Abstract
Background
The prevalence of risk factors for ischemic stroke may vary between different groups of stroke patients. We examined the distribution of individual well-established risk factors as well as the multiplicity of risk factors in different age groups and among subtypes.
Methods
In Lund Stroke Register, we consecutively enrolled 2505 patients with first-ever ischemic stroke from 2001 to 2009 and registered hypertension, diabetes mellitus, heart disease, current smoking, hypercholesterolemia as well as subtype.
Results
Among young patients (<55 years), at least 50% had ≥2 risk factors and 20–25% had ≥3 risk factors. In patients aged 55 years or older, the proportion with ≥2 risk factors was 70–80% and 35–45% with ≥3 risk factors. Men and women had similar burden of risk factors. Approximately 50% of the cases classified as cardioembolism (CE) and large artery atherosclerosis (LAA) had ≥3 risk factors, which was significantly more than the other TOAST subtypes (CE p<0.001, LAA p=0.001).
Conclusions
The prevalence of well-established risk factors is similar among young and old stroke patients with large proportions (50–80%) having ≥2 risk factors. Even though the prevalence of well-established risk factors differ between pathogenetic subtypes, these risk factors as well as the multiplicity of risk factors seem to be of clinical importance in all major subtypes of ischemic stroke.
Keywords: Ischemic stroke, Pathogenetic stroke subtypes, Risk factors, Hypertension, Diabetes, Mellitus, Heart disease, Smoking, Hypercholesterolemia
Introduction
Well-established vascular risk factors for stroke, including hypertension, diabetes mellitus, heart disease, current smoking and hypercholesterolemia, are common in the general population. Primary and secondary prevention of cerebrovascular disease, especially regarding hypertension and hypercholesterolemia, is believed to be related to the decline in stroke incidence over the last decades [1], although it has not been noted by others [2]. Hypertension is however still regarded as the most important risk factor for stroke [3–5].
Previous studies have described the total number of well-established vascular risk factors for ischemic stroke in young patients [6, 7] and between the ages 40 to 80 years [8], but less is known about the multiplicity of risk factors in cohorts of patients covering the full spectrum of age groups.
Individual stroke risk factors and their relation to the clinical subtypes of cerebral infarcts, e.g. OCSP (the Oxfordshire Community Stroke Project), a clinical classification system for acute ischemic stroke), have been reported previously [2, 3, 9]. However, there are less published data available concerning the multiplicity of risk factors for different pathogenetic subtypes of ischemic stroke, as defined e.g. according to the TOAST classification system [10–13].
In the present study, we therefore examined the distribution of individual well-established risk factors, as well as the multiplicity of risk factors, in first-ever ischemic stroke patients across the full spectrum of age groups and different pathogenetic subtypes.
Materials and Methods
Patients living within the primary catchment area of Skåne University Hospital Lund (total population of approximately 235 000 inhabitants) [14, 15] with a first-ever ischemic stroke were consecutively included in Lund Stroke Register (LSR) between March 2001 and June 2009. There is no other hospital for acute stroke patients in this catchment area. Patients from other areas treated at our hospital were excluded from this study.
The patients were divided into the following age groups: under 45, 45–54, 55–64, 65–74, 75–84 and 85 years or older.
Lund Stroke Register uses the definition of stroke suggested by the World Health Organization, which is “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death, with no apparent cause other than of vascular origin”. As of 2008, the definition was broadened to include patients with diffusion abnormalities on MRI, representing new brain ischemia, and with a location correlating to the clinical symptoms, irrespective of symptom duration. Patients who received rt-PA with no lasting symptoms after 24 hours were considered as having had a transient ischemic attack.
