV | SOF-LDV delayed | ≥F2 v. ≥F3: 8,687 ≥F3 v. ≥F2: 41,757 ≥F0 v. ≥F1: 105,167 |
US$ (2014) | Reporting values from sensitivity analysis with 46% price reduction (to $5,040/week) |
| Chidi, 201647 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | 3D for all fibrosis stages | SOF-LDV, 3D, standard of care for F4 first or ≥F3 first | Cost saving | US$ (2014) | US Veterans Affairs patient population and costs |
| Chidi, 201646 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | SOF-LDV for all patients | SOF-LDV for advanced fibrosis | Cost saving | US$ (2015) | Medicaid patient population |
| Cortesi, 201542 | Italy | 1 | Yes | Yes | M | No | No | No | No | No | No | TVR or BOC-based therapies for ≥F1 | Same therapies for ≥F2, ≥F3 patients | TVR: 5,132 BOC: 7,043 |
€ (2013) | |
| Crossan, 201544 | UK | 1–4 | Yes | Yes | M | No | No | No | No | No | No | TVR or BOC-based therapies for patients regardless of fibrosis | TVR or BOC-based therapies for patients after fibrosis monitoring for ≥F2 | 9,204 | £ (2011) | Includes many genotypes but does not report ICER by genotype. Considers many different fibrosis monitoring technologies and positivity cutoffs. |
| Deuffic-Burban, 201639 | France | 1–4 | Yes | Yes | M | No | No | No | No | No | No | IFN-free new DAAs with or without ribavirin for all (GT1 and GT4); IFN-based regimens for all patients (GT2 and GT3) | IFN-free new DAAs with or without ribavirin for ≥F3, ≥F2, IFN-using DAAs for ≥F3, ≥F2, or all, BOC or TVR regimens for ≥F3, ≥F2, or all | GT1: 40,400 GT2: 21,300 GT3: 19,400 GT4: 23,000 |
€ (2015) | |
| Ethgen, 201740 | France | 1–4 | Yes | Yes | M | No | No | No | No | No | No | IFN-free DAAs for stages F0–F4 | Various HCV screening scenarios combined with treatment strategies including no antiviral therapy | 25,832 | € (2015) | |
| Kim, 201537 | Egypt | 4 | Yes | No | M | No | No | No | No | No | Yes | Screening and treatment with SOF-PGN and ribavirin | Not screening and treating | Cost saving | US$ (2014) | |
| Kim, 201743 | Republic of Korea | 1, 2 | No | No | M | No | No | No | No | No | No | Onetime screening and treatment with an all-oral DAA for 40–70 year-olds | Not screening | 40–49 year olds: 5,714 50–59 year olds: 6,843 60–69 year olds: 8,889 |
US$ (2016) | Does not consider delaying screening to later ages for 40–49 or 50–59 |
| Leidner, 201548 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | Treatment at ≥F2 with DAA | Treatment at F4, ≥F3, ≥F2, ≥F1, ≥F0 | F2 patient, treat now v. at F3: 15,400 F1 patient, treat now v. at F2: 84,300 F0 patient, treat now v. at F2: 120,000 |
US$ (2016) | Reporting values from sensitivity analysis using $50,000 for course of treatment. Like Chahal,45 treating at ≥F0 compared to ≥F1 drops to below $100,000 per QALY gained if drug price drops to $42,400 for the total regimen and under $50,000 per QALY gained if drug price drops to $22,200 for the total regimen. |
| Linas, 201749 | US | 1 | Yes | Yes | MS | No | Yes | No | No | Yes | No | 3D + ribavirin or SOF-LDV to treat all patients depending on cirrhosis status and being treatment naïve versus experienced | Other treatment options and then treating ≥F2 with best regimen versus ≥F0 | <40,000 for all groups | US$ (2014) | |
| Linthicum, 201650 | US | 1, 2, 3 | Yes | Yes | DT | Yes | Yes | No | No | No | No | Screening and treatment of ≥F0 with all-oral DAA | Current Screening only and Screening and treatment of ≥F2 or ≥F3 with all-oral DAA | Current screening, ≥F0 v. ≥F2: Cost saving Universal screening and ≥F0: 20,175 |
US$ (2015) | The study reports its main results in terms of positive NMB using a WTP of $150,000 per QALY gained. In all screening scenarios QALYs are highest and costs lowest with treat ≥F0. |
| Liu, 201151 | US | 1, 2, 3 | Yes | Yes | M | No | No | No | No | Yes | No | Immediate treatment with TVR for >F0 | Various fibrosis monitoring techniques with treatment for ≥F2 | <32,000 across age 40–70 and sex subgroups | US$ (2009) | |
| Liu, 201352 | US | 1, 2, 3 | Yes | No | M | No | No | No | No | Yes | No | Birth cohort screening and treatment of ≥F0 with TVR or BOC-based therapies | No screening expansion or risk-based screening with various treatment regimens | <100,000 for 40–69 | US$ (2010) | Screening to expand treatment costs >150,000 per QALY gained for individuals aged 70+; Cost-effectiveness of screening within age groups depends on age-specific HCV prevalence. Cost-effectiveness also depends on the price and efficacy of treatment regimens. |
| Martin, 201658 | UK | 1–4 | Yes | Yes | DT | Yes | Yes | Yes | No | No | No | Treat all, targeting PWID/non- or ex-PWID | Delay treatment until advanced fibrosis and/or exclude PWID | Regardless of PWID HCV-prevalence, treatment of mild (F0–F1) Ex/non-PWID v. delay to moderate (≥F2) ICER between £20,000 and £30,000/QALY gained | £ (2014) | Treatment of general population at mild (F0/F1) v. moderate (≥F2) is cost effective if WTP = £30,000/QALY gained. |
| Moreno, 201753 | US | 1, 2, 3 | Yes | Yes | DT | Yes | Yes | Yes | No | Yes | No | Treatment of ≥F0 including PWIDs with all-oral DAA | Not including PWIDs and/or only ≥F3 in treatment | Non-PWID (Treat all v. ≥F3): Cost saving All non-PWID + PWID v. ≥F3 non-PWID + PWID: Cost saving |
US$ (2015) | With the information presented, comparing All non-PWID + PWID v. All non-PWID and no PWID increases net monetary benefit at WTP of $100,000 per QALY gained. |
| Obach, 201438 | Egypt | 4 | Yes | Yes | M | No | No | No | No | No | Yes | PGN and ribavirin for ≥F1 | Waiting until later fibrosis | F1, F2, F3 v. delay: Cost saving F4 v. never treat: 1,915 |
US$ (2012) | Analysis also considers when triple therapy becomes available as part of the waiting decision and it was cost-effective to wait until patients were F2 prior to the arrival of more effective therapy. This analysis is no longer relevant and hence the analysis shows that immediate treatment for ≥F1 is cost-effective. |
| Sbarigia, 201741 | Germany | 1–4 | Yes | No | DT | Yes | Yes | No | No | No | No | Increasing annual treatment capacity (treatment expansion) | Lower or no expansion scenarios | <30,000 | € (2015) | The study reports its main results in terms of positive NMB at 30,000 Euro per QALY gained. The most aggressive expansion has the highest NMB. |
| Tice, 201554 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | Treat all (F0–F4) using a range of all-oral DAA | Treat F3–F4 only | LDV/SOF (8/12 weeks): 35,975 | US$ (2014) | |
| Van Nuys, 201555 | US | 1, 2, 3 | Yes | Yes | DT | Yes | Yes | Yes | No | No | No | Treat all diagnosed patients | Treat advanced fibrosis; treat 5% of all patients annually | <100,000 | US$ (2014) | The study reports its main results in terms of NMB at WTP of $100,000 per QALY gained. The treat all policy has the highest NMB. |
| Wong, 201536 | Canada | 1–6 | Yes | No | M | No | No | No | No | No | No | Screen and treat with interferon-free DAAs | Screen and treat with older regimens or status quo (no screening) | 25–64 years old: 34,783 45–64 years old: 36,471 |
Can$ (2014) | DAA cost: 4,500/week |
| Younossi, 201756 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | SOF-LDV for all diagnosed patients | Treat advanced fibrosis | Cost saving | US$ (2014) | Medicaid patient population DAA cost: 4,000/week |
| Younossi, 201457 | US | 1 | Yes | Yes | M | No | No | No | No | No | No | Treat all-oral DAA for ≥F0 | Treat all-oral DAA for ≥F2; Treat triple therapy ≥F2 or ≥F0 | 15,709 | US$ (2012) | Base case age: 50 years DAA cost: 5,800/week |
3D, paritaprevir/ritonavir-ombitasvir and dasabuvir; BOC, boceprevir; DAA, direct-acting antiviral; DCV/ASV, daclatasvir/asunaprevir; EBR/GZR, elbasvir/grazoprevir; F0, F1, F2, F3, F4, indicates severity of patient’s liver disease using metavir fibrosis scale (“≥F2” indicates all patients with at least F2 fibrosis which is F2, F3, and F4); HCV, hepatitis C virus; HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; IFN, interferon; LMIC, low- and middle-income countries; NMB, net monetary benefit; PGN, pegylated interferon; PWID, people who inject drugs; QALY, quality-adjusted life year; SOF, sofosbuvir; SOF-DCV, sofosbuvir/daclatasvir; SOF-LDV, sofosbuvir/ledipasvir; SOF-PGN, sofosbuvir and pegylated interferon; SVR, sustained virologic response; TVR, telaprevir; WTP, willingness to pay.
Model type: M, Markov model; DT, dynamic transmission model; MS, microsimulation; AB, agent-based simulation.
Figure 1.
Cost-effectiveness analysis performed by Leidner and others48 comparing treat now versus waiting for disease progression in the general US population using a direct acting antiviral at a cost of $50,000 for a course of treatment for patients with (A) F2 fibrosis, (B) F1 fibrosis, and (C) F0 fibrosis. In a threshold analysis, the cost of treating patients with F0 fibrosis without delay falls to $100,000 per QALY gained at a treatment cost of $42,400 and falls to $50,000 per QALY gained at a treatment cost of $22,200.
Of the 23 articles that made comparisons of expanded access to earlier fibrosis stages compared to more restrictive treatment access policies, only five models included disease transmission;41,50,53,55,58 one additional model included a risk of reinfection after successful treatment.49 While nearly all studies examine the cost-effectiveness of treatment expansion to earlier fibrosis stages for genotype 1, several studies include multiple genotypes and/or focus explicitly on genotypes 2, 3, or 4.36–41,44,50–53,55 Though treatment expansion to earlier fibrosis stages in other genotypes costs more per QALY gained in general, it is still found to be cost-effective in non–genotype 1 patients.
These studies also consider a variety of DAA regimens. As efficacies of most of these regimens are high and reasonably similar, whichever regimen is the least costly (lowest price in each system) tends to be the one identified as the cost-effective alternative for immediate treatment.
