Abstract
Contraction-induced rapid vasodilation is attenuated similarly in the upper and lower limbs of older adults. In the forearm, this attenuation is in part due to a greater sympathetic vasoconstriction. We examined whether the age-related reduction in contraction-induced vasodilation in the leg is also due to a sympathetic vasoconstrictive mechanism. Thirteen young (24 ± 1 yr) and twelve older adults (67 ± 1 yr) performed single-leg knee extension at 20 and 40% of work-rate maximum (WRmax) during control and cold-pressor test (CPT) conditions. Femoral artery diameter and blood velocity were measured using Doppler ultrasound. Vascular conductance (VC; ml·min−1·mmHg−1) was calculated using blood flow (ml/min) and mean arterial pressure (mmHg). Peak (ΔVC from baseline) and total VC were blunted in older adults during control conditions across exercise intensities (P < 0.05). Peak and total VC were reduced during CPT in both age groups across exercise intensities (P < 0.05). The relative change (i.e., %reduction; CPT vs. control) in peak (−25 ± 5 vs. −22 ± 4% at 20% WRmax; and −21 ± 6 vs. −27 ± 5% at 40% WRmax; P = 0.42–0.55) and total VC (−28 ± 5 vs. −36 ± 6% at 20% WRmax; and −22 ± 8 vs. −33 ± 5% at 40% WRmax; P = 0.23–0.34) were similar between young and older adults. When matched for absolute workload (~10 W), age differences persisted in peak VC (P < 0.05) under both conditions, with similar relative changes in peak and total VC during CPT. Our data suggest that 1) sympathetic stimulation reduces contraction-induced rapid vasodilation in the leg of young and older adults similarly; and 2) enhanced sympathetic vasoconstriction does not fully explain age-related differences in contraction-induced vasodilation within the leg.
NEW & NOTEWORTHY Aging is associated with attenuated contraction-induced rapid onset vasodilation (ROV). Within the forearm, this attenuation is partially due to enhanced sympathetic vasoconstriction. In the current study, we found that sympathetic vasoconstriction reduces contraction-induced ROV within the leg of both young and older adults, with the magnitude of change being similar between age groups. Our current results suggest that age-related attenuations in contraction-induced ROV within the leg are not fully explained by a sympathetic vasoconstrictor mechanism.
Keywords: sympathetic vasoconstriction, contraction-induced rapid vasodilation, leg
advancing age is associated with a decline in skeletal muscle blood flow and vasodilation at rest and during dynamic exercise (9, 11, 22, 27, 31, 34, 35, 49). However, a large majority of the studies examining age-related alterations in blood flow of contracting skeletal muscle have focused primarily on responses during steady-state submaximal exercise (19, 36). By the nature of these studies and associated measurements, regulation of blood flow and vasodilation at the onset of exercise has been largely ignored, despite evidence to suggest rapid vasodilation is integral in initiating the increase in blood flow requisite for dynamic exercise (6, 7, 47). In this context, increases in skeletal muscle blood flow and vasodilation are apparent immediately following a single skeletal muscle contraction [i.e., contraction-induced rapid onset vasodilation (ROV)]. Evidence from our group as well as others suggests that older adults demonstrate a significantly lower hyperemic and vasodilator response to single muscle contractions (i.e., attenuated ROV) compared with their young counterparts (2, 3, 5, 16, 20). Although the majority of the human studies demonstrating age-related impairments in ROV have done so in the forearm (2, 3, 5, 16, 20), we have recently shown that ROV is also blunted in the leg of older adults and the magnitude of this impairment appears to be similar between limbs (16).
One mechanism potentially contributing to the impaired ROV in older adults is an enhanced sympathetic vasoconstriction. Advancing age is associated with a progressive increase in muscle sympathetic nerve activity (MSNA) and norepinephrine concentrations, acting to increase peripheral vascular resistance and thereby enhance vasoconstriction (1, 30, 43, 44). Along these lines, we have shown that sympathetic stimulation via lower body negative pressure attenuates the ROV response in the forearm of young but not older adults (3). Additionally, subsequent nonselective blockade of α-adrenergic receptors (via phentolamine) abolishes age-related differences in ROV (3). Moreover, ROV in animals is attenuated via α-adrenergic stimulation (42) and age-related differences are abolished with α-adrenergic blockade (18). Collectively, this evidence indicates that impairments in blood flow and vasodilation at the onset of exercise with aging are due in part to an enhanced sympathetic restraint at least in the forearm.
Within the context of human locomotion, the lower limbs represent a large vascular bed exposed to greater hydrostatic forces relative to the upper limbs and may be more representative of the physiological environment of normal activity. Interestingly, older adults exhibit reductions in resting leg blood flow and vascular conductance, which have been attributed to enhanced sympathetic vasoconstriction as well as increased oxidative stress (9, 11, 17, 27, 31). Furthermore, during dynamic exercise, leg blood flow and vasodilation are reduced with aging (29, 34, 35, 37, 49), and this attenuation does not appear to be due to a reduction in endothelial vasodilators but rather enhanced sympathetic vasoconstriction (21, 29, 39). Specifically, the leg vasculature of older adults appears to be more responsive to acute sympathetic stimulation during dynamic exercise (21). Despite evidence supporting sympathetic vasoconstriction contributing to age-related reductions in leg blood flow during dynamic steady-state exercise, it is unclear if this is apparent at the onset of exercise. Therefore, the aim of the present investigation was to determine whether enhanced sympathetic vasoconstriction contributes to the attenuated contraction-induced ROV response observed in the leg of older adults. We hypothesized that acute elevations in sympathetic nervous system activity would reduce ROV to a greater extent in young compared with older adults. Additionally, we hypothesized that the larger reduction in ROV with acute sympathetic stimulation in young adults would effectively abolish the age-related differences in contraction-induced rapid vasodilation in the leg of humans.