Risk factors
The five well-established vascular risk factors examined in this study were defined as follows: 1) Hypertension – previously diagnosed, including blood pressure examinations from the last year; newly diagnosed (≥160/90 mmHg) at time of discharge or at least one week of hospital care, or current treatment during the last two weeks before stroke onset; 2) Diabetes mellitus – previously diagnosed (dietary, oral pharmacological or insulin treatment); newly diagnosed at time of discharge or one week of care ≥6.1 mmol/L (capillary or whole blood) on two occasions or ≥7.0 mmol/L (plasma) on two occasions, >11 mmol/L (once + symptom) [16]; 3) Heart disease – angina pectoris, heart failure, myocardial infarction, medically treated heart disease, heart surgery (valve or bypass), atrial fibrillation or atrial flutter [17], as well as pacemaker (which was also regarded as an indicator of heart disease); 4) Hypercholesterolemia – previously diagnosed (within the last year); recently discovered (total cholesterol >5.0 mmol/L or LDL >3 mmol/L) or dietary or oral pharmacological treatment in the last two weeks before stroke onset; 5) Current smoking – current smoker, ≥1 cigarette/day during the last three months, i.e. ≥90 cigarettes the last three months. Those not classified as current smokers were either previous smokers or had never smoked at all.
For 312 of the 2505 patients, data was unavailable regarding one of the five risk factors. These patients were considered as not having that specific risk factor (Table 1). All other patients had available data regarding all risk factors.
Table 1.
Median age and risk factor prevalence in men and women
| Total (n=2505) | Men (n=1321) | Women (n=1184) | P-value* | Unavailable data (n)** | |
|---|---|---|---|---|---|
| Median age (years) | 76 | 72 | 80 | <0.001 | |
| Hypertension | 67.2% | 64.7% | 70.0% | 0.005 | 37 |
| Diabetes mellitus | 24.1% | 25.7% | 22.3% | 0.04 | 89 |
| Heart disease | 44.1% | 43.2% | 45.1% | n/s | 61 |
| Hypercholesterolemia | 66.4% | 64.8% | 68.2% | n/s | 92 |
| Current smoking | 18.8% | 21.6% | 15.6% | <0.001 | 33 |
Difference in prevalence between the sexes.
Data regarding prevalence of individual risk factors were unavailable for 312 patients. n/s (not significant).
Computed tomography (CT) was performed in 100% of the patients and approximately 96% underwent electrocardiography examination. A majority of the patients, ≈55%, were examined by Doppler ultrasonography of the carotid arteries, while 20–30% were examined by CT angiography and 20–30 % by ultrasound of the heart including the aortic arch. Fasting blood glucose levels was measured on at least two occasions during the hospital stay. Telemetry was made only in a minority (approximately 5%) of patients.
Pathogenetic subtype classification
The patients were classified in five major causative subtypes, as briefly described below: 1) Cardio-aortic embolism (CE), arterial occlusions presumably due to an embolus arising in the heart; 2) Supra-aortic large artery atherosclerosis (LAA), clinical and brain imaging findings of either significant stenosis or occlusion of clinically relevant extracranial or intracranial arteries judged to be due to atherosclerosis; 3) Small artery occlusion (SAO), one of the traditional clinical lacunar syndromes presumably caused by a single infarction within a territory supplied by a single penetrating end-artery and without evidence of cerebral cortical dysfunction; 4) Other causes (OC), rare causes of stroke, such as nonatherosclerotic vasculopathies, hypercoagulable states, or hematologic disorders; 5) Stroke of undetermined cause (UND). This category was further divided into subcategories: a. cause could not be determined because of incomplete evaluation (subcategory UND-IE), b. cause could not be determined despite relevant examination (subcategory UND-OTHER), and c. patients with two or more potential causes of stroke (subcategory UNCL).
Approximately 70% of the pathogenetic subtype classifications were made using the Causative Classification of Stroke System [12], approximately 20% of the cases were classified according to the Stop Stroke Study TOAST [11], 6% according to the Causative Classification System of Ischemic Stroke [13], and the original TOAST classification system [10] was used in <5%.
Ethics
This is a hospital based study. Informed consent was provided by the patients, or when necessary, their next of kin. Ethical permission for this project was obtained from the Regional Ethics Committee in Lund.
