Abstract
Objective
The respective contributions to endogenous glucose production (EGP) of the liver, kidney and intestine vary during fasting. We previously reported that the deficiency in either hepatic or intestinal gluconeogenesis modulates the repartition of EGP via glucagon secretion (humoral factor) and gut–brain–liver axis (neural factor), respectively. Considering renal gluconeogenesis reportedly accounted for approximately 50% of EGP during fasting, we examined whether a reduction in renal gluconeogenesis could promote alterations in the repartition of EGP in this situation.
Methods
We studied mice whose glucose-6-phosphatase (G6Pase) catalytic subunit (G6PC) is specifically knocked down in the kidneys (K-G6pc-/- mice) during fasting. We also examined the additional effects of intestinal G6pc deletion, renal denervation and vitamin D administration on the altered glucose metabolism in K-G6pc-/- mice.
Results
Compared with WT mice, K-G6pc-/- mice exhibited (1) lower glycemia, (2) enhanced intestinal but not hepatic G6Pase activity, (3) enhanced hepatic glucokinase (GK encoded by Gck) activity, (4) increased hepatic glucose-6-phosphate and (5) hepatic glycogen spared from exhaustion during fasting. Increased hepatic Gck expression in the post-absorptive state could be dependent on the enhancement of insulin signal (AKT phosphorylation) in K-G6pc-/- mice. In contrast, the increase in hepatic GK activity was not observed in mice with both kidney- and intestine-knockout (KI-G6pc-/- mice). Hepatic Gck gene expression and hepatic AKT phosphorylation were reduced in KI-G6pc-/- mice. Renal denervation by capsaicin did not induce any effect on glucose metabolism in K-G6pc-/- mice. Plasma level of 1,25 (OH)2 D3, an active form of vitamin D, was decreased in K-G6pc-/- mice. Interestingly, the administration of 1,25 (OH)2 D3 prevented the enhancement of intestinal gluconeogenesis and hepatic GK activity and blocked the accumulation of hepatic glycogen otherwise observed in K-G6pc-/- mice during fasting.
Conclusions
A diminution in renal gluconeogenesis that is accompanied by a decrease in blood vitamin D promotes a novel repartition of EGP among glucose producing organs during fasting, featured by increased intestinal gluconeogenesis that leads to sparing glycogen stores in the liver. Our data suggest a possible involvement of a crosstalk between the kidneys and intestine (via the vitamin D system) and the intestine and liver (via a neural gut-brain axis), which might take place in the situations of deficient renal glucose production, such as chronic kidney disease.
Keywords: Endogenous glucose production, Gluconeogenesis, Hypoglycemia, Glycogen, Chronic kidney disease, Vitamin D
Highlights
-
•
Reduced renal G6Pase activity promotes increased hepatic glycogen during fasting.
-
•
Reduced renal G6Pase activity enhances intestinal but not hepatic G6Pase activity.
-
•
Reduced renal G6Pase activity results in low vitamin D level.
-
•
Vitamin D injection restores metabolism in mice with reduced renal G6Pase activity.
1. Introduction
Endogenous glucose production (EGP), encompassing glycogenolysis and gluconeogenesis, is a fundamental physiological function to keep blood glucose level constant during the periods of food deprivation. Once glycogen is exhausted in the early phase of fasting, gluconeogenesis plays a central role in glucose supply. Only three organs, liver, kidney and intestine, perform gluconeogenesis since they have a specific enzyme for that, glucose-6-phosphatase (G6Pase). G6Pase dephosphorylates glucose 6-phosphate (G6P): a reaction that releases glucose and inorganic phosphate. Altered repartition of EGP among gluconeogenic organs takes place in various nutritional situations, such as during long-term fasting and under protein-enriched diet [1]. For example, the liver accounts for 70–75% of EGP in post-absorptive state, while renal gluconeogenesis contributes to up to 50% of EGP after 24 h of fasting in rats [2]. Consistent with the animal studies, results in humans showed that about 50% of EGP are contributed by the kidney after long-term fasting [3], [4]. Intestinal glucose production, making a small contribution to EGP at the fed to fasted transition, accounts to about 20%–25% of EGP after 24 h of fasting in the rat [5], [6]. These reports suggest that gluconeogenesis from the kidney and intestine is increasingly important during fasting. While hepatic gluconeogenesis from lactate and alanine is an endergonic process that consumes energy, renal and intestinal gluconeogenesis, utilizing glutamine as the main substrate, is exergonic and produces 4 ATP per mole of synthetized glucose [7]. This repartition of EGP allows the body to first maintain plasma glucose constant and simultaneously preserve the energetic status of the body for anabolic purposes [1]. The difference in glucose precursors according to gluconeogenic organs would also imply that the regulatory mechanisms of glucose production are not the same in the liver and/or kidney/intestine. Moreover, we previously found that the deficiency in hepatic glucose production induces upregulation of both renal and intestinal gluconeogenesis [8], while upregulation of intestinal gluconeogenesis decreases hepatic glucose production via a neural gut–brain–liver axis [9], [10]. These findings indicate the existence of inter-organ coordination of EGP to maintain glucose and energy homeostasis.