METHODS
Subjects
A total of 25 healthy normotensive adults participated in the study. All subjects were healthy, nonobese (body mass index: ≤30 kg/m2), nonsmokers, and not taking any vasoactive medications as self-reported via a health history screening questionnaire. Additionally, subjects who reported having Raynaud’s disease were excluded from participation in this study. Studies were performed in the morning after an overnight fast and refraining from exercise, alcohol, and caffeine for 24 h before reporting to the laboratory. Young female participants were studied during the early follicular phase of their natural menstrual cycle or the placebo/low hormone phase of oral contraceptives to control for the influence of sex hormones on exercise hyperemia (23, 26). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. Subjects completed written informed consent, and all study protocols were approved by the Institutional Review Board at the University of Iowa.
Systemic Hemodynamic Measurements
Following a 15-min rest period in a semirecumbent position, brachial blood pressure (BP) was measured in duplicate using an automated cuff (Cardiocap/5; Datex-Ohmeda, Louisville, CO). If BP values deviated by more than 5 mmHg, a third measurement was taken. Heart rate (HR) was measured via continuous three-lead electrocardiogram; and systemic BP was assessed (beat-to-beat) via finger plethysmography (Nexfin; Edwards Lifesciences, Irvine, CA) over the middle phalanx of the left hand.
Leg Blood Flow and Vascular Conductance
Common femoral artery diameter (~2 cm proximal to bifurcation) and blood velocity were determined with a 12-MHz linear array Doppler probe (model M12L; Vivid 7; General Electric, Milwaukee, WI). Blood velocity was measured with a probe insonation angle previously calibrated to 60°. Measured velocity waveforms were synchronized to a data acquisition system (WinDaq; DATAQ Instruments, Akron, OH) via a Doppler audiotransformer (14). Femoral artery diameter measurements were obtained during end diastole at rest (before contraction) and 1-min postcontraction. Leg blood flow (BF) was calculated as the product of mean blood velocity (cm/s) and artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min). Vascular conductance (VC) was calculated as the quotient of BF and mean arterial pressure (MAP) (expressed as ml·min−1·mmHg−1).
Single Muscle Contractions
Subjects performed dynamic single contractions in the right leg as previously described at 20 and 40% of their maximum work rate (WRmax) determined on a previous study day (15, 16). Subjects were seated in a semirecumbent position on a modified adjustable bucket seat that accommodates variable body and leg lengths allowing the subject’s lower leg to move through a full range of movement (90° flexion to ~0° extension). Both knees were flexed at 90° with a form-fitting orthopedic boot attached to the right ankle. The boot was attached to a leg shaft located behind the right knee that had a one-way clutch bearing allowing for no resistance as the leg returns to 90° flexion (eccentric). Resistance (torque) was developed by contracting (concentric) against the leg shaft with the device electronically developing the torque via an alternating current motor turning at a constant revolution per minute transferred to the leg shaft. A dedicated computer monitored the elapsed time and the angle of the leg controlling the actual torque presented to the leg during contraction. In this way, the subject was required to develop enough power to extend the leg through a full range of motion against a given torque eliminating any inertial load. Subjects were instructed to contract and relax through a full range of movement (90° flexion to ~0° extension) within ~1 s, on a verbal command from laboratory personnel. WRmax intensities were randomized before the experimental protocol, and each contraction intensity was performed in duplicate to calculate the average response for each subject for a given condition. Each contraction was visually observed by the laboratory personnel to ensure proper timing of contraction. One minute of relaxation was given between each contraction to allow continuous measures of limb hemodynamics postcontraction.
Cold-Pressor Test
The cold-pressor test (CPT) was used to examine the influence of increased sympathetic nervous system activity on ROV following single muscle contractions in the leg. Local cold stimulation has previously been shown to elicit a robust sympathetic stimulus, effectively increasing muscle sympathetic nerve activity twofold, within 1–2 min (41, 48). During each CPT trial, the subject’s contralateral (left) foot (at the level of ~5–7 cm above the malleolus) was lowered into the ice water (4°C) for a total of 4 min (13, 21).
Experimental Protocol/Design
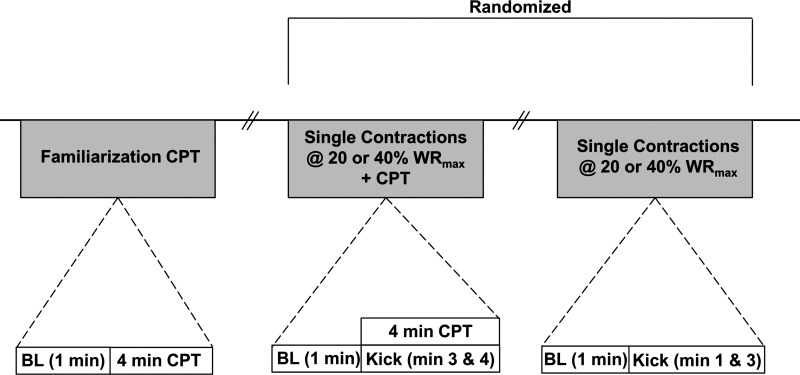
Following a 15-min rest period in a semirecumbent position and baseline brachial BP measurements, subjects underwent a familiarization CPT at the start of the study. The initial (familiarization) CPT was utilized because the first exposure to cold has been shown to cause exaggerated increases in MAP when compared with subsequent CPTs (13). Therefore, we aimed to avoid having the first exposure to cold and potential exaggerated pressor response occur during one of the leg-kicking trials. Following the CPT familiarization trial, subjects performed a total of four (2 CPT and 2 non-CPT/control) leg-kicking trials (Fig. 1). The order of control (no CPT) and CPT trials, as well as exercise intensity (20 vs. 40% WRmax), was randomized before the study day, and 20 min of quiet rest separated each trial that contained a CPT. Each trial consisted of two single contractions at either 20 or 40% WRmax. For the CPT trials, the contractions took place during the final 2 min of foot immersion within the ice bath. HR, systemic arterial BP, and femoral artery mean blood velocity were captured continuously throughout each trial.