Fewer studies consider both screening and treatment with DAAs, accounting for the additional costs to identify more undiagnosed infected individuals as a necessary step in treatment expansion given current low levels of diagnosis for those with chronic HCV.1 In general, these studies find that screening followed by broadening treatment availability (typically assuming that screening identifies those with milder fibrosis) is cost-effective.36,37,43,50,52
Patient age influences the cost-effectiveness of treatment expansion. Evidence supporting the cost-effectiveness of broad access to all fibrosis stages is strongest for studies of people currently aged 40 to 65. Analyses including individuals age 70 and older generally find that treating all individuals regardless of fibrosis stage yields smaller health benefits at costs too high to be considered cost-effective (e.g., Figure 2). This implies that the cost-effectiveness of treatment expansion for older individuals with mild fibrosis is highly sensitive to treatment price and in some settings, where relatively high prices remain, may not be cost-effective.45,48,52,59 Few articles consider the cost-effectiveness of treatment expansion for individuals who are currently between 20 and 40 years of age, though HCV treatment is generally found to be more cost-effective in younger individuals.
Figure 2.
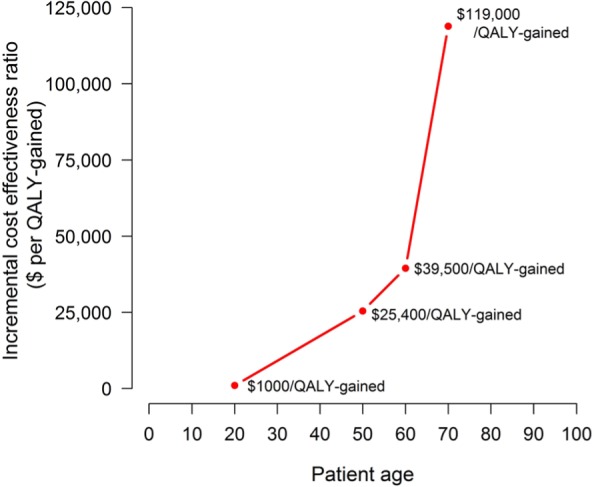
Cost-effectiveness analysis performed by Chahal and others45 comparing treat all (at any fibrosis stage) versus restricting treatment access to individuals with more severe liver disease (metavir stages F3 and F4) varying patient age. The analysis presented used a weekly treatment cost of $7,875 and a mix of treatment durations (8–12 weeks) depending on disease severity.
People Who Inject Drugs (PWID) and Incarcerated Individuals
The cost-effectiveness of HCV treatment in PWID is influenced by the high cost of treatment, the potential for preventing new infections, and by the risk of reinfection. Studies from Australia,60–62 the Netherlands,63 and the United Kingdom58,64,65 find that it is generally cost-effective to treat HCV-infected PWIDs (Table 4). Furthermore, some studies find that 1) intensified case finding in this group is cost-effective along with treatment scale-up;64,65 2) treatment for PWIDs regardless of fibrosis stage is cost-effective compared to delaying treatment;58,61 and 3) treatment can be cost-effective even in a declining epidemic.63 Of the seven studies that evaluated the cost-effectiveness of expanded treatment access to PWID, five included disease transmission,58,60,63–65 and all models included a risk of reinfection after successful treatment.
Table 4.
Cost-Effectiveness of Treating All Patients Versus Delaying Treatment to Later Fibrosis Stage or to Later Diagnosis and Fibrosis Stage: People Who Inject Drugs (PWID).
| Reference | Country | Genotype | Analysis |
Model Features |
Population |
LMIC | Newest Drug(s) | Comparator(s) | ICER ($/QALY Gained) | Currency (Year) | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis Stratified | Delay Considered | Model Typea | Transmission | Reinfection | PWID Focus | Incarcerated | Highly Stratified | |||||||||
| Bennett, 201664 | UK | 1, 3 | Yes | No | DT | Yes | Yes | Yes | No | No | No | SOF-DCV to treat PWIDs at high uptake rates | TVR-based treatment; PR treatment at different uptake rates | Cost saving | £ (2013) | Dependent on patient genotype, the cost-effectiveness of HCV treatment using daclatasvir plus sofosbuvir improved by 36% to 79% versus conventional analysis, at 10% to 100% treatment uptake in the PWID population. |
| Martin, 201665 | UK | 1–4 | Yes | Implicit | DT | Yes | Yes | Yes | Yes | No | No | Double testing rates and provide all-oral DAA in prison | Status quo | 15,090 | £ (2014) | Delay is implicit if lower screening rates imply later detection (and hence more advanced fibrosis progression). |
| Martin, 201658 | UK | 1–4 | Yes | Yes | DT | Yes | Yes | Yes | No | No | No | Treat all, targeting PWID/ex-PWID | Delay treatment until advanced fibrosis and/or exclude PWID | PWID HCV prevalence ≤40%, ≥F2 PWID and ex-PWID: <20,000 PWID HCV prevalence = 60%, ≥F2 ex-PWID: <20,000; including any PWID is not cost-effective |
£ (2014) | When reinfection risk is high and transmission not substantially cut due to high HCV prevalence, immediate treatment targeting PWID is less cost-effective. |
| Scott, 201660 | Australia | 1, 2, 3 | Yes | Yes | DT | Yes | Yes | Yes | No | No | No | Treatment expansion to active PWIDs with less advanced fibrosis | No expansion or expansion to reduce either mortality or incidence alone | 25,121 | Aus$ (2014) | |
| Scott, 201661 | Australia | 1, 2, 3 | Yes | Yes | DT | No | Yes | Yes | No | No | No | Treat early fibrosis | Treat late fibrosis; no treatment | 17,090 | Aus$ (2014) | Assumes an exogenous rate of reinfection (that treatment will not be scaled up sufficiently to impact infection risk to others). |
| Van Santen, 201663 | Netherlands | 1–4 | Yes | Implicit | MS with DT | Yes | Yes | Yes | No | No | No | Dual DAAs with 3× treatment uptake via screening | Dual DAAs or status quo treatment at lower screening/uptake levels | <4,115 | € (2014) | With an epidemic in decline (as in Amsterdam, Netherlands), the ICER is 4,115. With a stable epidemic (greater risk of transmission without treatment), the ICER is lower and treatment of PWID is more cost-effective. Delay is implicit if lower screening rates imply later detection (and hence more advanced fibrosis progression). |
| Visconti, 201362 | Australia | 1/non-1 | Yes | No | M | No | Yes | Yes | No | No | No | Treat ≥F1 | Treat ≥F4; treat ≥F2; best supportive care only | Non-injectors: 4,221 Active injectors: 7,719 Former injectors: 5,919 |
Aus$ (2011) | |
DAA, direct-acting antiviral; DCV/ASV, daclatasvir/asunaprevir; F0, F1, F2, F3, F4, indicates severity of patient’s liver disease using metavir fibrosis scale (“≥F2” indicates all patients with at least F2 fibrosis which is F2, F3, and F4); HCV, hepatitis C virus; ICER, incremental cost effectiveness ratio; LMIC, low- and middle-income countries; PWID, people who inject drugs; QALY, quality-adjusted life year; SOF, sofosbuvir; SOF-DCV, sofosbuvir/daclatasvir; TVR, telaprevir.
Model type: M, Markov model; DT, dynamic transmission model; MS, microsimulation; AB, agent-based simulation.
An important caveat to these observations is high-incidence/high-prevalence settings. When HCV prevalence is high (perhaps above 50%), the cost-effectiveness of preventing onward transmission via treatment is diminished by the high probability of reinfection.58 In these settings, there are too many possible paths to infection to disrupt transmission and so both the personal health benefits to the treated individual and the hoped-for prevention of secondary infections are difficult to realize without extremely high rates of diagnosis and treatment or substantial concurrent efforts with HCV-transmission prevention strategies.
Many of the insights obtained for PWIDs are applicable to communities of incarcerated individuals because rates of reinfection are high in prisons. However, treatment discontinuation and the associated loss of efficacy is a potential risk as individuals may be transferred or released before treatment completion. Studies from the United Kingdom,65 and the United States,66,67 two of which included disease transmission,65,66 find that it is generally cost-effective to treat HCV-infected incarcerated individuals and that screening to identify HCV-infected individuals on entry to prisons/jails is cost-effective (Table 5). Likewise, concurrent investments in HCV prevention programs complement treatment by reducing reinfection and secondary transmission from other infected individuals.
Table 5.
Cost-Effectiveness of Treating All Patients Versus Delaying Treatment to Later Fibrosis Stage or to Later Diagnosis and Fibrosis Stage: Incarcerated Individuals
| Reference | Country | Genotype | Analysis |
Model Features |
Population |
LMIC | Newest Drug(s) | Comparator(s) | ICER ($/QALY Gained) | Currency (Year) | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis Stratified | Delay Considered | Model Typea | Transmission | Reinfection | PWID Focus | Incarcerated | Highly Stratified | |||||||||
| He, 201666 | US | 1, 2, 3, 4 | Yes | Yes | AB | Yes | Yes | Yes | Yes | No | No | 10-year opt-out screening of incoming inmates with new DAA treatment | Risk-based screening or opt-out screening for shorter time periods | Treatment of ≥F3: 29,234 Treatment ≥F0: <50,000 |
US$ (2014) | The main analysis focuses on treatment access for ≥F3 but sensitivity analyses show that expanded sustained screening followed by treatment regardless of fibrosis stage also costs <$50,000 per QALY gained. |
| Liu, 201467 | US | 1 | Yes | Implicit | M | No | Yes | No | Yes | No | No | SOF-PGN and ribavirin treatment while incarcerated | No therapy or older therapies while incarcerated | <30,000 | US$ (2013) | Exact ICER depended on length of incarceration. Delay is implicit if lower screening rates imply later detection (and hence more advanced fibrosis progression). |
| Martin, 201665 | UK | 1, 2, 3, 4 | Yes | Implicit | DT | Yes | Yes | Yes | Yes | No | No | Double testing rates and provide all-oral DAA in prison | Status quo | 15,090 | £ (2014) | Delay is implicit if lower screening rates imply later detection (and hence more advanced fibrosis progression). |
DAA, direct-acting antiviral; F0, F1, F2, F3, F4, indicates severity of patient’s liver disease using metavir fibrosis scale (“≥F2” indicates all patients with at least F2 fibrosis which is F2, F3, and F4); ICER, incremental cost-effectiveness ratio; LMIC, low- and middle-income countries; PWID, people who inject drugs; QALY, quality-adjusted life year; SOF, sofosbuvir.
Model type: M, Markov model; DT, dynamic transmission model; MS, microsimulation; AB, agent-based simulation.
Highly Stratified Analyses
Cost-effectiveness analyses conducted in many highly stratified subpopulations can help identify subgroups for which treatment is not cost-effective. The intuition is that if certain characteristics determine the benefits, costs, and harms of treatment versus no treatment, value of treatment expansion can be uneven across groups, and even if, on average, for the population as a whole treatment expansion is cost-effective, this does not imply that it is in all subgroups. Studies considered subgroups defined by age (shorter remaining life expectancy); comorbidities and frailty (higher competing risks); and genotype (treatment alternatives have different costs and efficacy).
Studies performing highly stratified analysis tend to present cost-effectiveness analyses only on the question of whether it is cost-effective to treat the patient now versus not at all (Table 6).59,68–70 By not including the option to treat later, these analyses may be biased toward finding that “treat now” is cost-effective. However, comparing across the subpopulations within the analysis is still informative in showing which subpopulations have substantially higher costs per QALY gained (generally because the QALYs gained occur for lower proportions of these subpopulation and/or further in the future). Studies from countries including Israel,71 Italy,59 Scotland,72 and the United Kingdom68,69 find less benefit gained by treatment of older individuals, especially those who have more mild fibrosis or are frail. As the groups focused on tend to be the elderly, the omission of transmission from these studies is not likely a substantial problem if the primary mode of current transmission is illicit injection drug use as these subpopulations have very low prevalence of this risk behavior. However, in settings like Egypt where substantial transmission occurs through unsafe medical injection practices and/or limited access to sterilized medical equipment, treatment in older patients may have additional population health benefits that are not accounted for.