Increasing attention has been paid to the role of kidney in glucose homeostasis [11]. Disordered glucose metabolism is widely recognized in chronic kidney disease (CKD) patients. Fasting hypoglycemia, more common in CKD patients than in the general population [12], is generally attributed to diminished renal gluconeogenesis [13], [14]. It is not clear whether the repartition of EGP takes place and even participates in glycemic reduction in CKD with reduced renal gluconeogenesis. A lack of suitable animal model delayed the identification of factors responsible for pathophysiological complexity in CKD. Nephrectomy, often performed in CKD models, not only reduces renal glucose output [15] but surgically impairs almost all other kidney functions. An investigation into more selective pathophysiology caused by reduced renal gluconeogenesis could yield novel insights into the kidney's roles in whole body metabolism. To this end, we generated kidney-specific G6pc knockdown mice (K-G6pc-/- mice) in which renal G6Pase activity is reduced [16]. We examined the effect of this reduction in renal G6Pase activity on glucose metabolism during fasting in K-G6pc-/- mice.
In addition to renal gluconeogenesis, kidney-derived neural and/or humoral factors play essential roles in the whole body metabolism [17], [18]. For example, renal nerve activity and vitamin D deficiency were previously involved in whole body glucose homeostasis [19], [20]. We thus tested the possible involvement of kidney-derived factors, such as renal neural signaling or vitamin D, on the repartition of EGP in K-G6pc-/- mice.
We here report a previously unsuspected kidney's role in the repartition of EGP, which has the capacity to affect glucose metabolism.
2. Material and methods
2.1. Animals
Male adult (6–8 weeks old) B6.G6pclox/lox.KapcreERT2/w, B6.G6pclox/lox.KapCreERT2/w .VillCreERT2/w and C57Bl/6 J (Charles Rivers Laboratories, L'Arbresle, France) were intraperitoneally injected once daily with 1 mg of tamoxifen for 5 consecutive days, to obtain K-G6pc-/- mice, KI- G6pc-/- mice, wild-type (WT) mice, respectively. At the time of experiments, mice were 9–11 weeks of age. Mice were housed in the animal facility of Lyon 1 University (“Animalerie Lyon Est Conventionnelle” and “Specific Pathogen Free”) under controlled temperature (22 °C) conditions, with a 12-h light/dark cycle. All mice had free access to water and standard chow. All procedures were performed in accordance with the principles and guidelines established by the European Convention for the Protection of Laboratory Animals (2010/63/EU). The regional animal care committee (CEEA-55, Université Lyon I) approved all experiments.
2.2. Quantitative RT-PCR
Total RNA was extracted from frozen tissues using Trizol reagent (Invitrogen), according to the manufacturer's instructions. Reverse transcription was done on 1 μg of mRNA using the Qiagen QuantiTect Reverse Transcription kit. SsoAdvanced™ Universal SYBR® Green Supermix (BioRad) was used to determine mRNA levels. Ribosomal protein L19 (RPL19) was used as a housekeeping gene. Calculations were made based on the comparative cycle threshold method (2-ΔΔCt). Primer sequences are listed in Supplementary Table 1.
2.3. Western blot analysis
Tissues were rapidly sampled in liquid nitrogen and stored at −80 °C before analysis. Tissues were homogenized in lysis buffer (50 mM Tris pH 7.5, 5 mM MgCl2, 100 mM KCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, 1% NP40, protease and phosphatase inhibitors) by FastPrep® system.
Aliquots of 30 μg proteins were separated by 9%-SDS polyacrylamide gel electrophoresis and transferred to PVDF Immobilon membranes (Millipore). The membranes were probed with antibodies diluted in TBS/0.2% Tween/5% BSA against pAKT Ser473 or AKT (dilution 1:2000, Cell Signaling) and then with goat secondary anti-rabbit IgG linked to peroxidase (dilution 1:10000, BioRad). Membranes were exposed to ClarityTM Western ECL Substrate (BioRad). The visualization and quantification of proteins were performed using the BioRad ChemiDoc™ Touch Imaging system.
2.4. Metabolic studies
Body weight and blood glucose were measured before or 6 h, 10 h, 24 h, 30 h after the beginning of fasting. Blood glucose was measured with an Accu-Check Go glucometer (Roche Diagnostics, Meylan, France). Plasma samples were withdrawn by submandibular bleeding using a lancet and collected into the tube with EDTA after 6 h and 30 h of fasting, just before killing the animal. For the study of renal capsaicin treatment and vitamin D administration, mice were sacrificed at 30 h of fasting. Insulin (Mercodia), glucagon (Alpco Diagnostics), corticosterone (Arbor Assays), adrenaline and 1,25-dihydroxyvitamin D3 (1,25 (OH)2 D3) (LDN Labor Diagnostika Nord GmbH & Co.KG) were determined with mouse ELISA kits. Beta hydroxybutyrate and non-esterified fatty acids (NEFA) concentrations in plasma were assessed with an Abcam colorimetric kit and a Diasys colorimetric kit, respectively.