Fig. 1.
Experimental timeline. CPT, cold-pressor test; BL, baseline; WRmax, work-rate maximum; hashmarks = 20 min rest.
Data Analysis and Statistics
Data were collected at 250 Hz and analyzed offline with signal-processing software (WinDaq; DATAQ Instruments). Beat-to-beat MAP was derived from each Nexfin BP waveform and was synchronized to blood velocity (via Doppler ultrasound) measurements. Baseline BF and MAP represent an average of the last 30 s of the rest before each single contraction. To account for baseline changes in BF and VC with CPT, rapid flow and vasodilator responses after muscle contraction were adjusted (i.e., postcontraction value − baseline value) and expressed as the change (Δ) in BF and VC. Of particular interest to this study, the peak ΔVC and total VC responses postcontraction were analyzed between conditions. Total leg BF (ml) and VC (ml/mmHg) were defined as the area under the curve over 30 postcontraction cardiac cycles after subtracting respective baseline values for a given flow or conductance curve (24). To further investigate age-related differences in the hyperemic and vasodilator response to sympathetic stimulation (CPT), the percent reduction (%Δ) between age groups was compared. Given evidence for the potential confound of absolute work driving the age-related differences in exercise hyperemia and vasodilation (50), we performed additional analyses on a subset of subjects matched for an approximate absolute workload (~10 W). Additionally, due to the potential influence of sex on ROV (3), we also performed additional analyses to compare responses within and between age groups based on sex.
Group data are expressed as means ± SE. Baseline characteristics between groups were analyzed via one-way ANOVA. Hemodynamics before single-leg contractions were analyzed with a two-way repeated measures ANOVA. Age- and sex-related differences of the primary outcome variables postcontraction (peak ΔBF and ΔVC and total BF and VC) were analyzed using two-way repeated measures ANOVA. Percent reductions in the primary outcome variables were analyzed with independent one-way ANOVA. When significant F-ratios were detected, post hoc comparisons were made using Tukey’s post hoc test for pair-wise comparisons. Significance was set a priori at P < 0.05. All statistical analyses were performed using SigmaStat software version 12.0 (Systat Software, San Jose, CA).
RESULTS
Subject characteristics are shown in Table 1. Older adults had a higher body mass index and lower WRmax relative to young adults (P < 0.05). There were no age differences for any of the BP variables (P > 0.05).
Table 1.
Subject characteristics
| Young Adults (n = 13) | Older Adults (n = 12) | |
|---|---|---|
| Age, yr | 24 ± 1 | 67 ± 1* |
| Men/women | 7/6 | 6/6 |
| Height, cm | 176 ± 3 | 169 ± 2 |
| Weight, kg | 73 ± 3 | 73 ± 3 |
| Body mass index, kg⋅m2 | 23.4 ± 0.5 | 25.7 ± 1.0* |
| WRmax, W | 41 ± 4 | 26 ± 3* |
| Systolic pressure, mmHg | 121 ± 3 | 122 ± 3 |
| Diastolic pressure, mmHg | 74 ± 2 | 75 ± 2 |
| Mean arterial pressure, mmHg | 89 ± 2 | 91 ± 2 |
Values are means ± SE. WRmax, work rate maximum.
P < 0.05 vs. young adults.
Baseline (Resting) Hemodynamic Responses Across Trials
Baseline hemodynamics during control and CPT trials before single-leg contractions are shown in Table 2. Compared with control trials, exposure to the CPT increased baseline MAP in both young and older adults (P < 0.05). CPT also caused an increase in HR in the young adults (P < 0.05), whereas no chronotropic effect was observed in older adults. Baseline levels of VC were reduced during the CPT in young adults across exercise intensities and at 20% WRmax in older adults (P < 0.05). The magnitude of change (Δ) of MAP and HR in response to CPT was lower in older adults relative to young adults before both 20 and 40% WRmax trials (P < 0.05 for all). There were no differences in the impact of CPT on the magnitude of change in BF or VC between young and older adults (P = 0.21–0.45).
Table 2.
Baseline (resting) hemodynamics under each condition
| Young |
Older |
|||
|---|---|---|---|---|
| Control | CPT | Control | CPT | |
| Blood flow, ml/min | ||||
| 20% WRmax | 184 ± 15 | 171 ± 14 | 135 ± 27 | 108 ± 22* |
| 40% WRmax | 205 ± 27 | 181 ± 18 | 136 ± 27* | 114 ± 22 |
| MAP, mmHg | ||||
| 20% WRmax | 98 ± 3 | 112 ± 5† | 102 ± 4 | 107 ± 4† |
| 40% WRmax | 99 ± 3 | 111 ± 5† | 101 ± 3 | 108 ± 3† |
| HR, beats/min | ||||
| 20% WRmax | 70 ± 4 | 76 ± 4† | 63 ± 2 | 64 ± 2* |
| 40% WRmax | 69 ± 4 | 76 ± 4† | 62 ± 2 | 64 ± 2* |
| VC, ml⋅min−1⋅mmHg−1 | ||||
| 20% WRmax | 1.9 ± 0.1 | 1.6 ± 0.2† | 1.3 ± 0.3 | 0.9 ± 0.2*† |
| 40% WRmax | 2.1 ± 0.3 | 1.7 ± 0.2† | 1.3 ± 0.3* | 1.1 ± 0.02 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate; VC, vascular conductance.
P < 0.05 vs. young adults;
P < 0.05 vs. control.
