Abstract
The underlying mechanism as to why some hypotensive preterm infants do not respond to inotropic medications remains unclear. For these infants, we hypothesize that impaired vasomotor function is a significant factor and is manifested through a decrease in low-frequency blood pressure variability across regulatory components of vascular tone. Infants born ≤28 wk estimated gestational age underwent prospective recording of mean arterial blood pressure for 72 h after birth. After error correction, root-mean-square spectral power was calculated for each valid 10-min data frame across each of four frequency bands (B1, 0.005–0.0095 Hz; B2, 0.0095–0.02 Hz; B3, 0.02–0.06 Hz; and B4, 0.06–0.16) corresponding to different components of vasomotion control. Forty infants (twenty-nine normotensive control and eleven inotrope-exposed) were included with a mean ± SD estimated gestational age of 25.2 ± 1.6 wk and birth weight 790 ± 211 g. 9.7/11.8 Million (82%) data points were error-free and used for analysis. Spectral power across all frequency bands increased with time, although the magnitude was 20% less in the inotrope-exposed infants. A statistically significant increase in spectral power in response to inotrope initiation was noted across all frequency bands. Infants with robust blood pressure response to inotropes had a greater increase compared with those who had limited or no blood pressure response. In this study, hypotensive infants who require inotropes have decreased low-frequency variability at baseline compared with normotensive infants, which increases after inotrope initiation. Low-frequency spectral power does not change for those with inotrope treatment failure, suggesting dysfunctional regulation of vascular tone as a potential mechanism of treatment failure.
NEW & NOTEWORTHY In this study, we examine patterns of low-frequency oscillations in blood pressure variability across regulatory components of vascular tone in normotensive and hypotensive infants exposed to inotropic medications. We found that hypotensive infants who require inotropes have decreased low-frequency variability at baseline, which increases after inotrope initiation. Low-frequency spectral power does not change for those with inotrope treatment failure, suggesting dysfunctional regulation of vascular tone as a potential mechanism of treatment failure.
Keywords: blood pressure, inotropes, prematurity, spectral analysis
the optimal blood pressure of preterm infants remains ill-defined. Although there is evidence that a mean arterial blood pressure persistently <30 mmHg may be associated with an increased risk of intraventricular hemorrhage (IVH), a devastating complication of prematurity associated with adverse neurodevelopmental outcomes (12), the threshold at which interventions (e.g., fluid resuscitation, initiation of inotropic agents) should be made remains unclear (16). Recent work by us (27) suggests that there is a differential response in blood pressure to inotropes among hypotensive preterm infants that can be divided into three categories, nonresponse, nominal response, and superresponse, but the underlying mechanism of this differential response is not known.
The variability of minute fluctuations in blood vessel caliber, termed vasomotion, represents the composite influence of autonomic (2), myogenic (8, 20), and cellular (19) control mechanisms. Crucially, the intensity of response of these control mechanisms is modulated by circulating catecholamines (22), vasoactive intestinal peptides (4), nitric oxide (2, 10, 25), hypovolemia (21), and the renin-angiotensin system (3). Through spectral analysis, the relative contributions of each domain of vasomotion can be interrogated based on their distinctive periodicity: endothelial (0.005–0.0095 Hz), endothelial NO-dependent (0.0095–0.02 Hz), neurogenic (0.02–0.06 Hz), and myogenic (0.06–0.16 Hz; Ref. 7). As a result, blood pressure variability may offer a potential adjunctive measure of cardiovascular status, beyond the absolute measured value of the mean arterial blood pressure, and could serve as a potential biomarker of cardiovascular health in the setting of injury. However, despite this potential promise, blood pressure variability has received limited evaluation in the preterm infant, with preliminary evidence suggesting that less variability is associated with worse outcomes (6, 11, 28).
We hypothesize that hypotensive, inotrope-exposed infants will demonstrate impaired vasomotor function compared with normotensive peers. To investigate, we conducted a prospective observational study, first to examine the longitudinal evolution of blood pressure variability, across four different frequency bands in a normative population of preterm infants who were not hypotensive and did not receive inotropic medications. Next, to evaluate the mechanism of inotrope treatment failure, we evaluated frequency-dependent differences in blood pressure variability in a second group of hypotensive infants, comparing those with no response, nominal response, and superresponse to inotropic medications.
METHODS
Cohort selection.