Hepatic glycogen and G6P determinations were carried out as previously described [21].
G6Pase activity was directly assayed in the homogenates for 10 min at 30 °C at pH 7.3 in the presence of a saturating G6P concentration (20 mmol/L). The inorganic phosphate release was determined by complexometry [22].
GK activity was measured by using a spectrophotometric method with some modifications, as previously described [23]. Briefly, enzyme activity was assayed at 37 °C using 200 mM Hepes-NaOH, 100 mM KCl, 5 mM MgCl2, 1 mM NADP+, 5 mM ATP, 1 mM DTT, 0.5 g/L BSA, pH 7.6, and excess glucose 6-phosphate dehydrogenase. GK activity in crude homogenates was estimated subtracting the rate of NADPH formation (at 340 nm) in the presence of 1 mM glucose (scoring low-Km HK activity) from that obtained in the presence of 40 mM glucose (scoring total HK activity).
2.5. Renal capsaicin treatment
WT mice and K-G6pc-/- mice were assigned to sham-treated (Sham) or capsaicin-treated (Cap) groups: WT-Sham, WT-Cap, K-G6pc-/--Sham, K-G6pc-/--Cap. Ten days after the tamoxifen injection, sham- or capsaicin-treatments were performed as previously described in rats [24] with some modifications. Treated mice were allowed to recover for 10 days and examined during fasting. Briefly, Cap mice were anesthetized under isoflurane (2%). Following a midline abdominal incision, the left kidney was exposed and renal arteries and veins were gently isolated from connective tissue including fat. Two small pieces of gauze soaked in a capsaicin solution (33 mM in 10% ethanol, 10% Tween 20 and 80% normal saline) were wrapped around the renal artery and vein for 15 min. A small piece of parafilm was placed beneath the renal artery and vein prior to the placement of capsaicin-soaked gauze to prevent any capsaicin exposure outside of the kidney. Following 15 min of capsaicin exposure, the gauze and parafilm were removed, and the procedure was repeated on the right side. At the end of the procedure, the abdominal muscles and skin were closed separately with 6-0 silk suture. In Sham mice, left renal arteries and veins were isolated and, after 15 min, the same procedure was repeated on the right side.
2.6. Histological analysis
For histological examination, 4 μm-thick tissue sections of formalin-fixed and paraffin-embedded tissue were prepared according to conventional procedures. Sections were then treated with hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS) staining and examined with a light microscope.
Immunohistochemistry was performed with an automated immunostainer (Ventana Discovery XT, Roche, Meylan, France) using Ultramap Kit according to the manufacturer's instructions. Sections were incubated 1 h with a rabbit anti-calcitonin gene-related peptide (CGRP, diluted at 1:50, Cell Signaling). Staining was visualized with DAB solution with 3,3′-diaminobenzidine as a chromogenic substrate. Finally, the sections were counterstained with Gill's hematoxylin.
The CGRP content analysis was performed using a light microscope (Eclipse E400, Nikon France, Champigny, France) equipped with a tri-CDD video camera (Sony, Japan). CGRP positive nerves were quantified as the area of CGRP positive labeling (μm2) per length of pelvic wall (mm) determined by morphometric analysis (Histolab, Microvision Instruments, Evry, France), as previously reported [24].
2.7. Vitamin D administration
WT mice and K-G6pc-/- mice were assigned to vehicle (Vh) or vitamin D (VD) groups: WT-Vh, WT-VD, K-G6pc-/--Vh, K-G6pc-/--VD.
The dilution vehicle for 1,25 (OH)2 D3 (Sigma) injections consisted of 20% EtOH, 30% propylene glycol and 50% H2O. 1,25 (OH)2 D3 (2.5 μg/kg) was intraperitoneally injected into mice at the beginning and after 10 h of fasting. A single acute dose of 2.5 μg/kg was reported to induce desired pharmacological effects without adverse toxicity [25], [26].
2.8. Statistics
All data are presented as mean ± SEM. Two-group comparison was analyzed using unpaired t test. Two-way ANOVA followed by Tukey–Kramer was used for the comparison between WT and K-G6pc-/- mice or Sham and Cap in capsaicin treatment study. One-way ANOVA followed by Tukey–Kramer was used for the comparison among four groups in vitamin D injection study.