Table 6.
Highly Stratifying the Population and the Cost-Effectiveness of Treat All
| Reference | Country | Genotype | Analysis |
Model Features |
Population |
LMIC | Newest Drug(s) | Comparator(s) | ICER ($/QALY Gained) | Currency (Year) | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis Stratified | Delay Considered | Model Typea | Transmission | Reinfection | PWID Focus | Incarcerated | Highly Stratified | |||||||||
| Ciaccio, 201759 | Italy | 1–4 | Yes | No | M | No | No | No | No | Yes | NO | All-oral DAA treatment for people 65–85 by fibrosis stage and frailty | No treatment | <40,000 for younger, less, frail, more advanced fibrosis, and GT1 | € (2013) | Older individuals, especially when frail, with F1 or F2 and with GT2 or 3 have ICERs >40,000 per QALY gained. All ICERs are likely is biased toward cost-effectiveness in general because the analysis does not consider delaying treatment to later fibrosis stages. |
| Cure, 201568 | Italy | 1–6 | No | No | M | No | Yes | No | No | Yes | NO | SOF-based therapy relative to others defined by cirrhosis, prior treatment, and genotype | Appropriate treatment regimens or no treatment | <40,000 for most groups | € (2013) | Above the WTP threshold for treatment naïve GT4, 5, 6 and for some noncirrhotic groups. Given that delay is not considered it may be the case that a greater number of non-cirrhotic groups would have ICERs above the WTP threshold. |
| Cure, 201569 | UK | 1–6 | No | No | M | No | Yes | No | No | Yes | NO | SOF-based therapy relative to others defined by prior treatment, interferon eligible, and genotype | Appropriate treatment regimens or no treatment | <20,000 for most groups | £ (2011) | For GT2, 3, 4, 5, 6 who are treatment naïve and unable to take interferon SOF triple therapy had an ICER >20,000/QALY gained. Given that delay is not considered it may be the case that a greater number of treatment experienced and/or interferon eligible groups would have ICERs above the threshold. |
| Elbasha, 201770 | US | 1 | Yes | No | M | No | Yes | No | No | Yes | NO | EBR/GZR ± ribavirin for noncirrhotic/cirrhotic, treatment experienced/naïve patients | Other all oral DAAs (SOF-LDV, 3D) | <26,000 | US$ (2015) | Subgroup analyses showed that even at F0, the ICER was <$60,000 per QALY gained. Given that delay is not considered it may be the case that the ICERs estimated are overly favorable. |
3D, paritaprevir/ritonavir-ombitasvir and dasabuvir; DAA, direct-acting antiviral; EBR/GZR, elbasvir/grazoprevir; F0, F1, F2, F3, F4, indicates severity of patient’s liver disease using metavir fibrosis scale (“≥F2” indicates all patients with at least F2 fibrosis which is F2, F3, and F4); ICER, incremental cost-effectiveness ratio; LMIC, low- and middle-income countries; PWID, people who inject drugs; QALY, quality-adjusted life year; SOF, sofosbuvir; SOF-LDV, sofosbuvir/ledipasvir; WTP, willingness to pay.
Model type: M, Markov model; DT, dynamic transmission model; MS, microsimulation; AB, agent-based simulation.
Low- and Middle-Income Countries
Evidence on the cost-effectiveness of expanded versus more restricted treatment is substantially more limited for low and middle-income countries.73 Our review identified cost-effectiveness analyses related to access to HCV treatment for Chile,74 China,6,7 Egypt,37,38 India,75 Iran,76 South Africa,19 and Thailand,24 although these studies did not generally address our question of interest directly. The overall finding was that newer drugs offered substantial health benefits especially when broadly delivered but that sufficiently low drug prices were critical for cost-effectiveness (Table 7). None of the cost-effectiveness evaluations of HCV treatment focused on low- and middle-income countries included disease transmission or the possibility of reinfection.
Table 7.
Evidence From Low- and Middle-Income Countries
| Reference | Country | Genotype | Analysis |
Model Features |
Population |
LMIC | Newest Drug(s) | Comparator(s) | ICER ($/QALY Gained) | Currency (Year) | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis Stratified | Delay Considered | Model Typea | Transmission | Reinfection | PWID Focus | Incarcerated | Highly Stratified | |||||||||
| Aggarwal, 201775 | India | 1, 3, 4 | No | No | MS | No | No | No | No | Yes | Yes | DAA treatment | No treatment | Cost saving | US$ (2016) | Finding consistent across cirrhosis/no cirrhosis, ages 20–70 given a very low drug price. |
| Alavian, 201676 | Iran | 1 | No | No | MS | No | No | No | No | No | Yes | SOF-LDV treatment | SOF-based triple therapy; PGN and ribavirin | 190,335 | Int $b (2014) | SOF+PGN-RBV is lowest cost option. |
| Chen, 20166 | China | 1 | Yes | No | M | No | No | No | No | Yes | Yes | SOF-LDV by region and cirrhosis/no cirrhosis and treatment experienced/naïve | PGN and ribavirin |
Treatment naïve
No cirrhosis: 85,588 Cirrhosis: 15,975 Treatment experienced No cirrhosis: 25,067 Cirrhosis: 9,947 |
US$ (2014) | Only for cirrhotic patients does the ICER fall below $22,770 per QALY gained, the country specific willingness to pay threshold. ICERs may be high because the SVR rate in PGN and ribavirin for patients without cirrhosis is 75%. |
| Chen, 20177 | China | 1 | Yes | No | M | No | No | No | No | No | Yes | All oral DAA | PGN and ribavirin | 12,536 | US$ (2016) | May not be cost-effective to treat people with F0 fibrosis age 50+. The analysis includes waiting for new treatments but not in the sense of treat now with a given drug versus waiting like others reviewed. |
| Fraser, 201619 | South Africa | 5 | No | No | M | No | No | No | No | No | Yes | SOF-LDV | SOF-based triple therapy; PGN and ribavirin | Cost saving | US$ (2015) | |
| Kapol, 201624 | Thailand | 1, 6 | No | No | M | No | No | No | No | No | Yes | PGN and ribavirin | Palliative care | Cost saving | Baht (2013) | |
| Kim, 201537 | Egypt | 4 | Yes | No | M | No | No | No | No | No | Yes | Screening and treatment with SOF-PGN and ribavirin | Not screening and treating | Cost Saving | US$ (2014) | |
| Obach, 201438 | Egypt | 4 | Yes | Yes | M | No | No | No | No | No | Yes | PGN and ribavirin for ≥F1 | Waiting until later fibrosis | F1, F2, F3 v. delay: Cost saving F4 v. never treat: 1,915 |
US$ (2012) | Analysis also considers when triple therapy becomes available as part of the waiting decision and it was cost-effective to wait until patients were F2 prior to the arrival of more effective therapy. This analysis is no longer relevant and hence the analysis shows that immediate treatment for ≥F1 is cost-effective. |
| Vargas, 201574 | Chile | 1 | Yes | No | M | No | No | No | No | No | Yes | DCV/ASV | DAA, PGN and ribavirin | 6,375 | US$ (2014) | |
ASV, daclatasvir; DAA, direct-acting antiviral; ASV, asunaprevir; F0, F1, F2, F3, F4, indicates severity of patient’s liver disease using metavir fibrosis scale (“≥F2” indicates all patients with at least F2 fibrosis which is F2, F3, and F4); ICER, incremental cost-effectiveness ratio; LMIC, low- and middle-income countries; PGN, pegylated interferon; PWID, people who inject drugs; QALY, quality-adjusted life year; SOF, sofosbuvir; SOF-LDV, sofosbuvir/ledipasvir; SOF-PGN, sofosbuvir and pegylated interferon; SVR, sustained virologic response.
Model type: M, Markov model; DT, dynamic transmission model; MS, microsimulation; AB, agent-based simulation.
This article used international dollars. “To make international comparisons we tried to convert the costs to international dollars using the purchasing power parity (PPP) with an exchange rate of 8565.41 Rials per $1.”76
A cost-effectiveness analysis for Egypt examined immediate treatment of patients with earlier stages of fibrosis relative to waiting for progression to later stages, considering patients with F1 to F4 fibrosis.38 In general, this study found that it was cost-effective to treat patients at their current fibrosis stage except in patients with the mildest fibrosis stages. Sensitivity analyses revealed treatment at F1 fibrosis should also be cost-effective given efficacy of newer drugs and prices offered in Egypt. A cost-effectiveness analysis for India did not examine treating now versus delay but did consider the cost-effectiveness of treatment highly stratified subpopulations by fibrosis stage and genotype as well as for many age groups (20–70 years).75 At a price of $100 for 4 weeks of treatment, this analysis found that treatment of all groups compared to no treatment was cost-effective; this remained the case for prices approaching $900 per 4 weeks.
Several recent articles detail epidemiological modeling for multiple low- and middle-income countries concerning the impact of broad treatment expansions and their effect on reduced epidemics and gains in population health in terms of life years, QALY, prevalence, and mortality.77–80 A number of these analyses include transmission and reinfection effects.78–80 Broadly expanded treatment has the potential to provide substantial health gains including secondary health gains due to reduced transmission risks and reinfection risks, which, at substantial coverage levels and a sufficiently low drug price, could be cost-effective. However, for low- and middle-income countries, we did not identify any studies including the potential benefits of reduced disease transmission that also quantified the incremental cost-effectiveness ratio of treatment expansion—either to a greater number of individuals with later stage disease or to individuals with earlier stage disease—in comparison to a more restricted treatment access.
Population Health Benefits
Overview
The sum of the evidence on the population health benefits of “treat all” indicates that achieving prevalence reductions aiming toward elimination requires very high rates of diagnosis, unrestricted and timely access to highly effective treatment, and concurrent investments in other HCV prevention strategies such as access to sterile medical equipment, clean needles, and opioid substitution therapy, depending on the dominant transmission routes in a given country. Individuals with HCV who are unlikely to infect others in the absence of treatment or to be reinfected if they are successfully treated (e.g., individuals who may have previously engaged in risk behaviors but no longer do so) contribute to prevalence but not to incidence. Hence, scaling up treatment for such individuals has a linear relationship with reducing prevalence. For individuals who may infect others in the absence of treatment or who face a nonnegligible risk of being reinfected after successful treatment, “treat all” has the potential to avert future infections. For this reason, the vast majority of epidemiological modeling focuses on treatment as prevention in populations of PWIDs.81–92 There are also several studies that examine the impact of scaling up treatment on the overall population prevalence, though the models that underlie, either explicitly or implicitly, capture the direct impacts of treatment on prevalence for all individuals and the secondary-prevention benefits specific to treating those at high risk of contributing to disease transmission.53,58,78–80,93–98
When treatment capacity is limited—either by health system capacity or capitated treatment budgets—a “treat all” strategy can result in using limited treatment resources on patients who, in the short term, are least at-risk for HCV-related morbidity or mortality and least at-risk for transmitting the virus to others. With limited resources, allocating treatment to individuals with more advanced disease, who are more likely to experience HCV-related morbidity or mortality in the near-term, will have the greatest impact on reducing near-term HCV-related mortality. Allocating treatment to individuals who are more likely to transmit the virus to others (PWID or individuals who require many injections for medical purposes in settings with health care–driven epidemics) will have the greatest impact on reducing the disease incidence and prevalence.