All infants admitted to the Neonatal Intensive Care Unit at St. Louis Children’s Hospital, a Level IV unit serving urban, suburban, and rural populations, have a complete set of physiological data [heart rate, respiratory rate, blood pressure (invasive and/or noninvasive), and pulse oximetry] prospectively captured from the patient monitor and stored in an electronic database (BedMasterEx; ExcelMedical, Jupiter, FL). This database was searched to identify infants who 1) were born at or before 28 completed wk of gestation, 2) had an umbilical arterial catheter placed for clinical indications, and 3) had ≥72 h of continuous data. Infants were then divided into those who were hypotensive and received inotropes and a normotensive control group (defined as no exposure to inotropes or volume resuscitation with saline or blood products in the 1st 72 h of life).
Comprehensive maternal/perinatal, patient, and clinical factors were collected for the included infants. Maternal/perinatal factors included mode of delivery, antenatal magnesium sulfate exposure, antenatal corticosteroid exposure, diagnosis of preeclampsia, chorioamnionitis (histopathological diagnosis), arterial cord blood gas pH, and the 5-min Apgar score. Patient factors included estimated gestational age (EGA) in completed weeks, birth weight, intrauterine growth restriction status (defined as birth weight <10th centile), sex, and race/ethnicity. Clinical factors included clinical risk index for babies (CRIB) II score [using the algorithm developed by Parry et al. (18)], IVH [based on cranial ultrasound in the 1st wk of life, graded using the Papile scoring system (17)], inotrope use in the 1st 72 h of life (inclusive of dopamine, dobutamine, epinephrine, and norepinephrine), volume expansion in the 1st 72 h of life, use of hydrocortisone for treatment of refractory hypotension, culture-positive sepsis, and mortality.
Institutional guidelines for the management of hypotension.
For blood pressure support, our institutional practice is to consider inotrope initiation if 1) mean arterial blood pressure (MABP) < EGA in weeks, 2) there is poor urine output (<1 ml·kg−1·h−1), and/or 3) there are clinical signs of poor perfusion (e.g., prolonged central capillary refill time or tachycardia). Dopamine is used as the first-line agent, with a starting dose between 2.5 and 5 µg·kg−1·min−1. Epinephrine is used as an alternative first-line treatment (starting dose 0.05 µg·kg−1·min−1) or as a second-line treatment when there is inadequate response to dopamine (when dosing exceeds 10 µg·kg−1·min−1). Additionally, hydrocortisone, dosed at 1 mg/kg every 8 h, can be added as an alternative second-line treatment for refractory hypotension. Volume resuscitation with normal saline (NS) solution or packed red blood cells (pRBCs) can be given before or in conjunction with inotropic support and is administered in 10 ml/kg (NS) or 15 ml/kg (pRBC) aliquots.
Data acquisition.
Per clinical practice, invasive umbilical blood pressure measurements are made using a pressure transducer (TruWave; Edwards Lifesciences, Irvine, CA), which interfaces the umbilical arterial catheter (3.5-Fr Argyle single-lumen umbilical vessel catheter; Medtronic, Minneapolis, MN) with the patient monitor (IntelliVue MP70; Philips Medical, Andover, MA). To make continuous measurements of the arterial blood pressure and to provide an access point for blood sampling, umbilical arterial catheters are placed in the “high-lying” position in the thoracic aorta, located between the sixth and eight vertebral body on chest radiograph. All included infants had umbilical lines placed at the discretion of the clinical team and were not placed or maintained solely for research purposes. The mean arterial blood pressure was recorded at 1 Hz by taking the time-integrated mean of the beat-to-beat arterial blood pressure.
For those infants who were exposed to inotropes, the mean MABP in the hour preceding inotrope initiation (designated t−1) was calculated as well as the mean MABP 3 h after initiation (designated t+3). The ΔMABP was calculated as the difference between MABP at baseline and at 3 h.
Error correction.
The raw MABP signal underwent a two-step error correction process by identifying 1) regions of the recording flagged by the patient monitor as invalid (e.g., when the pressure transducer is briefly disconnected or when an infusion is given through the line) and 2) motion artifact, defined as periods of sudden, nonphysiological change in blood pressure (an increase in MABP >5 mmHg over a 1-s interval). The error correction algorithm reviewed the entire recording in 10-min data frames and excluded those that contained errors from further analysis.
Power calculation.
