Highlights
-
•
Training of the pelvic floor muscles (PFM) is essential in the treatment of pelvic floor dysfunctions.
-
•
Only women who are able to contract the PFM are eligible for PFM training.
-
•
There is no consensus as to the best method to facilitate PFM contraction.
-
•
Vaginal palpation with posterior pelvic tilt and vaginal palpation showed the larger effect to facilitate a PFM contraction.
-
•
There was significant improvement among all of the groups in UI and the largest changes were respectively noted in the PG, PTG, ESG and CG.
Keywords: Pelvic floor, Physical therapy, Facilitation, Muscle contraction, Electrical stimulation, Vaginal palpation
Abstract
Objective
To evaluate the effect of vaginal palpation, vaginal palpation associated with posterior pelvic tilt, and intravaginal electrical stimulation in facilitating voluntary contraction of the pelvic floor muscles in women.
Methods
A randomized controlled trial in which 132 women with pelvic floor muscles function graded at 0 or 1 using the Modified Oxford Scale were randomized into four groups: vaginal palpation (n = 33); vaginal palpation with posterior pelvic tilt (n = 33); intravaginal electrical stimulation (n = 33) and a control group (n = 33) that only received verbal instructions. The primary outcome was evaluated by the Modified Oxford Scale and the secondary using the ICIQ-UI-SF. The assessment was performed at baseline with follow-up assessment after eight weeks.
Results
A total of 69.7% of the women from posterior pelvic tilt; 63.6% from vaginal palpation; 33.3% from intravaginal electrical stimulation; and 18.2% from control group (p < 0.001) were able to attain Modified Oxford Scale greater than or equal to 2 after eight weeks. In comparison with control group, the posterior pelvic tilt (OR = 10.35; 95% CI = 3.26–32.84) and vaginal palpation (OR = 7.87; 95% CI = 2.53–24.47) had the most significant improvement as opposed to intravaginal electrical stimulation (OR = 2.25; 95% CI = 0.72–7.06). There was significant improvement among all of the groups in UI. The largest changes respectively were noted in the vaginal palpation, posterior pelvic tilt, intravaginal electrical stimulation and control group. There were no reports of adverse effects.
Conclusion
Vaginal palpation with posterior pelvic tilt and vaginal palpation were more effective interventions to facilitate pelvic floor muscles contraction when compared with intravaginal electrical stimulation and controls. Vaginal palpation was the most effective in improving urinary incontinence.
Clinical Trials Identifier: ClinicalTrial.gov: NCT02062242.
Introduction
Pelvic floor muscle training (PFMT) plays an essential role in the treatment of various pelvic floor dysfunctions.1 The highest level of evidence has been related to treatment for urinary incontinence.2 Only women who are able to properly contract the pelvic floor muscles (PFM) are eligible for PFM training,3 but the prevalence of women unable to contract PFM appears to be high.4, 5 Two studies have indicated a prevalence of 30%–40% of women who were unable to perform a proper voluntary PFM contraction.4, 5 Tibaek and Dehlendorff6 also found that 70% of women with pelvic floor dysfunction were unable to correctly perform a voluntary PFM contraction.
Clinically, a correct PFM contraction is felt during vaginal palpation as compression and elevation of the examiner's fingers.7 However, there is no consensus as to the best method to facilitate voluntary PFM contraction in women who have difficulty in performing this task. Different methods such as biofeedback are recommended in the literature. Biofeedback is considered an adjunct to training that might help the patient be more aware of muscle function.8 According to Bø and Morkved,9 electrical stimulation and vaginal palpation can be also used to facilitate a PFM contraction.
To date, there is no randomized controlled trial (RCT) whose primary objective was to investigate the effectiveness of methods to facilitate a voluntary PFM contraction in women not able to perform a voluntary PFM contraction. Therefore, the primary objective of this trial was to assess the effect of vaginal palpation (PG), vaginal palpation associated with posterior pelvic tilt (PTG), and intravaginal electrical stimulation (ESG) compared to a control group (CG). Our secondary objective was to assess the effect of these interventions on urinary incontinence and their impact on quality of life.
Methods
This is a randomized controlled trial conducted at the Rehabilitation Center of Clinical Hospital of the Ribeirão Preto Medical School at the Universidade de São Paulo (CER-HCFMRP-USP), Brazil. This trial was ethically approved by the Research Ethics Committee of Clinical Hospital of the Ribeirão Preto Medical School at the Universidade de São Paulo (USP), Ribeirão Preto, SP, Brazil (HCRP Process No. 1918/2013) and it was registered at ClinicalTrials.gov (NCT02062242). All those who agreed to participate signed a Consent Form.
Participants
Recruitment was done verbally among women routinely referred to the Physical Therapy Service of CER-HCFMRP-USP for physical therapy treatment of pelvic floor dysfunctions. The research assistant who recruited the participants was blinded to the treatment allocation. Women, who were older than 18 years of age, with PFM function graded at 0 or 1 by the Modified Oxford Scale (MOS) for pelvic floor muscle strength, were considered eligible. Patients with neurological diseases, symptoms of vaginal or urinary tract infections, pelvic organ prolapse ≥ stage 2, suspected or confirmed pregnancy, or cognitive impairment that would hinder or affect the procedures were excluded. The women who did not meet the inclusion criteria also received care according to the institution's protocol.
