Abstract
Haemorrhagic cholecystitis is a rare entity of acute cholecystitis that carries a high morbidity and mortality rate if management is delayed. Its clinical course can mirror that of acute cholecystitis. Characteristic findings on ultrasound or CT scan are useful clues to early diagnosis. Urgent cholecystectomy is required prior to progressing to perforation of gallbladder. Most of the literature are case reports with causes associated with anticoagulation. Herein, we described a morbidly obese patient with poorly controlled diabetes presenting with non-specific right upper quadrant pain and was subsequently diagnosed with haemorrhagic cholecystitis. A review of the literature was also performed to summarise the potential clinical presentations, distinctive imaging findings and management options available for this rare condition.
Keywords: general surgery, surgery, biliary intervention
Background
Haemorrhagic cholecystitis (HC) is a rare complication of acute cholecystitis. It usually occurs in the setting of blunt trauma, bleeding diathesis in patients with renal failure, cirrhosis and on anticoagulation.1–3 Its symptoms mimic those of acute cholecystitis such as right upper quadrant pain, fever and leucocytosis.3 4 Rarer presentations have also been reported in the literature including obstructive jaundice and/or cholangitis from the clot causing common bile duct obstruction5 and features of upper gastrointestinal bleeding (haematemesis and melaena).1 Due to its non-specific features, late detection carries a significant morbidity and mortality up to 20%.4 Most of the literature on HC is limited to case report or small case series. Herein, we describe a case of HC in a morbidly obese patient with poorly controlled diabetes mellitus. We also aimed to review the literature to describe the various clinical presentations, imaging findings and management options for HC.
Case presentation
A 68-year-old morbidly obese female presented with 3-day history of right upper quadrant pain. The abdominal pain was described as sudden onset, sharp and progressively worsening with associated nausea and vomiting. She denied any fever. There is no change in bowel habit or urinary symptom. Her medical history includes poorly controlled type 2 diabetes mellitus, depression and open appendicectomy.
On presentation, she was tachycardic with heart rate of 110 beats per minute and she was hypotensive with a blood pressure of 90/60 mm Hg. She was afebrile. Clinical examination of her abdomen was difficult due to her body habitus. Abdomen was soft but there was right upper quadrant tenderness and a mass could be felt just below the right costal area. Bedside urinalysis was positive for ketones. Bedside blood sugar level (BSL) reading was ‘high’. Venous blood gas subsequently reported her glucose level as 40 mmol/L.
Investigations
Biochemistry examination showed a haemoglobin level of 142 g/L, raised inflammatory markers—white cell count of 40.1×109/L and C-reactive protein of 406.5 mg/L. Her electrolyte profile showed hyponatraemia with sodium 124 mmol/L (135–145). There was also acute kidney injury with raised creatinine 148 μmol/L (45–90), estimated glomerular filtration rate 31 mL/min/1.73 m. Liver function test showed bilirubin 12 μmol/L (0–20), alanine transaminase 36 U/L (0–34), alkaline phosphatase 93 U/L (30–110), gamma glutamyl transferase 36 U/L (0–38). Coagulation profile and lipase were normal.
CT was organised instead of ultrasound abdomen due to her large body habitus which may limit the sonographic views. Due to her acute kidney injury, a non-contrast CT abdomen and pelvis was performed which demonstrated thickened gallbladder wall with high density, raising the likelihood of HC (figures 1 and 2). Two small locules of gas were also observed, concerning for mural necrosis (figure 1).
Figure 1.
