Abstract
Objective
Cementless total hip arthroplasty (THA) is associated with reliable clinical results and high patient satisfaction. Short-stem prostheses (SS) were designed to achieve superior preservation of proximal bone stock and stability compared with those of conventional-stem prostheses (CS). This meta-analysis was conducted to determine the proximal bone remodelling, revision rate, Harris Hip Score, radiolucent line and maximum total point motion values of both SS and CS for primary THA.
Method
Relevant randomised controlled trials (RCTs) involving SS and CS in primary THA were identified from electronic databases, such as EMBASE, PubMed and the Cochrane Library.
Result
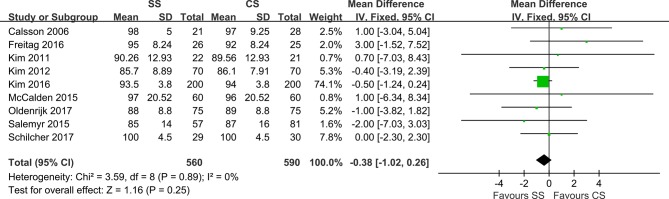
Ultimately, 12 RCTs involving 1130 patients (1387 hips) were included. The results showed that compared with CS, SS resulted in less bone mineral density (BMD) changes in Gruen zone 7 at 1 year and 2 years postoperatively (mean difference (MD)=5.11; 95% CI, 1.61, 8.61; P=0.30; and MD=4.90; 95% CI, 1.01, 8.79; P=0.17, respectively). No difference in BMD changes was found for Gruen zone 1 (MD=2.66; 95% CI, −3.31, 8.64; P<0.00001), and no differences were observed for the revision rate (relative risk (RR)=1.52; 95% CI, 0.71, 3.26; P=0.94), Harris Hip Score (MD=−0.38; 95% CI, −1.02, 0.26; P=0.89) or stem migration (MD=0.02; 95% CI, −0.07, 0.11; P=0.04).
Conclusion
Our results suggest that compared with CS, SS may provide superior bone remodelling and similar survival rates and clinical outcomes. However, the short-term follow-up of the included studies was inadequate to determine the long-term performance of SS.
Keywords: total hip arthroplasty, short stem, conventional stem, meta-analysis
Strengths and limitations of this study.
The present results derived from all available comparative data are the first to document the differences in postoperative bone remodelling, implant survival, complications and radiological performance between SS and CS.
Although the most recent evidence regarding the use of SS and CS in primary THA were provided in our study, the average follow-up of 3.9 years (range, 0.115–11.8 years) was relatively short for evaluating the outcomes of femoral prostheses.
The effects of the lateral flare design and the extent and type of surface coating for bone conservation in primary THA have not been fully defined.
Introduction
Total hip arthroplasty (THA) is an effective treatment method for hip disorders. Along with improved techniques and prosthetic designs, the number of THAs performed has increased worldwide.1 Moreover, the overall burden of revision has increased,2 particularly in patients who are young and thus have the potential for revision. The THA revision rate has significantly increased in patients aged between 45 and 64 years in the United States.3 Therefore, optimal implant survival with reliable clinical outcomes and bone stock preservation are essential for facilitating future revisions. Conventional cementless femoral stems may provide satisfactory clinical and radiographic outcomes at long-term follow-up.4–6 However, despite excellent results, stress shielding and thigh pain may occur.7 8
Stress shielding is associated with periprosthetic bone loss, which may contribute to late-occurring periprosthetic fractures in uncemented femoral stems.9 10 First introduced in 1989,11 short-stem prostheses (SS) were developed to preserve proximal bone stock and allow more physiological proximal loading.12 13 The design of SS requires less resection of the femoral neck and less reaming of the femoral shaft. Although no exact definition of SS exists, these prostheses are characterised by their fixation principles and the location of proximal loading, with stem lengths ranging from 40 to 135 mm.14 According to some research, short stems typically do not extend beyond 120 mm in a majority of cases and a trochanter-sparing design is employed, which is uncemented with a proximal metaphyseal and short diaphyseal anchorage.15 Previous studies on SS have shown excellent clinical results and fixation at short-term to mid-term follow-up.16–18
Some clinical studies compared the effectiveness and radiographical outcomes between SS and conventional stem prostheses (CS) in primary THA, and several studies reported superior bone preservation for SS after evaluating bone mineral density (BMD) changes around the prostheses.19–21 However, other studies have reported different results.22–24 A meta-analysis conducted by Huo et al25 showed that SS achieved the same clinical and radiological outcomes as CS and additionally reduced thigh pain. However, the meta-analysis included only six studies with a small number of subjects: 275 hips in the SS group and 297 in the CS group. Their analysis did not evaluate bone remodelling outcomes, such as periprosthetic bone density and radiolucent lines and migration. Jahnke et al26 observed that BMD changes did not directly correlate with clinical outcomes despite significant improvements in the Harris Hip Score (HHS). Because additional clinical trials were recently published, such results warrant re-evaluation. To survey superior survival rates and clinical outcomes in primary THA, we conducted a systematic review and meta-analysis to evaluate the evidence from all available randomised controlled trials (RCTs) that compared SS and CS in patients undergoing primary THA.
Methods
Search strategy
We conducted a meta-analysis to identify relevant RCTs involving SS and CS in primary THA in electronic databases. Two reviewers independently searched electronic databases, including the Web of Science, EMBASE, PubMed, the Cochrane Controlled Trials Register and the Cochrane Library, through July 2017 using the following keywords and their combinations: short stem, conventional stem, arthroplasty, hip and randomised controlled trial. The reviewers first screened the titles and abstracts to identify the relevant studies. Only RCTs performed with humans were included. The search strategy is presented in online supplementary table 1. The PRISMA guidelines27 and Cochrane Handbook were applied to assess the quality of the results published in all the included studies to ensure that the results of our meta-analysis were reliable and verifiable. The reference lists of the selected articles were also reviewed manually to identify additional relevant studies.
bmjopen-2018-021649supp001.pdf (15.6KB, pdf)
Inclusion and exclusion criteria
The inclusion criteria were defined before searching the databases, and study inclusion eligibility was determined by the following population, intervention, comparator, outcome and study design criteria: (1) patients with hip disorders, such as osteoarthritis, osteonecrosis, traumatic arthritis or femoral neck fracture and scheduled for primary THA; (2) RCTs comparing SS and CS; and (3) the age of the patients and follow-up periods were not restricted. The publication language was also limited to English. The exclusion criteria were as follows: observational studies, noncontrolled clinical trials, animal studies or revision THA.
Data extraction and quality assessment
Two investigators independently extracted the relevant data from each study, which included the first author’s name, year of publication, country, study design, company, details of the intervention and control, and the follow-up duration and outcome measurements for each study. Any uncertainty was discussed by the two reviewers and resolved by consensus through discussion with other reviewers. We contacted the corresponding authors of the included RCTs when necessary to obtain any missing data. Revision rates were recalculated based on the number of revisions due to all causes provided in the article. The Cochrane Collaboration tool28 was used to assess the methodological quality and risk of bias of the included studies, including randomisation, allocation concealment, blinding method, selective reporting, group similarity at baseline, incomplete outcome data, compliance, timing of outcome assessments and intention-to-treat analysis.