For each error-free, 10-min (600-sample) data frame, the total spectral power between 0 and 0.5 Hz was calculated using Welch modified power spectral estimate, which estimates the power spectrum by time-averaging the fast Fourier transformation of short segments of data. The total spectral power for each 10-min data frame was then calculated as the root mean square (RMS) for each of 4 frequency bands (B1, 0.005–0.0095 Hz; B2, 0.0095–0.02 Hz; B3, 0.02–0.06 Hz; and B4, 0.06–0.16) corresponding to the endothelial, endothelial NO-dependent, neurogenic, and myogenic components, respectively.
For both groups of infants, the mean hourly RMS power for each of the 4 frequency bands was calculated for the 1st 72 h of life. For those who were exposed to inotropes, the mean RMS spectral power was calculated for the 1-h period preceding inotrope initiation (t−1) as well as the mean spectral power 3 h after initiation (t+3) for all 4 frequency bands.
Error correction and spectral analysis were conducted using in-house software written for MATLAB version 9.1.0 (The MathWorks, Natick, MA).
Statistical approach.
Univariate comparisons of the sample characteristics were made between the inotrope-exposed and the control group using the Mann-Whitney U test for continuous variables or Fisher exact test (2-sided) for categorical variables. Between-group comparisons were made using a 1-way ANOVA test. Statistical comparisons were made using R version 3.3.0 (The R Foundation for Statistical Computing, Vienna, Austria).
The study design and procedures were reviewed and approved by the Human Research Protection Office at Washington University. Parents of the infants in the study provided written, informed consent.
RESULTS
Sample characteristics.
A total of 40 infants were included in the study with a mean ± SD EGA of 25.2 ± 1.6 wk and birth weight of 790 ± 211 g; 19/40 (48%) were male; 11/40 (28%) infants were in the inotrope-exposed group, and 29 (73%) were in the control group. In general, the hypotensive, inotrope-exposed and normotensive control infants were similar in terms of demographic and perinatal factors. Clinical factors differed between the inotrope-exposed and control groups with a higher median CRIB II score (14.5 vs. 12; P = 0.02), greater rates of IVH (72 vs. 28%; P = 0.01), and high-grade IVH (55 vs. 17%; P = 0.04), lower initial MABP (28.6 vs. 23.9 mmHg; P = 0.02), and greater mortality (36 vs. 7%; P = 0.04). A complete overview of the sample characteristics, divided by group, is shown in Table 1.
Table 1.
Sample characteristics
| Control (n = 29) | Inotrope-Exposed (n = 11) | P Value | |
|---|---|---|---|
| Patient factors | |||
| Gestational age, mean (SD) wk | 25.4 (1.5) | 24.8 (1.8) | 0.26 |
| Birth weight, mean (SD) g | 808 (197) | 745 (250) | 0.17 |
| IUGR status†, n (%) | 1 (3) | 0 (0) | 1.00 |
| Male sex, n (%) | 15 (52) | 4 (36) | 0.48 |
| Race/ethnicity | |||
| Asian, n (%) | 1 (3) | 1 (9) | 0.28 |
| Black, n (%) | 10 (35) | 4 (37) | |
| Hispanic, n (%) | 0 (0) | 1 (9) | |
| White, n (%) | 18 (62) | 5 (45) | |
| Perinatal factors | |||
| Vaginal delivery, n (%) | 8 (28) | 1 (9) | 0.39 |
| Antenatal MgSO4 exposure, n (%) | 19 (66) | 8 (72) | 1.00 |
| Antenatal steroid exposure | |||
| Any exposure, n (%) | 25 (86) | 9 (82) | 1.00 |
| Complete course‡, n (%) | 17 (59) | 9 (82) | 0.27 |
| Preeclampsia, n (%) | 7 (24) | 3 (27) | 1.00 |
| Chorioamnionitis§, n (%) | 4 (14) | 3 (27) | 0.37 |
| Cord pH, mean (SD) | 7.30 (0.09) | 7.34 (0.07) | 0.53 |
| Apgar score 5 min, median (range) | 6 (1–9) | 5 (2–9) | 0.24 |
| Clinical factors | |||
| Initial MABP, mean (SD) mmHg | 28.6 (6.3) | 23.9 (3.9) | 0.02* |
| CRIB II score, median (range) | 12 (8–17) | 14.5 (8–17) | 0.02* |
| Culture-positive sepsis, n (%) | 0 (0) | 2 (18) | 0.07 |
| Intraventricular hemorrhage | |||
| Any grade, n (%) | 8 (28) | 8 (72) | 0.01* |
| Grade III/IV, n (%) | 5 (17) | 6 (55) | 0.04* |
| Died, n (%) | 2 (7) | 4 (36) | 0.04* |
IUGR, intrauterine growth restriction; MABP, mean arterial blood pressure.