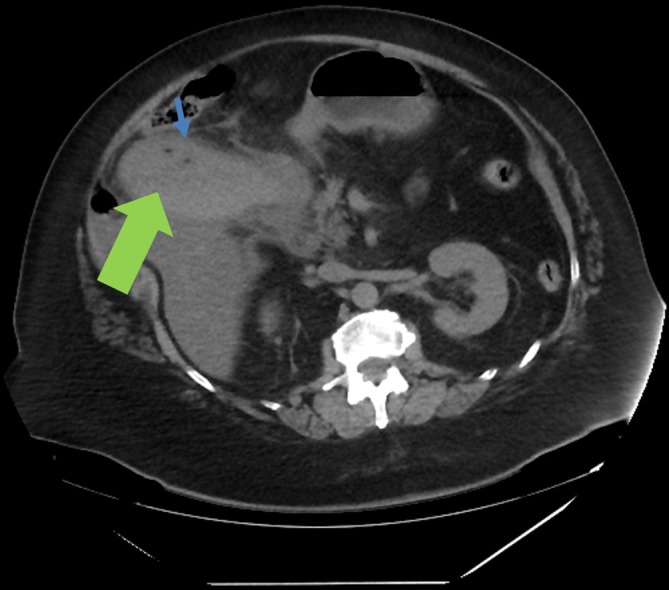
Sagittal film of CT abdomen/pelvis showing severe cholecystitis. The high density of thickened gallbladder wall (green thick arrow) is highly suggestive of haemorrhagic cholecystitis. The two tiny foci of low density (blue thin arrow) within the gallbladder lumen could represent locules of gas, concerning for mural necrosis.
Figure 2.
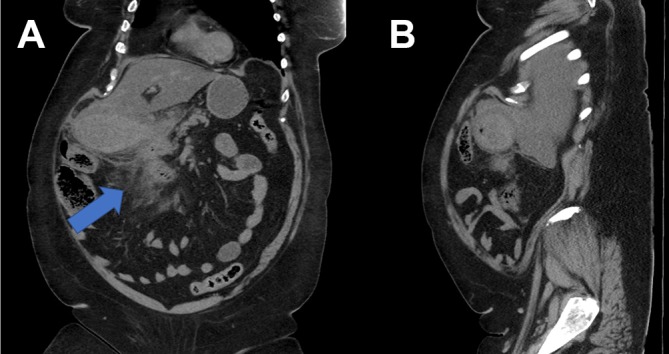
(A and B) Coronal and axial films of CT abdomen/pelvis demonstrating the thickened gallbladder wall with high density and induration of the surrounding fat (thick arrow) extending into the porta hepatis and adjacent to the second part of duodenum.
Treatment
Fluid resuscitation was commenced and intravenous tazocin (piperacillin/tazobactam) 4.5 g was administered. Insulin and dextrose infusion was also commenced. She was brought to theatre in the next hour for open cholecystectomy. A right subcostal incision was made to access to the gallbladder. Intraoperatively, the findings were consistent with the CT findings—there was necrosis of the gallbladder at part of the fundus and there was old clot in the lumen of gallbladder with multiple gallstones. A subtotal cholecystectomy was performed with ‘fundus-down’ approach. The cystic duct was closed with 3/0 polydioxanone sutures. A 24 Fr bore drain was left in situ at the gallbladder bed.
Outcome and follow-up
Postoperatively, her BSLs improved and she recovered progressively. Her postoperative recovery was complicated with right lower lobe pneumonia which was treated with intravenous tazocin and later stepped down to oral Augmentin duo forte. She was discharged home on day 7.
Histopathology
The histopathological features (figures 3 and 4) show marked denudation of the surface epithelium with degenerate material within the lumen and transmural mixed acute and chronic inflammation with marked vascular congestion and red cell extravasation into the surrounding stroma and focal necrosis of the muscularis, consistent with HC given the clinical context; however, the features are non-specific in isolation.
Figure 3.
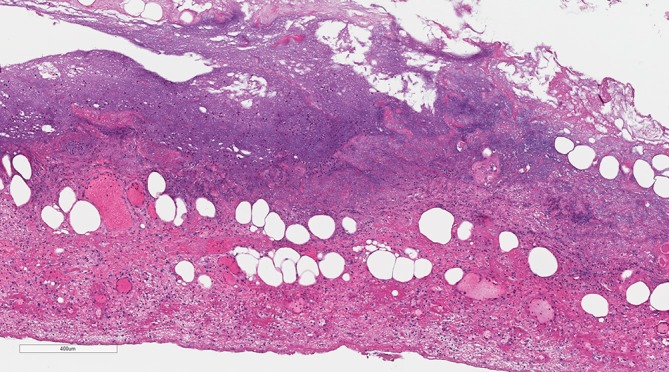
Within the low power image of the histopathology slide, there is basophilic degenerate material towards the mucosal surface (top) and marked vascular congestion and red cell extravasation of the obliterated muscularis, subserosa and serosa (towards the bottom of the image).