Outcome measurements
The primary outcome measurements evaluated in our meta-analysis included BMD changes in different femoral zones to assess the efficacy of short stems by evaluating whether they can achieve better bone remodelling, postoperatively measured by dual-energy X-ray absorptiometry (DEXA), and the revision rate to evaluate the safety of short-stem prostheses. The secondary outcomes were as follows: the Harris Hip Score to evaluate the functional outcome; the presence of radiolucent lines, which suggests loosening of the prosthesis; and the maximum total point motion to evaluate stem migration in three axial directions.
Statistical analysis and data synthesis
The meta-analyses were performed using Review Manager (Revman Version 5.3., the Cochrane Collaboration, Oxford, UK). Given the characteristics of the data extracted for the review, continuous outcomes were expressed as the mean difference with 95% CIs. An assumption that the standard deviations (SDs) of the outcome measurements were the same in both groups was required in all cases, and the SD would then be used for both intervention groups. Where actual P values obtained from t-tests are quoted, the corresponding t value may be obtained from a table of the t distributions. The df are given by NE +NC – 2, where NE and NC are the sample sizes in the experimental and control groups, respectively. The t value is the ratio of the difference in means to the SE of the difference in means. The SE of the difference in means can therefore be obtained by dividing the difference in means by the t value: SE=MD/t. The within-group SD can be obtained from the SE of the difference in means using the following formula: SD=SE/√((1/NE)+(1/NC)). If authors do not report exact P-values but a 95% CI is available for the difference in means, then the same SE can be calculated as SE=(upper limit – lower limit)/3.92, as long as the trial is large. For 90% CIs, 3.92 should be replaced by 3.29, and for 99% CIs, this value should be replaced by 5.15. If the sample size is small, then confidence intervals should have been calculated using a t distribution. The numbers 3.92, 3.29 and 5.15 need to be replaced with larger numbers specific to both the t distribution and the sample size and can be obtained from tables of the t distribution with df equal to NE +NC – 2, where NE and NC are the sample sizes in the two groups, respectively. Relevant details related to the t distribution are available as appendices in many statistical textbooks or in standard computer spreadsheet packages. Heterogeneity was assessed using the I² statistic. I²≥50% represented high heterogeneity. To detect the impact of each data set on the overall effects of the analyses, sensitivity analysis was performed by sequentially deleting a single study involved in the meta-analysis. Subgroup analysis was performed based on the different follow-up periods. Risk ratios with a 95% CI were used to assess dichotomous outcomes. The inverse variance and Mantel–Haenszel methods were used to combine separate statistics. We evaluated whether asymmetry was due to publication bias or to a relationship between the trial size and effect size using funnel plots. A P value <0.05 was considered statistically significant.
Patient and public involvement
No patients or public were involved in this study.
Results
Description of the included studies
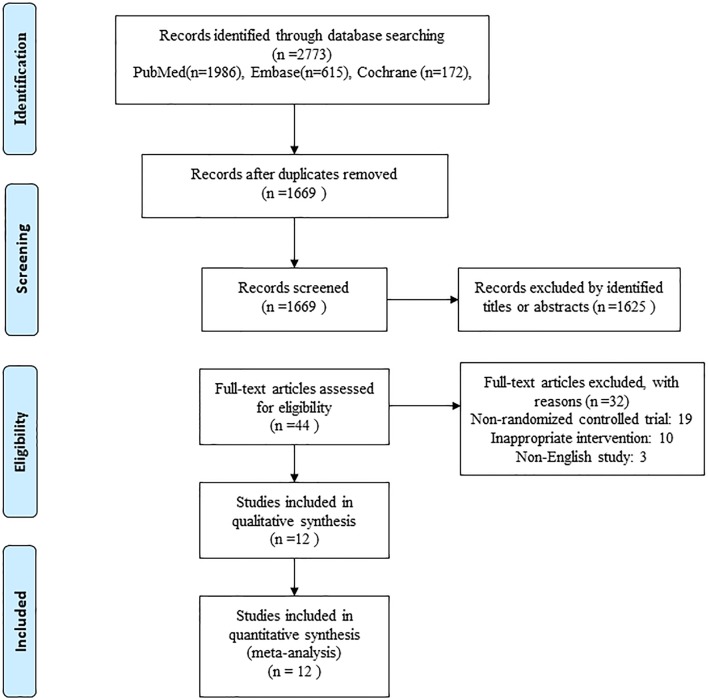
An initial literature search yielded a total of 2773 relevant studies, of which 1104 were excluded as duplicates. After screening the titles and abstracts of the remaining studies, we identified 44 potentially relevant studies. After excluding 32 publications by full-text screening, 12 RCTs published from 2006 to 2017 involving eight different SS types and 1130 participants were ultimately included in the study (figure 1). The characteristics of the included studies are presented in table 1. The total sample size was 680 for the SS group and 707 for the CS group. The populations of the included studies ranged from 21 to 200 participants. The follow-up durations ranged from 6 weeks to 11.8 years (mean 3.9 years). All the femoral components were uncemented designs except for one that was cemented.29 Ceramic-on-ceramic bearing surfaces were reported in five studies,22–24 29–31 while other studies did not report this information. A one-stage bilateral THA was performed in two studies.21 24 The mean patient age was 62.7 years (range, 51.7–76.0 years), and 42.2% (range, 11%–63%) of the patients were men.
Figure 1.
Flow diagram of the relevant study selection process.
Table 1.