Statistical significance at P < 0.05.
Birth weight <10th centile;
2 doses of β-methasone over a 48-h period;
based on histopathological diagnosis.
Hemodynamic characteristics.
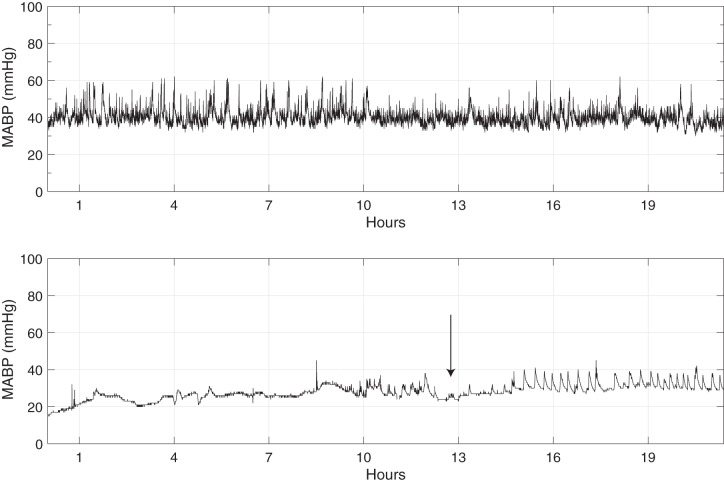
Of the 11 infants who were in the inotrope-exposed group, 10/11 (91%) received dopamine, 3/11 (27%) received hydrocortisone, 7/11 (64%) received volume resuscitation with NS, and 7/11 (64%) received volume resuscitation with pRBCs. Five of the eleven infants failed to respond to treatment (defined as a ΔMABP ≤1 mmHg at t+3), 4/11 infants had a nominal response (defined as ΔMABP between 2 and 7 mmHg at t+3), and 2/11 infants had a superresponse (defined as ΔMABP >7 mmHg at t+3). See Table 2 for a complete listing of the hemodynamic characteristics. An example of the greater variability in MABP in a control infant, compared with a hypotensive, inotrope-exposed infant, can be readily visualized on the raw MABP traces shown in Fig. 1.
Table 2.
Hemodynamic support for inotrope-exposed group
| N = 11 | |
|---|---|
| Inotropic agent | |
| Dopamine, n (%) | 10 (91) |
| Epinephrine, n (%) | 1 (9) |
| Dopamine starting dose, median (range) µg·kg−1·min−1 | 2.5 (0.5–4) |
| Dopamine maximum dose, median (range) µg·kg−1·min−1 | 3.25 (0.5–10) |
| Proportion receiving hydrocortisone for resistant hypotension, n (%) | 3 (27) |
| MABP before inotrope initiation (t−1), mean (SD) | 25.0 (4.9) |
| MABP after 3 h of treatment (t+3), mean (SD) | 28.5 (6.5) |
| NS resuscitation | |
| Any fluid bolus, n (%) | 7 (64) |
| No. fluid boluses, median (range) | 1 (0–5) |
| pRBC resuscitation | |
| Any transfusion, n (%) | 7 (64) |
| No. transfusions, median (range) | 2 (0–5) |
Fig. 1.
Two examples traces of the raw MABP are shown, each ~20 h in length. The difference in variability is visually apparent between a control group infant who did not require inotropes (top) and 1 who did (bottom). The arrow at bottom denotes initiation of inotropic medications; note, over the next several hours, there is increased variability in the blood pressure that emerges.
Data quality.
Mean recording start time was 5.7 ± 3.4 h after birth; all included infants had continuous MABP measurements until the end of the recording at 72 h of life. A total of 11.8 million data points were collected, 9.7 million of which (82%) were error-free and could be used for spectral analysis. Motion artifact was the most common reason for data exclusion, accounting for ~65% of the excluded data frames.
Longitudinal comparison.