3. Results
3.1. Reduced renal glucose-6 phosphatase activity lowers blood glucose level with the enhancement of intestinal G6Pase activity during fasting
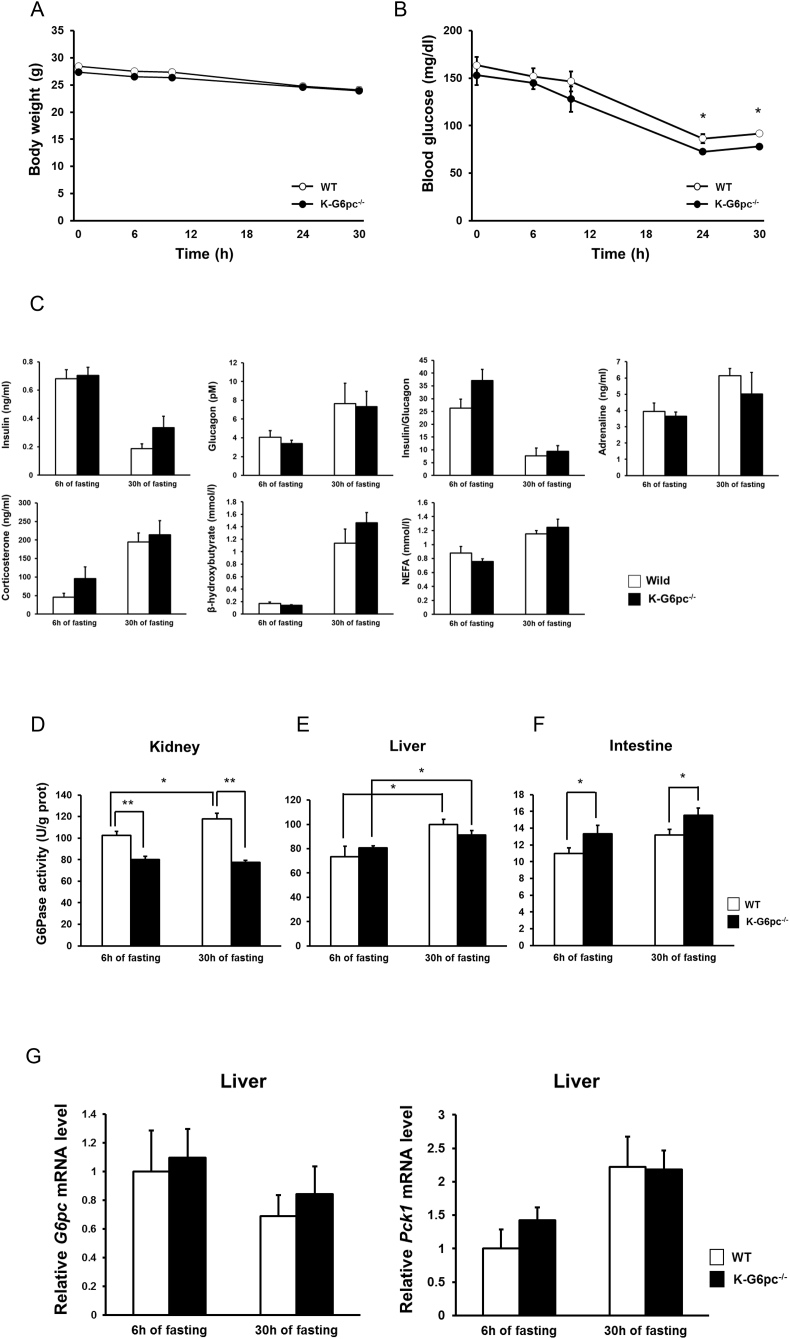
We examined glucose metabolism during fasting in K-G6pc-/- mice. Since K-G6pc-/- mice developed first signs of CKD after 6 months of G6pc deletion [16], we here used mice three weeks after tamoxifen injections. Body, liver and epididymal white adipose tissue (eWAT) weights did not differ between WT and K-G6pc-/- mice during fasting (Figure 1A and Supplementary Figs. 1A and B). Interestingly, blood glucose level was lower at 24 h (WT mice: 86.4 ± 4.94, K-G6pc-/- mice: 72.6 ± 2.50 mg/dl) and 30 h (WT mice: 91.8 ± 2.41, K-G6pc-/- mice: 78.0 ± 2.59 mg/dl) of fasting in K-G6pc-/- mice compared to WT mice (Figure 1B). However, plasma insulin and counter-regulatory hormones such as glucagon, corticosterone and adrenaline, and the insulin/glucagon ratio were not different in WT and K-G6pc-/- mice (Figure 1C). Consistent with the absence of body and eWAT weight difference in K-G6pc-/- mice and WT mice at 6 h and 30 h of fasting, plasma β-hydroxybutyrate and NEFA were also not different between WT and K-G6pc-/- mice during fasting (Figure 1C). As expected, the renal G6Pase activity at 6 h and 30 h of fasting were significantly lower in K-G6pc-/- mice than WT mice. Moreover, enhancement of renal G6Pase activity at 30 h of fasting compared to 6 h of fasting in WT was prevented in K-G6pc-/- mice (Figure 1D). Accordingly, renal glycogen contents during fasting and G6P content at 30 h of fasting were increased in K-G6pc-/- mice compared to WT mice (Supplementary Fig. 2A). As we previously reported, H&E staining of the kidney in K-G6pc-/- mice showed that morphological alterations took place the kidney cortex with a clarification and enlargement of tubular epithelial cells due to the accumulation of glycogen (Supplementary Fig. 2B) [16]. Hepatic G6Pase activity at 30 h of fasting was significantly increased in both WT and K-G6pc-/- mice compared to that at 6 h of fasting. There were, however, no difference in hepatic G6Pase activity as well as in hepatic G6pc and Pck1 (encoding PEPCK-c) gene expression levels between WT and K-G6pc-/- mice (Figure 1E,G). In contrast, intestinal G6Pase activity at 6 h and 30 h of fasting was significantly higher in K-G6pc-/- mice than WT mice (Figure 1F).