Interventions
All of the interventions were conducted by the same physical therapist (ECLMV), who had 17 years of experience in women's health physical therapy and who had no contact with the assessments and their results. Women who were unable to perform voluntary PFM contraction, verified by bidigital vaginal palpation (i.e., with PFM function graded at 0 or 1 by the MOS), were randomly allocated to one of the four groups: electrical stimulation group (ESG), palpation group (PG), palpation with posterior pelvic tilt group (PTG) and control group (CG), defined as follows:
Electrical stimulation group (ESG): were composed of women who received intravaginal electrical stimulation. The subjects were positioned in supine with the lower limbs extended. The Dualpex 961 and urogynecological electrode (19 cm long and 2 cm wide), both from Quark®, were used for treatment. The equipment was calibrated. The current used was symmetrical biphasic, rectangular pulse, and the stimulation parameters were: frequency of 50 Hz, pulse time of 200 ms, contraction time (Ton) of 5 s, relaxation time (Toff) of 10 s, and current intensity defined by the motor threshold and adjusted according to the occurrence of accommodation. Total stimulation time was 20 min.10, 11 The women were instructed not to voluntarily contract their PFM while they were receiving the electrical stimulation.
Palpation group (PG): PG was composed of women who received vaginal palpation. The participants were placed in a supine position, with the lower limbs flexed and feet resting on the stretcher. The examiner performed bidigital vaginal palpation in order to provide proprioceptive stimulation and to feel the requested PFM contraction, using the command: “Squeeze around my fingers and lift your PFM as if you were trying not to urinate”. The protocol consisted of three series of ten contractions each held for 6 s, followed by a rest period of 6 s between each contraction. At the end of each series, six quick contractions were requested. Vaginal palpation was maintained throughout the sequence to ensure contractions. The PFM training protocol was described by Bø et al.10
Palpation with posterior pelvic tilt (PTG): This intervention consisted of vaginal palpation associated with posterior pelvic tilt. The participant's position and the protocol were identical to PG,10 except that the patient was asked to perform a posterior pelvic tilt movement during their trial to do a PFM contractions. Control of the pelvic position was observed visually by the physical therapist who applied the interventions.
Control group (CG): The participants were instructed only once to execute the PFM contractions daily at home, following the protocol of three series of 10 contractions held for 6 s, with a rest period of 6 s between each contraction. Between each series, they were told to do six quick contractions.
The ESG, PG and PTG participants were not instructed to perform any training at home. They were seen individually once each week for treatment, and the assessment was performed at baseline with follow-up assessment right after eight weeks. The CG participants did not receive any supervision other than the instructions on the first day, did not have any contact with the physical therapist after the first instruction/session and were also reassessed after eight weeks.
After the RCT follow-up period, the participants were offered other treatment options, based on their need.
Outcomes
The assessments were performed at baseline and right after eight weeks in all groups. The data collection methods were the same for all of the women. The assessments were all performed by the same trained physical therapist (FIA), with 12 years of experience in women's health physical therapy, not involved with any other part of the research.
Primary outcome measure
Assessment of voluntary PFM contraction capacity
PFM contraction capacity was assessed through bidigital vaginal palpation.12 All women received individual verbal instructions about female anatomy, pelvic floor functions, as well as how to perform a correct PFM contraction. They were then placed in supine position with knees and hips in a flexed and abducted position, and with their feet on a bench. Participants were instructed to breathe normally and the examiner then carefully inserted the index and middle fingers into the vaginal canal. Participants were told to perform the maximum voluntary contraction of the PFM, lifting and squeezing the examiner's fingers. The verbal instruction used to request a contraction during the assessment was: ““Squeeze around my fingers and lift your PFM as if you were trying not to urinate” right.” At this time, the PFM function was graded, and participants were then instructed to completely relax the PFM.
The MOS, developed by Laycock,13 was used to grade PFM function. The MOS is considered a reliable and valid scale for assessing PFM contractions.14, 15 The intra-rater reliability has been shown to range from moderate to very good.16 To characterize the women in terms of acquisition of PFM contraction capacity, two distinct analyses were performed: one that considered the change as MOS ≥ 2 and the other as MOS ≥ 3.
Secondary outcome measure
Assessment of urinary incontinence in terms of frequency, severity and impact on quality of life
For urinary incontinence assessment, the International Consultation on Incontinence Questionnaire Short Form (ICIQ-UI-SF) was used. This form evaluated the presence of urinary complaints and urinary continence status. The ICIQ-UI-SF was composed of three questions related to frequency, severity of urinary loss and impact of incontinence on quality of life. The ICIQ-UI-SF score was the sum of the values from Questions 3–5, and varied from 0 to 21 (the higher the score, the greater the severity and impact of urinary incontinence on daily life).17
Adherence assessment
Recording of attendance at the physical therapy sessions by the participants in the three groups was performed by the same researcher who applied the interventions.
Sample size
The sample size was calculated using a pilot sample of 55 patients who had completed the intervention. The pilot sample was included in the study. The proportions between each group were used, where change was considered as MOS ≥ 2 in the reassessment. The sample size calculation was based on the test of differences between proportions and Fisher's exact test, with a p-value of 0.05. The change percentage in each group was 64.7% in PTG; 56.32% in PG; 35.32% in ESG and 13.63% in CG. Based on a significance level of 5% and power of the test of 90%, in the comparison between PG and CG, the minimum number indicated was 25 participants; and in the comparison between PTG and CG, the minimum number indicated was 19 participants. To calculate sample size and power of the test, the SAS version 9.0 statistical software was used.