Hyperemic and Vasodilator Responses to Single Muscle Contractions with Age
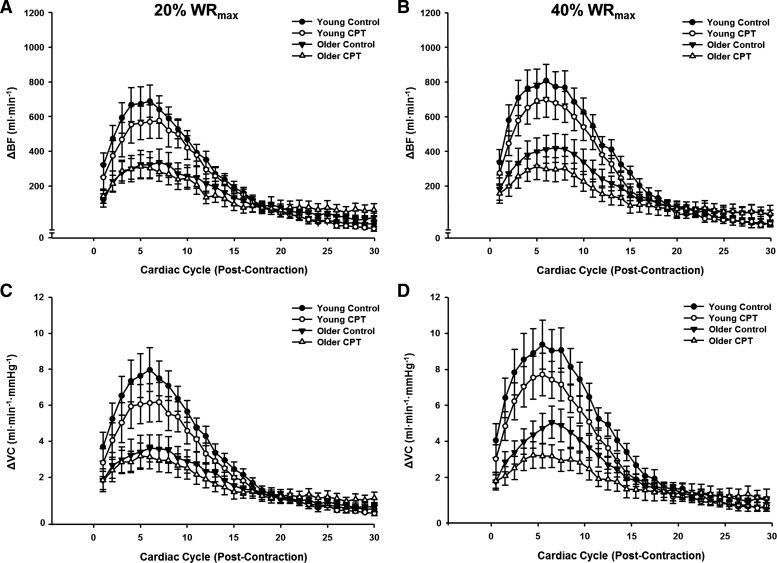
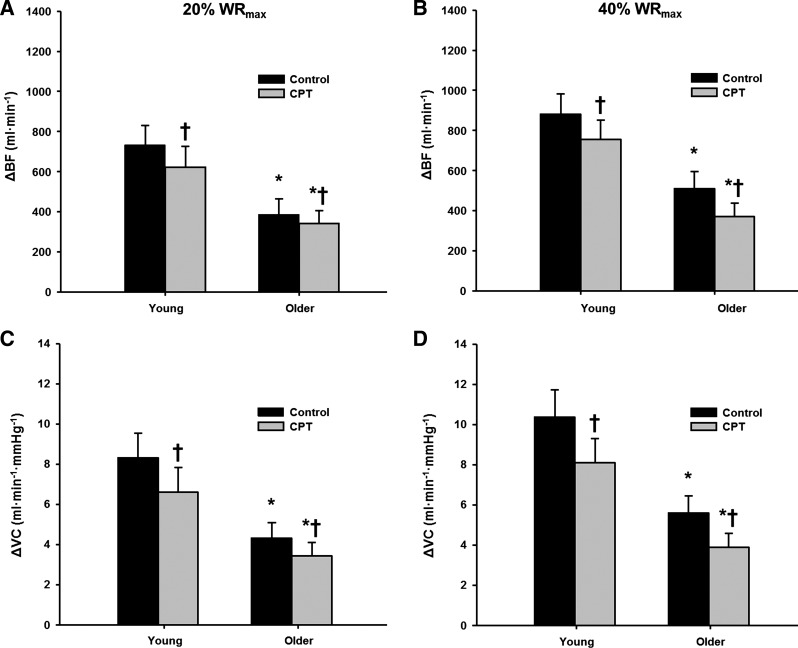
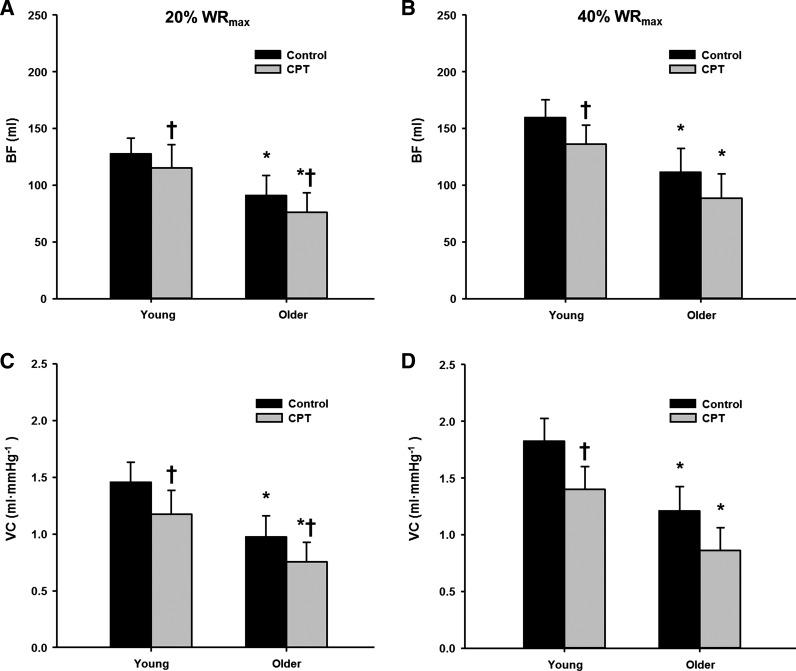
Figure 2 illustrates the temporal rapid hyperemic (Fig. 2, A and B) and vasodilator (Fig. 2, C and D) responses in young and older adults following single contractions during control and CPT trials at 20 and 40% WRmax. Peak hyperemic and vasodilator responses tended to occur during the sixth cardiac cycle postcontraction in both young and older adults. Under control conditions, peak (Fig. 3) and total (Fig. 4) hyperemic and vasodilator responses following a single skeletal muscle contraction were attenuated in older compared with young adults at 20 and 40% WRmax (main effect of age P < 0.05).
Fig. 2.