Overview of the characteristics of the included studies
| Study/year (country) |
SS group/CS group | |||||||||
| Number of patients | Number of hips | Age (mean), years |
Gender (% male) |
Follow-up (mean), years | Type of implant (company) | Dorr type | Outcome assessment | |||
| Type A | Type B | Type C | ||||||||
| Sluimer et al20 /2006(Netherlands) | 80 | 40/40 | 53/56 | 37/43 | 2.0/2.0 | Omnifit-HA1090 (Osteonics)/ Omnifit-HA1017 (Osteonics) |
NA | HHS, BMD, Engh score | ||
| Calsson et al29/2006 (Sweden) |
52 | 24/29 | 59 | NA | 3.0/3.0 | GOT (Astra Tech AB)/Spectrum (Smith & Nephew) | NA | HHS, VAS, radiograph (RSA) | ||
| Kim23/2011 (Korea) |
100 | 60/60 | 54.3/51.8 | 44/48 | 3.3/3.4 | Proxima (DePuy)/ Profile (DePuy) |
58/55 | 2/5 | 0 | HHS, BMD, radiograph, activity score |
| Kim24/2012 (Korea) |
140 | 70/70 | 74.9/76 | 27/24 | 4.1/4.8 | Proxima (DePuy)/ AML (DePuy) |
6/8 | 21/18 | 43/44 | HHS, WOMAC, UCLA activity score |
| Roth et al33/2014 (Germany) |
80 | 40/40 | 60.1/64.8 | 58/48 | 0.115/0.115 | Fitmore (Zimmer)/ CLS (Zimmer) |
NA | HHS, WOMAC, SF-36, radiograph | ||
| Salemyr et al19
/2015 (Sweden) |
51 | 26/25 | 62/62 | 59/43 | 2 | Proxima (DePuy)/ Bimetric (Biomet) |
26 | 25 | 0 | HHS, BMD, WOMAC, radiograph (RSA), EQ-5D, thigh pain |
| McCalden et al32/ 2015 (Canada) |
43 | 22/21 | 62.8/66.6 | 37/38 | 2 | SMF (Smith & Nephew)/ Synergy (Smith & Nephew) |
NA | 0 | HHS, WOMAC, SF-12, radiograph |
|
| Freitag et al22/2016 (Germany) |
138 | 57/81 | 56.8/59.1 | 63/63 | 1.0/1.0 | Fitmore (Zimmer)/ CLS (Zimmer) |
NA | 0 | HHS, BMD, WOMAC, | |
| Kim30/2016 (Korea) |
200 | 200/200 | 52.5/52.5 | 69/69 | 11.8 | Proxima (DePuy)/ Profile (DePuy) |
102/100 | 62/64 | 36/36 | HHS, BMD, WOMAC, UCLA activity score, radiograph |
| Koyano et al21/2017 (Japan) |
36 | 36/36 | 51.7 | 11/11 | 9.2 | Super Secur-Fit (Stryker)/CentPillar GBHA (Stryker) | 10/8 | 26/28 | 0 | JOA hip score, BMD |
| Schilcher et al31/ 2017 (Sweden) |
60 | 30/30 | 59.4/60.6 | 43/37 | 2.0/2.0 | Taperloc Microplasty (Biomet)/Taperloc (Biomet) | 21 | 39 | 0 | HHS, BMD, WOMAC, radiograph (RSA) |
| Oldenrijk et al36/ 2017 (Netherlands) |
150 | 75/75 | 60.3/60.5 | 28/29 | 2.0/2.0 | CFP (Link)/ Alloclassic Zweymuller (Zimmer-Biomet) |
NA | HOOS, HHS, PCS-12, Pain-NRS Score, TUG, Trendelenburg test, EQ5D | ||
AML, anatomical medullary locking fully porous coated cementless femoral component; BMD, bone mineral density; CFP, collum femoris preserving stem; CLS, cementless straight stem; CS, conventional stem prostheses; GOT, Gothenburg osseointegrated titanium hip; HA, hydroxyapatite; HHS, Harris Hip Score; HOOS, hip disability and osteoarthritis outcome score; JOA, Japanese Orthopaedic Association; MTPM, maximum total point motion; NA, not available; PCS-12: physical component scale of the SF12; RSA, radiostereometric analysis; SF-36: short form 36-item health survey; SMF, short metaphyseal fixation stem; SS, short-stem prostheses; TUG, timed up-and-go test; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Risk of bias of the included studies
All included studies were RCTs, and two were performed at multiple centres, one of which was a double-blind RCT. Eight trials reported random sequence generation clearly and were defined as having a low risk of bias, while the remaining trials did not report their sequence generation. Thus, the risk of selection bias was unclear in the latter trials.19 21 32 33 The blinding methods of two studies were considered to have a high risk of bias; in one trial,20 the clinical and radiographical outcomes were assessed by the researchers and surgeon, and in another trial,19 the participants were not blinded. Selective reporting, incomplete outcome data and other obvious biases were not observed, and these factors were judged as having a low risk of bias. Details of the bias assessment are shown in figure 2. Based on the high quality of the methodology used in registered trials published in the English-language literature, we hypothesised that the possible source of such bias might be affect size differences among the variously sized samples in our study.
Figure 2.
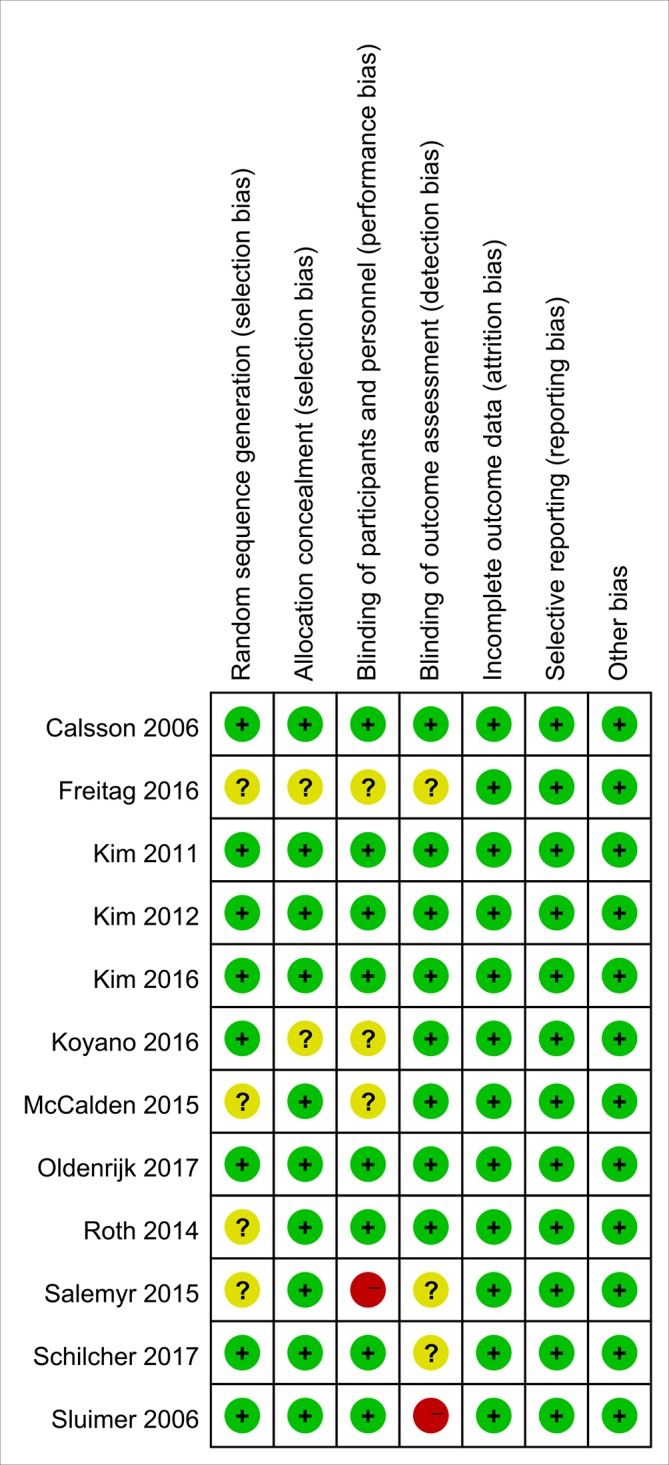
Risk of bias summary for each included study.