Figure 1.
Alteration of blood glucose level and intestinal G6Pase activity in K-G6pc-/- mice during fasting. (A) Body weight and (B) blood glucose level in WT and K-G6pc-/- mice during fasting. n = 5 per group. (C) Circulating levels of insulin, glucagon, adrenaline, corticosterone, β-hydroxybutyrate, NEFA and insulin/glucagon ratio at 6 h and 30 h of fasting in WT and K-G6pc-/- mice. n = 5–6 per group. (D) Renal, (E) hepatic and (F) intestinal G6Pase activity at 6 h and 30 h of fasting in WT and K-G6pc-/- mice. n = 5–7 per group. (G) Hepatic gene expressions of G6pc and Pck1 at 6 h and 30 h of fasting. n = 5–6 per group. The results are expressed as the mean ± SEM Significant differences between WT and K-G6pc-/- mice are indicated. (*P < 0.05, **P < 0.01).
These data showed that reduced renal G6Pase activity during fasting in K-G6pc-/- mice resulted in decreased blood glucose level without further counter-regulatory process and induced differential responses in hepatic and intestinal G6Pase activity compared with WT mice.
3.2. Reduced renal glucose-6 phosphatase activity promotes sparing in hepatic glycogen during fasting
To further examine hepatic glucose production, we next examined whether hepatic glycogen metabolism was affected in response to reduced renal G6Pase activity. At 6 h of fasting, hepatic glycogen content was not different in WT and K-G6pc-/- mice (Figure 2A). Unexpectedly, hepatic glycogen, exhausted at 30 h of fasting in WT mice, was not totally depleted in K-G6pc-/- mice (p = 0.004) (Figure 2A,C). We also found that hepatic G6P content after 30 h of fasting was higher in K-G6pc-/- mice than WT mice (p = 0.002), which was not observed at 6 h of fasting (Figure 2B). Increased G6P content in the absence of substantial decrease in G6Pase activity (Figure 1E) prompted us to examine hepatic GK, whose activity operates phosphorylation of glucose in G6P. Hepatic GK activity at 30 h of fasting was higher in K-G6pc-/- mice than WT (p = 0.04) (Figure 2D). These findings indicate that reduced renal G6Pase activity promoted enhanced GK activity and thereby increased G6P content in the liver, resulting in sparing glycogen stores during fasting. To further clarify the mechanism of enhanced hepatic GK activity in K-G6pc-/- mice, we investigated Gck gene, whose expression is controlled by hepatic insulin signaling [27]. Gck expression and AKT phosphorylation were significantly increased in K-G6pc-/- mice at 6 h of fasting (p = 0.049 and p = 0.04, respectively) (Figure 2E,F), indicating that enhanced hepatic insulin signaling participates in GK activation in K-G6pc-/- mice during fasting.
Figure 2.
Spared glycogen with hepatic GK activation after 30h of fasting in K-G6pc-/-mice and prevention of hepatic GK activation in KI-G6pc−/−mice. (A) Glycogen and (B) G6P contents at 6 h and 30 h of fasting in WT and K-G6pc-/- mice. n = 13–15 per group. (C) PAS staining at 30 h of fasting in WT and K-G6pc-/- mice. (D) Hepatic GK activity and at 6 h and 30 h of fasting in WT and K-G6pc−/− mice. n = 5–6 per group. (E) Hepatic Gck expression of at 6 h and 30 h of fasting in WT and K-G6pc-/- mice. n = 5–6 per group. (F) Hepatic phosphorylation state of AKT at 6 h of fasting in WT and K-G6pc-/- mice. (G) Hepatic GK activity and at 6 h and 30 h of fasting in WT and KI-G6pc-/- mice. n = 5–6 per group. (H) Hepatic Gck expression at 6 h and 30 h of fasting in WT and KI-G6pc-/- mice. n = 5–6 per group. (I) Hepatic phosphorylation state of AKT at 6 h of fasting in WT and KI-G6pc−/− mice. n = 5 per group. The results are expressed as the mean ± SEM. Significant differences between WT and K-G6pc-/- mice or KI-G6pc-/- mice are indicated. (*P < 0.05, **P < 0.01).