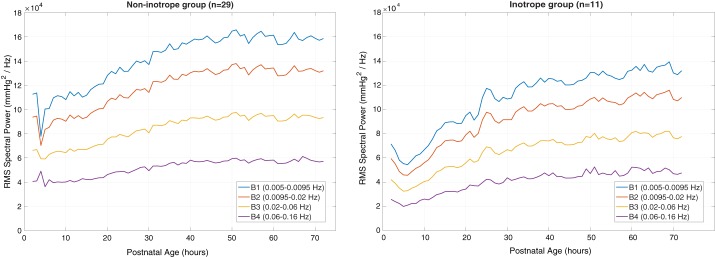
For the infants who did not receive inotropes, spectral power across all four bands increased with time before achieving a plateau by ~48 h of life. Endothelial vasomotion, represented by the lowest frequency bands (B1 and B2), demonstrated three- to fourfold greater RMS spectral power than that in the highest frequency band (B4, myogenic). The hypotensive infants who received inotropes demonstrated a similar distribution of spectral power to the normotensive infants (with the lowest frequency bands having the greatest power). However, there was an ~20% reduction in power in each of the four frequency bands, compared with the normotensive group, and the spectral power did not plateau at 48 h but rather was continuing to increase even at the end of the monitoring period. The change in spectral power by band and time for both groups is shown in Fig. 2.
Fig. 2.
RMS spectral power over the 1st 72 h following birth across all 4 frequency bands. Infants who were not hypotensive and did not require inotropes are shown on the left, whereas those who were exposed to inotropes are shown on the right. Note an ~20% reduction in power between the groups.
Differential inotrope response and VLF power.
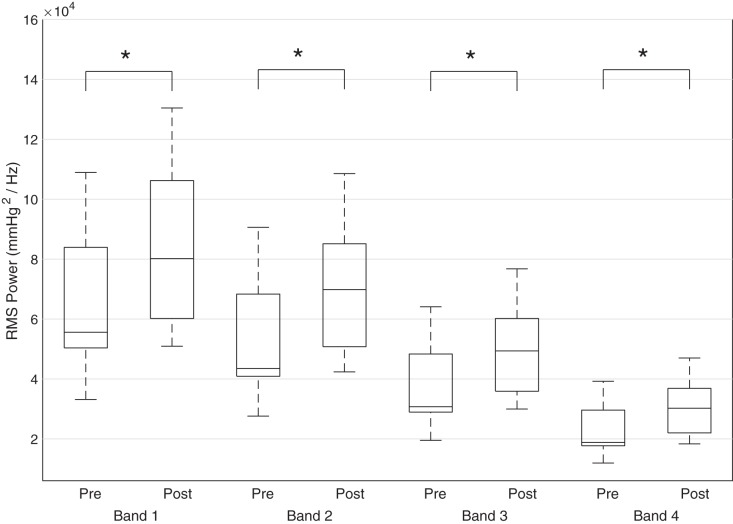
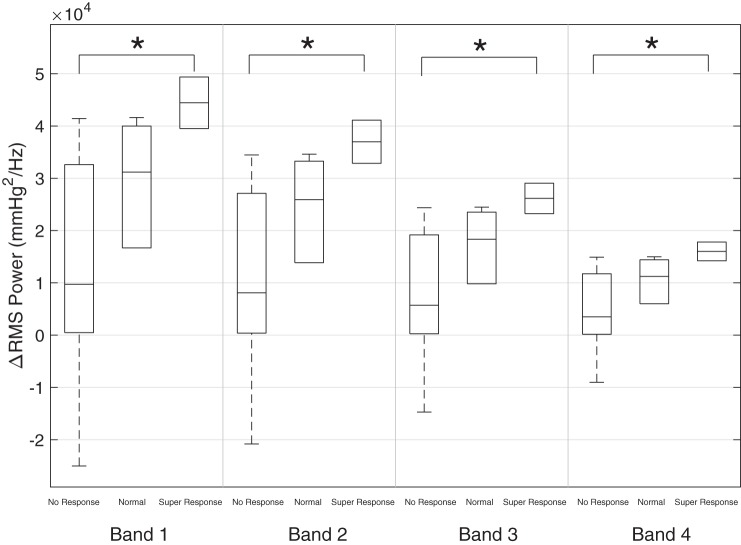
For the hypotensive, inotrope-exposed infants, the mean blood pressure at t−1 (1 h before inotrope initiation) was 25.0 ± 4.9 mmHg and increased to a mean of 28.5 ± 6.5 at t+3 for a mean ΔMABP of 3.5 ± 3.3 mmHg. There was a statistically significant difference in RMS spectral power, when comparing the pre- and postinotrope data, across all four frequency bands (B1, P < 0.01; B2, P < 0.01; B3, P < 0.01; and B4, P < 0.01) as shown in Fig. 3. There was also a statistically significant difference in ΔRMS spectral power across all four bands between the nonresponders and the superresponders (B1, P = 0.04; B2, P = 0.04; B3, P = 0.04; and B4, P = 0.04) but not the nominal responders as shown in Fig. 4. There was no relationship between the starting dose (r = 0.12, P = 0.71) or peak dose (r = 0.07, P = 0.84) of dopamine and ΔMABP at t+3. The absolute value and relative change from preinotrope baseline for RMS spectral power is displayed in Table 3.