Statistics
The Mann-Whitney U-test was used for the analysis of the number of risk factors when comparing the sum of risk factors in the different age groups, between the sexes as well as between the pathogenetic subtypes. To examine the difference in prevalence of individual risk factors between men and women, we used the Pearson chi-square test and linear-by-linear association for examining trends in individual risk factor prevalence in different ages. A logistic regression analysis was made to examine the distribution of TOAST subtypes among men and women and the total number of risk factors for the pathogenetic subtypes in different ages. The linear-by-linear association and logistic regression analyses involving age, was performed using continuous age (years). The age distribution results are however presented in age groups. Confidence intervals for calculated proportions of risk factors were estimated according to Gardner and Altman [18]. Two-tailed probability values of <0.05 were considered to be statistically significant. Where appropriate, this was adjusted for multiple comparisons according to Bonferroni. The processing of data, including statistical calculations and preparing of tables, was made using the SPSS software version 20.
Results
Our sample consisted initially of 2763 patients. Patients were excluded in cases where no pathogenetic subtype classification had been performed (n=196), when no exact date for stroke onset could be determined and therefore missing data regarding age at the time of stroke onset (n=9), and also in cases lacking information regarding two or more of the risk factors (n=53). Thus, the final number of patients for this study was 2505.
Baseline characteristics of the 2505 patients are presented in Table 1. The mean age was 74 years. The age distribution ranged from 17 to 101 years in males and 18 to 102 in female patients.
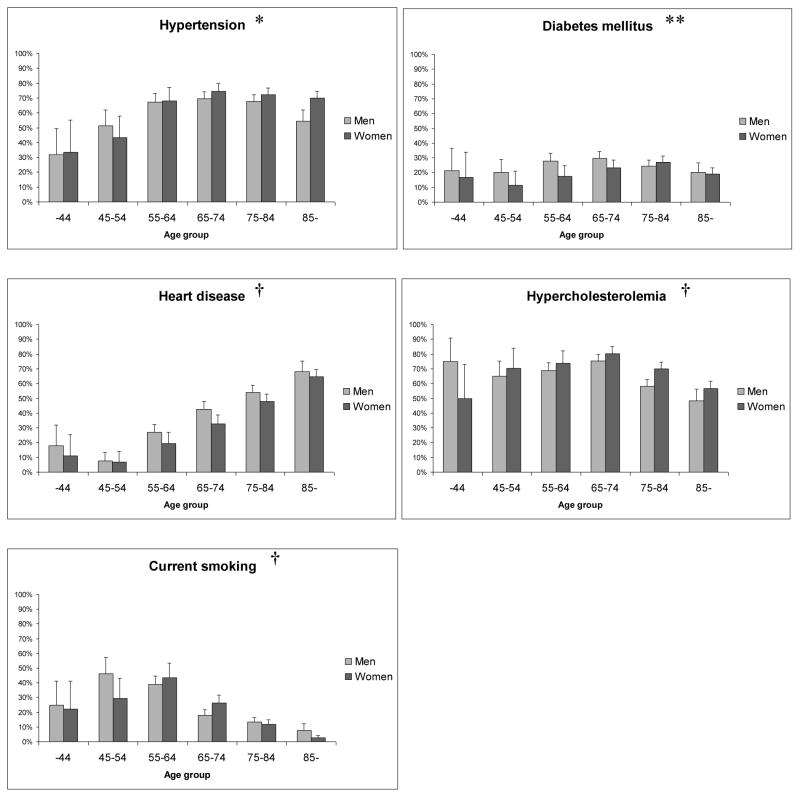
The prevalence of individual risk factors in different age groups is shown in Figure 1. It can be noted that heart disease was more common among the older patients (p<0.001). The highest proportion of current smokers was seen between the ages 45–64.
Figure 1. Prevalence of individual risk factors in different age groups.
Error bars indicate upper limits of 95% confidence intervals. * p=0.006 indicates a significant trend in risk factor prevalence in relation to age among women (n/s for men), ** n/s for both sexes, † p<0.001 for both sexes (linear-by-linear association). n/s (not significant).
Hypertension was more common among women than men (p=0.005), while there was a male dominance for current smoking (p<0.001) and diabetes mellitus (p=0.04). The prevalence of the other risk factors did not differ significantly between the sexes (Table 1).