General Population
In some countries, historical modes of transmission have been virtually eliminated through a safe transfusion blood supply and near universal systems to properly sterilize medical treatment.99 Therefore, even in the absence of treatment or substantial treatment scale-up, reductions in prevalence can be expected. For example, Kabiri and others93 developed a microsimulation model initially populated with individuals estimated to be HCV-infected in 2001 (stratified by age, sex, and awareness of infection status) based on the US National Health and Nutrition Examination Survey and exogenous incidence based on the annual number of new HCV cases reported by the US Centers for Disease Control and Prevention. Using this model, Kabiri and others estimate that in the absence of treatment, the prevalence of HCV in the United States will decrease from approximately 2.2 million in 2015 to 1.8 million in 2030 (an 18% reduction).93 As a result, continuing with the status quo level of diagnosis and treatment can appear to have substantial effects on disease prevalence in these settings compared to similar levels of investment in settings with stable or increasing epidemics. Similarly, in an analysis that combined all European Union countries using a Markov model with projections of future incidence based on past incidence,94 maintaining the status quo (150,000 treatments per year ≈ 4.6% of HCV-infected individuals) achieves a 50% reduction in HCV-related mortality and a 40% reduction in HCV prevalence by 2030.
Several studies identify one or more strategies to achieve targets aimed at elimination on a 10- to 15-year horizon in high-income countries (Belgium,95 the European Union,94 Spain,96 and the United States93). Each of these models initialized their population in the past, at a time when some information was available about the prevalence of HCV in their population, modeled past incidence using reported rates, and projected future incidence using a constant rate based on the most recently available data or using a trend curve fit to recent incidence data. Even in these settings, the required additional investments in diagnosis and treatment are substantial to achieve reductions in prevalence exceeding 50% (Table 8). All of these studies emphasize the important role of increasing diagnostic capacity to unlock the population health benefits of treatment expansion.93–96 As one example, in the Belgian analysis,95 comparing strategies with greater investments in screening and diagnosis to those with greater investments in treatment demonstrates the importance of the former for reducing prevalence (Figure 3).
Table 8.
Estimated Reductions in HCV Mortality and Prevalence Corresponding to Increased Investments in HCV Diagnosis, Treatment, and Access
| Reference | Country | Current Prevalence | Current Treatment Level (Annual) | Modelled Intervention (Annual) | Effect in 2030 (% Reduction Compared to Current Level) |
|---|---|---|---|---|---|
| Bourgeois, 201695 | Belgium | HCV+: 66,200 Undiagnosed: 57% |
Diagnoses: 2,280 Treatments: 1,350 Access: ≥F2 |
Diagnoses: 3,030 (37% ↑) Treatments: 4,060 (200% ↑) Access: All |
Mortality: 50% ↓ Prevalence: 90% ↓ |
| Diagnoses: 2,280 (no change) Treatments: 4,060 (200% ↑) Access: All |
Mortality: 29% ↓ Prevalence: 59% ↓ |
||||
| Diagnoses: 3,030 (37% ↑) Treatments: 4,060 (200% ↑) Access: ≥F2 |
Mortality: 65% ↓ Prevalence: 67% ↓ |
||||
| Diagnoses: 3,030 (37% ↑) Treatments: 3,000 (122% ↑) Access: All |
Mortality: 32% ↓ Prevalence: 75% ↓ |
||||
| Buti, 201796 | Spain | HCV+: 426,998 | Diagnoses: 5,500 Treatments: 20,000 Access: ≥ F2 |
Status quo | Prevalence (2025): 32% ↓ |
| Diagnoses: 40,000 (627% ↑) Treatments: 50,000 (150% ↑) Access: ≥F1 until 2022, then all |
Prevalence (2025): 95% ↓ | ||||
| Diagnoses: 15,000 (172% ↑) Treatments: 38,000 (90% ↑) Access: ≥F2 |
Prevalence (2025): 44% ↓ | ||||
| Kabiri, 201493 | US | HCV+: 2.2 million Undiagnosed: 43% |
Screening: 0 Treatment: 0 |
Prevalence: 18% ↓ | |
| Screening: Risk based Treatments: 83,270 Average SVR: 36% to 54% |
Prevalence: 50% ↓ | ||||
| Screening: Risk based and birth years 1945–1965 Treatments: 83,270 Average SVR: 90% |
Prevalence: 73% ↓ | ||||
| The European Union HCV Collaborators, 201794 | All EU | HCV+: 3,238,000 Undiagnosed: 64% (10–88% undiagnosed across countries) |
Diagnoses: 88,800 Treatments: 150,000 Access: ≥F2 Average SVR: 89% |
Continuing with status quo | Mortality: 50% ↓ Prevalence: 40% ↓ |
| Diagnoses: 180,000 (100% ↑) Treatments: 187,000 (25% ↑) Access: All Average SVR: 95% |
Mortality: 65% ↓ Prevalence: 90% ↓ |
||||
| Diagnoses: 180,000 (100% ↑) Treatments: 192,000 (28% ↑) Access: All Average SVR: 85% |
Mortality: 65% ↓ Prevalence: 90% ↓ |
||||
| Van Nuys, 201555 | US | Undiagnosed: 50% | Treatments: 296,000 Access: ≥F3 |
Prevalence: 62% ↓ | |
| Treatments: 125,000 Access: All |
Prevalence: 70% ↓ | ||||
| Woode, 201697 | Low-income | HCV+: 10.8 milliona | Diagnoses: 1% × (1.2 × year) Treatments (10 years): 7,081 Access: ≥F3 |
Diagnoses: 1% × (1.2 × year) Treatments (10 yearsb): 3.6 M Access: ≥F3 |
Mortality (2025): 19% ↓ Incidence (2025): 7.8% ↓ |
| Diagnoses: 1% × (1.2 × year) Treatments (10 yearsb): 9.5 M Access: All |
Mortality (2025): 19% ↓ Incidence (2025): 19% ↓ |
||||
| Low-middle | HCV+: 33.3 milliona | Diagnoses: 3% × (1.2 × year) Treatments (10 years): 787,535 Access: ≥F3 |
Diagnoses: 3% × (1.2 × year) Treatments (10 yearsb): 13.1 M Access: ≥F3 |
Mortality (2025): 40% ↓ Incidence (2025): 15% ↓ |
|
| Diagnoses: 3% × (1.2 × year) Treatments (10 yearsb): 31.4 M Access: All |
Mortality (2025): 40% ↓ Incidence (2025): 19% ↓ |
||||
| Upper-middle | HCV+: 25.3 milliona | Diagnoses: 5% × (1.2 × year) Treatments (10 years): 1.38 M Access: ≥F3 |
Diagnoses: 5% × (1.2 × year) Treatments (10 yearsb): 9.0 M Access: ≥F3 |
Mortality (2025): 46% ↓ Incidence (2025): 17% ↓ |
|
| Diagnoses: 5% × (1.2 × year) Treatments (10 yearsb): 20.6 M Access: All |
Mortality (2025): 46% ↓ Incidence (2025): 39% ↓ |
HCV, hepatitis C virus; HCV+, HCV-infected; SVR, sustained virologic response.
Personal communication (Maame E. Woode, October 15, 2017).
Cumulative over 10 years, but not evenly as treatment capacity increases by a factor of 1.2 per year.
Figure 3.
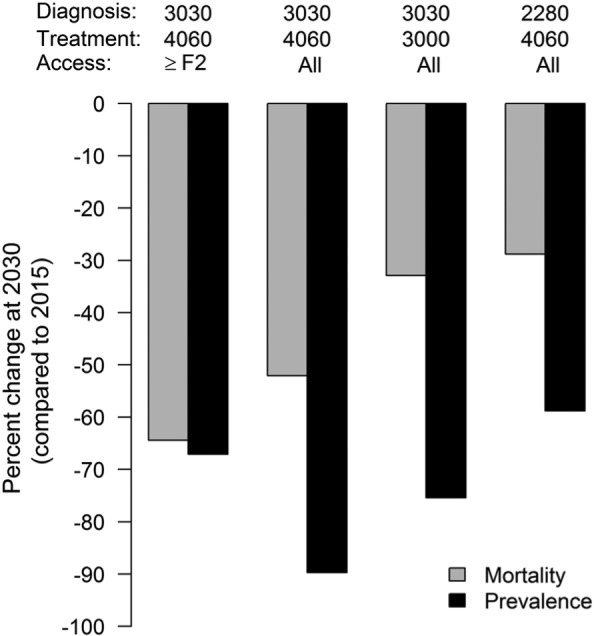
Population health model of HCV in Belgium performed by Bourgeouis and others.95 Percent change in the annual mortality rate and HCV prevalence compared to 2015 estimates (in 2015, there were an estimated 365 annual deaths and a total of 66,200 HCV-infected individuals) under four policies with differing treatment access restrictions and different investments in diagnosis and treatment.
Confirming the important linkage between increasing screening/diagnosis as well as expanding treatment for achieving HCV elimination targets, a series of 2014–2015 studies presented Markov model analyses with exogenous HCV incidence rates for 46 countries (including countries of all income levels).78–80 For 45 of the countries, country-specific strategies comprising diagnosis rates, treatment rates, and treatment access were described, which in most cases achieved goals of >50% reductions in HCV-related morbidity and mortality and >90% reductions in prevalence compared to 2015 levels. The countries differed in terms of prevalence; whether their HCV epidemic is increasing, stable, or declining without further intervention; the fraction of HCV-infected individuals who are PWID and the size of the PWID population; the fraction of HCV-infected individuals aware of their status; and current diagnosis and treatment capacity. To achieve target outcomes, some country-specific strategies required increases in diagnosis of up to 11-times the current rate and increases in treatment of up to 15-times the current rate including use of highly effective DAAs and expansion of treatment to all fibrosis stages. Hence, investment levels to achieve elimination may be infeasible for some countries—often, but not exclusively, low- and middle-income countries (i.e., Brazil, Czech Republic, India, Lithuania, Mongolia, Pakistan, Portugal, Russia, Slovak Republic). In general, while these analyses illustrate HCV-control targets can be achieved in all nations, in countries with limited health care resources or a limited HCV treatment budget, these analyses do not identify how countries should efficiently allocate resources.
Low- and Middle-Income Countries
In settings where most people have yet to be diagnosed and competing mortality risks are high, as is the case with many low- and middle-income countries, population health benefits are likely more difficult to achieve. Few studies model paths to HCV elimination in lower-income countries. Using a dynamic transmission model, Woode and others projected the effects of greater treatment uptake on mortality and incidence rates over 10 years in 32 low-income countries, 42 lower-middle income countries, and 42 upper-middle income countries.97 Large expansions in treatment of individuals with advanced disease (≥F3) result in 19% to 46% reductions in HCV-related mortality and 7% to 17% reductions in the number of new infections over 10 years (Table 8). Even extremely large expansions of treatment to include individuals with less advanced disease (F0–F2) do little to prevent additional HCV mortality over this timeframe but do have substantive impacts on incidence (augmenting reductions in new infections from 7% to 17% to 19$ to 39%). Comparing across country-income level, the marginal reduction in HCV mortality and incidence per additional treated individual increases with the wealth of the country.97 Though the analysis of Woode and others does not directly explore the reasons for these differences, it suggests that they may be due in part to the initial fraction of HCV-infected individuals who are aware of their infection status, screening and diagnosis capacity, and initial treatment capacity, all generally lower in low resource settings.