Fig. 3.
Box plots demonstrating the mean RMS power before (Pre) and after (Post) inotrope initiation, clustered by frequency band. Asterisk denotes significance at P < 0.05.
Fig. 4.
Box plots demonstrating the mean ΔRMS power for each of the 3 inotrope response groups, clustered by frequency band. Asterisk denotes significance at P < 0.05.
Table 3.
Mean and change in RMS power by inotrope response
|
Band 1 |
Band 2 |
Band 3 |
Band 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| X̄ RMS | ΔRMS | X̄ RMS | ΔRMS | X̄ RMS | ΔRMS | X̄ RMS | ΔRMS | |
| No response | 73,079 | 18,795 | 60,790 | 15,627 | 42,987 | 11,050 | 26,324 | 6,767 |
| Nominal response | 90,701 | 35,232 | 75,456 | 29,311 | 53,358 | 20,727 | 32,675 | 12,692 |
| Superresponse | 1,10,778 | 44,437 | 92,165 | 36,980 | 65,174 | 26,151 | 39,911 | 16,014 |
All values have units of mmHg2/Hz. X̄, sample mean.
DISCUSSION
In this study, we demonstrate that low-frequency oscillations in arterial blood pressure, across regulatory components of vascular tone, in preterm infants can be quantified. The predominate source of spectral power arises from the endothelial component, with neurogenic and myogenic components contributing a smaller fraction. The strength of oscillations in a population of nonhypotensive infants increases steadily after birth and plateaus at ~48 h.
Additionally, we have demonstrated that hypotensive, inotrope-exposed infants have significantly less low-frequency blood pressure variability across all frequency bands, compared with normotensive control infants who did not receive inotropes. Potential mechanisms of diminished variability could include a decreased level of vasoactive compounds such as catecholamines, vasoactive intestinal peptides, or NO and/or a diminished response by the vascular regulatory system. The second comparison in this study suggests that both components of this hypothesis are likely correct: some infants respond with a significant increase in MABP and low-frequency variability after the introduction of additional catecholamines (in the form of dopamine or epinephrine), whereas others remain unresponsive. It is important to note, however, that even those infants with a robust response to inotropes fail to achieve the same spectral power in any of the bands as the infants who were never hypotensive. Also of note is that, whereas spectral power increases across all frequency bands in response to inotropes, the greatest absolute change occurs in the lowest frequency bands, perhaps suggesting a predominate effect at the endothelial level. This finding may be the result of blood pressure measurements made in the aorta, a large-caliber blood vessel with relatively limited innervation, compared with smaller arterial vessels (15).
These findings have important implications for clinical management; an adjunctive measure of response to inotropic medications may allow for more rapid, optimal titration, decreasing the possible risk of ischemia-reperfusion and hypertension-induced IVH (26). Indeed, there is significant evidence in the cerebral autoregulation literature to suggest that merely improving the MABP does not necessarily indicate that autoregulation has been restored or is more active (9, 13).
Also of interest are the clinical implications for those infants who remain unresponsive to inotropic treatment. Seri and colleagues (24) evaluated plasma catecholamines in 31 preterm infants who required dopamine treatment due to hypotension, finding that serum catecholamine levels were elevated compared with control infants, even before dopamine initiation, and appear to be asymptotically limited, reaching a ceiling at a dopamine infusion rate of 4 µg·kg−1·min−1. This is further reinforced in the present study, given the lack of relationship between the dose of dopamine and subsequent change in blood pressure. Although serum catecholamines were not measured in the present study, one might hypothesize that the limited change in low-frequency variability in those infants who fail to respond to inotrope might be the result of a lack of response to endogenous or exogenous catecholamines rather than an absence of these compounds.
Indeed, poor adrenocortical function has been associated with “inotrope-resistant” hypotension. A few small studies (1, 14) have described hypotensive preterm infants with relative adrenal insufficiency (cortisol levels <15 μg/dl) who demonstrated no improvement in their heart rate or blood pressure until hydrocortisone was added as an adjunctive agent to the dopamine already being given. Although the authors were not able to ascertain the underlying mechanism for the preterm infants, Hoen et al. (5) were able to measure directly the increased sensitivity to α1-adrenergic receptors after hydrocortisone administration in a group of trauma patients unresponsive to inotropic treatment. These preliminary findings could be extrapolated to preterm infants who did not respond to inotropes, suggesting an alternative pharmacological approach to increase the level of catecholamine receptors (i.e., corticosteroids; Ref. 23), perhaps guided by measurement of low-frequency blood pressure oscillations.