Multiplicity of risk factors
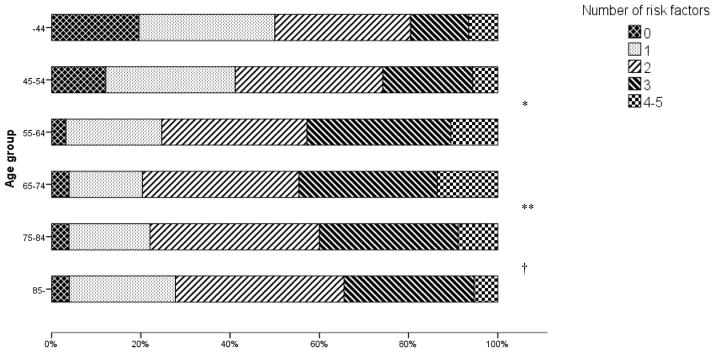
Among the younger patient groups (<55 years), at least 50% had ≥2 risk factors and 20–25% had ≥3 risk factors. In patient groups aged 55 years or older, the proportion with ≥2 risk factors was 70–80% and 35–45% with ≥3 risk factors (Table 2, Figure 2).
Table 2.
Number of risk factors within each age group
| Total number of risk factors
|
|||||||
|---|---|---|---|---|---|---|---|
| Age (years) | 0 | 1 | 2 | 3 | 4–5 | Total | Mean number of risk factors |
| 15–44, n (%) | 9 (19.6) | 14 (30.4) | 14 (30.4) | 6 (13.0) | 3 (6.5) | 46 (100) | 1.6 |
| 45–54, n (%) | 15 (12.1) | 36 (29.0) | 41 (33.1) | 25 (20.2) | 7 (5.6) | 124 (100) | 1.8* |
| 55–64, n (%) | 12 (3.1) | 82 (21.5) | 124 (32.5) | 123 (32.3) | 40 (10.5) | 381 (100) | 2.3 |
| 65–74, n (%) | 24 (3.9) | 102 (16.5) | 217 (35.1) | 192 (31.0) | 84 (13.6) | 619 (100) | 2.4** |
| 75–84, n (%) | 32 (3.9) | 151 (18.2) | 315 (38.0) | 258 (31.1) | 74 (8.9) | 830 (100) | 2.2† |
| 85-, n (%) | 20 (4.0) | 120 (23.8) | 191 (37.8) | 147 (29.1) | 27 (5.3) | 505 (100) | 2.1 |
| Total, n (%) | 112 (4.5) | 505 (20.2) | 902 (36.0) | 751 (30.0) | 235 (9.4) | 2505 (100) | 2.2 |
The median number of risk factors was 2.0 for all age groups, except for the youngest patients, 15–44 years, where the median number was 1.5.
p<0.001 difference in number of risk factors between age groups 45–54 and 55–64,
p=0.04 between age groups 65–74 and 75–84,
p=0.006 between age groups 75–84 and over 85 years. There was no significant difference in risk factor burden between the sexes in any age group.
Figure 2. Proportions of number of risk factors in different age groups.
* p<0.001 difference in number of risk factors between age groups 45–54 and 55–64, ** p=0.04 between age groups 65–74 and 75–84, † p=0.006 between age groups 75–84 and over 85 years.
The total number of risk factors increased significantly (p<0.001) between the ages 45–54 and 55–64 and peaked in the 65–74 age group (mean number of risk factors 2.4) (Table 2, Figure 2). However, the risk factor burden was lower among the older patients, with fewer risk factors among patients aged 85 years and older compared to the age group 75–84 years (p=0.006) as well as between the age groups 75–84 and 65–74 (p=0.04). There was no significant difference in the total risk factor burden between men and women in any age group (Table S 1).
