In 1986, Gawin and Kleber1 proposed that cue-induced cocaine craving increases progressively during early abstinence and remains high during extended periods. However, subsequent inpatient clinical studies in the late 1980s have shown that baseline (nonprovoked) craving for cocaine decreases progressively during the first month of abstinence.2 Consequently, the hypothesis by Gawin and Kleber1 was not empirically tested in clinical studies on the time course of cue-induced cocaine craving during abstinence and was largely forgotten. More than a decade later, and largely independent of the early clinical literature, studies using animal models of relapse3 have shown that the rat’s drug-seeking response to cues associated with cocaine (Figure)4–7 and other abused drugs8 increases progressively after cessation of drug self-administration, a phenomenon termed incubation of drug craving.7
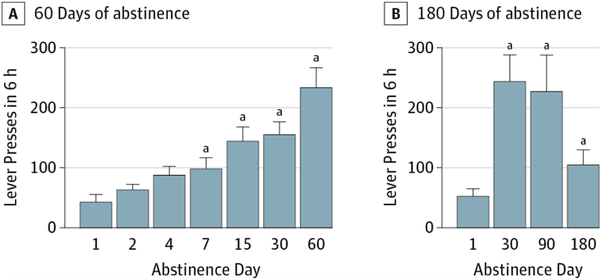
Figure. Incubation of Cocaine Craving in Rats.
A, Number (mean [SEM]) of nonreinforced lever presses during the extinction tests in the presence of cocaine-associated contexts and cues during 60 days of forced abstinence from intravenous self-administration of cocaine. B, Number (mean [SEM]) of nonreinforced lever presses during the extinction tests in the presence of cocaine-associated contexts and cues during 180 days of forced abstinence from intravenous self-administration of cocaine. During the extinction tests, the rats were reexposed to the cocaine-associated contexts and cues but lever presses did not lead to the delivery of cocaine infusions.
These observations led to renewed interest in the time course of cue-induced drug craving in humans, and subsequently to the demonstration of incubation of self-reported cue-induced cravings for nicotine,9 methamphetamine,10 and alcohol,11 but not for heroin.12 (The failure to detect incubation of cue-induced craving for heroin may be owing to the lack of assessment of craving during the first week of abstinence.) In this issue of JAMA Psychiatry, Parvaz et al13 extended these studies by describing the time course of incubation of cue-induced craving for cocaine during the first year of abstinence using an objective measure of drug cue reactivity: the late positive potential (LPP) component of electroencephalography (EEG).14
Parvaz et al13 recruited 76 long-term cocaine users with different durations of abstinence (2 days, 1 week, 1 month, 6 months, and 1 year) and first measured their baseline self-reported craving using the Cocaine Craving Questionnaire. Next, the authors assessed cue-induced craving for cocaine by both EEG recording and subjective self-reports of cocaine “wanting” (craving for cocaine) and “liking” (hedonic feelings toward cocaine). On the study day, the authors recorded the participants’ EEG while they were passively viewing 4 types of pictures (30 pictures per category, each viewed for 2 seconds): pleasant, unpleasant, neutral, and cocaine-associated pictures. Immediately after the EEG recording, the participants rated on a Likert scale their level of cocaine wanting and liking for the different pictures.
The main finding of the study was that the LPP amplitude followed an inverted U-shaped curve with higher amplitude after 1 month or 6 months of abstinence than after 2 days or 1 year of abstinence.13 In agreement with previous studies on the incubation of craving9–11 and the early study by Weddington et al,2 the authors found that self-reports of baseline drug craving decreased during abstinence.13 However, unlike the data from previous studies on the incubation of drug craving,9–11 Parvaz et al13 found that self-reports of cue-induced cocaine wanting and liking also decreased during abstinence.
This human translational study provides empirical support to the 30-year-old hypothesis by Gawin and Kleber1 on the time course of cue-induced craving for cocaine, introduces the late LPP component of EEG as a measure of the time course of drug cue reactivity and craving in humans, and supports the translational utility of the rodent model of incubation of drug craving.7,15 The results of this study, however, also raise some questions for future research.