There are a number of important limitations to this study. First, although this study used >9 million MABP measurements for analysis, the group of inotrope-exposed infants was relatively small. Although sufficient for examination of patterns in low-frequency variability, it precludes further subdivision to evaluate differences between inotropic agents. Second, as the study design was observational and “open-label,” the decision to start inotropes, dosing, and duration of treatment were at the discretion of the individual physician caring for the patient. Although institutional guidelines are in place, they allow for some individual flexibility. Third, the data management strategy in this study was intentionally conservative, discarding data frames with error rather than imputing data. Future evaluation of this approach should explore techniques for recovering some of these data.
In conclusion, the outlined technique for evaluating low-frequency blood pressure variability demonstrates a clear distinction in the baseline state of vascular regulation in preterm infants who go on to receive inotropes for hypotension compared with those who do not. Furthermore, within the inotrope-exposed infants, there is a strong association between improvement in blood pressure and an increase in low-frequency blood pressure oscillations. These observations suggest that a lack of response to modulatory signals by the myogenic vascular control system may form the underlying mechanism for preterm infants who remain hypotensive despite treatment with inotropic medications. More research into this mechanism, and potential changes in management to manipulate this mechanism, is needed. Additional study in a larger sample size cohort is also needed to relate changes in low-frequency variability to important clinical outcomes, particularly intraventricular hemorrhage.
GRANTS
This work was supported by the Intellectual and Developmental Disabilities Research Center at Washington University [National Institutes of Health (NIH)-National Institute of Child Health and Human Development Grant P30-HD-062171], Washington University Institute of Clinical and Translational Sciences KL2 Training Program [NIH-National Center for Advancing Translational Sciences (NCATS) Grant KL2-TR-000450], and the Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH-NCATS Grant UL1-TR-000448).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.A.V., C.M., and A.M.M. conceived and designed research; Z.A.V., J.H., and N.M.E.T. performed experiments; Z.A.V., J.H., and N.M.E.T. analyzed data; Z.A.V., C.M., and A.M.M. interpreted results of experiments; Z.A.V. prepared figures; Z.A.V. drafted manuscript; Z.A.V., J.H., C.M., N.M.E.T., and A.M.M. edited and revised manuscript; Z.A.V., J.H., C.M., N.M.E.T., and A.M.M. approved final version of manuscript.
REFERENCES
- 1.Fernandez E, Schrader R, Watterberg K. Prevalence of low cortisol values in term and near-term infants with vasopressor-resistant hypotension. J Perinatol : 114–118, 2005. doi: 10.1038/sj.jp.7211211. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ. Autonomic nervous system control of the cerebral circulation. In: Handbook of Clinical Neurology. Amsterdam: Elsevier, 2013, vol. 117, chapt. 16, p. 193–201. doi: 10.1016/B978-0-444-53491-0.00016-X. [DOI] [PubMed] [Google Scholar]
- 3.Gouédard O, Blanc J, Gaudet E, Ponchon P, Elghozi JL. Contribution of the renin-angiotensin system to short-term blood pressure variability during blockade of nitric oxide synthesis in the rat. Br J Pharmacol : 1085–1092, 1996. doi: 10.1111/j.1476-5381.1996.tb16008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henning RJ, Sawmiller DR. Vasoactive intestinal peptide: cardiovascular effects. Cardiovasc Res : 27–37, 2001. doi: 10.1016/S0008-6363(00)00229-7. [DOI] [PubMed] [Google Scholar]
- 5.Hoen S, Mazoit JX, Asehnoune K, Brailly-Tabard S, Benhamou D, Moine P, Edouard AR. Hydrocortisone increases the sensitivity to alpha1-adrenoceptor stimulation in humans following hemorrhagic shock. Crit Care Med : 2737–2743, 2005. doi: 10.1097/01.CCM.0000189743.55352.0E. [DOI] [PubMed] [Google Scholar]
- 6.Jones C, Pereira SS, Wertheim D, Shah DK, Kempley S. PC.51 variability of blood pressure in extremely preterm infants in the first 72 hrs of postnatal adaptation. Arch Dis Child Fetal Neonatal Ed , Suppl 1: A53–A54, 2014. doi: 10.1136/archdischild-2014-306576.152. [DOI] [Google Scholar]