Pathogenetic stroke subtypes
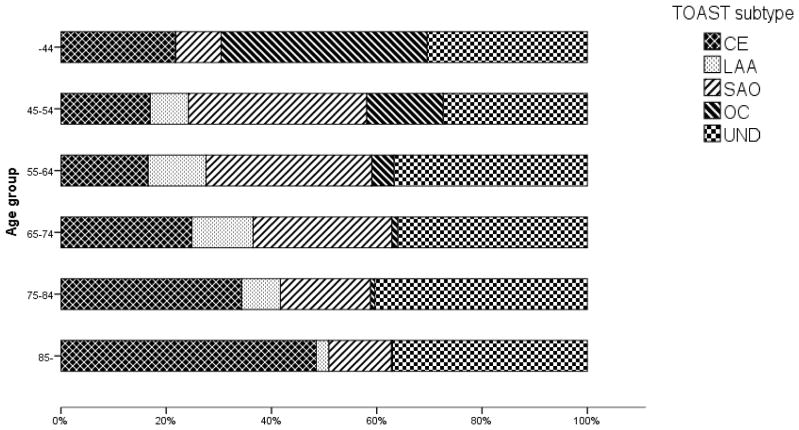
A decrease in proportion with increasing age was seen for SAO (p<0.001) and OC (p<0.001), while strokes in older age groups were more frequently classified as CE (p<0.001) and UND (p=0.03) (Figure 3). LAA was uncommon both among the youngest and the very oldest patients (p=0.001). A large percentage of all age groups were classified as UND, ranging from 27% in the 45–54 age group, to 40% among the patients aged 75–84 years. Of these undetermined cases, the proportion classified as UND-IE was 13–15% among patients under 55 years and 34–35% in patients 75 years or older. The proportion of patients in the UND subtype despite relevant examinations (UND-OTHER), ranged from 11–15% in patients under 55 years to 1–4% in patients 75 years or older.
Figure 3. Proportions of pathogenetic stroke subtypes in different age groups.
CE (cardioembolism), LAA (large artery atherosclerosis, SAO (small artery occlusion), OC (other causes), UND (stroke of undetermined cause).
There was a high prevalence of all the well-established individual risk factors, except for heart disease in the LAA subtype. In the CE subtype, the lowest number of current smokers was observed (11%, average 19%). Hypertension, hypercholesterolemia and current smoking were common in the SAO subtype. As expected, cases classified as OC had a low prevalence of the individual risk factors, with the exception of current smoking (22%). Among the UNCL cases, there was a high prevalence of hypertension, diabetes and heart disease, while the prevalence of risk factors in the UND-IE category was near the general average in all risk factors apart from heart disease (26%).
The risk factor burden was heavier among the CE (total mean 2.5) and LAA (total mean 2.5) subtypes compared to the other pathogenetic subtypes (CE p<0.001, LAA p=0.001) (Figure S 1). Approximately 50% of the cases classified as CE and LAA, and 44% of the CE cases aged over 85 years (mean 2.3 risk factors), had ≥3 risk factors. The overall mean number of risk factors in the over 85 year age group was 2.1. The higher number of risk factors among the CE and LAA subtypes remained when heart disease was excluded from the risk factor burden (p<0.001).
When sex distribution for the different pathogenetic subtypes was weighted against age in a logistic regression analysis, no significant difference could be seen between the sexes for CE, SAO and UND. However, the male dominance in the LAA subtype remained after the logistic regression analysis (p=0.02), while there was a tendency towards female dominance for OC (p=0.04).
Discussion
Our study showed that in all age groups, a large proportion of stroke patients, ranging from 50 to 80%, had ≥2 well-established vascular risk factors. This suggests that the influence of these risk factors on developing an ischemic stroke may often be similar in young patients compared to older patients. This is in line with previous studies where it has been reported that well-established risk factors are not uncommon among young stroke patients [6, 7]. In the Sifap study [7] for example, 56% of the patients younger than 56 years were smoking and 47% had hypertension. Less than one fifth of the youngest patients, i.e. those under 45 years, did not have any of the examined risk factors. This indicates that these risk factors are important in all ages. Our method using assessment of the multiplicity of risk factors is supported by the observation that several simultaneous comorbidities are frequent in the population, indicating a need for a comprehensive health care [19].
Somewhat surprisingly, the very oldest stroke patients (aged >85 years) had fewer risk factors than the average of all patients (Table 2) even though old age can be regarded as a non-modifiable risk factor per se. One explanation may be that it is less likely to reach the age of 85 and older if many of these risk factors are present. Our observation that current smoking was less frequent among the elderly may be explained by that some of the patients had quit smoking or died of tobacco-related causes.