The first question is how to interpret the different time courses of cue-induced changes in the LPP component of EEG and the self-reports of cue-induced cocaine wanting and liking in light of the previous human studies showing incubation of cue-induced self-reported craving for nicotine, alcohol, and methamphetamine, as well as the persistent and stable time-independent cue-induced craving for heroin during abstinence. Parvaz et al13 argued that the LPP component provides an objective measure of cue reactivity and, by extension, cue-induced craving, because unlike subjective self-reports, the LPP component is not prone to biases owing to the influence of demand characteristics, social desirability, and limited self-awareness. They also argued that it is important to use objective measures of drug craving and cue reactivity in human translational studies, because the studies in rodents use an objective measure—operant lever presses—as the operational measure of the drug craving and relapse.3 These are valid arguments, but they do not provide an explanation for the differences in the time course of cue-induced self-reports of drug craving in the study by Parvaz et al13 vs the previous studies.
In accounting for these time course differences, 2 factors should be considered. The first is that in the previous studies, the participants were randomly assigned to different durations of abstinence, which was validated over time using objective drug-screening measures. In contrast, in the study by Parvaz et al,13 the participants were not randomly assigned to different durations of abstinence and instead were assigned to the different groups based on historical self-reports of duration of abstinence. The second factor is that the procedures used in previous studies to elicit cue-induced craving, as well as the measures of craving, were different from those in the study by Parvaz et al.13 Thus, the authors assessed cue-induced self-reports of craving by asking the participants to rate their wanting and liking based on cocaine-associated pictures. In contrast, in the previous studies, in which self-reports of cue-induced drug craving incubated over time, the cue manipulations were more salient and included not only pictures of drug-associated cues but also actual physical cues such as a lit cigarette9 or other drug paraphernalia.10,11
The degree to which these differences in the experimental procedures can account for the different pattern of results for cue-induced self-reports of drug craving between the study by Parvaz et al13 and previous studies is a subject for future research. In this regard, an important question for future studies that Parvaz et al13 are qualified to answer is whether the time course of cue-induced incubation of drug craving, as assessed by the LPP component of EEG, is similar or different across different drugs of abuse. Based on the preclinical literature,8 we suspect that the time courses will not be the same.
In conclusion, in a clinical translational study, Parvaz et al13 have introduced the LPP component of EEG as an operational measure of the time course of incubation of drug craving during abstinence. They showed that, as in the rat model,6 the response to the cocaine-associated cues increases progressively during abstinence. Their data have important implications for the duration of treatments for relapse prevention and for the time course of vulnerability to cocaine relapse. Finally, their translational data illustrate the importance of using animal models to identify important addiction-related phenomena relevant to humans and the importance of continued discussion and validation efforts between basic and clinical researchers.
Acknowledgments
Conflict of Interest Disclosures: The authors reported having received research funding from the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health. No other disclosures were reported.
References
- 1.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. [DOI] [PubMed] [Google Scholar]
- 2.Weddington WW, Brown BS, Haertzen CA, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts: a controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861–868. [DOI] [PubMed] [Google Scholar]
- 3.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming–induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. [DOI] [PubMed] [Google Scholar]
- 4.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 2000;20(2):798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl). 2004;176(1):101–108. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(suppl 1):214–226. [DOI] [PubMed] [Google Scholar]
- 8.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci 2011;34(8):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedi G, Preston KL, Epstein DH, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69(7):708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Wu P, Xin X, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol 2015;20(3):513–522. [DOI] [PubMed] [Google Scholar]
- 12.Wang GB, Zhang XL, Zhao LY, et al. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl). 2012;221(4):701–708. [DOI] [PubMed] [Google Scholar]
- 13.Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography [published online September 7, 2016]. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken IH, Dietvorst RC, Hesselmans M, Franzek EJ, van de Wetering BJ, Van Strien JW. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict Biol 2008;13(3–4):386–392. [DOI] [PubMed] [Google Scholar]
- 15.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 2016;17(6):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]