Randomization, allocation and blinding
The randomization of the participants was done using a computer-generated list of numbers, on the website: www.randomization.com. The allocation of the participants in the study was concealed. The researcher who decided if the women were eligible for inclusion in the study was unaware, of which group they would be allocated to. The research assistant who performed the patients allocation in the groups was “off site” (i.e. not involved in the recruitment, assessment of participants or interventions) with the randomization schedule.
The assessments were all performed by the same trained physical therapist (FIA), not involved in doing any of the interventions. The ICIQ-UI-SF was administered to all the participants by an assistant researcher (TDS), who was specifically trained to perform this data collection.
All of the interventions were conducted by the same physical therapist (ECLMV), who had no contact with the assessments and their results.
Statistical analysis
The data was first analyzed through descriptive statistics that enabled characterizing the sample according to the variables collected, and then presented using frequency tables and descriptive means. Analysis of variance (ANOVA) was used to confirm homogeneity between the groups of quantitative variables (i.e. age and number of deliveries). The Fisher's exact test was used to verify the association between the categorical variables. The between-group differences for the primary outcomes were measured using logistic regression models, in which the crude odds ratio was calculated with respective confidence intervals of 95%. The linear mixed-effects model (i.e. random and fixed effects) was used to analyze between-group differences for continuous outcomes such as the ICIQ-UI-SF instrument. In the mixed effects model used, individuals were considered as random effects and groups, times and their interaction as fixed effects. The adjustment of the model was performed using with the PROC MIXED procedure from SAS statistical software.
All the statistical analyses were performed using SAS 9.0 statistical software. The significance level was set at 5%.
Results
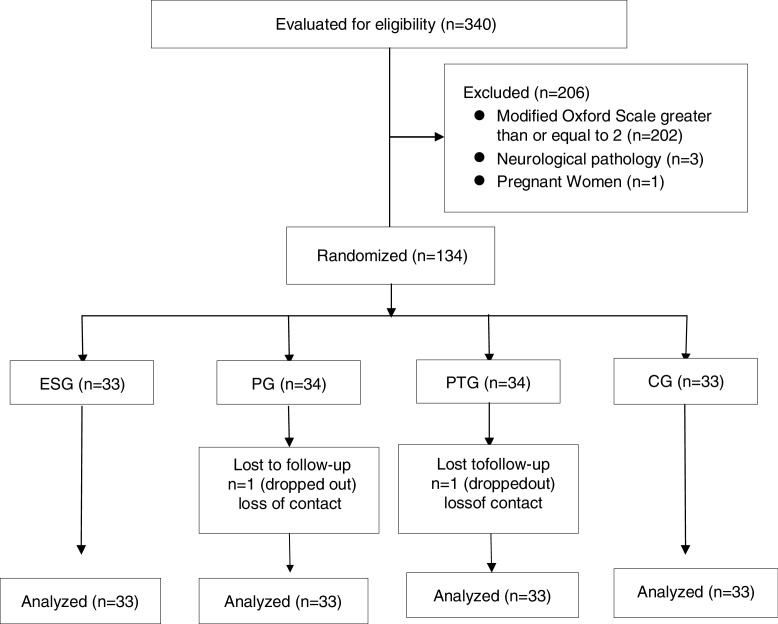
Recruitment and data collection took place between March 2013 and December 2015. Of the 340 women assessed in the Physical Therapy Service of CER-HCFMRP-USP during this period, 134 (38.6%) met the inclusion criteria and agreed to participate in the study. Of these, 132 completed the study (98.5%), as shown in Fig. 1.
Figure 1.
Flowchart of the 4 patient groups included and analyzed in the study to determine the effects of 3 interventions in facilitating voluntary pelvic floor muscle contractions in women.
Baseline
The mean age of the participants was 53.1 years (SD 12.6) and the mean body mass index (BMI) was 29.7 kg/m2 (SD 5.4). The groups were homogeneous in relation to age; parity; classification between nullipara, primipara and multipara; type of delivery; and reproductive status. All participants had some kind of pelvic floor dysfunction; the most prevalent of which was urinary incontinence in 126 women (95.4%). Ninety-five women (72%) had a urodynamic diagnosis with a predominance of detrusor hyperactivity.
The data regarding the characteristics of the participants is presented in Table 1.
Table 1.
Characteristics of the study participants.