- 7.Kvandal P, Landsverk SA, Bernjak A, Stefanovska A, Kvernmo HD, Kirkebøen KA. Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc Res : 120–127, 2006. doi: 10.1016/j.mvr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Langager AM, Hammerberg BE, Rotella DL, Stauss HM. Very low-frequency blood pressure variability depends on voltage-gated l-type Ca2+ channels in conscious rats. Am J Physiol Heart Circ Physiol : H1321–H1327, 2007. doi: 10.1152/ajpheart.00874.2006. [DOI] [PubMed] [Google Scholar]
- 9.Lightburn MH, Gauss CH, Williams DK, Kaiser JR. Observational study of cerebral hemodynamics during dopamine treatment in hypotensive ELBW infants on the first day of life. J Perinatol : 698–702, 2013. doi: 10.1038/jp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lymperopoulos A. (editor). The Cardiovascular Adrenergic System. Cham, Switzerland: Springer International Publishing, 2015. [Google Scholar]
- 11.Miall-Allen VM, de Vries LS, Dubowitz LM, Whitelaw AG. Blood pressure fluctuation and intraventricular hemorrhage in the preterm infant of less than 31 weeks’ gestation. Pediatrics : 657–661, 1989. [PubMed] [Google Scholar]
- 12.Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child : 1068–1069, 1987. doi: 10.1136/adc.62.10.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics : 1591–1596, 2004. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 14.Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, Wong E. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed : F119–F126, 2004. doi: 10.1136/adc.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson H, Goldstein M, Nilsson O. Adrenergic innervation and neurogenic response in large and small arteries and veins from the rat. Acta Physiol Scand : 121–133, 1986. doi: 10.1111/j.1748-1716.1986.tb07795.x. [DOI] [PubMed] [Google Scholar]
- 16.Noori S, Seri I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin Perinatol : 221–238, 2012. doi: 10.1016/j.clp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr : 529–534, 1978. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 18.Parry G, Tucker J, Tarnow-Mordi W; UK Neonatal Staffing Study Collaborative Group . CRIB II: an update of the clinical risk index for babies score. Lancet : 1789–1791, 2003. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 19.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res : 833–841, 2010. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ping P, Johnson PC. Mechanism of enhanced myogenic response in arterioles during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol : H1185–H1189, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Ponchon P, Elghozi JL. Contribution of humoral systems to the short-term variability of blood pressure after severe hemorrhage. Am J Physiol Regul Integr Comp Physiol : R58–R69, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Radaelli A, Castiglioni P, Centola M, Cesana F, Balestri G, Ferrari AU, Di Rienzo M. Adrenergic origin of very low-frequency blood pressure oscillations in the unanesthetized rat. Am J Physiol Heart Circ Physiol : H357–H364, 2006. doi: 10.1152/ajpheart.00773.2005. [DOI] [PubMed] [Google Scholar]
- 23.Sakaue M, Hoffman BB. Glucocorticoids induce transcription and expression of the alpha 1B adrenergic receptor gene in DTT1 MF-2 smooth muscle cells. J Clin Invest : 385–389, 1991. doi: 10.1172/JCI115315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seri I, Rudas G, Bors Z, Kanyicska B, Tulassay T. Effects of low-dose dopamine infusion on cardiovascular and renal functions, cerebral blood flow, and plasma catecholamine levels in sick preterm neonates. Pediatr Res : 742–749, 1993. doi: 10.1203/00006450-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Siu KL, Sung B, Moore LC, Birzgalis A, Chon KH. Very low frequency modulation in renal autoregulation. Conf Proc IEEE Eng Med Biol Soc : 771–774, 2006. doi: 10.1109/IEMBS.2006.259620. [DOI] [PubMed] [Google Scholar]
- 26.Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Aetiological rôle of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Dev Med Child Neurol : 601–614, 1987. doi: 10.1111/j.1469-8749.1987.tb08502.x. [DOI] [PubMed] [Google Scholar]
- 27.Vesoulis ZA, Ters NE, Foster A, Trivedi SB, Liao SM, Mathur AM. Response to dopamine in prematurity: a biomarker for brain injury? J Perinatol : 453–458, 2016. doi: 10.1038/jp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One : e43165, 2012. doi: 10.1371/journal.pone.0043165. [DOI] [PMC free article] [PubMed] [Google Scholar]