Figure 4.
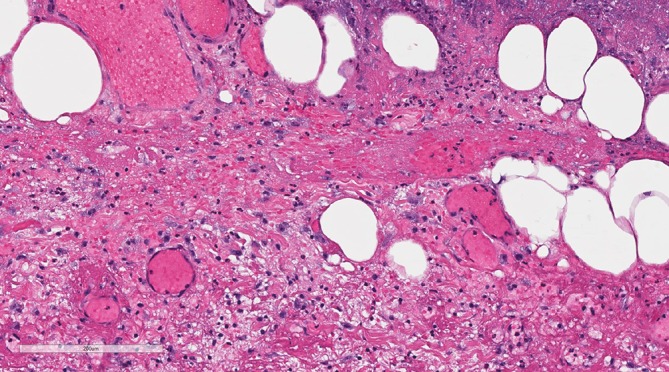
This high power histopathology slide image highlights the marked red cell extravasation and vascular congestion.
Discussion
Haemorrhagic cholecystitis was first described by 1979 by Shah and Clegg in a case of haemobilia.6 It is a rare disease with an estimated incidence of 7%.3 Most of the literature have been limited to case reports or small case series. Chinn and colleagues’ work in 1987 remains the biggest series investigating the patterns of presentation for HC.3
Various causes have been described to be associated with HC including trauma, malignancy, the use of anticoagulation and higher bleeding tendency in dialysis patient.1 2 7 8
HC is believed to be part of the continuum spectrum of cholecystitis, ranging from simple acute cholecystitis to HC prior to gangrenous cholecystitis and lastly gallbladder perforation.4 Very rarely, in the end spectrum of gallbladder perforation, a bilioenteric fistula can occur which leads to gallstone ileus.9 The pathogenesis of HC in the setting of calculous cholecystitis results from transmural inflammatory process causing infarction and necrosis of gallbladder mucosa, which results in the destruction of vessel walls and bleeding. Infrequently, the haemorrhage can stem from the development of cystic artery pseudoaneurysm likely from either early thrombosis of the cystic artery from inflammation or erosion of the cystic artery wall by a large gallstone.
The morbidity and mortality are significant as HC is often overlooked due to the symptoms often mimicking those of acute cholecystitis, viz, fever, right upper quadrant pain and leucocytosis. In diabetic or immunocompromised patients, the symptoms may also be masked and appear ‘less severe’ such as our patient. In rare circumstances, upper gastrointestinal bleeding can present in the form of haematemesis or malaena or obstructive jaundice/cholangitis secondary to clot obstructing the common bile duct.3 If the patient presents with upper gastrointestinal bleeding coupled with upper abdominal pain and jaundice, it is known as Quinke’s triad.10 Nevertheless, it occurs only in 22% of case presentations.10
Biochemistry examination findings may not differ significantly from what would be expected from a typical acute cholecystitis. In the setting of haemorrhage, a fall in haemoglobin may be observed; an obstructive picture of liver function test may represent a clot in the biliary tree. Imaging is essential in the diagnosis of HC as swift management is required. Ultrasound abdomen typically demonstrates one or more of the following sonographic features: focal gallbladder wall irregularity, intraluminal membranes and coarse non-mobile, non-shadowing, intraluminal echoes.3 However, ultrasound may not be readily available and the views maybe limited in obese patients. CT aids to exclude other pathologies and provides certain findings such as high attenuation material within gallbladder lumen with fluid-fluid level.4 11 In arterial phase as emphasised by Pandya and O’Malley, active extravasation of the contrast into the gallbladder lumen maybe appreciated.11 MRI maybe less helpful in the acute setting. Typically on T1 imaging, the haemorrhagic components are hyperintense and the signal intensity varies on T2 imaging depending on the oxygenation state of the haemoglobin.4
Surgery remains the gold standard for the management of HC.4 It should be performed swiftly in either laparoscopic or open approach prior to gallbladder perforation. In our patient, we elected to deal with the open approach due to her large body habitus which will be challenging from a laparoscopic perspective. Moreover, given her unstable haemodynamics, a longer operative time in laparoscopic surgery will not be beneficial for her. In patients presenting with obstructive jaundice, an urgent endoscopic retrograde cholangiopancreatography will be required to relieve the haemobilia prior to proceeding to cholecystectomy.8 In the unfit and frail cohort of patients, a cholecystostomy tube is an alternative option. There has been a report of HC managed with a cholecystostomy tube with urokinase therapy.12
Learning points.