| ESG (n = 33) | PG (n = 33) | PTG (n = 33) | CG (n = 33) | |
|---|---|---|---|---|
| Age (years) | 55.6 (10.3) | 53.7 (14.0) | 49.6 (11.3) | 53.5 (14.0) |
| BMI (kg/m2) | 28.1 (5.1) | 28.3 (4.3) | 30.4 (5.4) | 32.0 (5.8) |
| Has a conjugal partner | 22 (66.8%) | 22 (66.7%) | 27 (81.8%) | 19 (57.6%) |
| Has no conjugal partner | 11 (33.3%) | 11 (33.3%) | 6 (18.2%) | 14 (42.4%) |
| Education | ||||
| Illiterate | 4 (12.1%) | 5 (15.1%) | 1 (3.0%) | 2 (6.1%) |
| Elementary incomplete | 15 (45.4%) | 18 (54.5%) | 18 (54.5%) | 24 (72.7%) |
| Elementary complete | 4 (12.1%) | 4 (12.1%) | 7 (21.2%) | 1 (3.0%) |
| Secondary incomplete | 3 (9.1%) | 1 (3.0%) | 1 (3.0%) | 0 (0%) |
| Secondary complete | 2 (6.1%) | 4 (12.1%) | 4 (12.1%) | 5 (15.1%) |
| University complete | 5 (15.1%) | 1 (3.0%) | 2 (6.1%) | 1 (3.0%) |
| Number of pregnancies | 3.5 (2.8) (0–12) |
4.1 (3.1) (0–16) |
3.5 (2.2) (0–11) |
4.0 (2.4) (0–12) |
| Parity | 3.2 (2.3) | 3.8 (3.0) | 3.0 (2.1) | 3.4 (2.5) |
| Classification by number of births | ||||
| Nullipara | 3 (9.1%) | 1 (3.0%) | 3 (9.1%) | 2 (6.1%) |
| Primipara | 3 (9.1%) | 1 (3.0%) | 6 (18.2%) | 3 (9.1%) |
| Multipara | 27 (81.8%) | 31 (93.9%) | 24 (72.7%) | 28 (84.8%) |
| Type of delivery | ||||
| Vaginal | 23 (69.7%) | 27 (81.3%) | 23 (69.7%) | 27 (81.8%) |
| Assisted | 21 (63.6%) | 21 (63.6%) | 20 (60.6%) | 22 (66.7%) |
| C-section | 16 (48.5%) | 14 (42.4%) | 19 (57.6%) | 13 (39.4%) |
| Reproductive status | ||||
| Menacme | 6 (18.2%) | 9 (27.3%) | 12 (36.4%) | 10 (30.3%) |
| Menopause | 4 (12.1%) | 3 (9.1%) | 2 (6.1%) | 0 (0%) |
| Postmenopause | 23 (69.7%) | 21 (63.6%) | 19 (57.6%) | 23 (69.7%) |
| Pelvic floor dysfunctiona | ||||
| Urinary incontinence | 31 (93.9%) | 31 (93.9%) | 32 (97.0%) | 32 (97.0%) |
| Anal incontinence | 1 (3.0%) | 1 (3.0%) | 1 (3.0%) | 0 (0%) |
| Interstitial cystitis | 1 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pelvic organ prolapse | 0 (0%) | 1 (3.0%) | 0 (0%) | 1 (3.0%) |
| Urodynamic diagnosis | ||||
| Normal | 3 (9.1%) | 5 (15.1%) | 3 (9.1%) | 2 (6.1%) |
| Urinary stress incontinence | 7 (21.2%) | 6 (18.2%) | 5 (15.1%) | 4 (12.1%) |
| Detrusor hyperactivity | 6 (18.2%) | 7 (21.2%) | 10 (30.3%) | 14 (42.4%) |
| Mixed urinary incontinence | 3 (9.1%) | 2 (6.1%) | 5 (15.1%) | 5 (15.1%) |
| Detrusor hypocontractility | 1 (3.0%) | 0 (0%) | 1 (3.0%) | 1 (3.0%) |
| Infravesical obstruction | 1 (3.0%) | 3 (9.1%) | 1 (3.0%) | 0 (0%) |
| Without urodynamics | 12 (36.4%) | 10 (30.3%) | 8 (24.2%) | 7 (21.2%) |
Absolute values with percentages are presented as: n (%).
Means with standard deviation are presented as: mean (SD).
ESG, electrical stimulation group; PG, palpitation group; PTG, palpation with posterior pelvic tilt group; CG, control group; BMI, body mass index.
Medical diagnosis with referral to physical therapy.
Primary outcome
Assessment by vaginal palpation, and change in MOS from grades 0 and 1 to ≥ 2
All the groups studied demonstrated improved PFM contraction capacity. The groups that experienced greatest changes were the PTG (69.7%) and the PG (63.6%); and the groups with fewer changes were the ESG (33.3%) and the CG (18.2%). (Table 2).
Table 2.
Assessment of pelvic floor muscles function by digital palpation, according to the Modified Oxford Scale for pelvic floor muscles, after intervention, considering change as FPFA grade ≥2 in the study to determine the effects of 3 interventions in facilitating voluntary pelvic floor muscle contractions in women.
| Modified Oxford Scale (grade) | ESG (n = 33) | PG (n = 33) | PTG (n = 33) | CG (n = 33) | p-valuea |
|---|---|---|---|---|---|
| 0 | 11 (33.3%) | 6 (18.2%) | 4 (12.1%) | 11 (33.3%) | |
| 1 | 11 (33.3%) | 6 (18.2%) | 6 (18.2%) | 16 (48.5%) | |
| 2 | 8 (24.2%) | 11 (33.3%) | 10 (30.3%) | 6 (18.2%) | <0.001 |
| 3 | 3 (9.1%) | 7 (21.2%) | 10 (30.3%) | 0 (0%) | |
| 4 | 0 (0%) | 3 (9.1%) | 3 (9.1%) | 0 (0%) | |
| Total change | 11 (33.3%) | 21 (63.6%) | 23 (69.7%) | 6 (18.2%) | |
Analyzed with Fisher's exact test.
Absolute values with percentages are presented as: n (%).
ESG, electrical stimulation group; PG, palpation group; PTG, palpation with posterior pelvic tilt group; CG, control group; FPFA, functional pelvic floor assessment.
In comparison to the CG, logistic regression analysis showed that the PTG (OR = 10.35; 95% CI = 3.26–32.84) and the PG (OR = 7.87; 95% CI = 2.53–24.47) had the most significant improvement in PFM contraction capacity. This improvement was greater in the PTG than the ESG (OR = 2.25; 95% CI = 0.72–7.06).