It is of interest to compare our findings with a background population. In Lund Stroke Register, we also examined 970 control subjects without stroke (57% men, median age 76 years). For all the age groups of this study, the number of risk factors was significantly lower (p<0.001 Mann-Whitney U-test) among these control subjects when compared with the included patients.
The pathogenetic stroke subtypes varied considerably regarding the total risk factor burden as well as regarding the prevalence of the different specific well-established risk factors. The highest sums of risk factors were seen in CE and LAA, whereas the SAO subtype had a higher prevalence of hypertension (74% vs 65%), hypercholesterolemia (70% vs 66%) and current smoking (26% vs 17%) compared with the average of the other subtypes combined. Even though an insufficient diagnostic work-up in some patients may have an effect on the results, this could suggest that the examined risk factors vary in influence on different subtypes of ischemic stroke [20]. Other risk factors not examined in our study may also be of importance.
Within the UND subtype, the mean number of risk factors was 2.0 for the UND-IE subcategory, 2.4 for UNCL and 2.0 for the other undetermined strokes, UND-OTHER.
When excluding strokes classified as OC, the mean number of risk factors increased from 1.6 to 1.7 among patients under 45 years and from 1.8 to 1.9 in the 45–54 age group. It must be mentioned though, that when removing OC, there were only 28 patients left in the youngest age group.
There are some limitations in our study. Even though it was most common among the older patients, a large percentage of all age groups were diagnosed as UND (Figure 3). The reason why the oldest patients were more frequent in the UND subtype, may partly be because the diagnostic work-up in these cases is generally not as comprehensive as for the younger patients. Also, older patients are more likely to have severe strokes that may inhibit further diagnostic evaluation [21, 22]. The proportion of UND-OTHER was higher in the younger age groups. The greater percentage of UND in our study compared to previous reports [3, 9, 23] might also be explained by that the TOAST classifications were using results from the routine diagnostic methods for evaluating stroke.
Other suggested modifiable risk factors, such as obesity and physical inactivity, were not included in the analysis. Patients who received treatment for hypercholesterolemia and therefore had cholesterol levels within the recommended limits, were still considered as having this risk factor, which might be disputed [24]. The current definition of hypertension is >140 mmHg systolic blood pressure (SBP), but when the study started in 2001, the mostly used threshold was >160 mmHg SBP. Since we were not able to apply the current definition on patients that had already been included, we used the older threshold for this study. Lund Stroke Register has gathered data consecutively from patients with first-ever strokes since 2001. The chosen methods of classifying ischemic stroke into pathogenetic subtypes have changed over the years and it cannot be ruled out that the different methods may have affected our results. In our material, the collection of data did not allow for a distinction made between atrial fibrillation and other types of heart disease. However, approximately 65–70% of the cases classified as CE were diagnosed as having AF. On the other hand, even though we only included patients who were treated at Skåne University Hospital in Lund, where approximately 8% of all stroke patients are estimated to never arrive at hospital [25], this hospital-based study consists of a large material. The method for including patients has been consistent through the entire study period and the uptake area has remained unaltered and homogeneous.
When describing stroke in terms of pathogenetic subtypes, our results indicate that it is important to be aware of the difference in age distribution, since some subtypes are dominated by older patients and others by younger patients (Figure 3). Designing a treatment study, for example, with a limited age range might lead to a bias in what subtypes will be included in the study. But if the study is designed to focus on a specific subtype, age selection can be used to maximize the number of included patients.
In conclusion, concurrent multiple well-established risk factors are highly prevalent in all age groups of ischemic stroke patients and the pathogenetic subtypes with the heaviest risk factor burden are CE and LAA. A shift in focus from single risk factors to the total burden of risk appears warranted in initial assessment and long term follow-up after stroke.
Supplementary Material
Acknowledgments
We are grateful for the cooperation of the SiGN consortium in letting us use CCS classification data for 160 LSR patients participating in the SiGN project [26]. We thank Björn Hansen for technical input.
Sources of funding
This study was supported by grants from the Swedish Research Council (K2010-61X-20378-04-3), Region Skåne, the Freemasons Lodge of Instruction EOS in Lund, King Gustaf V’s and Queen Victoria’s Foundation, US federal funds from the NINDS U01NS069208, Lund University, and the Swedish Stroke Association.