Hyperemic [change (Δ) in blood flow (BF); A and B] and vasodilator [Δ vascular conductance (VC); C and D] responses over 30 cardiac cycles following a single-leg knee extension at 20% (A and C) and 40% WRmax (B and D) during control and sympathetic stimulation (CPT) conditions in young and older adults.
Fig. 3.
Peak hyperemic (A and B) and vasodilator (C and D) responses in young and older adults at 20% (A and C) and 40% WRmax (B and D). *P < 0.05 vs. young; †P < 0.05 vs control.
Fig. 4.
Total hyperemic (A and B) and vasodilator (C and D) responses in young and older adults at 20% (A and C) and 40% WRmax (B and D). *P < 0.05 vs. young; †P < 0.05 vs. control.
Vasoconstrictor Responsiveness to Single-Leg Contractions
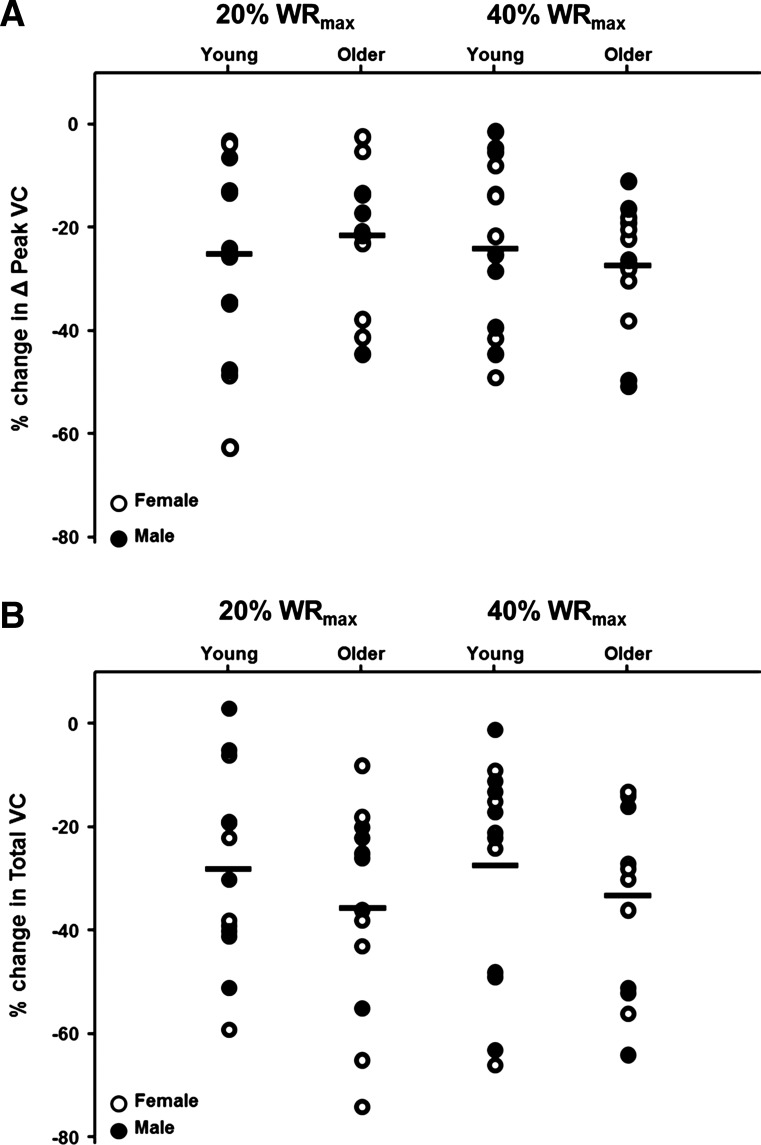
During the CPT trials, peak hyperemic and vasodilator responses were reduced in both young and older adults at 20 and 40% WRmax (P < 0.05, Fig. 3). Additionally, sympathetic stimulation via CPT reduced the total hyperemic and vasodilator responses in young and older adults at 20% WRmax (P < 0.05), whereas the total responses were only reduced in the young adults at 40% WRmax (P < 0.05, Fig. 4). When expressed as a percent reduction (%change in VC during CPT vs. control), there were no age-related differences for peak (P = 0.55 at 20% WRmax; P = 0.42 at 40% WRmax) or total (P = 0.34 at 20% WRmax; P = 0.23 at 40% WRmax) vasodilator responses (Fig. 5).
Fig. 5.
Percent reduction (%Δ) in peak (A) and total (B) vasodilator responses to single-leg knee extension at 20 and 40% WRmax in response to sympathetic stimulation in young and older adults. Open circles represent females and closed circles represent males of each age group.
Absolute Workload Subanalysis
The mean ± SE workloads for the subset of young (n = 9) and older (n = 9) subjects were 9.7 ± 0.4 and 9.6 ± 0.6 W, respectively. Table 3 shows BF and VC data at baseline and in response to single-leg contractions during control and CPT trials. In agreement with relative workload responses, young adults demonstrated greater peak vasodilator responses compared with older adults during control and CPT trials (P < 0.05 for both). However, total vasodilator responses were not significantly different between young and older adults across conditions (P = 0.08–0.1) at a comparable absolute workload. Additionally, the magnitude of vasoconstriction (% change in VC during CPT vs. control) was similar for the peak (−20 ± 7 vs. −25 ± 4%; P = 0.53) and total (−22 ± 8 vs. −28 ± 4%; P = 0.47) responses between young and older adults.
Table 3.