The analyses above include treatment expansion to all fibrosis stages, but only a few55,95 explicitly consider the tradeoffs created in terms of who has access to treatment when treatment budgets are limited (e.g., Figure 3). Restricting treatment access to the patients with the most severe disease has the greatest impact on near-term HCV-related mortality. Expanding treatment access to patients with early stage disease generally prevents more new infections but has less impact on near-term HCV mortality. The smaller the treatment budget, the larger is the tradeoff between reducing HCV mortality and HCV incidence.
PWIDs and General Population Treatment Prioritization
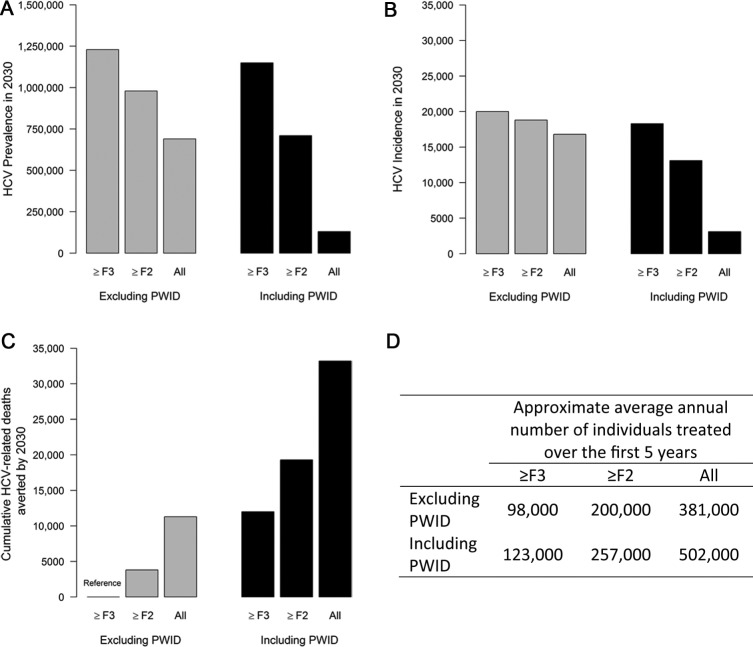
The net benefits of treatment expansion in individuals engaging in transmission behaviors depends on a balance between averting secondary transmissions and the rate of subsequent reinfection. This has implications both for personal health benefits of treatment to these individuals and for the hoped-for population health effects. Some analyses have directly considered which patient groups to prioritize:53,98 individuals who are at low risk for HCV transmission to others and themselves for reinfection but in whom the personal benefits of treatment are more certain or individuals who are at high risk for HCV transmission in whom the benefits could include disease prevention but who are themselves at risk for the benefits to be lost as a consequence of reinfection. Using a dynamic transmission model, a US-based analysis compared treatment access strategies using different minimum fibrosis-stage thresholds (≥F3, ≥F2, and treat all) and the inclusion or exclusion of PWID.53 It found that, at a similar number of total treatments, offering treatment to all individuals (PWIDs and non-PWIDs) with ≥F2 fibrosis prevented more deaths and reduced incidence more than a policy of expansion to all fibrosis stages for non-PWIDs only (Figure 4). Similarly, using a dynamic transmission model, Innes and others98 identified that shifting treatments from non-PWID with less advanced disease to PWID has little impact on HCV-related liver morbidity and mortality in 2030, but has a relatively large impact on the HCV incidence. These tradeoffs are not required with sufficiently expanded treatment resources.98
Figure 4.
Population health and health economic model of HCV in the US performed by Moreno and others.53 (A) HCV prevalence in 2030; (B) Annual HCV incidence in 2030; (C) Cumulative HCV-related deaths averted comparing HCV treatment expansion policies using different minimum fibrosis-stage thresholds (≥F3, ≥F2, and treat all) and the inclusion or exclusion of PWID; (D) Approximate average annual number of individuals treated over the first 5 years. Note that the total number of people treated each year differs across scenarios.
PWID Communities
Models of HCV treatment in PWID have shown that HCV treatment access expansion combined with harm reduction programs are required to achieve substantial HCV prevalence reductions in Australia,81,82 Canada,82,83 Greece,84 the United Kingdom,82,87 the United States,85,86 and Viet Nam,88 as well as in illustrative communities.89 All of the models were deterministic dynamic transmission models, except one that was a microsimulation model with dynamic transmission.84 Harm reduction programs complement HCV treatment by reducing the risk of reinfection for HCV-infected PWIDs who are successfully treated and hence not transmitting to others. Because communities of PWIDs vary in their network structure, frequency of injecting, and tendency to share injecting equipment, the rapidity with which HCV spreads through these communities and the number of transmission paths that must be simultaneously reduced varies substantially.90,100 Hence, the level of simultaneous interventions required to achieve a declining epidemic varies.90
The level of investment required to control the epidemic increases nonlinearly with the community prevalence of HCV. In high-prevalence/high-incidence communities of PWID, small levels of treatment or prevention investment may not appreciably alter reinfection risks.58,89,101 Thus, models focusing on the role of HCV treatment as prevention have identified very high rates of treatment (10% to 12% of HCV-infected PWID annually) necessary to reduce the HCV prevalence by 50% or 90% even in the presence of substantial harm reduction programs (Tables 9 and 10). In contrast, in lower prevalence communities, with stable or declining HCV prevalence of 20% to 25%,87,89 lower treatment rates of 15 to 18/1,000 PWID per year (approximately 7.5% of HCV-infected PWID) are able to achieve 50% and 90% reductions in prevalence in 10 to 15 years.
Table 9.
Estimated Time to Achieve a 50% Reduction in HCV Prevalence Among PWID Given the Community Prevalence, Access to Harm Reduction Programs, and Access to HCV Treatment
| Reference | Country/City | Prevalence of HCV in PWID | Level of Harm Reduction Effort | 50% Prevalence Decline Achieved by | Treatment Level Required (Annual) |
|
|---|---|---|---|---|---|---|
| Rate per 1,000 PWID | % OF HCV-Infected PWIDa | |||||
| Bennett, 201587 | Edinburgh, UK | 25% | 57% OST | 15 years | 15.4 | 6% |
| Cousien, 201783 | Montreal, Canada | 53% | Implicitly included and not described | 10 years | 106 | 20% of HCV-infected PWID with 1 year average time from infection to treatment |
| Durier, 201288 | Viet Nam | 60% | 10% NSP | 15 years | 150–180 | 25% to 30% |
| Echevarria, 201586 | Chicago, US | 47% | 69% in harm reduction program | 10 years | 35 | 7.4% |
| Martin, 201382 | Edinburgh, UK | 25% | 57% in OST and status quo NSP | 15 years | 15 | 6% |
| Martin, 201382 | Melbourne, Australia | 50% | 48% in OST and status quo NSP | 15 years | 40 | 8% |
| Martin, 201382 | Vancouver, Canada | 65% | 45% in OST and status quo NSP | 15 years | 76 | 12% |
| Martin, 201389 | Illustrative community | 20% | 50% OST and NSP | 10 years | 18 | 9% |
| 20% | 60% OST and NSP | 10 years | 15 | 7.5% | ||
| 20% | 70% OST and NSP | 10 years | 12 | 6% | ||
| 20% | 80% OST and NSP | 10 years | 9.6 | 5% | ||
| Martin, 201389 | Illustrative community | 40% | 50% OST and NSP | 10 years | 38 | 9.5% |
| 40% | 60% OST and NSP | 10 years | 34 | 8.4% | ||
| 40% | 70% OST and NSP | 10 years | 29 | 7.2% | ||
| 40% | 80% OST and NSP | 10 years | 21 | 5.3% | ||
| Martin, 201389 | Illustrative community | 60% | 50% OST and NSP | 10 years | 68 | 11.4% |
| 60% | 60% OST and NSP | 10 years | 59 | 9.8% | ||
| 60% | 70% OST and NSP | 10 years | 48 | 8% | ||
| 60% | 80% OST and NSP | 10 years | 38 | 6.4% | ||
| Zeiler, 201081 | Australia | 60% | 38% in OST | 3.3 years | 204 | 34% |
HCV, hepatitis C virus; NSP, needle-syringe exchange program; OST, opioid substitution therapy; PWID, people who inject drugs.
In most cases this is estimated from the treatment rate per 1,000 PWID using the current prevalence rate and so it represents the fraction of HCV-positive PWID who would be treated in the first year.
Table 10.
Estimated Time to Achieve a 90% Reduction in HCV Prevalence Among PWID Given the Community Prevalence, Access to Harm Reduction Programs, and Access to HCV Treatment
| Reference | Country/City | Prevalence of HCV in PWID | Level of Harm Reduction Effort | 90% Prevalence Decline Achieved by | Treatment Level Required (Annual) |
|
|---|---|---|---|---|---|---|
| Rate per 1,000 PWID | % of HCV-Infected PWIDa | |||||
| Bennett, 201587 | Edinburgh, UK | 25% | 57% OST | 15 years | 40 | 16% |
| Fraser, 201785 | US | 55.3% | 40% NSP | 10 years | 213 | 34.1% |
| Fraser, 201785 | US | 55.3% | 50% NSP and OST | 10 years | 121 | 20% |
| Fraser, 201785 | US | 55.3% | 40% NSP | 15 years | 159 | 25% |
| Fraser, 201785 | US | 55.3% | 50% NSP and OST | 15 years | 89 | 14.5% |
| Gountas, 201784 | Greece | 64% | 44% OST or NSP | 10 years | 258 | 40% |
| Gountas, 201784 | Greece | 64% | 44% OST or NSP | 15 years | 125 | 19.5% |
OST, opioid substitution therapy; NSP, needle-syringe exchange program.
In most cases this is estimated from the treatment rate per 1,000 PWID using the current prevalence rate and so it represents the fraction of HCV-positive PWID who would be treated in the first year.
Likewise, achieving HCV epidemic decline involves combinations of investments in treatment and harm reduction.84,85,88,89 To achieve a given level of HCV prevalence reduction, lower levels of investment in treatment expansion are required with greater investments in harm reduction (e.g., Figure 5). A US analysis found that to achieve a 90% reduction in prevalence, the treatment rate needs to be nearly double current levels without a concurrent increase in harm reduction: 159/1,000 PWID (25% of HCV-infected PWID) without an increase in harm reduction efforts versus 89/1,000 PWID (14.5% of HCV-infected PWID) with an increase in harm reduction efforts.85
Figure 5.