3.3. Enhanced intestinal glucose-6 phosphatase activity contributes to increased hepatic GK activity in K-G6pc-/- mice through alteration of hepatic insulin signaling
We recently showed that deficit in intestinal gluconeogenesis was involved in the attenuation of hepatic insulin signaling via a modulation of sympathetic tone [28]. Moreover, fiber-enriched diets were shown to up-regulate intestinal gluconeogenesis, resulting in enhancement of hepatic insulin signaling [10]. A gut-brain-liver neural axis mediated by portal glucose sensing was suggested to be involved in this mechanism [29]. We next examined whether the upregulation of intestinal gluconeogenesis contributed to the GK activation in K-G6pc-/- mice. We generated mice whose G6pc exon 3 was excised specifically both in the kidney and intestine (KI-G6pc-/-mice). As we previously reported [16], [21], exon 3 in the intestine was almost completely excised, while that of the kidney was only partially excised (Supplementary Fig. 3A). As a consequence, intestinal G6Pase activity was substantially decreased and renal G6Pase activity was significantly, but more slightly decreased. This is due to a compensatory process, taking place in the intact proximal tubules in KI-G6pc-/-mice compared to WT mice (Supplementary Fig. 3B) [16]. Hepatic G6Pase activity, whose exon 3 was unaffected, was not changed in KI-G6pc-/-mice.
Body weight during fasting was not different in WT and KI-G6pc-/-mice (Supplementary Fig. 3C). Blood glucose level was decreased at 10 h, 24 h and 30 h of fasting in KI-G6pc-/- mice (Supplementary Fig. 3D). Interestingly, hepatic GK activity was not different in WT and KI-G6pc-/- mice during fasting (Figure 2G). Moreover, contrary to K-G6pc-/- mice, KI-G6pc-/- mice exhibited decreased hepatic Gck gene expression and AKT phosphorylation (Figure 2H,I). These findings strongly suggest that up-regulation of intestinal gluconeogenesis in K-G6pc-/- mice is involved in the enhanced hepatic GK activation, which could be linked to increased hepatic Gck gene expression and insulin signaling.
3.4. The disruption of sensitive renal fibers by capsaicin treatment does not affect intestinal gluconeogenesis and hepatic glycogen metabolism
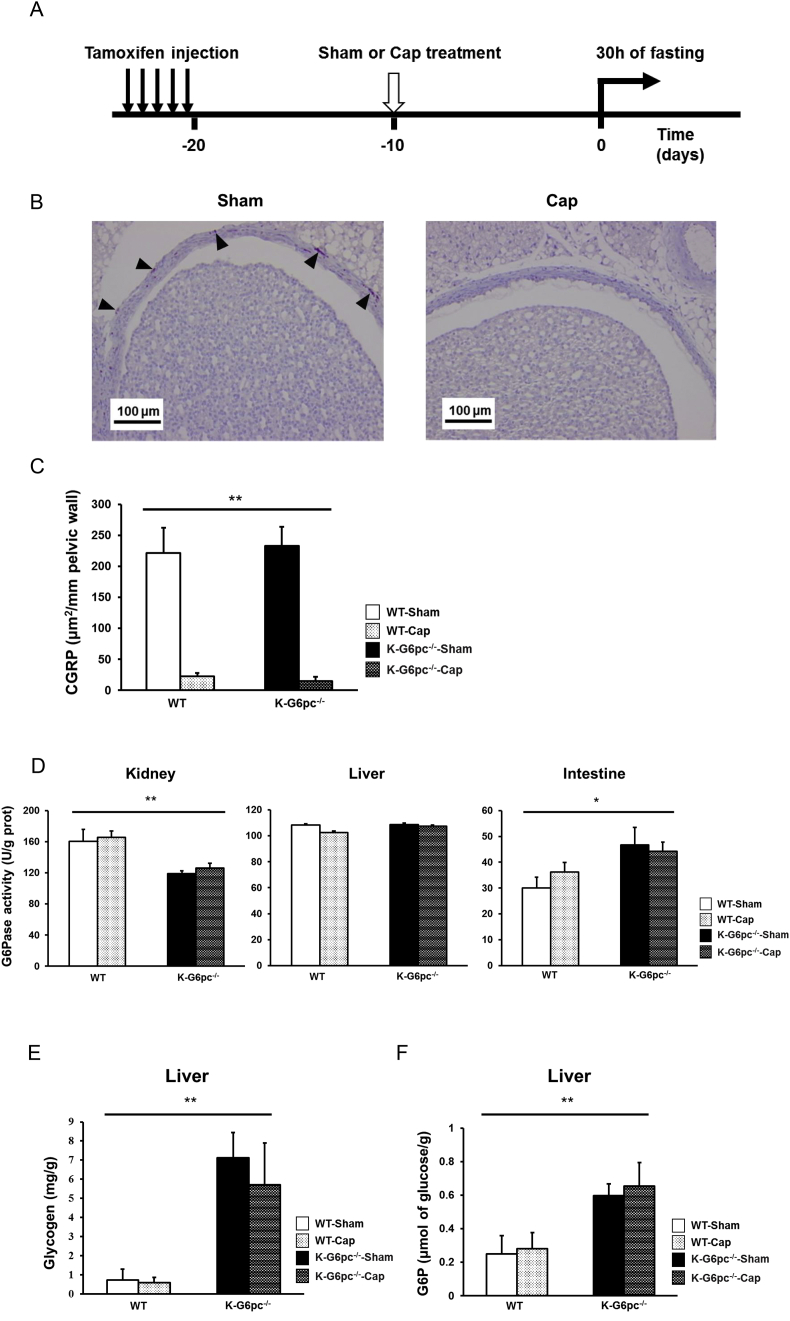
Next, we tested the hypothesis according to which renal gluconeogenesis could be sensed by the renal sensitive neurons (as we previously reported for intestinal gluconeogenesis) and interfere with the inter-organ repartition of EGP via a central signalization process. To test this possibility, we performed specific sensitive renal denervation by using capsaicin (Figure 3A).