Assessment by vaginal palpation, and change in MOS from grades 0, 1 and 2 to MOS ≥ 3
In this analysis, all 3 treatment groups (i.e. ESG, PG and PTG) showed improved PFM contraction capacity. The groups with the greatest changes were PTG (39.4%) and PG (30.3%). The group with less change was ESG (9.1%). No CG participants moved to MOS ≥ 3 (Table 3).
Table 3.
Assessment of pelvic floor muscles function by digital palpation, according to the Modified Oxford Scale for pelvic floor muscles, after intervention, considering change as FPFA grade ≥3 in the study to determine the effects of 3 interventions in facilitating voluntary pelvic floor muscle contractions in women.
| Modified Oxford Scale (grade) | ESG (n = 33) | PG (n = 33) | PTG (n = 33) | CG (n = 33) | p-valuea |
|---|---|---|---|---|---|
| 0 | 11 (33.3%) | 6 (18.2%) | 4 (12.1%) | 11 (33.3%) | |
| 1 | 11 (33.3%) | 6 (18.2%) | 6 (18.2%) | 16 (48.5%) | |
| 2 | 8 (24.2%) | 11 (33.3%) | 10 (30.3%) | 6 (18.2%) | <0.001 |
| 3 | 3 (9.1%) | 7 (21.2%) | 10 (30.3%) | 0 (0%) | |
| 4 | 0 (0%) | 3 (9.1%) | 3 (9.1%) | 0 (0%) | |
| Total change | 3 (9.1%) | 10 (30.3%) | 13 (39.4%) | 0 (0%) | |
Analyzed with Fisher's exact test.
Absolute values with percentages are presented as: n (%).
ESG, electrical stimulation group; PG, palpation group; PTG, palpation with posterior pelvic tilt group; CG, control group; FPFA, functional pelvic floor assessment.
For logistic regression analysis, since there was no change in MOS to grade 3 or higher, ESG was used as the reference group, since its results were the closest to those of CG. It was found that PTG (OR = 4.35; 95% CI = 1.07–17.63) and PG (OR = 6.50; 95% CI = 1.64–25.76) had the most significantly improved PFM contraction capacity, when compared with ESG, with the improvement being greater in the PTG.
Secondary outcome
Urinary incontinence
Table 4 presents the results related to reports of urinary incontinence with regards to frequency, severity, and impact on quality of life, assessed using the ICIQ-UI-SF before and after the intervention. In the intragroup assessment of total ICIQ-UI-SF scores, there was significant improvement among all of the groups. The largest changes respectively were noted in the PG, PTG, ESG and CG.
Table 4.
Effects of the intervention in reports of urinary incontinence regarding frequency, severity, and impact on quality of life, assessed using the ICIQ-IU-SF in the study to determine the effects of 3 interventions in facilitating voluntary pelvic floor muscle contractions in women.
| ICIQ-SF | ESG (n = 33) |
PG (n = 33) |
PTG (n = 33) |
CG (n = 33) |
||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Frequency | ||||||||
| Never | 2 (6.1%) | 10 (30.3%) | 2 (6.1%) | 15 (45.4%) | 2 (6.1%) | 9 (27.3%) | 4 (12.1%) | 8 (24.2%) |
| ≤Once a week | 3 (9.1%) | 6 (18.2%) | 6 (18.2%) | 12 (36.4%) | 2 (6.1%) | 6 (18.2%) | 3 (9.1%) | 5 (15.1%) |
| 2–3 times a week | 5 (15.1%) | 2 (6.1%) | 4 (12.1%) | 1 (3.0%) | 4 (12.1%) | 6 (18.2%) | 4 (12.1%) | 7 (21.2%) |
| Once a day | 3 (9.1%) | 5 (15.1%) | 2 (6.1%) | 4 (12.1%) | 4 (12.1%) | 5 (15.1%) | 4 (12.1%) | 4 (12.1%) |
| Several times a day | 15 (45.4%) | 9 (27.3%) | 14 (42.4%) | 0 (0%) | 11 (33.3%) | 5 (15.1%) | 11 (33.3%) | 5 (15.1%) |
| All the time | 5 (15.1%) | 1 (3.0%) | 5 (15.1%) | 1 (3.0%) | 10 (30.3%) | 2 (6.1%) | 7 (21.2%) | 4 (12.1%) |
| Amount | ||||||||
| None | 2 (6.1%) | 10 (30.3%) | 2 (6.1%) | 15 (45.4%) | 2 (6.1%) | 9 (27.3%) | 4 (12.1%) | 8 (24.2%) |
| Small | 16 (48.5%) | 14 (42.4%) | 10 (30.3%) | 15 (45.4%) | 12 (36.4%) | 13 (39.4%) | 7 (21.2%) | 12 (36.4%) |
| Moderate | 6 (18.2%) | 4 (12.1%) | 12 (36.4%) | 2 (6.1%) | 14 (42.4%) | 8 (24.2%) | 9 (27.3%) | 7 (21.2%) |
| Large | 9 (27.3%) | 5 (15.1%) | 9 (27.3%) | 1 (3.0%) | 5 (15.1%) | 3 (9.1%) | 13 (39.4%) | 6 (18.2%) |
| Impact | ||||||||
| None | 2 (6.1%) | 7 (21.2%) | 2 (6.1%) | 13 (39.4%) | 2 (6.1%) | 11 (33.3%) | 3 (9.1%) | 8 (24.2%) |
| Slight | 2 (6.1%) | 7 (21.2%) | 1 (3.0%) | 6 (18.2%) | 2 (6.1%) | 2 (6.1%) | 2 (6.1%) | 4 (12.1%) |
| Moderate | 4 (12.1%) | 5 (15.1%) | 6 (18.2%) | 5 (15.1%) | 6 (18.2%) | 8 (24.2%) | 7 (21.2%) | 3 (9.1%) |
| Severe | 13 (39.4%) | 6 (18.2%) | 10 (30.3%) | 7 (21.2%) | 11 (33.3%) | 5 (15.1%) | 6 (18.2%) | 8 (24.2%) |
| Very severe | 12 (36.4%) | 8 (24.2%) | 14 (42.4%) | 2 (6.1%) | 12 (36.4%) | 7 (21.2%) | 15 (45.4%) | 10 (30.3%) |
| Total ICIQ-SF (0–21) |
14.12 (5.59)b | 9.42 (7.09) | 14.55 (4.94)b | 5.79 (5.58) | 14.24 (5.35)b | 8.85 (6.93) | 14.15 (6.25)b | 10.52 (7.37) |
| aWithin-group differences (95% CI) | 4.70 (1.77–7.65) | 8.76 (5.80–11.71) | 5.39 (2.43–8.35) | 3.64 (0.68–6.59) | ||||
| p-value | p = 0.002 | p < 0.0001 | p = 0.0004 | p = 0.016 | ||||
Analyzed using linear mixed-effects model.
Absolute values with percentages are presented as: n (%).
Means with standard deviation are presented as: mean (SD).