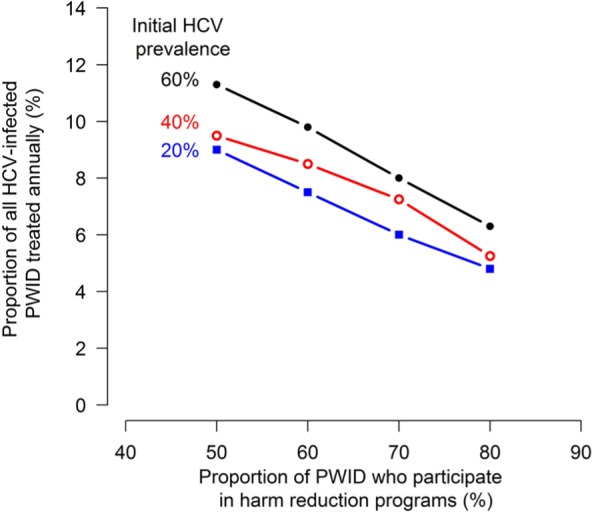
Population health model of HCV in hypothetical PWID communities presented in Martin and others.89 The proportion of HCV-infected PWID that must be treated annually to achieve a 50% reduction in the HCV prevalence in 10 years at various levels of initial HCV prevalence in the PWID population (20%, 40%, and 60%) and fractions of the PWID population who participate in harm reduction programs (from 50% to 80%).
Only one study in the review modeled the population health benefits of HCV treatment in PWID in a low- or middle-income country (Viet Nam88). However, the findings in this study are consistent with the insights gained from modeling higher-income country PWID communities—substantial reductions in HCV prevalence require that time from infection to diagnosis and treatment be short, implying readily accessible screening, diagnosis, and treatment.88
Treatment Prioritization Within PWIDs
Some analyses have focused on whom to target within the PWID community to ensure the greatest impact on incidence for settings in which system capacity or budgets are insufficient to treat all PWID.81,83,86,91,102–104 Though a less common question than in the general population, treatment prioritization in terms of fibrosis stage within PWID communities is the topic of two Canadian studies, using dynamic transmission models, that show that a “treat all” approach has a greater impact on HCV prevalence.83,91 In a model of PWID in Vancouver, Canada, for example, a treatment rate to 80/1,000 PWID (approximately 12.3% of HCV-positive PWID) only reduces the prevalence by 15% (from 65% to 55%) over 10 years when focusing treatment exclusively on individuals with ≥F2 fibrosis. Without increasing the total number of patients treated, widening eligibility to all fibrosis stages augments reductions in incidence and prevalence (estimated prevalence at 10 years is reduced from 65% to 46%).91 Other studies consider treatment prioritization by risk behavior in PWID communities81,86 and by network features.102–104 In models assuming homogenous mixing, targeting HCV treatments to patients not using harm reduction services maximized societal treatment benefits by focusing on individuals at highest risk of transmitting the virus to others.81,86 However, models that specifically consider network structure identify that prioritizing HCV treatment to clusters of individuals, particularly to those clusters in which HCV prevalence is relatively lower, achieves the maximum reductions in prevalence.102–104 Of note, analyses that assume homogenous mixing overestimate the benefits of treatment on prevalence reduction compared to those that explicitly incorporate network structure.104
Incarceration
The prevalence of HCV in incarcerated persons is high: a meta-analysis combining regionally diverse studies estimated an overall prevalence of 26% in the general incarcerated population and 64% among incarcerated individuals with a history of injection drug use.105 The frequency of injection drug use and other risk behaviors such as unsafe tattooing inside and outside of prison after release is high.106–108 In some settings, PWID have high incarceration rates.109 When PWIDs incarceration status acts as a bridge for transmission, the model-based evidence indicates that treatment of PWID during incarceration can have substantial impacts on the HCV prevalence in the entire PWID community.92
Health Care–Driven Epidemics
Only one study, using a dynamic transmission model, focused on treatment as a means of controlling a health care–driven HCV epidemic (Egypt).110 Health care–driven epidemics have many similarities to epidemics in communities of PWID. In both settings, transmission and risks of reinfection can be reduced through other interventions (e.g., access to sterilization equipment, clean needles). In a health care–driven epidemic, transmission network clusters are formed by sharing a common medical facility. In both, rapid diagnosis and access to highly effective treatment are essential.110 Extending insights from PWID communities, concurrent investments in incidence reductions can prevent reinfection enhancing treatment expansion’s transmission reduction benefits.
Models That Reported Both Cost-Effectiveness and Population Health Outcomes
Three studies included in our report performed a population-based cost-effectiveness analysis and reported population health outcomes.53,55,58 All of these models include dynamic transmission and separate compartments for high-risk groups (i.e., PWID). At a willingness to pay threshold of $100,000 per QALY gained, Van Nuys and others55 find that a policy of treating all individuals with a HCV-infection diagnosis in the United States would be cost saving and would nearly eliminate HCV within 10 years although they note that this strategy “would require greater treatment capacity than is currently available.”55 Moreno and others,53 using the same model, present a cost-effectiveness analysis of less restrictive treatment access policies (treatment access to non-PWID with milder fibrosis v. delayed treatment after progression to later fibrosis and whether or not to include PWID in treatment access strategies). Despite relatively moderate reductions in prevalence, incidence, and mortality, providing treatment to all non-PWID is cost saving and improves quality of life. Expanding treatment access further to include PWID has a greater impact on prevalence, incidence, and mortality, and compared to similar fibrosis-stage access policies focusing only on non-PWID, policies that include PWID have higher net monetary benefit at a willingness to pay threshold of $100,000 per QALY gained. Notably, the analysis of Moreno and others assumes the prevalence of HCV in the PWID community is 36%. This makes their findings consistent with the third population-based cost-effectiveness study, which found that it is cost-effective to expand treatment from ex- and non-PWID with ≥F2 fibrosis to include PWID only when the prevalence of HCV in the PWID community was 40% or less.58 This analysis found that the population health impacts of expanding treatment to PWID—the number of new infections averted, HCV-related deaths, decompensated cirrhosis, and HCC—was substantially diminished when the prevalence of HCV in PWID was higher.58
Discussion
Our review focused on analyses that assessed the cost-effectiveness of offering treatment to all individuals chronically infected with HCV compared to providing treatment to only a subgroup of such individuals or delaying treatment conditional on an event (e.g., fibrosis progression or ceasing injection drug use). Modeling studies from many different countries find that expanding treatment more broadly to include individuals with milder liver fibrosis, PWID, or people who are incarcerated is generally cost-effective at country-specific current treatment prices. Treatment price sensitivity is greatest in low- and middle-income countries.
We note that, globally, a minority of people are aware that they are chronically infected with HCV (20%), and treatment capacity and coverage are also relatively low.1 The feasibility and cost-effectiveness of a “treat all” policy are practically linked to efforts to screen broadly in the population to identify/diagnose HCV-infected individuals and to scale-up treatment so that the total time from infection to diagnosis and then to treatment is relatively short. This was an issue that many cost-effectiveness analyses did not specifically consider. Several epidemic modeling analyses identified the levels of diagnosis, treatment, treatment efficacy, and access necessary to achieve elimination targets (>90% reduction in incidence or prevalence by 2030). The sum of the evidence indicates that to achieve these targets, there need to be very high rates of diagnosis,78 unrestricted and timely access to highly effective treatment, and concurrent investments in other HCV-prevention strategies such as access to sterile medical equipment, clean needles, and opioid substitution therapy.
Importantly, the vast majority of published cost-effectiveness analyses, across many countries, do not include HCV transmission or reinfection risk, which is a potentially significant limitation when considering a “treat all” approach. Curing a person with HCV who would have otherwise gone on to infect others has the potential to avert the costs and health harms of these other infections. However, in high-incidence communities these secondary infections may occur via someone else, thus attenuating the secondary benefits of curing the index case. Furthermore, cured individuals may also be reinfected; hence, ignoring the risk of reinfection may overcount health benefits and downstream cost savings. The omission of disease transmission and the risk of re-infection may, therefore, result in an over- or underestimation of long-term costs and benefits. The net effect of these forces on the cost-effectiveness of treatment or delayed treatment is unclear and likely depends on both individual and community factors. This limitation may be more pronounced in models of low- and middle-income countries where HCV epidemics may be growing or persisting and where the lack of multiple high-quality studies makes the incremental cost-effectiveness ratio for “treat all” strategies less certain.
Treatment expansion to some subpopulations may be less valuable than to others. Highly stratified analyses identify cohorts of patients—those who are older, with mild disease, and infected with some HCV genotypes—for which treatment with an all-oral DAA may not be cost-effective at current prices. Though treatment expansion to PWIDs appears consistently cost-effective and important for achieving sustainable reductions in incidence and prevalence,53,58,98 concurrent investments in transmission reduction interventions may be required, especially in high-incidence/high-prevalence PWID communities.58,89,111
If diagnosis or treatment resources are limited and treatment cannot be made available to all HCV-infected individuals, then who is prioritized for treatment influences the expected outcomes.53,98 Prioritizing non-PWIDs with more advanced disease will reduce HCV-related mortality in the near-term but do little to reduce incidence and prevalence. Prioritizing PWIDs or others at high risk of transmission will do less to reduce near-term mortality but has the opportunity to have a greater impact on incidence and prevalence (particularly if coupled with other HCV prevention strategies).
Our review has limitations. We exclusively searched PubMed using our search terms, which may have resulted in missing some relevant published articles. However, we also scanned the reference sections of review articles as well as selected articles, and we searched the gray literature (primarily for government-funded or research institute reports) manually using Google. The questions under study were not always the primary research question of the articles but instead were included as a secondary analysis or a sensitivity analysis. We read articles looking for these analyses, but may have missed situations when the analyses were only presented or discussed in supplementary materials or were discussed very briefly.
In summary, a broad literature review reveals that greatly expanding the use of new HCV treatment in many countries may achieve population health goals toward elimination cost-effectively—all the more so toward “treat all” as larger reductions in treatment prices can be secured. Given that not all HCV-infected individuals are currently diagnosed and that not all diagnosed individuals can be treated immediately, expansions of screening, diagnosis, and treatment are required.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support for this study was provided by the World Health Organization and Unitaid. The authors had independence in designing the study, interpreting the data, writing, and publishing the report.
ORCID iD: Lauren E. Cipriano  https://orcid.org/0000-0001-5568-4516
https://orcid.org/0000-0001-5568-4516
Contributor Information
Lauren E. Cipriano, Ivey Business School and the Department of Biostatistics and Epidemiology, Western University, London, Ontario, Canada.