Figure 3.
Afferent renal denervation has no effect on the glucose metabolism in K-G6pc-/- mice. (A) Time line of the study of capsaicin treatment. (B) Immunohistochemical CGRP staining at the renal pelvis level in WT-Sham and WT-Cap mice. Arrowheads indicate CGRP staining in brown. (C) The area of CGRP positive labeling per length of pelvic wall analyzed. n = 3–6 per group. (D) Renal, hepatic and intestinal G6Pase activity in WT-Sham, WT-Cap, K-G6pc-/--Sham and K-G6pc-/--Cap mice at 30 h of fasting. n = 3–6 per group. (E) Hepatic glycogen and (F) G6P content at 30 h of fasting in WT-Sham, WT-Cap, K-G6pc-/--Sham and K-G6pc-/--Cap mice at 30 h of fasting. n = 3–6 per group. The results are expressed as the mean ± SEM. Significant differences between WT and K-G6pc-/- mice are indicated. (*P < 0.05, **P < 0.01).
The renal sensory fibers originate from the renal pelvis and cortex [30]. Immunohistochemical staining of CGRP, a major neuronal marker of sensory fibers, at the renal pelvis level, revealed that CGRP labeled fibers were virtually eliminated in CAP mice (Figure 3B,C). In addition, while small amounts of CGRP positive neuronal fibers could be observed at the renal cortex in Sham mice, no CGRP labeling was detected in CAP mice (Supplementary Fig. 4).
It is noteworthy that sensitive renal denervation had no effect on renal, hepatic and intestinal G6Pase activities at 30 h of fasting (Figure 3D) in both WT and K-G6pc-/- mice. Similarly, hepatic glycogen and G6P content, which were increased in K-G6pc-/- mice, were also increased after sensitive renal denervation (Figure 3E,F). These results strongly suggest that signals from renal sensitive nerves are not involved in the alteration of intestinal gluconeogenesis and hepatic glycogen metabolism observed in K-G6pc-/- mice.
3.5. Vitamin D is involved in intestinal gluconeogenesis induction and modulation of hepatic glycogen metabolism during fasting
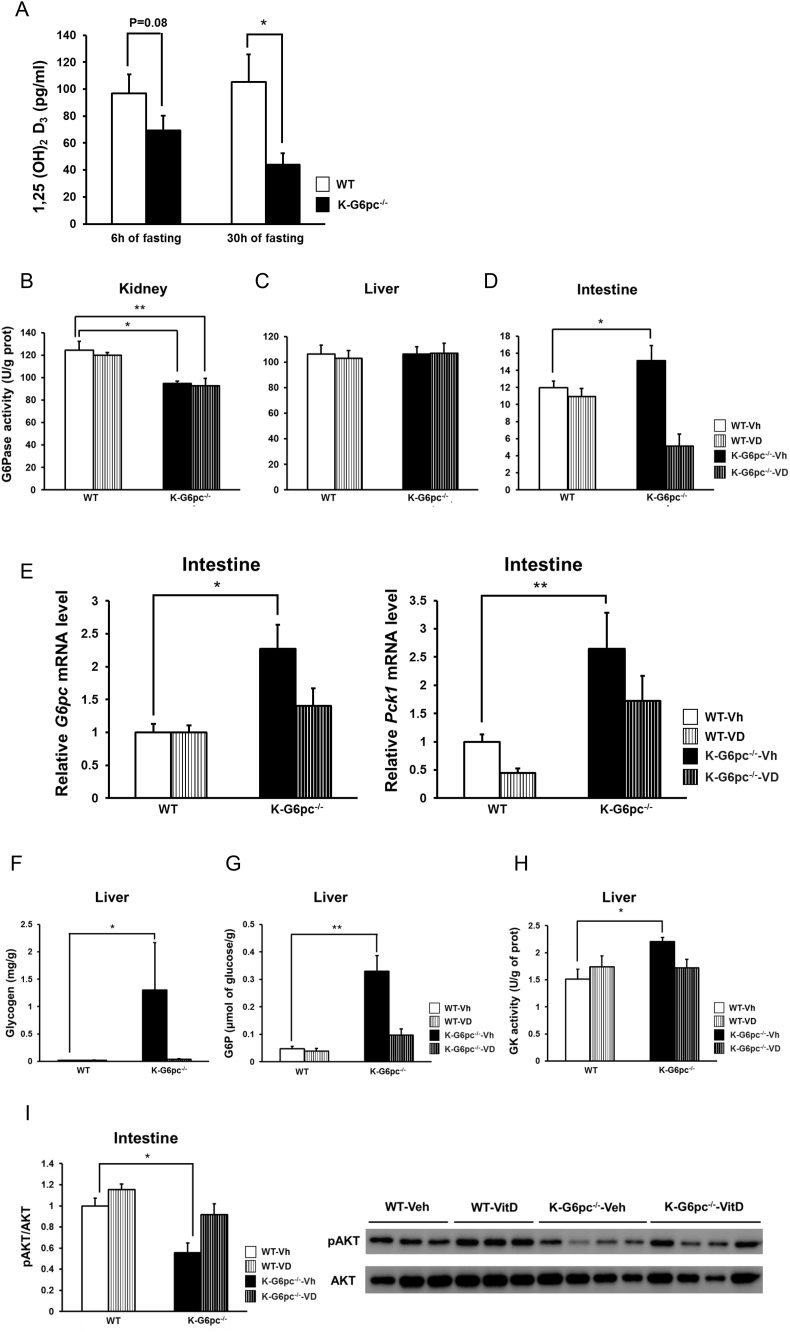