Leg hemodynamics at rest and following a single contraction at ~10 W
| Young (n = 9) |
Older (n = 9) |
|||||
|---|---|---|---|---|---|---|
| Baseline | ΔPeak | ΔTotal | Baseline | ΔPeak | ΔTotal | |
| Control | ||||||
| Blood flow, ml/min | 226 ± 36 | 876 ± 113 | 141 ± 17 | 140 ± 29* | 560 ± 99* | 122 ± 19 |
| VC, ml⋅min−1⋅mmHg−1 | 2.0 ± 0.2 | 9.9 ± 2 | 1.6 ± 0.2 | 1.4 ± 0.3* | 5.9 ± 1.0* | 1.3 ± 0.2 |
| CPT | ||||||
| Blood flow, ml/min | 192 ± 23 | 741 ± 127† | 136 ± 27 | 128 ± 23* | 426 ± 74*† | 97 ± 19 |
| VC, ml⋅min−1⋅mmHg−1 | 1.7 ± 0.3† | 8.3 ± 2† | 1.5 ± 0.3† | 1.1 ± 0.3* | 4.4 ± 0.8*† | 0.9 ± 0.2† |
Values are means ± SE. CPT, cold-pressor test; VC, vascular conductance.
P < 0.05 vs. young adults;
P < 0.05 vs. control.
Potential Sex Differences in Rapid Hyperemic and Vasodilator Responses to Single-Leg Knee Extension with Acute Sympathetic Stimulation
Within groups.
Young men demonstrated greater peak hyperemic and vasodilator responses than young women during both control (20 and 40% WRmax; P < 0.05) and CPT (20% WRmax only; P < 0.05). Total hyperemic and vasodilator responses were not different between young men and women during control or CPT across exercise intensities (P = 0.06–0.27). Importantly, there were no sex differences evident in the percent reduction due to CPT in peak (P = 0.44–0.88) or total VC (P = 0.70–0.96) at 20 and 40% WRmax.
There were no sex differences observed in any measure of ROV between older men and women during control or CPT (P = 0.18–0.79). Additionally, the percent reduction due to CPT in peak and total hyperemic and vasodilator responses within the leg was not different between older men and women across exercise intensities (P = 0.40–0.79).
Between groups.
Young males demonstrated greater peak hyperemic and vasodilator responses during both control and CPT compared with older men across exercise intensities (P < 0.05 for all). There were no statistically significant differences between young and older men for total hyperemic or vasodilator responses during control or CPT across exercise intensities (P = 0.07–0.14). Furthermore, the percent reduction due to CPT was not different between young and older men across exercise intensities for peak VC (P = 0.39–0.90) or total VC (P = 0.34–0.66).
Older women demonstrated attenuated peak hyperemic (P < 0.05) but not vasodilator responses at 20 and 40% WRmax (P = 0.13–0.18) relative to young females during control trials. There were no differences between the two groups during CPT trials (P = 0.17–0.47). No age-associated differences were observed within young and older females for total hyperemic or vasodilator responses during control or CPT trials across exercise intensities (P = 0.08–0.23). Furthermore, there were no age-associated differences between young and older females for the percent reduction due to CPT across exercise intensities for peak (P = 0.38–0.65) or total VC (P = 0.43–0.51).
DISCUSSION
This is the first study to examine the influence of sympathetic vasoconstriction on the contraction-induced rapid hyperemic and vasodilator responses in the leg of young and older adults. The novel finding of this study is that acute sympathetic stimulation via a CPT significantly blunts ROV in the contracting leg of both young and older adults and the magnitude of change is similar between age groups. Additionally, age-related differences in ROV still persist in the face of acute sympathetic stimulation. Taken together, our present findings suggest that sympathetic vasoconstriction does not appear to fully explain age-associated reductions in contraction-induced rapid hyperemia and vasodilation in the lower limbs of humans.
Lower Limb Vasoconstrictor Responses and Age-Associated Reductions in Contraction-Induced ROV
We have previously demonstrated that contraction-induced ROV is attenuated to a similar extent in the upper and lower limbs of older adults across a range of exercise intensities (16). Furthermore, we have demonstrated that within the forearm, sympathetic stimulation reduces contraction-induced ROV in young but not older adults (3). Moreover, nonselective α-adrenergic blockade effectively abolishes the age-related differences in ROV in the forearm (3). In the present study, contraction-induced ROV in the leg was blunted during acute sympathetic stimulation to a similar degree between age groups (Figs. 3 and 4). While contraction-induced ROV is not reduced in the forearm of older adults during sympathetic stimulation (3), previous evidence has shown that older adults demonstrate greater vascular tone within the leg at rest (43) as well as reduced blood flow and vasodilation during dynamic exercise (21, 29). Taken together, this evidence might suggest that while regulation of vascular tone at the onset of exercise is altered with age, the regulatory mechanisms may differ between limbs. However, it should be noted that the contrasting ROV responses between limbs during sympathetic stimulation with aging might not truly reflect limb-specific differences but rather methodological differences. Along these lines, our previous study in the arm (3) utilized lower body negative pressure to induce acute sympathetic stimulation whereas a CPT was used in the current study. Differences in the magnitude of sympathetic stimulation and consequent hemodynamic changes between the two experimental conditions could influence ROV independent of potential inherent limb differences. In support of this notion, lower body negative pressure and CPT have been shown to elicit divergent hemodynamic responses as well as arterial blood flow patterns at rest (33).
Sympathetic Vasoconstriction, ROV, and Sex Differences
Previous work from our group indicates sex may play a role in the ROV responses in the forearm during sympathetic stimulation (3). Specifically, young men exhibited greater sympathetically mediated reductions in ROV during low-intensity contractions but not during moderate to higher intensity contractions, suggestive of greater vasoconstrictive responsiveness that is abolished with increasing exercise intensity. In contrast to what we previously reported in the forearm, our current findings in the leg suggest that the reduction in ROV during acute sympathetic stimulation is similar between young men and women regardless of exercise intensity. Moreover, the magnitude of reduction in ROV (expressed as a relative or absolute change) during sympathetic stimulation did not differ between age groups within each sex. It is important to note that these statistical comparisons and conclusions regarding potential sex-related differences are derived from a relatively small sample size (n = 6–7 for each group), which could contribute to the lack of differences observed within and between groups.