Jeremy D. Goldhaber-Fiebert, Center for Health Policy and Center for Primary Care and Outcomes Research, Department of Medicine, Stanford University, Stanford, California
References
- 1. World Health Organization. Global hepatitis report, 2017. Available from: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Available from: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
- 3. World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection (Updated version, April 2016). Available from: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/ [PubMed]
- 4. Wong WW, Lee KM, Singh S, Wells G, Feld JJ, Krahn M. Drug therapies for chronic hepatitis C infection: a cost-effectiveness analysis. CMAJ Open. 2017;5(1):E97–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moshyk A, Martel MJ, Monfared AAT, Goeree R. Cost-effectiveness of daclatasvir plus sofosbuvir-based regimen for treatment of hepatitis C virus genotype 3 infection in Canada. J Med Econ. 2016;19(2):181–92. [DOI] [PubMed] [Google Scholar]
- 6. Chen GF, Wei L, Chen J, et al. Will sofosbuvir/ledipasvir (Harvoni) be cost-effective and affordable for Chinese patients infected with hepatitis C virus? An economic analysis using real-world data. PLoS One. 2016;11(6):e0155934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H, Chen L. Estimating cost-effectiveness associated with all-oral regimen for chronic hepatitis C in China. PLoS One. 2017;12(4):e0175189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elsisi GH, Aburawash A, Waked E. Cost-effectiveness analysis of new HCV treatments in Egyptian cirrhotic and non-cirrhotic patients: a societal perspective. Value Health Reg Issues. 2017;13:7–15. [DOI] [PubMed] [Google Scholar]
- 9. Muhlbacher AC, Sadler A. The probabilistic efficiency frontier: a framework for cost-effectiveness analysis in Germany put into practice for hepatitis C treatment options. Value Health. 2017;20(2):266–72. [DOI] [PubMed] [Google Scholar]
- 10. Restelli U, Alberti A, Lazzarin A, Bonfanti M, Nappi C, Croce D. Cost-effectiveness analysis of the use of daclatasvir + sofosbuvir + ribavirin (16 weeks and 12 weeks) vs sofosbuvir + ribavirin (16 weeks and 24 weeks) for the treatment of cirrhotic patients affected with hepatitis C virus genotype 3 in Italy. Eur J Health Econ. 2016;19(1):37–44. [DOI] [PubMed] [Google Scholar]
- 11. Restelli U, Bonfanti M, Alberti A, Lazzarin A, Nappi C, Croce D. Cost effectiveness analysis of The use of daclatasvir for the treatment of hepatitis C virus (HCV) genotypes 3 in cirrhotic patients within the Italian National Health Service. Value Health. 2015;18(7):A587. [Google Scholar]
- 12. Igarashi A, Tang W, Cure S, et al. Cost-utility analysis of sofosbuvir for the treatment of genotype 2 chronic hepatitis C in Japan. Curr Med Res Opin. 2017;33(1):1–10. [DOI] [PubMed] [Google Scholar]
- 13. Igarashi A, Tang W, Guerra I, Marié L, Cure S, Lopresti M. Cost-utility analysis of ledipasvir/sofosbuvir for the treatment of genotype 1 chronic hepatitis C in Japan. Curr Med Res Opin. 2017;33(1):11–21. [DOI] [PubMed] [Google Scholar]
- 14. McEwan P, Ward T, Webster S, et al. Estimating the cost-effectiveness of daclatasvir plus asunaprevir in difficult to treat Japanese patients chronically infected with hepatitis C genotype 1b. Hepatol Res. 2016;46(5):423–33. [DOI] [PubMed] [Google Scholar]
- 15. Virabhak S, Yasui K, Yamazaki K, et al. Cost-effectiveness of direct-acting antiviral regimen ombitasvir/paritaprevir/ritonavir in treatment-naïve and treatment-experienced patients infected with chronic hepatitis C virus genotype 1b in Japan. J Med Econ. 2016;19(12):1144–56. [DOI] [PubMed] [Google Scholar]
- 16. Wisløff T, White R, Dalgard O, et al. Economic evaluation of direct-acting antivirals for hepatitis C in Norway. Pharmacoeconomics. 2018;36(5):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao YJ, Khoo AL, Lin L, et al. Cost-effectiveness of strategy-based approach to treatment of genotype 1 chronic hepatitis C. J Gastroenterol Hepatol. 2016;31(9):1628–37. [DOI] [PubMed] [Google Scholar]
- 18. Dan YY, Ferrante SA, Elbasha EH, Hsu TY. Cost-effectiveness of boceprevir co-administration versus pegylated interferon-α2b and ribavirin only for patients with hepatitis C genotype 1 in Singapore. Antivir Ther. 2015;20(2):209–16. [DOI] [PubMed] [Google Scholar]
- 19. Fraser I, Burger J, Lubbe M, Dranitsaris G, Sonderup M, Stander T. Cost-effectiveness modelling of sofosbuvir-containing regimens for chronic genotype 5 hepatitis C virus infection in South Africa. Pharmacoeconomics. 2016;34(4):403–17. [DOI] [PubMed] [Google Scholar]
- 20. Gimeno-Ballester V, Mar J, O’Leary A, Adams R, Miguel RS. Cost-effectiveness analysis of therapeutic options for chronic hepatitis C genotype 3 infected patients. Expert Rev Gastroenterol Hepatol. 2017;11(1):85–93. [DOI] [PubMed] [Google Scholar]
- 21. Gimeno-Ballester V, Mar J, Miguel RS. Cost-effectiveness analysis of simeprevir with daclatasvir for non-cirrhotic genotype-1b-naïve patients plus chronic hepatitis C. Expert Rev Pharmacoecon Outcomes Res. 2016;16(2):285–94. [DOI] [PubMed] [Google Scholar]
- 22. Miguel RS, Gimeno-Ballester V, Blázquez A, Mar J. Cost-effectiveness analysis of sofosbuvir-based regimens for chronic hepatitis C. Gut. 2015;64(8):1277–88. [DOI] [PubMed] [Google Scholar]
- 23. Pfeil AM, Reich O, Guerra IM, et al. Cost-effectiveness analysis of sofosbuvir compared to current standard treatment in Swiss patients with chronic hepatitis C. PLoS One. 2015;10(5):e0126984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapol N, Lochid-Amnuay S, Teerawattananon Y. Economic evaluation of pegylated interferon plus ribavirin for treatment of chronic hepatitis C in Thailand: genotype 1 and 6. BMC Gastroenterol. 2016;16(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ward T, Gordon J, Jones B, et al. Value of sustained virologic response in patients with hepatitis C as a function of time to progression of end-stage liver disease. Clin Drug Investig. 2017;37(1):61–70. [DOI] [PubMed] [Google Scholar]
- 26. McEwan P, Bennett H, Ward T, et al. The cost-effectiveness of daclatasvir-based regimens for the treatment of hepatitis C virus genotypes 1 and 4 in the UK. Eur J Gastroenterol Hepatol. 2016;28(2):173–80. [DOI] [PubMed] [Google Scholar]
- 27. Johnson SJ, Parisé H, Virabhak S, Filipovic I, Samp JC, Misurski D. Economic evaluation of ombitasvir/paritaprevir/ritonavir and dasabuvir for the treatment of chronic genotype 1 hepatitis c virus infection. J Med Econ. 2016;19(10):983–94. [DOI] [PubMed] [Google Scholar]
- 28. Saint-Laurent Thibault C, Moorjaney D, Ganz ML, et al. Cost-effectiveness of combination daclatasvir-sofosbuvir for treatment of genotype 3 chronic hepatitis C infection in the United States. J Med Econ. 2017;20(7):692–702. [DOI] [PubMed] [Google Scholar]
- 29. Younossi ZM, Park H, Dieterich D, Saab S, Ahmed A, Gordon SC. The value of cure associated with treating treatment-naive chronic hepatitis C genotype 1: are the new all-oral regimens good value to society? Liver Int. 2017;37(5):662–68. [DOI] [PubMed] [Google Scholar]
- 30. Saab S, Parisé H, Virabhak S, et al. Cost-effectiveness of currently recommended direct-acting antiviral treatments in patients infected with genotypes 1 or 4 hepatitis C virus in the US. J Med Econ. 2016;19(8):795–805. [DOI] [PubMed] [Google Scholar]
- 31. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–19. [DOI] [PubMed] [Google Scholar]
- 32. Linas BP, Barter DM, Morgan JR, et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med. 2015;162(9):619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luhnen M, Waffenschmidt S, Gerber-Grote A, Hanke G. Health economic evaluations of sofosbuvir for treatment of chronic hepatitis C: a systematic review. Appl Health Econ Health Policy. 2016;14(5):527–43. [DOI] [PubMed] [Google Scholar]
- 34. Chhatwal J, He T, Lopez-Olivo MA. Systematic review of modelling approaches for the cost effectiveness of hepatitis C treatment with direct-acting antivirals. Pharmacoeconomics. 2016;34(6):551–67. [DOI] [PubMed] [Google Scholar]
- 35. Hickman M, De Angelis D, Vickerman P, Hutchinson S, Martin NK. Hepatitis C virus treatment as prevention in people who inject drugs: testing the evidence. Curr Opin Infect Dis. 2015;28(6):576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong WW, Tu HA, Feld JJ, Wong T, Krahn M. Cost-effectiveness of screening for hepatitis C in Canada. CMAJ. 2015;187(3):E110–E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim DD, Hutton DW, Raouf AA, et al. Cost-effectiveness model for hepatitis C screening and treatment: implications for Egypt and other countries with high prevalence. Glob Public Health. 2015;10(3):296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Obach D, Deuffic-Burban S, Esmat G, et al. Effectiveness and cost-effectiveness of immediate versus delayed treatment of hepatitis C virus-infected patients in a country with limited resources: the case of Egypt. Clin Infect Dis. 2014;58(8):1064–71. [DOI] [PubMed] [Google Scholar]
- 39. Deuffic-Burban S, Obach D, Canva V, et al. Cost-effectiveness and budget impact of interferon-free direct-acting antiviral-based regimens for hepatitis C treatment: the French case. J Viral Hepat. 2016;23(10):767–79. [DOI] [PubMed] [Google Scholar]
- 40. Ethgen O, Sanchez Gonzalez Y, Jeanblanc G, Duguet A, Misurski D, Juday T. Public health impact of comprehensive hepatitis C screening and treatment in the French baby-boomer population. J Med Econ. 2017;20(2):162–70. [DOI] [PubMed] [Google Scholar]
- 41. Sbarigia U, Wirth D, Van Nuys K, et al. Economic study of the value of expanding HCV treatment capacity in Germany. BMJ Open Gastroenterol. 2017;4(1):e000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cortesi PA, Ciaccio A, Rota M, et al. Management of treatment-naïve chronic hepatitis C genotype 1 patients: a cost-effectiveness analysis of treatment options. J Viral Hepat. 2015;22(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim DY, Han KH, Jun B, et al. Estimating the cost-effectiveness of one-time screening and treatment for hepatitis C in Korea. PLoS One. 2017;12(1):e0167770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crossan C, Tsochatzis EA, Longworth L, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015;19(9):1–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med. 2016;176(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chidi AP, Bryce CL, Donohue JM, et al. Economic and public health impacts of policies restricting access to hepatitis C treatment for Medicaid patients. Value Health. 2016;19(4):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chidi AP, Rogal S, Bryce CL, et al. Cost-effectiveness of new antiviral regimens for treatment-naïve US veterans with hepatitis C. Hepatology. 2016;63(2):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61(6):1860–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Linas BP, Morgan JR, Pho MT, et al. Cost effectiveness and cost containment in the era of interferon-free therapies to treat hepatitis C virus genotype 1. Open Forum Infect Dis. 2017;4(1):ofw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linthicum MT, Gonzalez YS, Mulligan K, et al. Value of expanding HCV screening and treatment policies in the United States. Am J Manag Care. 2016;22(6 Spec No.):Sp227-Sp235. [PubMed] [Google Scholar]
- 51. Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS One. 2011;6(12):e26783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8(3):e58975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moreno GA, Wang A, Sánchez González Y, et al. Value of comprehensive HCV treatment among vulnerable, high-risk populations. Value Health. 2017;20(6):736–44. [DOI] [PubMed] [Google Scholar]