ESG (n = 33), electrical stimulation group; PG (n = 33), palpation group; PTG (n = 33), palpation with posterior pelvic tilt group; CG (n = 33), control group; CI, confidence interval; ICIQ-UI-SF, International Consultation on Incontinence Questionnaire Short Form.
The groups were homogeneous at baseline for the total ICIQ-SF variable.
There was no significant difference in the initial assessment between the four groups in the study. In the analysis between the groups in relation to total ICIQ-UI-SF score, there was a significant differnce in the reassessment between the ESG and PG (mean difference = 3.64; 95% CI = 0.68–6.59; p = 0.0162); between PG and PTG (mean difference = −3.06; 95% CI = −6.02 to −0.10; p = 0.0426); and between PG and CG (mean difference = −4.73; 95% CI = −7.68 to −1.77; p = 0.0018), all of which had the best response in the PG. There was no significant difference in the total ICIQ-SF score in the reassessment among the other groups (Table 5).
Table 5.
Between-groups differences after interventions in relation to total ICIQ-SF score in the study to determine the effects of 3 interventions in facilitating voluntary pelvic floor muscle contractions in women.
| Comparisons between groups | Between-group differences | 95% CI | p-valuea |
|---|---|---|---|
| ESG vs PG | 3.64 | 0.68 to 6.59 | 0.02 |
| ESG vs PTG | 0.57 | −2.38 to 3.53 | 0.71 |
| ESG vs CG | −1.09 | −4.05 to 1.87 | 0.47 |
| PG vs PTG | −3.06 | −6.02 to −0.10 | 0.04 |
| PG vs CG | −4.73 | −7.68 to −1.77 | 0.01 |
| PTG vs CG | −1.67 | −4.62 to 1.29 | 0.27 |
Analyzed using linear mixed-effects model.
ESG (n = 33), electrical stimulation group; PG (n = 33), palpation group; PTG (n = 33), palpation with posterior pelvic tilt group; CG (n = 33), control group; CI, confidence interval.
ICIQ-IU-SF, International Consultation on Incontinence Questionnaire Short Form.
Adherence and adverse effects
All the study completers from the three intervention groups attended to all eight planned supervised sessions. During the follow-up period, there were no reports of adverse effects or complications resulting from any of the interventions.
Discussion
PFMT has shown level 1 scientific evidence to be effective in treating urinary incontinence. However, the capacity to perform a correct voluntary PFM contraction is essential to allow women to perform PFMT. Frequently, the RCTs assessing the impact of PFMT on urinary incontinence excluded women unable to voluntary contract their PFM. This RCT was the first in the literature to include only women who were unable to voluntarily contract their PFM and to study the effects of electrical stimulation, vaginal palpation, and vaginal palpation associated with posterior pelvic tilt to facilitate a voluntary PFM contraction. The two most effective interventions were found to be vaginal palpation and vaginal palpation associated with posterior pelvic tilt. The primary outcome was PFM contraction capacity, which was graded using the MOS.13 Only women with MOS grades 0 or 1 were included in the study, since women with MOS grade 2 may not need an intravaginal method to specifically promote a voluntary PFM contraction.18, 19
There have been few studies whose primary objective was to assess physical therapy interventions to facilitate PFM contraction; and it seems that none of them included only women unable to perform a voluntary PFM contraction.20 Vaginal palpation is a proprioceptive stimulus that can be used as a method to promote learning and facilitation of the correct voluntary contraction of PFM.21 Changes in pelvic positioning are also reported in the literature as a factor that may help with the activation and the ability to contract the PFM.22, 23 The rationale for testing the hypothesis that posterior pelvic tilt associated to vaginal palpation could strengthen the effect of vaginal palpation alone was based on a study showing a significantly higher PFM tonic electromyographic activity in a posterior pelvic tilt posture.23
Also used as an intervention in our study, electrical stimulation has been a widely used therapeutic resource in clinical practice for feedback in women unable to contract the PFM. According to Price et al.,24 electrical stimulation is an intervention that should be considered in women unable to actively contract their PFM and by so doing aiding in motivation and adherence to therapy. In the present study, women treated with electrical stimulation had the smallest effect in PFM contraction capacity, compared to women in the PTG and the PG and was not significantly different from the CG. This result could potentially be explained by the fact that they were not told to voluntarily contract the PFM when the current was active, although it is not known whether this instruction would have changed the result.