Outcomes of the meta-analyses
Primary outcomes
BMD
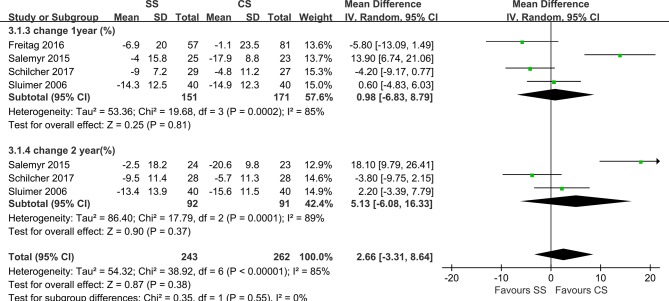
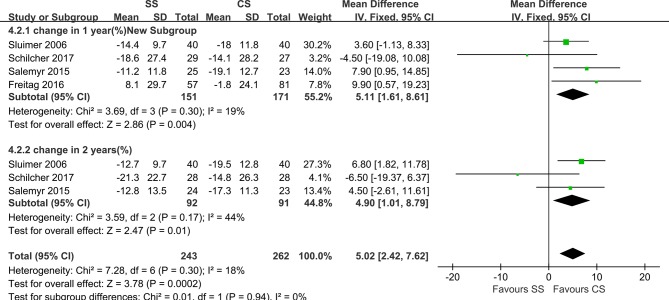
In total, seven studies reported on the BMD of different zones according to Gruen’s description, representing a total of 322 hips. The BMD was determined using DEXA in all of these studies. Five studies had a DEXA scan within the first week after surgery.19–22 31 Sluimer et al20 reported that DEXA scans were conducted at 4 to 7 days, 7 weeks and 3, 6, 12 and 24 months postoperatively, and all femoral prosthetic zones were measured. The results showed that the BMD around both femoral components decreased immediately after surgery in all the Gruen zones. After 2 years, this decrease was followed by an increase to 94% to 100% of the original BMD, except in Gruen zones 1 (G1) and 7. Freitag et al22 reported that the BMD data were collected at 1 week and 3 and 12 months after surgery for all the zones. The result showed that on evaluation at 1 year, the BMD was lower than at the 7-day postoperative assessment for both implants in all zones, aside from G3 in the short-stem group, which showed a small increase. In addition, there was a significant difference in BMD changes at 1 year between the groups in G1 and (G6. The periprosthetic decrease in the BMD was most pronounced in G7 for both implants. Salemyr et al reported that DEXA scans were performed at 1 and 2 days and 3, 6, 12 and 24 months postoperatively, with reported BMD values in G1 and G7. Additionally, they reported the BMD changes of Gruen zones 1–7 together as an entity and found 5% lower bone resorption in the ultra-short group relative to the conventional group. Kim et al24 reported that DEXA scans were performed at 1 week, 1 year, 10 years and at the final follow-up after surgery. BMD values were available in G1 and G7. Koyano et al21 only measured BMD values at the final follow-up, and the result showed that BMD values in G2, G3 and G6 on the anatomic-stem side were significantly lower than those on the straight-stem side. DEXA scans were performed within 3 days after surgery and 1 and 2 years postoperatively. Schilcher et al31 reported the BMD values of G1, G5 and G7, and their results showed no significant difference between the two groups at 1 year and 2 years after surgery. However, G5 was created by the authors within G1, which was different from the original description of the zones. Kim et al23 reported the BMD values of G1 and G7, and the first DEXA scan was performed 1 week after surgery, with further scans obtained at the final follow-up (3 years postoperatively). The results indicated that in the short-stem group, the BMD at 3 years after surgery was significantly increased in G1 but slightly decreased in G7, and the BMD at 3 years after surgery was significantly decreased in G1 and G7. In the profile group, data recorded as BMD changes between 1 week postoperatively and 1–2 years postoperatively were available in five studies. Heterogeneity analysis demonstrated statistical evidence for variation within the study (I²=82%). The BMD of both groups decreased in G1 and G7. Data pooled by random effects indicated no significant difference between SS and CS at both year 1 and year 2 postoperatively in zone 1 (MD=2.66; 95% CI, −3.31, 8.64; P<0.00001; figure 3). BMD changes in G7 differed significantly both at 1 year (MD=5.11; 95% CI, 1.61, 8.61; P=0.30) and at 2 years postoperatively (MD=4.90, 95% CI, 1.01, 8.79; P=0.17; figure 4) without any heterogeneity (I²=0%), indicating more proximal bone loss in G7 in the CS group.
Figure 3.
Forest plot of BMD changes in Gruen zone 1.
Figure 4.
Forest plot of BMD changes in Gruen zone 7.
Revision rate
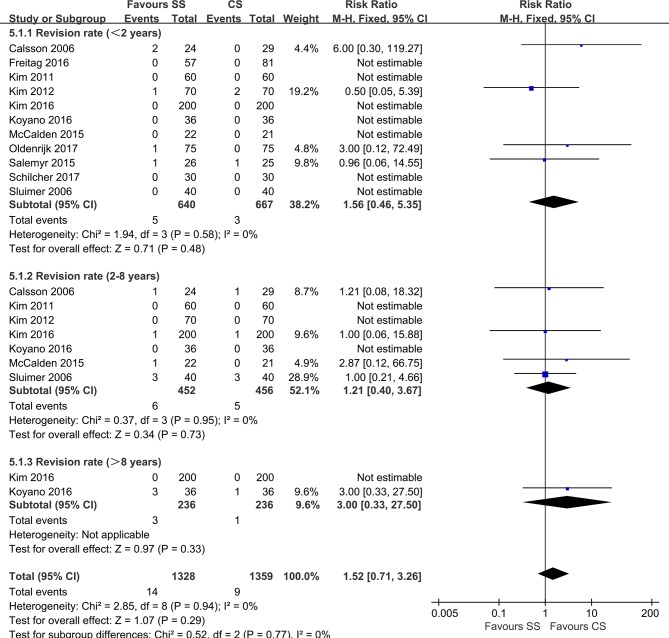
All included trials except one reported the revision rate as an outcome. Thus, 11 studies with 1307 hips involving 23 revisions were analysed (figure 5). The major reasons for revision were related to femoral stems with periprosthetic fractures, aseptic loosening and recurrent dislocations. We divided the revision rate in these studies into three groups (<2 years, 2–8 years and >8 years) according to the timepoint at which the revision occurred. Heterogeneity analysis demonstrated no statistical evidence for variation within the study (I²=0%). No significant differences were observed regarding the revision rate results in any subgroup (<2 years: RR=1.56; 95% CI, 0.46, 5.35; P=0.58, 2–8 years: RR=1.21; 95% CI, 0.40, 3.67; P=0.95) or in total (RR=1.52; 95% CI, 0.71, 3.26; P=0.94), indicating that, at an average follow-up of 3.9 years, revision for all causes was comparable between the two groups.
Figure 5.
Forest plot of revision rates.
Secondary outcomes
HHS
A total of nine studies reported the HHS, encompassing 1150 hips, although HHS reporting for patients in the SS groups was more common (mean difference=−0.38). The combined data demonstrated no significant differences between the two groups (95% CI, −1.02, 0.26; P=0.89), with low heterogeneity (I²=0%)) at the latest follow-up (figure 6).
Figure 6.
Forest plot of Harris Hip Scores.
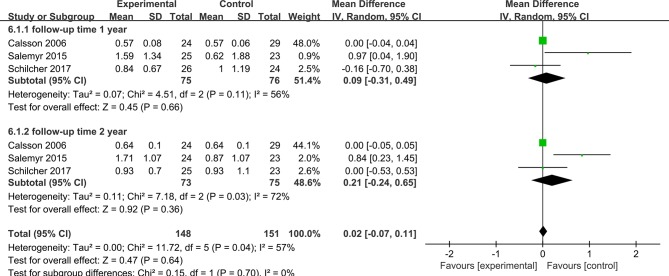
Maximum Total Point Motion
Three studies included 151 hips at the 1-year follow-up and 148 hips at the 2-year follow-up, and they reported the MTPM to evaluate femoral stem migration (figure 7). Pooled analyses were conducted using a random-effects model because of significant heterogeneity (I²=57%). No significant difference was found between the two groups at the different follow-up times (MD=0.09; 95% CI, −0.31, 0.49; P=0.11; and MD=0.21; 95% CI, −0.24, 0.56; P=0.56, respectively).