- 54. Tice JA, Ollendorf DA, Chahal HS, et al. The Comparative Clinical Effectiveness and Value of Novel Combination Therapies for the Treatment of Patients With Genotype 1 Chronic Hepatitis C Infection. A Technology Assessment Final Report. Boston: Institute for Clinical and Economic Review; 2015. [Google Scholar]
- 55. Van Nuys K, Brookmeyer R, Chou JW, Dreyfus D, Dieterich D, Goldman DP. Broad hepatitis C treatment scenarios return substantial health gains, but capacity is a concern. Health Aff (Millwood). 2015;34(10):1666–74. [DOI] [PubMed] [Google Scholar]
- 56. Younossi Z, Gordon SC, Ahmed A, Dieterich D, Saab S, Beckerman R. Treating Medicaid patients with hepatitis C: clinical and economic impact. Am J Manag Care. 2017;23(2):107–12. [PubMed] [Google Scholar]
- 57. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530–37. [DOI] [PubMed] [Google Scholar]
- 58. Martin NK, Vickerman P, Dore GJ, et al. Prioritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. J Hepatol. 2016;65(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ciaccio A, Cortesi PA, Bellelli G, et al. Direct-acting antivirals combination for elderly patients with chronic hepatitis C: a cost-effectiveness analysis. Liver Int. 2017;37:982–94. [DOI] [PubMed] [Google Scholar]
- 60. Scott N, McBryde ES, Thompson A, Doyle JS, Hellard ME. Treatment scale-up to achieve global HCV incidence and mortality elimination targets: a cost-effectiveness model. Gut. 2016;66(8):1507–15. [DOI] [PubMed] [Google Scholar]
- 61. Scott N, Iser DM, Thompson AJ, Doyle JS, Hellard ME. Cost-effectiveness of treating chronic hepatitis C virus with direct-acting antivirals in people who inject drugs in Australia. J Gastroenterol Hepatol. 2016;31(4):872–82. [DOI] [PubMed] [Google Scholar]
- 62. Visconti AJ, Doyle JS, Weir A, Shiell AM, Hellard ME. Assessing the cost-effectiveness of treating chronic hepatitis C virus in people who inject drugs in Australia. J Gastroenterol Hepatol. 2013;28(4):707–16. [DOI] [PubMed] [Google Scholar]
- 63. van Santen DK, de Vos AS, Matser A, et al. Cost-effectiveness of hepatitis C treatment for people who inject drugs and the impact of the type of epidemic; extrapolating from Amsterdam, the Netherlands. PLoS One. 2016;11(10):e0163488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bennett H, Gordon J, Jones B, et al. Hepatitis C disease transmission and treatment uptake: impact on the cost-effectiveness of new direct-acting antiviral therapies. Eur J Health Econ. 2017;18(8):1001–11. [DOI] [PubMed] [Google Scholar]
- 65. Martin NK, Vickerman P, Brew IF, et al. Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. Hepatology. 2016;63(6):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in US prisons. Ann Intern Med. 2016;164(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD. Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in US incarcerated populations: a cost-effectiveness analysis. Ann Intern Med. 2014;161(8):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cure S, Guerra I, Cammà C, Craxì A, Carosi G. Cost-effectiveness of sofosbuvir plus ribavirin with or without pegylated interferon for the treatment of chronic hepatitis C in Italy. J Med Econ. 2015;18(9):678–90. [DOI] [PubMed] [Google Scholar]
- 69. Cure S, Guerra I, Dusheiko G. Cost-effectiveness of sofosbuvir for the treatment of chronic hepatitis C-infected patients. J Viral Hepat. 2015;22(11):882–89. [DOI] [PubMed] [Google Scholar]
- 70. Elbasha EH, Robertson MN, Nwankwo C. The cost-effectiveness of testing for NS5a resistance-associated polymorphisms at baseline in genotype 1a-infected (treatment-naïve and treatment-experienced) subjects treated with all-oral elbasvir/grazoprevir regimens in the United States. Aliment Pharmacol Ther. 2017;45(3):455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maor Y, Malnick SD, Melzer E, Leshno M. Treatment of chronic hepatitis C in the aged—does it impact life expectancy? A decision analysis. PLoS One. 2016;11(7):e0157832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Innes H, Goldberg D, Dusheiko G, et al. Patient-important benefits of clearing the hepatitis C virus through treatment: a simulation model. J Hepatol. 2014;60(6):1118–26. [DOI] [PubMed] [Google Scholar]
- 73.World Bank Group. DataBank: Low & middle income. Accessed September 3, 2017. Available at: https://data.worldbank.org/income-level/low-and-middle-income
- 74. Vargas CL, Espinoza MA, Giglio A, Soza A. Cost effectiveness of daclatasvir/asunaprevir versus peginterferon/ribavirin and protease inhibitors for the treatment of hepatitis C genotype 1b naïve patients in Chile. PLoS One. 2015;10(11):e0141660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aggarwal R, Chen Q, Goel A, et al. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLoS One. 2017;12(5):e0176503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alavian SM, Nikfar S, Kebriaeezadeh A, et al. A cost-utility analysis of different antiviral medicine regimens in patients with chronic hepatitis C virus genotype 1 infection. Iran Red Crescent Med J. 2016;18(11):e37094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Obach D, Yazdanpanah Y, Esmat G, et al. How to optimize hepatitis C virus treatment impact on life years saved in resource-constrained countries. Hepatology. 2015;62(1):31–9. [DOI] [PubMed] [Google Scholar]
- 78. Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21(Suppl. 1):60–89. [DOI] [PubMed] [Google Scholar]
- 79. Gane E, Kershenobich D, Seguin-Devaux C, et al. Strategies to manage hepatitis C virus (HCV) infection disease burden—volume 2. J Viral Hepat. 2015;22(Suppl. 1):46–73. [DOI] [PubMed] [Google Scholar]
- 80. Alfaleh FZ, Nugrahini N, Matiĉiĉ M, et al. Strategies to manage hepatitis C virus infection disease burden—volume 3. J Viral Hepat. 2015;22(Suppl. 4):42–65. [DOI] [PubMed] [Google Scholar]
- 81. Zeiler I, Langlands T, Murray JM, Ritter A. Optimal targeting of hepatitis C virus treatment among injecting drug users to those not enrolled in methadone maintenance programs. Drug Alcohol Depend. 2010;110(3):228–33. [DOI] [PubMed] [Google Scholar]
- 82. Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cousien A, Leclerc P, Morissette C, et al. The need for treatment scale-up to impact HCV transmission in people who inject drugs in Montreal, Canada: a modelling study. BMC Infect Dis. 2017;17(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gountas I, Sypsa V, Anagnostou O, et al. Treatment and primary prevention in people who inject drugs for chronic hepatitis C infection: is elimination possible in a high-prevalence setting? Addiction. 2017;112(7):1290–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fraser H, Zibbell J, Hoerger T, et al. Scaling up HCV prevention and treatment interventions in rural United States—model projections for tackling an increasing epidemic. Addiction. 2018;113(1):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Echevarria D, Gutfraind A, Boodram B, et al. Mathematical modeling of hepatitis C prevalence reduction with antiviral treatment scale-up in persons who inject drugs in metropolitan Chicago. PLoS One. 2015;10(8):e0135901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bennett H, McEwan P, Sugrue D, Kalsekar A, Yuan Y. Assessing the long-term impact of treating hepatitis C virus (HCV)-infected people who inject dugs in the UK and the relationship between treatment uptake and efficacy on future infections. PLoS One. 2015;10(5):e0125846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Durier N, Nguyen C, White LJ. Treatment of hepatitis C as prevention: a modeling case study in Vietnam. PLoS One. 2012;7(4):e34548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl. 2):S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Corson S, Greenhalgh D, Hutchinson S. Mathematically modelling the spread of hepatitis C in injecting drug users. Math Med Biol. 2012;29(3):205–30. [DOI] [PubMed] [Google Scholar]
- 91. Lima VD, Rozada I, Grebely J, et al. Are interferon-free direct-acting antivirals for the treatment of HCV enough to control the epidemic among people who inject drugs? PLoS One. 2015;10(12):e0143836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stone J, Martin NK, Hickman M, et al. Modelling the impact of incarceration and prison-based hepatitis C virus (HCV) treatment on HCV transmission among people who inject drugs in Scotland. Addiction. 2017;112(7):1302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161(3):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(5):325–36. [DOI] [PubMed] [Google Scholar]
- 95. Bourgeois S, Blach S, Blach C, et al. Achieving WHO recommendations for hepatitis C virus elimination in Belgium. Acta Gastroenterol Belg. 2016;79(2):222–6. [PubMed] [Google Scholar]
- 96. Buti M, Calleja JL, García-Samaniego J, et al. Elimination of hepatitis C in Spain: adaptation of a mathematical model based on the public health strategic plan for addressing hepatitis C in the National Health System. Med Clin (Barc). 2017;148(6):277–82. [DOI] [PubMed] [Google Scholar]
- 97. Woode ME, Abu-Zaineh M, Perriëns J, Renaud F, Wiktor S, Moatti JP. Potential market size and impact of hepatitis C treatment in low- and middle-income countries. J Viral Hepat. 2016;23(7):522–34. [DOI] [PubMed] [Google Scholar]
- 98. Innes H, Goldberg D, Dillon J, Hutchinson SJ. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 2015;64(11):1800–9. [DOI] [PubMed] [Google Scholar]
- 99. Joy JB, McCloskey RM, Nguyen T, et al. The spread of hepatitis C virus genotype 1a in North America: a retrospective phylogenetic study. Lancet Infect Dis. 2016;16(6):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Elbasha EH. Model for hepatitis C virus transmissions. Math Biosci Eng. 2013;10(4):1045–65. [DOI] [PubMed] [Google Scholar]
- 101. Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver’s supervised injection facility. CMAJ. 2008;179(11):1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rolls DA, Sacks-Davis R, Jenkinson R, et al. Hepatitis C transmission and treatment in contact networks of people who inject drugs. PLoS One. 2013;8(11):e78286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–70. [DOI] [PubMed] [Google Scholar]
- 104. Metzig C, Surey J, Francis M, Conneely J, Abubakar I, White PJ. Impact of hepatitis C treatment as prevention for people who inject drugs is sensitive to contact network structure. Sci Rep. 2017;7(1):1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Larney S, Kopinski H, Beckwith CG, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58(4):1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Snow KJ, Young JT, Preen DB, Lennox NG, Kinner SA. Incidence and correlates of hepatitis C virus infection in a large cohort of prisoners who have injected drugs. BMC Public Health. 2014;14:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bretana NA, Boelen L, Bull R, et al. Transmission of hepatitis C virus among prisoners, Australia, 2005–2012. Emerg Infect Dis. 2015;21(5):765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cunningham EB, Hajarizadeh B, Bretana NA, et al. Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an Australian prison setting, 2005–2014: the HITS-p study. J Viral Hepat. 2017;24(9):733–41. [DOI] [PubMed] [Google Scholar]
- 109. DeBeck K, Cheng T, Montaner JS, et al. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV. 2017;4(8):e357–e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Breban R, Arafa N, Leroy S, et al. Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Glob Health. 2014;2(9):e541–e549. [DOI] [PubMed] [Google Scholar]
- 111. Grebely J, Dore GJ, Morin S, Rockstroh JK, Klein MB. Elimination of HCV as a public health concern among people who inject drugs by 2030—what will it take to get there? J Int AIDS Soc. 2017;20(1):22146. [DOI] [PMC free article] [PubMed] [Google Scholar]