Although most of participants in this study reported urinary incontinence, a small percentage had a urodynamic diagnosis of detrusor hypocontractility and infravesical obstruction. Despite the differences in their diagnosis, all of them were initially unable to perform a PFM contraction, which was our primary aim. The total ICIQ-SF score showed that all of the groups experienced decreased urinary incontinence after their intervention treatment with the best effect found in the PG, and the least in the CG.PFM training programs for urinary incontinence treatment generally last from three to six months.25 The length of the intervention in the present study was eight weeks, with one session per week. Increased PFM contraction capacity, especially in women receiving vaginal palpation, is probably related to neuromuscular adaptation through motor leaning.26 This would explain improved maximum voluntary contraction before the occurrence of muscle hypertrophy.26
The group that received electrical stimulation also experienced decreased urinary incontinence. The normal mechanism for decreasing urinary incontinence would be to start voluntarily or automatically activating the PFM to prevent urinary losses and possibly detrusor inhibition activity by an electrical stimulation-induced PFM contraction. However, the number, duration, intensity and length of PFM contractions required to inhibit detrusor muscle contractions is not known.27 The CG, also showed decreased urinary incontinence, the possible result of a placebo effect in relation to the urinary incontinence also needs to be considered.
One limitation of the present study was the absence of neurophysiological tests such as needle electromyography to exclude women with PFM nerve injury. However, even though these women were not excluded, the interventions investigated still showed a positive effect. Another limitation was the absence of imaging methods such as ultrasound to analyze the PFM contractions. The intra-examiner reliability of the method used to assess our primary outcome (MOS) has been shown to range from moderate to very good.14, 16 More than one study in the literature has indicated a moderate to strong positive correlation between MOS and ultrasound evaluation of PFM function.28, 29 Thus, from a clinical point of view, the use of vaginal palpation could be considered a sufficiently satisfactory outcome for assessing voluntary PFM contraction capacity.21, 30
The strong points of this study included its study design, the concealed allocation, the use of reliable outcome measures, and a blinded experienced assessor. In addition, adherence of the three intervention groups was excellent and the participants did not report any adverse effects or complications.
Conclusion
Vaginal palpation with or without posterior pelvic tilt was the most effective intervention for facilitating voluntary PFM contraction in women, when compared with intravaginal electrical stimulation and a control group. Vaginal palpation was the most effective in improving urinary incontinence compared to the other interventions.
Funding sources
Funding: This work was supported by the Foundation for Support to Teaching, Research and Assistance (FAEPA) of the University of São Paulo.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Martinho N.M., Silva V.R., Marques J., Carvalho L.C., Iunes D.H., Botelho S. The effects of training by virtual reality or gym ball on pelvic floor muscle strength in postmenopausal women: a randomized controlled trial. Braz J Phys Ther. 2016;20(3):248–257. doi: 10.1590/bjpt-rbf.2014.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumoulin C., Hay-Smith E.J., Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2014;(5):CD005654. doi: 10.1002/14651858.CD005654.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Dias L.A., Driusso P., Aita D.L., Quintana S.M., Bø K., Ferreira C.H. Effect of pelvic floor muscle training on labour and newborn outcomes: a randomized controlledtrial. Rev Bras Fisioter. 2011;15(6):487–493. doi: 10.1590/s1413-35552011005000011. [DOI] [PubMed] [Google Scholar]
- 4.Bø K., Stien R., Needle E.M.G. registration of striated urethral wall and pelvic floor muscle activity patterns during cough, valsalva, abdominal, hip adductor, and gluteal muscles contractions in nulliparous healthy females. Neurourol Urodyn. 1994;13(1):35–41. doi: 10.1002/nau.1930130106. [DOI] [PubMed] [Google Scholar]
- 5.Talasz H., Himmer-Perschak G., Marth E., Fischer-Colbrie J., Hoefner E., Lechleitner M. Evaluation of pelvic floor muscle function in a random group of adult women in Austria. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):131–135. doi: 10.1007/s00192-007-0404-y. [DOI] [PubMed] [Google Scholar]
- 6.Tibaek S., Dehlendorff C. Pelvic floor muscle function in women with pelvic floor dysfunction: a retrospective chart review, 1992–2008. Int Urogynecol J. 2014;25(5):663–669. doi: 10.1007/s00192-013-2277-6. [DOI] [PubMed] [Google Scholar]
- 7.Messelink B., Benson T., Berghmans B. Standardisation of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24(4):374–380. doi: 10.1002/nau.20144. [DOI] [PubMed] [Google Scholar]