Figure 7.
Forest plot of maximum total point motion.
Radiolucent lines
A total of six studies21–23 29 30 32 indicated that radiolucent lines existed around the femoral prostheses. Sufficient data were not available to conduct a meta-analysis for this variable. Radiolucent lines were observed in only two trials involving 15 subjects in the SS group and none in the CS group without clinical symptoms. Calsson et al29 reported radiolucent lines in a thin zone adjacent to the proximal cranial fixation region of the femoral component in 14 patients. McCalden et al32 reported aseptic loosening in one patient 3 years postoperatively who underwent revision because of pain and radiographical features with complete radiolucent lines around the proximal porous coating.
Sensitivity analysis
Sensitivity analysis was performed by individually removing each study to identify whether the pooled results changed. All results were stable except the BMD change in G7. At 2 years postoperatively, the difference between groups lost significance after one study was removed20; additionally, although the removal of the data from another study31 at 1 year and 2 years postoperatively reduced the I² to 0%, it also resulted in a significant difference between the groups. Thus, based on the results of our analysis, SS is equally effective for preserving proximal bone stock.
Discussion
This meta-analysis of 12 RCTs including 1130 patients and comparing the efficacy and safety of SS and CS showed more proximal bone loss in G7 in the CS group but no significant difference in G1 within 2 years postsurgery. We found no significant differences in the revision rate, HHS, maximum total point motion and radiolucent line.
Recently, the performance of SS in primary THA has been investigated, and the results have been promising.21 24 31 Several meta-analyses have evaluated the effectiveness of SS in primary THA. Decking et al34 reported unsatisfactory SS survival rates. Huo et al25 reported that SS might achieve the same clinical and radiological outcomes as CS in addition to reducing thigh pain after surgery. However, limited trials were pooled for the analysis in the review due to incomplete search strategies. Subsequent studies were published recently. In this systematic review and meta-analysis, we sought to address whether, compared with CS, SS in primary THA might provide superior bone remodelling and lower revision rates with improved clinical outcomes. To the best of our knowledge, the present results derived from all the available comparative data are the first to document the differences in postoperative bone remodelling, implant survival, complications and radiological performance between SS and CS.
However, as no clear definition of short stem exists, there are different classification systems for short stems. Gulow et al35 divided short stems into three types according to the different anchoring principles, including (1) resurfacing endoprostheses anchoring on the epiphysis; (2) collum endoprostheses solely anchoring on the metaphysis; and (3) short collum-preserving stems anchoring on the metaphysis with short anchorage on the diaphysis. Oldenrijk et al36 classified short stems into three categories: (1) ‘collum’: conical or cylindrical ultra-short stems, with complete anchorage in the femoral neck; (2) ‘partial collum’: partial femoral neck-sparing curved designs; and (3) ‘trochanter-sparing’: trochanter-sparing but not neck-sparing, with a shortened tapered stem. The differentiation of short stems within a single classification system was clear. However, there was little difference between the short stem of the metaphyseal anchorage (partial neck preserving) group and shortened standard stems according to the classification principles mentioned above. According to the classification system of Khanuja et al,14 which categorised SS into four categories based on fixation principles and the location of proximal loading, metaphyseal anchorage short stems were those that retained the femoral neck by using partial collum short stems, with a curved or angulated distal end of the stem contacting the proximal lateral cortex and potentially improving biomechanical reconstruction. Shortened standard stems were similar to conventional, proximally porous-coated tapered designs with a shorter length or a reduced distal end of the stem, which were designed for proximal stress transfer. This type of stem is rarely neck-preserving and often extends to the upper diaphysis. With their tapered-wedge design and proximal porous coating, these stems achieve fixation proximally.37
In terms of BMD changes, significant bone loss of the proximal femur was found in both groups compared to before surgery, which was consistent with the results of previously reported studies.38–40 The pooled results of the meta-analysis of four studies with BMD changes revealed that SS and CS were comparable for G1 at both the 1- and 2-year follow-ups. However, a significant decrease was found for G7 at these two different time points. Some studies21 24 30 provided only comparisons between the endpoints and 1 week postoperatively and no comparison was performed in other studies. We therefore excluded these studies from the analysis. Although only two trials in the analysis found significantly different BMD changes between the groups for G7, significant differences were also found in the previous studies.23 24 Because bone remodelling after femoral implantation is most dynamic within the first postoperative year,41 which is also the period during which most periprosthetic BMD changes occur after femoral stem implantation,42 43 the short-term (>1 year) evaluation of BMD changes might objectively ascertain bone remodelling after THA.
Longer-term follow-up showed that BMD increased in G1 and that it decreased less in G7 at 1 year postoperatively in SS subjects when compared with the decreased BMD observed for both G1 and G7 in CS subjects,44 which might lead to further proximal femoral bone loss in the CS group with longer-term follow-up. These findings suggested that, compared with CS, SS may achieve superior biomechanical loading, more pronounced proximal load transfer to reduce stress shielding and may preserve more bone stock in primary THA to some extent. Because the BMDs of other zones were insufficient to evaluate bone remodelling of the distal femur, distal loading after implantation remains unclear, considering that migration and fracture might occur should loading become excessive,45 46 although some researchers34 observed decreased bone density in other zones. The BMD values in some of the included studies varied, which might have been due to different measurements and varied patient positioning when the assessments were performed. Additionally, Jahnke et al26 found that BMD changes were related to gender and age and that greater BMD changes occurred in men and older participants. However, we observed no pronounced differences in our analysis. Although the reduction in proximal BMD reduces the area of implant-bone support, potentially leading to late failure or periprosthetic fatigue fractures,47 whether mid-term bone remodelling necessarily reflects long-term consequences remains unknown.
According to Oldenrijk et al, most of the short stems included in our review were trochanter-sparing stems.19 20 23 24 30–32 One was classified as a collum stem, and another one as a partial collum stem. However, BMD values were available only for the trochanter-sparing stems included in our review. We tried to individually assess each short stem of the seven studies, which reported the BMD values of different zones. Three studies evaluated the BMD changes of the Proxima stem (DePuy), and the others reported on Fitmore (Zimmer), Omnifit-HA1090 (Osteonics), Super Secur-Fit (Stryker) and Taperloc Microplasty (Biomet). However, limited data and variations in follow-up did not allow for further assessments.
Some non-randomised studies have evaluated BMD changes with DEXA. Lerch et al48 examined BMD changes following Metha short -stem implantation, and the result showed that compared with baseline values measured immediately postoperatively, there was about a 10% periprosthetic bone loss in G7 after 1 year, which was consistent with the result of another study performed by Jahnke et al.26 However, Lerch et al reported that a regain of BMD in the G7 was found 2 years post-surgery. Chen et al49 performed a retrospective BMD analysis following implantation of the Mayo short stem and indicated that compared with the contralateral hip, the decrease (14.4%–17.9%) in the BMD was significant in G1, G6 and G7 and was much less apparent in other zones. Lazarinis et al50 carried out a prospective cohort study of collum femoris-preserving stems, and DEXA after 1 year showed a substantial loss of the BMD of between 13% and 31% in G7, G6 and G2. Nevertheless, some research has indicted contradicting results. A prospective study evaluated the periprosthetic BMD change of the Fitmore stem and Trabecular Metal Primary stem, and a lower periprosthetic bone loss was observed in all Gruen zones.51 Moreover, a retrospective study of within 10-year follow-ups found no significant difference in the periprosthetic BMD between short anatomical stems and metaphyseal anchored short stems. The bone mineral density significantly increased in G1 at each follow-up but slightly decreased in G7 in both groups. However, the results of these studies were insufficient to provide a power of comparability, as they were non-randomised or lacked a direct comparison between short and conventional stems.