Haemorrhagic cholecystitis remains a rare entity of acute cholecystitis.
Its clinical presentation is often non-specific and maybe masked in diabetic or immunocompromised patients.
CT scan is often the imaging of choice. Urgent cholecystectomy should be performed as any delay will lead to perforation of gallbladder and this carries a high morbidity and mortality rate.
Footnotes
Contributors: ZQN designed the study, collected the data and drafted the article. KC prepared the histopathology slides. RW co-designed the study and supervised the study. SP, KC and RW critically reviewed the article. All authors analysed the data and approved the final version of the article to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hicks N. Haemorrhagic cholecystitis: an unusual cause of upper gastrointestinal bleeding. BMJ Case Rep 2014;2014:bcr2013202437 10.1136/bcr-2013-202437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinnear N, Hennessey DB, Thomas R. Haemorrhagic cholecystitis in a newly anticoagulated patient. BMJ Case Rep 2017;2017:bcr-2016-214617 10.1136/bcr-2016-214617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinn DH, Miller EI, Piper N. Hemorrhagic cholecystitis. Sonographic appearance and clinical presentation. J Ultrasound Med 1987;6:313–7. 10.7863/jum.1987.6.6.313 [DOI] [PubMed] [Google Scholar]
- 4.Revzin MV, Scoutt L, Smitaman E, et al. The gallbladder: uncommon gallbladder conditions and unusual presentations of the common gallbladder pathological processes. Abdom Imaging 2015;40:385–99. 10.1007/s00261-014-0203-0 [DOI] [PubMed] [Google Scholar]
- 5.Seok DK, Ki SS, Wang JH, et al. Hemorrhagic cholecystitis presenting as obstructive jaundice. Korean J Intern Med 2013;28:384–5. 10.3904/kjim.2013.28.3.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VR, Clegg JF. Haemorrhagic cholecystitis. Br J Surg 1979;66:404–5. 10.1002/bjs.1800660608 [DOI] [PubMed] [Google Scholar]
- 7.Parekh J, Corvera CU. Hemorrhagic cholecystitis. Arch Surg 2010;145:202–4. 10.1001/archsurg.2009.265 [DOI] [PubMed] [Google Scholar]
- 8.Shishida M, Ikeda M, Karakuchi N, et al. Hemorrhagic cholecystitis in a patient on maintenance dialysis. Case Rep Gastroenterol 2017;11:488–93. 10.1159/000479497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conzo G, Mauriello C, Gambardella C, et al. Gallstone ileus: One-stage surgery in an elderly patient: One-stage surgery in gallstone ileus. Int J Surg Case Rep 2013;4:316–8. 10.1016/j.ijscr.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nana GR, Gibson M, Speirs A, et al. Upper gastrointestinal bleeding: a rare complication of acute cholecystitis. Int J Surg Case Rep 2013;4:761–4. 10.1016/j.ijscr.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandya R, O’Malley C. Hemorrhagic cholecystitis as a complication of anticoagulant therapy: role of CT in its diagnosis. Abdom Imaging 2008;33:652–3. 10.1007/s00261-007-9358-2 [DOI] [PubMed] [Google Scholar]
- 12.Stempel LR, Vogelzang RL. Hemorrhagic cholecystitis with hemobilia: treatment with percutaneous cholecystostomy and transcatheter urokinase. J Vasc Interv Radiol 1993;4:377–80. 10.1016/S1051-0443(93)71882-5 [DOI] [PubMed] [Google Scholar]