- 8.Bo K., Frawley H.C., Haylen B.T. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J. 2017;28(2):191–213. doi: 10.1007/s00192-016-3123-4. [DOI] [PubMed] [Google Scholar]
- 9.Bø K., Morkved S. Motor learning. In: Bø K., Berghmans B., Morkved S., Van Kampen M., editors. Evidence-based Physical Therapy Pelvic Floor: Bridging Science and Clinical Practice. 2nd ed. Elsevier; London: 2015. pp. 111–117. [Google Scholar]
- 10.Bø K., Talseth T., Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ. 1999;318(7182):487–493. doi: 10.1136/bmj.318.7182.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro R.A., Arruda R.M., Zanetti M.R., Santos P.D., Sartori M.G., Girão M.J. Single-blind, randomized, controlled trial of pelvic floor muscle training, electrical stimulation, vaginal cones, and no active treatment in the management of stress urinary incontinence. Clinics (Sao Paulo) 2008;63(4):465–472. doi: 10.1590/S1807-59322008000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bø K. Visual observation and palpation. In: Bø K., Berghmans B., Morkved S., Van Kampen M., editors. Evidence-based Physical Therapy Pelvic Floor: Bridging Science and Clinical Practice. 2nd ed. Elsevier; London: 2015. pp. 47–53. [Google Scholar]
- 13.Laycock J. Pelvic muscle exercise: physiotherapy for the pelvic floor. Urol Nurs. 1994;14(3):136–140. [PubMed] [Google Scholar]
- 14.Frawley H.C., Galea M.P., Phillips B.A., Sherburn M., Bø K. Reliability of pelvic floor muscle strength assessment using different test positions and tools. Neurourol Urodyn. 2006;25(3):236–242. doi: 10.1002/nau.20201. [DOI] [PubMed] [Google Scholar]
- 15.Isherwood P.J., Rane A. Comparative assessment of pelvic floor strength using a perineometer and digital examination. BJOG. 2000;107(8):1007–1011. doi: 10.1111/j.1471-0528.2000.tb10404.x. [DOI] [PubMed] [Google Scholar]
- 16.Frawley H. Pelvic floor muscle strength testing. Aust J Physiother. 2006;52(4):307. doi: 10.1016/s0004-9514(06)70016-2. [DOI] [PubMed] [Google Scholar]
- 17.Tamanini J.T., Dambros M., D’Ancona C.A., Palma P.C., Rodrigues Netto N., Jr. Validation of the “International Consultation on Incontinence Questionnaire – Short Form” (ICIQ-SF) for Portuguese. Rev Saude Publica. 2004;38(3):438–444. doi: 10.1590/s0034-89102004000300015. [DOI] [PubMed] [Google Scholar]
- 18.Alves F.K., Riccetto C., Adami D.B. A pelvic floor muscle training program in postmenopausal women: a randomized controlled trial. Maturitas. 2015;81(2):300–305. doi: 10.1016/j.maturitas.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Huebner M., Riegel K., Hinninghofen H., Wallwiener D., Tunn R., Reisenauer C. Pelvic floor muscle training for stress urinary incontinence: a randomized, controlled trial comparing different conservative therapies. Physiother Res Int. 2011;16(3):133–140. doi: 10.1002/pri.489. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro B.F., Franco G.R., Feitosa S.M., Yuaso D.R., Castro R.A., MJBC Physiotherapy for perineal awareness: a comparison between kinesiotherapy with digital palpation and with biofeedback aid. Fisioter Mov. 2012;25(3):639–648. [in Portuguese] [Google Scholar]
- 21.Bø K., Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85(3):269–282. [PubMed] [Google Scholar]
- 22.Enck P., Vodusek D.B. Electromyography of pelvic floor muscles. J Electromyogr Kinesiol. 2006;16(6):568–577. doi: 10.1016/j.jelekin.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Capson A.C., Nashed J., McLean L. The role of lumbopelvic posture in pelvic floor muscle activation in continent women. J Electromyogr Kinesiol. 2011;21(1):166–177. doi: 10.1016/j.jelekin.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Price N., Dawood R., Jackson S.R. Pelvic floor exercise for urinary incontinence: a systematic literature review. Maturitas. 2010;67(4):309–315. doi: 10.1016/j.maturitas.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Hay-Smith E.J., Bø K., Berghmans L.C., Hendriks H.J., de Bie R.A., van Waalwijk van Doorn E.S. Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2001;(1):CD001407. doi: 10.1002/14651858.CD001407. [DOI] [PubMed] [Google Scholar]
- 26.Remple M.S., Bruneau R.M., VandenBerg P.M., Goertzen C., Kleim J.A. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res. 2001;123(2):133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 27.Dumoulin C., Hay-Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2010;(1):CD005654. doi: 10.1002/14651858.CD005654.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Arab A.M., Behbahani R.B., Lorestani L., Azari A. Correlation of digital palpation and transabdominal ultrasound for assessment of pelvic floor muscle contraction. J Man Manip Ther. 2009;17(3):e75–e79. doi: 10.1179/jmt.2009.17.3.75E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volløyhaug I., Mørkved S., Salvesen Ø., Salvesen K.Å. Assessment of pelvic floor muscle contraction with palpation, perineometry and transperineal ultrasound: a cross-sectional study. Ultrasound Obstet Gynecol. 2016;47(6):768–773. doi: 10.1002/uog.15731. [DOI] [PubMed] [Google Scholar]
- 30.Moen M.D., Noone M.B., Vassallo B.J., Elser D.M. Pelvic floor muscle function in women presenting with pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):843–846. doi: 10.1007/s00192-009-0853-6. [DOI] [PubMed] [Google Scholar]