Revision rates were comparable regardless of femoral stem length. Four studies reported no revisions observed during the research period and the remaining studies were pooled for the analysis with an average follow-up of 4.1 years. The revision rate was similar between the groups and was within the range of previous studies.1 52 Therefore, based on the available evidence, SS was as durable as CS according to short-term to mid-term follow-up in primary THA. The reasons for the revision found in the included studies encompassed recurrent dislocation, loosening, periprosthetic fracture, idiopathic osteonecrosis, painful iliac pseudotumour and deep periprosthetic infection. Subgroup analysis was performed based on revision for the reasons of periprosthetic fracture and loosening, and the results were consistent in the pooled analysis. Thus, we concluded that both SS and CS might achieve similar revision rates at the early stage after primary THA. The revision rate was high in one trial with two of the revisions due to aseptic loosening that occurred within 2 years, and a third undertaken for femoral neck resorption. The short-stem femoral prostheses of this study were metaphyseal loading, threaded, cylindrical designs with a collar, which led to a noted increase in strains on the lateral side of the greater trochanter and assessment of the risk of failure.53 Notably, this type of stem is no longer available. Towle et al54 observed a higher rate of implant revision in men after primary THA compared with that in women. However, limited data were available to evaluate the gender-specific risk of revision in our study.
Significant HHS improvement was found for both types of stems compared with the preoperative values. Increased HHS may be realised shortly after surgery and maintained at a high level. Additionally, strong evidence indicated no difference between SS and CS groups, which was consistent with a previous meta-analysis25 and cohort studies with long-term follow-ups.55 56 However, we were unable to evaluate other factors that might have had a potential influence, and whether longer-term clinical performance is maintained warrants concern. MTPM measured by radiostereometric analysis) may critically assess stem micromovement.55 56 Although the results of the analysis revealed no significant difference between the two stems at 1 year and 2 years of follow-up, a concern exists that more migration might arise in the CS group in the long term, which might predict implant loosening.
The most common complications related to surgery reported in the included studies were recurrent dislocation20 21 29 31; venous thromboembolism, including deep vein thrombosis and pulmonary embolism; and deep periprosthetic infection.19 31 Reduced thigh pain in SS was reported in four studies,19 23 24 30 although the aetiology of thigh pain remains unclear and whether the occurrence of thigh pain is related to periprosthetic loosening remains unknown. Rehabilitation programmes and bearing surfaces varied in the included studies. Ceramic-on-ceramic bearing surfaces of the prostheses were reported in most included studies; only one study reported a ceramic-on-polyethylene bearing,22 with no increased migration observed.57 Most studies reported full weight-bearing after surgery using two crutches. Others included full weight-bearing after 2 days, partial (50%) weight-bearing and full weight-bearing postoperatively for 6 weeks. Early research considered it a crucial factor for early fixation. However, a recent study indicated that early full weight-bearing and partial-bearing rehabilitation did not have a significant impact on clinical outcomes and complications in THA.58 A radiolucent line was reported in only two studies. McCalden et al32 reported one subject with a radiolucent line in the SS group and revision surgery was performed. However, 14 cases of a radiolucent line adjacent to the proximal cranial fixation region of the femoral component were reported in the SS group without detailed descriptions in another report. Calsson et al29 used a Type-2C stem design, which is no longer available. Despite potential improvement in proximal loading, stress shielding in the calcar and greater trochanter regions remains a challenge at short- to intermediate-term follow-up for most designs. Although stress shielding was observed in both groups in some studies, we were unable to determine whether SS might cause a higher rate of stress shielding.
Interestingly, substantial differences in femoral morphology were observed among patients in different studies using the Dorr classification,59 which was reported in limited studies. Of these studies, five trials19 21 23 31 32 excluded participants who had a type C femur. Schilcher et al31 found a significant effect of Dorr type on prostheses migration. More migration was observed in the CS group, particularly in type A femurs. However, two studies24 30 that included patients with type C femora achieved similar clinical and radiological results in that subtype compared with other femoral types by using Proxima SS. Thus, more trials are needed to determine whether SS might obtain similar outcomes in patients with different types of femora.
Strength and limitations
The present results derived from all available comparative data are the first to document the differences in postoperative bone remodelling, implant survival, complications and radiological performance between SS and CS. Our study has several limitations. First, sample size heterogeneities existed and the moderate methodological quality of some of the included studies might have biased the meta-analysis. Second, although the most recent evidence regarding the use of SS and CS in primary THA were provided in our study, the average follow-up length of 3.9 years (range, 0.115–11.8 years) was relatively short for evaluating the outcomes of femoral prostheses. Third, in addition to the different surgical indications, such as osteoarthritis, osteonecrosis and femoral fractures, that were included, basic parameters, such as patient age, gender and BMI, which may influence bone remodelling progression and stress shielding, varied in the studies, although comparable performance characteristics were observed among younger patients in the SS group and CS groups.24 Fourth, in addition to the materials, the stem designs were inconsistent across the studies. However, the effects of the lateral flare design and the extent and type of surface coating for bone conservation in primary THA have not been fully defined. Moreover, based on the limited data, we only evaluated bone remodelling of trochanter-sparing SS, while other categories were not assessed and require future study. Finally, although reasons for revision plans were evaluated in included studies, details of the failures were not known. We only evaluated the revision rates for any reason, without subgroup analysis of the different mechanisms, and we cannot make any statements regarding patient satisfaction. Despite the limitations, the present meta-analysis demonstrated that compared with CS, SS might achieve superior bone remodelling for proximal fixation and bone preservation, with similar revision rates and functional outcomes.
Conclusion
In conclusion, compared with CS, SS showed similar prosthesis survival rates, functional outcomes and migration in primary THA. However, SS may achieve superior bone remodelling and preserve more proximal bone stock in the short term. Additionally, SS may be applied to any type of femoral morphology. Considering these outcomes, the use of SS in primary THA may have several subtle clinical advantages over CS. However, because of the short-term follow-up and stem variation in our study, more high-quality RCTs are needed to further identify the optimal femoral prostheses for primary THA.
Supplementary Material
Acknowledgments
We thank American Journal Experts for linguistic assistance during the preparation of this manuscript.
Footnotes
H-DL, W-YY and J-KP contributed equally.
Contributors: H-DL and JL conceived and designed the study; J-KP, W-YY and H-TH performed the literature searches; H-DL, J-KP, L-FZ and M-HL analysed the data; and H-DL prepared the manuscript.
Funding: This study was supported by the TCM Standardization Projects of the State Administration of Traditional Chinese Medicine of China (No. SATCM-2015-BZ115, SATCM-2015-BZ173), the Science and Technology Planning Project of Guangdong Province, China (No. 2011B031700027), the Project of Guangdong Provincial Department of Finance (No. [2014] 157), Guangdong Provincial Medical Science and Technology Research (No. A2017215), the Administration of Traditional Chinese Medicine of Guangdong Province (No. 20164020), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YK2013B2N19, YN2015MS15).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We retrieved all data for the meta-analyses from published material. Therefore, the data are available in the respective articles.