Feedforward Mechanisms to Dynamic Exercise
The capacity to rapidly increase blood flow and vasodilation at the onset of contractions has important implications for the regulation of skeletal muscle blood flow during dynamic exercise. Rapid vasodilation at the onset of exercise contributes to the biphasic nature of exercise hyperemia (46) and presumably facilitates the transition from rest to steady-state dynamic exercise. While it is well documented that vascular tone is altered with aging at rest and during repeated muscle contractions (10, 39), far less is known regarding the dynamics of vasodilation leading up to steady-state dynamic exercise. However, emerging evidence points to age-related impairments in the local control of blood flow in contracting human skeletal muscle occurring as early as the first contraction (2, 3, 5, 16, 20), which is due in part to enhanced α-adrenergic-mediated vasoconstriction and reductions in nitric oxide (NO)-mediated local vasodilation (3). Decreases in NO bioavailability have also been implicated in the blunted transient leg blood flow response to movement-induced hyperemia in older adults (12, 25, 28, 45). Interestingly, the age-related reductions in forearm blood flow during steady-state exercise do not appear to be due to an enhanced sympathetic vasoconstriction (38). This evidence suggests that sympathetically mediated vasoconstriction possibly plays a greater role in the age-related impairments in exercise hyperemia at the very onset of exercise compared with under steady-state conditions. Furthermore, this discrepancy also highlights a knowledge gap related to the vasodilator and vasoconstrictor mechanisms involved in the regulation of muscle blood flow during the transition from rest to steady-state exercise. Although we recently demonstrated the speed (i.e., kinetics) of skeletal muscle blood flow and vasodilation during rhythmic forearm contractions are slower in otherwise healthy aging humans, possibly due to blunted NO signaling (4), the role sympathetic vasoconstriction plays in slowing vasodilator kinetics with age has not been explored.
Attenuation of sympathetic vasoconstriction during exercise is critical in the matching of oxygen supply to demand during dynamic exercise via redistribution of blood flow to contracting tissues (e.g., functional sympatholysis) (39). Previous evidence in the forearm of young adults indicates that attenuation of sympathetic vasoconstriction is immediate (after a single muscle contraction) (8). Conversely, sympathetic vasoconstriction appears to limit rapid vasodilation in the forearm of older adults (3) as well as contributes to the age-related reductions in muscle blood flow during steady-state dynamic exercise in the leg (10, 29, 50). In the present study, acute sympathetic stimulation reduced ROV in the leg ~20–30% of both young and older adults, with the magnitude of reduction being similar between age groups and across exercise intensities (Fig. 5). Noting previous evidence from our laboratory of a similar attenuation in ROV between the arm and leg with age following a single muscle contraction (16), it does not appear that a greater tonic sympathetic vasoconstriction explains the drastic age-related differences in contraction-induced rapid vasodilation within the leg. Considering the idea that ROV is thought to play a critical role in modulating skeletal muscle blood flow during steady-state dynamic exercise conditions, the ability to attenuate sympathetic vasoconstriction within the leg after a single contraction may play a critical role in oxygen delivery and exercise tolerance (40).
Experimental Considerations
There are a few experimental considerations that warrant mention. First, we used a CPT to increase sympathetic activity with the goal of enhancing vasoconstriction in the contracting leg. Although we did not directly assess sympathetic outflow via direct measurement of MSNA or norepinephrine spillover, previous evidence has clearly demonstrated that a local CPT is capable of doubling MSNA in both young and older adults (21, 32, 41, 48). Along with inducing sympathetic vasoconstriction, the CPT also resulted in concomitant increases in systemic arterial pressure, which in turn could potentially confound our results. However, the calculation of vascular conductance takes into account changes in arterial pressure, and our method of calculating ROV (Δ from respective baseline) allows for comparison despite baseline differences between age groups as well as between trials (control vs. CPT).
The conclusion that there are no age-associated differences in the reduction of ROV in response to acute sympathetic stimulation is in contrast to our original hypothesis. Our conclusion is based off the finding that the relative reduction in ROV during CPT (compared with control conditions) is similar between young and older adults. However, it could be argued that based on the calculation of percent reduction in VC due to CPT [(CPT-control)/control × 100], the much lower VC in older adults during control conditions results in a smaller denominator and thereby possibly makes the percent reduction appear greater than it actually is. Although this is possible, close examination of our data reveals that this is likely not the case. When examined as an absolute difference (i.e., CPT-control), there are still no differences in the peak and total VC between young and older adults (P = 0.12–0.63). These results, taken in context with the percent reduction due to CPT, suggest that sympathetic stimulation reduces ROV within the leg independent of age.
Similar to our previous studies (15, 16), the older adults demonstrated a lower WRmax in the leg than their young counterparts. In turn, their absolute work rates at each respective workload (5 ± 1 vs. 8 ± 1 W at 20% WRmax and 11 ± 1 vs. 16 ± 1 W at 40% WRmax) were lower, which could have influenced our current findings. Also, previous evidence suggests that any age-related difference in the ability to inhibit sympathetic vasoconstriction in the exercising leg is more closely associated with work performed rather than age per se (50). Therefore, we addressed this potential confound by comparing ROV responses under control and CPT conditions in a subset of young and older adults matched for a similar absolute workload (~10 W). Results from this subanalysis clearly demonstrate 1) blunted ROV responses with age still persist when matched for an absolute workload, and 2) the magnitude of reduction in the ROV response during acute sympathetic stimulation is still similar between young and older adults. Finally, using the current study design, we were unable to examine whether α-adrenergic blockade abolishes the age-related differences in ROV in the leg, similar to our previous work in the forearm (3). However, removal of sympathetic α-adrenergic vasoconstriction via phentolamine abolishes age-related differences in femoral blood flow and vasodilation at rest, as well as during a CPT (11).