Footnotes
Disclosures
None.
References
- 1.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 2.Béjot Y, Osseby GV, Gremeaux V, Durier J, Rouaud O, Moreau T, et al. Changes in risk factors and preventive treatments by stroke subtypes over 20 years: A population-based study. J Neurol Sci. 2009;287:84–8. doi: 10.1016/j.jns.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 5.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putaala J, Haapaniemi E, Kaste M, Tatlisumak T. How does number of risk factors affect prognosis in young patients with ischemic stroke? Stroke. 2012;43:356–61. doi: 10.1161/STROKEAHA.111.635276. [DOI] [PubMed] [Google Scholar]
- 7.von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, et al. Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the Stroke in Young Fabry Patients study. Stroke. 2013;44:119–25. doi: 10.1161/STROKEAHA.112.665190. [DOI] [PubMed] [Google Scholar]
- 8.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–9. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, et al. Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama study. Stroke. 2000;31:2616–22. doi: 10.1161/01.str.31.11.2616. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–97. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 12.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: The Causative Classification of Stroke System. Stroke. 2007;38:2979–84. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 13.Arsava EM, Ballabio E, Benner T, Cole JW, Delgado-Martinez MP, Dichgans M, et al. The Causative Classification of Stroke system: An international reliability and optimization study. Neurology. 2010;75:1277–84. doi: 10.1212/WNL.0b013e3181f612ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallström B, Jönsson A-C, Nerbrand C, Norrving B, Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: Comparisons with the late 20th century and projections into the future. Stroke. 2008;39:10–5. doi: 10.1161/STROKEAHA.107.491779. [DOI] [PubMed] [Google Scholar]
- 15.Jönsson A-C, Lindgren I, Hallström B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: A population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77:590–5. doi: 10.1136/jnnp.2005.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Hart RG. Cardiogenic embolism to the brain. Lancet. 1992;339:589–94. doi: 10.1016/0140-6736(92)90873-2. [DOI] [PubMed] [Google Scholar]
- 18.Gardner MJ, Altman DG. Calculating confidence intervals for proportions and their differences. In: Gardner MJ, Altman DG, editors. Statistics with confidence. London: BMJ Publishing Group; 1989. pp. 28–9. [Google Scholar]
- 19.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes: Evidence for a distinct lacunar arteriopathy? Stroke. 2010;41:624–9. doi: 10.1161/STROKEAHA.109.558809. [DOI] [PubMed] [Google Scholar]
- 21.Marini C, Baldassarre M, Russo T, De Santis F, Sacco S, Ciancarelli I, et al. Burden of first-ever ischemic stroke in the oldest old: Evidence from a population-based study. Neurology. 2004;62:77–81. doi: 10.1212/01.wnl.0000101461.61501.65. [DOI] [PubMed] [Google Scholar]
- 22.Saposnik G, Hill MD, O’Donnell M, Fang J, Hachinski V, Kapral MK. Variables associated with 7-day, 30-day, and 1-year fatality after ischemic stroke. Stroke. 2008;39:2318–24. doi: 10.1161/STROKEAHA.107.510362. [DOI] [PubMed] [Google Scholar]
- 23.Leyden JM, Kleinig TJ, Newbury J, Castle S, Cranefield J, Anderson CS, et al. Adelaide stroke incidence study: Declining stroke rates but many preventable cardioembolic strokes. Stroke. 2013;44:1226–31. doi: 10.1161/STROKEAHA.113.675140. [DOI] [PubMed] [Google Scholar]
- 24.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–63. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 25.Hallström B, Jönsson A-C, Nerbrand C, Petersen B, Norrving B, Lindgren A. Lund Stroke Register: Hospitalization pattern and yield of different screening methods for first-ever stroke. Acta Neurol Scand. 2007;115:49–54. doi: 10.1111/j.1600-0404.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 26.Meschia JF, Arnett DK, Ay H, Brown RD, Jr, Benavente OR, Cole JW, et al. Stroke Genetics Network (SiGN) study: Design and rationale for a genome-wide association study of ischemic stroke subtypes. Stroke. 2013;44:2694–702. doi: 10.1161/STROKEAHA.113.001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.