References
- 1. Cavagnaro L, Formica M, Basso M, et al. Femoral revision with primary cementless stems: a systematic review of the literature. Musculoskelet Surg 2018;102 10.1007/s12306-017-0487-7 [DOI] [PubMed] [Google Scholar]
- 2. Kurtz SM, Lau E, Ong K, et al. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res 2009;467:2606–12. 10.1007/s11999-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajaee SS, Campbell JC, Mirocha J, et al. Increasing burden of total hip arthroplasty revisions in patients between 45 and 64 years of age. J Bone Joint Surg Am 2018;100:449–58. 10.2106/JBJS.17.00470 [DOI] [PubMed] [Google Scholar]
- 4. Capello WN, D’Antonio JA, Jaffe WL, et al. Hydroxyapatite-coated femoral components: 15-year minimum followup. Clin Orthop Relat Res 2006;453:75–80. 10.1097/01.blo.0000246534.44629.b2 [DOI] [PubMed] [Google Scholar]
- 5. Aldinger PR, Breusch SJ, Lukoschek M, et al. A ten- to 15-year follow-up of the cementless spotorno stem. J Bone Joint Surg Br 2003;85:209–14. 10.1302/0301-620X.85B2.13216 [DOI] [PubMed] [Google Scholar]
- 6. Hennessy DW, Callaghan JJ, Liu SS. Second-generation extensively porous-coated THA stems at minimum 10-year followup. Clin Orthop Relat Res 2009;467:2290–6. 10.1007/s11999-009-0831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 1987;69:45–55. 10.1302/0301-620X.69B1.3818732 [DOI] [PubMed] [Google Scholar]
- 8. Lavernia C, D’Apuzzo M, Hernandez V, et al. Thigh pain in primary total hip arthroplasty: the effects of elastic moduli. J Arthroplasty 2004;19(7 Suppl 2):10–16. [DOI] [PubMed] [Google Scholar]
- 9. Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 2007;38:651–4. 10.1016/j.injury.2007.02.048 [DOI] [PubMed] [Google Scholar]
- 10. Streit MR, Merle C, Clarius M, et al. Late peri-prosthetic femoral fracture as a major mode of failure in uncemented primary hip replacement. J Bone Joint Surg Br 2011;93:178–83. 10.1302/0301-620X.93B2.24329 [DOI] [PubMed] [Google Scholar]
- 11. Morrey BF. Short-stemmed uncemented femoral component for primary hip arthroplasty. Clin Orthop Relat Res 1989;249:169–75. 10.1097/00003086-198912000-00018 [DOI] [PubMed] [Google Scholar]
- 12. Leali A, Fetto J, Insler H, et al. The effect of a lateral flare feature on implant stability. Int Orthop 2002;26:166–9. 10.1007/s00264-002-0355-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dabirrahmani D, Hogg M, Kohan L, et al. Primary and long-term stability of a short-stem hip implant. Proc Inst Mech Eng H 2010;224:1109–19. 10.1243/09544119JEIM704 [DOI] [PubMed] [Google Scholar]
- 14. Khanuja HS, Banerjee S, Jain D, et al. Short bone-conserving stems in cementless hip arthroplasty. J Bone Joint Surg Am 2014;96:1742–52. 10.2106/JBJS.M.00780 [DOI] [PubMed] [Google Scholar]
- 15. Rometsch E, Bos PK, Koes BW. Survival of short hip stems with a "modern", trochanter-sparing design - a systematic literature review. Hip Int 2012;22:344–54. 10.5301/HIP.2012.9472 [DOI] [PubMed] [Google Scholar]
- 16. Patel RM, Lo WM, Cayo MA, et al. Stable, dependable fixation of short-stem femoral implants at 5 years. Orthopedics 2013;36:e301–7. 10.3928/01477447-20130222-18 [DOI] [PubMed] [Google Scholar]
- 17. Santori FS, Santori N. Mid-term results of a custom-made short proximal loading femoral component. J Bone Joint Surg Br 2010;92:1231–7. 10.1302/0301-620X.92B9.24605 [DOI] [PubMed] [Google Scholar]
- 18. Renkawitz T, Santori FS, Grifka J, et al. A new short uncemented, proximally fixed anatomic femoral implant with a prominent lateral flare: design rationals and study design of an international clinical trial. BMC Musculoskelet Disord 2008;9:147 10.1186/1471-2474-9-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salemyr M, Muren O, Ahl T, et al. Lower periprosthetic bone loss and good fixation of an ultra-short stem compared to a conventional stem in uncemented total hip arthroplasty. Acta Orthop 2015;86:659–66. 10.3109/17453674.2015.1067087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sluimer JC, Hoefnagels NH, Emans PJ, et al. Comparison of two hydroxyapatite-coated femoral stems: clinical, functional, and bone densitometry evaluation of patients randomized to a regular or modified hydroxyapatite-coated stem aimed at proximal fixation. J Arthroplasty 2006;21:344–52. 10.1016/j.arth.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 21. Koyano G, Jinno T, Koga D, et al. Comparison of bone remodeling between an anatomic short stem and a straight stem in 1-stage bilateral total hip arthroplasty. J Arthroplasty 2017;32:594–600. 10.1016/j.arth.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 22. Freitag T, Hein MA, Wernerus D, et al. Bone remodelling after femoral short stem implantation in total hip arthroplasty: 1-year results from a randomized DEXA study. Arch Orthop Trauma Surg 2016;136:125–30. 10.1007/s00402-015-2370-z [DOI] [PubMed] [Google Scholar]
- 23. Kim YH, Choi Y, Kim JS. Comparison of bone mineral density changes around short, metaphyseal-fitting, and conventional cementless anatomical femoral components. J Arthroplasty 2011;26:931–40. 10.1016/j.arth.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 24. Kim YH, Park JW, Kim JS. Ultrashort versus conventional anatomic cementless femoral stems in the same patients younger than 55 years. Clin Orthop Relat Res 2016;474:2008–17. 10.1007/s11999-016-4902-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huo SC, Wang F, Dong LJ, et al. Short-stem prostheses in primary total hip arthroplasty: a meta-analysis of randomized controlled trials. Medicine 2016;95:e5215 10.1097/MD.0000000000005215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jahnke A, Engl S, Altmeyer C, et al. Changes of periprosthetic bone density after a cementless short hip stem: a clinical and radiological analysis. Int Orthop 2014;38:2045–50. 10.1007/s00264-014-2370-6 [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carlsson LV, Albrektsson BE, Albrektsson BG, et al. Stepwise introduction of a bone-conserving osseointegrated hip arthroplasty using RSA and a randomized study: I. Preliminary investigations--52 patients followed for 3 years. Acta Orthop 2006;77:549–58. 10.1080/17453670610012601 [DOI] [PubMed] [Google Scholar]
- 30. Kim YH, Oh JH. A comparison of a conventional versus a short, anatomical metaphyseal-fitting cementless femoral stem in the treatment of patients with a fracture of the femoral neck. J Bone Joint Surg Br 2012;94:774–81. 10.1302/0301-620X.94B6.29152 [DOI] [PubMed] [Google Scholar]