Conclusion
The results from the present study are the first to provide experimental evidence that contraction-induced rapid vasodilation within the leg is reduced by sympathetic stimulation, independent of age as well as sex. Furthermore, while sympathetic stimulation reduces rapid vasodilation following a single muscle contraction in the leg of young adults, it does not fully explain the blunted ROV response commonly observed in the leg of older adults.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-105467 (to D. P. Casey) and T32-HL-007121 (to N. T. Kruse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.E.H. and D.P.C. conceived and designed research; W.E.H. and N.T.K. performed experiments; W.E.H. analyzed data; W.E.H. and D.P.C. interpreted results of experiments; W.E.H. prepared figures; W.E.H. drafted manuscript; W.E.H., N.T.K., and D.P.C. edited and revised manuscript; W.E.H., N.T.K., and D.P.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for participation. Extended appreciation goes out to Charles Ganger IV, Aaron Schneider, and Samuel Norton for technical assistance.
REFERENCES
- 1.Best SA, Okada Y, Galbreath MM, Jarvis SS, Bivens TB, Adams-Huet B, Fu Q. Age and sex differences in muscle sympathetic nerve activity in relation to haemodynamics, blood volume and left ventricular size. Exp Physiol : 839–848, 2014. doi: 10.1113/expphysiol.2013.077248. [DOI] [PubMed] [Google Scholar]
- 2.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol : H1963–H1970, 2008. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- 3.Casey DP, Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol (1985) : 1201–1212, 2012. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Ranadive SM, Joyner MJ. Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: role of nitric oxide. J Appl Physiol (1985) : 232–241, 2015. doi: 10.1152/japplphysiol.00787.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol (1985) : 446–455, 2013. doi: 10.1152/japplphysiol.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol : 825–833, 2007. doi: 10.1113/jphysiol.2007.135673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifford PS, Tschakovsky ME. Rapid vascular responses to muscle contraction. Exerc Sport Sci Rev : 25–29, 2008. doi: 10.1097/jes.0b013e31815ddba4. [DOI] [PubMed] [Google Scholar]
- 8.DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol (1985) : 555–560, 2002. doi: 10.1152/japplphysiol.00245.2002. [DOI] [PubMed] [Google Scholar]
- 9.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation : 164–170, 1999. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- 10.Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation : 329–341, 2006. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 11.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol : 977–983, 2001. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol : H610–H619, 2013. doi: 10.1152/ajpheart.00656.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwich D, Fowler KL, Wynn LJ, Fisher JP. Differential responses to sympathetic stimulation in the cerebral and brachial circulations during rhythmic handgrip exercise in humans. Exp Physiol : 1089–1097, 2010. doi: 10.1113/expphysiol.2010.054387. [DOI] [PubMed] [Google Scholar]
- 14.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol : H1626–H1632, 2010. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes WE, Ueda K, Casey DP. Chronic endurance exercise training offsets the age-related attenuation in contraction-induced rapid vasodilation. J Appl Physiol (1985) : 1335–1342, 2016. doi: 10.1152/japplphysiol.00057.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes WE, Ueda K, Treichler DP, Casey DP. Rapid onset vasodilation with single muscle contractions in the leg: influence of age. Physiol Rep : e12516, 2015. doi: 10.14814/phy2.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol (1985) : 1715–1721, 2007. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 18.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol : 2269–2282, 2010. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev : 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol : 1989–2003, 2009. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol : 337–344, 2003. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol : H1023–H1031, 2003. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 23.Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol (1985) : 1360–1368, 2010. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ : 230–235, 1990. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol : 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation : 862–868, 2000. doi: 10.1161/01.CIR.101.8.862. [DOI] [PubMed] [Google Scholar]
- 27.Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol : 309–316, 2003. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol : 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol : 6227–6236, 2012. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension : 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 31.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol : H308–H315, 2005. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol : H344–H353, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol : H1128–H1135, 2010. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol : H1251–H1259, 2003. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 35.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol (1985) : 1963–1970, 2003. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- 36.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation : 315–327, 2006. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- 37.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol (1985) : 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Role of α-adrenergic vasoconstriction in regulating skeletal muscle blood flow and vascular conductance during forearm exercise in ageing humans. J Physiol : 4775–4788, 2014. doi: 10.1113/jphysiol.2014.278358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol : 6269–6275, 2012. doi: 10.1113/jphysiol.2012.241026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand : 421–436, 1998. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 41.Seals DR. Sympathetic activation during the cold pressor test: influence of stimulus area. Clin Physiol : 123–129, 1990. doi: 10.1111/j.1475-097X.1990.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 42.Sinkler SY, Fernando CA, Segal SS. Differential α-adrenergic modulation of rapid onset vasodilatation along resistance networks of skeletal muscle in old versus young mice. J Physiol : 6987–7004, 2016. doi: 10.1113/JP272409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol : 63–71, 2007. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundlöf G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol : 525–532, 1978. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol : 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol (1985) : 639–644, 2004. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 47.Tschakovsky ME, Saunders NR, Webb KA, O’Donnell DE. Muscle blood-flow dynamics at exercise onset: do the limbs differ? Med Sci Sports Exerc : 1811–1818, 2006. doi: 10.1249/01.mss.0000230341.86870.4f. [DOI] [PubMed] [Google Scholar]
- 48.Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension : 429–436, 1987. doi: 10.1161/01.HYP.9.5.429. [DOI] [PubMed] [Google Scholar]
- 49.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest : 79–86, 1974. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- 50.Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol : H497–H504, 2009. doi: 10.1152/ajpheart.01016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]