- 31. Schilcher J, Ivarsson I, Perlbach R, et al. No Difference in periprosthetic bone loss and fixation between a standard-length stem and a shorter version in cementless total hip arthroplasty. A randomized controlled trial. J Arthroplasty 2017;32:1220–6. 10.1016/j.arth.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 32. McCalden RW, Korczak A, Somerville L, et al. A randomised trial comparing a short and a standard-length metaphyseal engaging cementless femoral stem using radiostereometric analysis. Bone Joint J 2015;97-B:595–602. 10.1302/0301-620X.97B5.34994 [DOI] [PubMed] [Google Scholar]
- 33. von Roth P, Perka C, Mayr HO, et al. Reproducibility of femoral offset following short stem and straight stem total hip arthroplasty. Orthopedics 2014;37:e678–84. 10.3928/01477447-20140626-61 [DOI] [PubMed] [Google Scholar]
- 34. Decking R, Rokahr C, Zurstegge M, et al. Maintenance of bone mineral density after implantation of a femoral neck hip prosthesis. BMC Musculoskelet Disord 2008;9:17 10.1186/1471-2474-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gulow J, Scholz R, Freiherr von Salis-Soglio G. [Short-stemmed endoprostheses in total hip arthroplasty]. Orthopade 2007;36:353–9. 10.1007/s00132-007-1071-x [DOI] [PubMed] [Google Scholar]
- 36. van Oldenrijk J, Molleman J, Klaver M, et al. Revision rate after short-stem total hip arthroplasty: a systematic review of 49 studies. Acta Orthop 2014;85:250–8. 10.3109/17453674.2014.908343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molli RG, Lombardi AV, Berend KR, et al. A short tapered stem reduces intraoperative complications in primary total hip arthroplasty. Clin Orthop Relat Res 2012;470:450–61. 10.1007/s11999-011-2068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inaba Y, Kobayashi N, Oba M, et al. Difference in Postoperative Periprosthetic Bone Mineral Density Changes Between 3 Major Designs of Uncemented Stems: A 3-Year Follow-Up Study. J Arthroplasty 2016;31:1836–41. 10.1016/j.arth.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 39. Albanese CV, Santori FS, Pavan L, et al. Periprosthetic DXA after total hip arthroplasty with short vs. ultra-short custom-made femoral stems: 37 patients followed for 3 years. Acta Orthop 2009;80:291–7. 10.3109/17453670903074467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Synder M, Krajewski K, Sibinski M, et al. Periprosthetic bone remodeling around short stem. Orthopedics 2015;38(3 Suppl):S40–5. 10.3928/01477447-20150215-55 [DOI] [PubMed] [Google Scholar]
- 41. Kim YH, Yoon SH, Kim JS. Changes in the bone mineral density in the acetabulum and proximal femur after cementless total hip replacement: alumina-on-alumina versus alumina-on-polyethylene articulation. J Bone Joint Surg Br 2007;89:174–9. 10.1302/0301-620X.89B2.18634 [DOI] [PubMed] [Google Scholar]
- 42. Venesmaa PK, Kröger HP, Miettinen HJ, et al. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res 2001;16:1056–61. 10.1359/jbmr.2001.16.6.1056 [DOI] [PubMed] [Google Scholar]
- 43. Brodner W, Bitzan P, Lomoschitz F, et al. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg Br 2004;86:20–6. [PubMed] [Google Scholar]
- 44. Kim YH, Oh SH, Kim JS. Primary total hip arthroplasty with a second-generation cementless total hip prosthesis in patients younger than fifty years of age. J Bone Joint Surg Am 2003;85-A:109–14. 10.2106/00004623-200301000-00017 [DOI] [PubMed] [Google Scholar]
- 45. Ender SA, Machner A, Pap G, et al. Cementless CUT femoral neck prosthesis: increased rate of aseptic loosening after 5 years. Acta Orthop 2007;78:616–21. 10.1080/17453670710014301 [DOI] [PubMed] [Google Scholar]
- 46. Joshi MG, Advani SG, Miller F, et al. Analysis of a femoral hip prosthesis designed to reduce stress shielding. J Biomech 2000;33:1655–62. 10.1016/S0021-9290(00)00110-X [DOI] [PubMed] [Google Scholar]
- 47. Engh CA, Glassman AH, Suthers KE. The case for porous-coated hip implants. The femoral side. Clin Orthop Relat Res 1990;261:63–81. [PubMed] [Google Scholar]
- 48. Lerch M, von der Haar-Tran A, Windhagen H, et al. Bone remodelling around the Metha short stem in total hip arthroplasty: a prospective dual-energy X-ray absorptiometry study. Int Orthop 2012;36:533–8. 10.1007/s00264-011-1361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen HH, Morrey BF, An KN, et al. Bone remodeling characteristics of a short-stemmed total hip replacement. J Arthroplasty 2009;24:945–50. 10.1016/j.arth.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 50. Lazarinis S, Mattsson P, Milbrink J, et al. A prospective cohort study on the short collum femoris-preserving (CFP) stem using RSA and DXA. Primary stability but no prevention of proximal bone loss in 27 patients followed for 2 years. Acta Orthop 2013;84:32–9. 10.3109/17453674.2013.765623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gasbarra E, Celi M, Perrone FL, et al. Osseointegration of Fitmore stem in total hip arthroplasty. J Clin Densitom 2014;17:307–13. 10.1016/j.jocd.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 52. Kendoff DO, Citak M, Egidy CC, et al. Eleven-year results of the anatomic coated CFP stem in primary total hip arthroplasty. J Arthroplasty 2013;28:1047–51. 10.1016/j.arth.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 53. Decking R, Puhl W, Simon U, et al. Changes in strain distribution of loaded proximal femora caused by different types of cementless femoral stems. Clin Biomech 2006;21:495–501. 10.1016/j.clinbiomech.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 54. Towle KM, Monnot AD. An assessment of gender-specific risk of implant revision after primary total hip arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2016;31:2941–8. 10.1016/j.arth.2016.07.047 [DOI] [PubMed] [Google Scholar]
- 55. Cinotti G, Della Rocca A, Sessa P, et al. Thigh pain, subsidence and survival using a short cementless femoral stem with pure metaphyseal fixation at minimum 9-year follow-up. Orthop Traumatol Surg Res 2013;99:30–6. 10.1016/j.otsr.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 56. Patel RM, Smith MC, Woodward CC, et al. Stable fixation of short-stem femoral implants in patients 70 years and older. Clin Orthop Relat Res 2012;470:442–9. 10.1007/s11999-011-2063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou ZK, Li MG, Börlin N, et al. No increased migration in cups with ceramic-on-ceramic bearing: an RSA study. Clin Orthop Relat Res 2006;448:39–45. 10.1097/01.blo.0000223999.10389.c9 [DOI] [PubMed] [Google Scholar]
- 58. Tian P, Li ZJ, Xu GJ, et al. Partial versus early full weight bearing after uncemented total hip arthroplasty: a meta-analysis. J Orthop Surg Res 2017;12:31 10.1186/s13018-017-0527-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dorr LD, Faugere MC, Mackel AM, et al. Structural and cellular assessment of bone quality of proximal femur. Bone 1993;14:231–42. 10.1016/8756-3282(93)90146-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-021649supp001.pdf (15.6KB, pdf)