We next examined whether vitamin D, which is reportedly decreased in CKD patients [31], participates in regulation of glucose metabolism. Deficient vitamin D receptor signaling was recently reported to be involved in insulin resistance at tissue and cellular level [20], [32]. Moreover, vitamin D receptor is most abundant in the intestine and kidneys while expressed at low levels in the liver [33]. This prompted us to hypothesize that vitamin D signaling could be involved in the differential regulation in G6Pase activity in gluconeogenic organs during fasting in K-G6pc-/- mice. Interestingly, 1,25 (OH)2 D3, the active metabolite of vitamin D, was decreased during fasting in K-G6pc-/- mice compared to WT mice (Figure 4A).
Figure 4.
Involvement of vitamin D in the regulation of intestinal gluconeogenesis and hepatic glycogen metabolism in K-G6pc-/- mice. (A) Plasma 1,25(OH)2 D at 6 h and 30 h of fasting in K-G6pc-/- mice. n = 5–6 per group. (B) Renal, (C) hepatic and (D) intestinal G6Pase activity at 30 h of fasting in WT-Vh, WT-VD, K-G6pc-/--Vh and K-G6pc-/--VD mice. n = 4–6 per group. (E) Intestinal gene expressions of G6pc and Pck1 at 30 h of fasting in WT-Vh, WT-VD, K-G6pc-/--Vh and K-G6pc-/--VD mice. n = 4–6 per group. (F) Hepatic glycogen and (G) G6P content at 30 h of fasting in WT-Vh, WT-VD, K-G6pc-/--Vh and K-G6pc-/--VD mice at 30 h of fasting. n = 4–6 per group. (H) Hepatic GK activity at 30 h of fasting in WT-Vh, WT-VD, K-G6pc-/--Vh and K-G6pc-/--VD mice at 30 h of fasting. n = 4–6 per group. (I) Intestinal phosphorylation state of AKT at 30 h of fasting in WT-Vh, WT-VD, K-G6pc-/--Vh and K-G6pc-/--VD mice. n = 3–4 per group. Significant differences between WT-Vh and K-G6pc-/--Vh mice are indicated. (*P < 0.05, **P < 0.01).
To investigate whether the vitamin D deficiency is involved in the increase in intestinal gluconeogenesis, we performed intraperitoneal injections of 1,25 (OH)2 D3 into WT and K-G6pc-/- mice during fasting. The reduction in renal G6Pase activity in K-G6pc-/--vitamin D (VD) injected mice was comparable as that in K-G6pc-/--Vehicle (Vh) injected mice (Figure 4B). There was no difference in hepatic G6Pase activity among four groups (Figure 4C). Interestingly, the enhancement in intestinal G6Pase activity at 30 h of fasting in K-G6pc-/--Vh mice compared to WT-Vh mice was completely blocked in K-G6pc-/--VD mice (Figure 4D). Consistently, increased intestinal G6pc and Pck1 gene expressions in K-G6pc-/--Vh mice were suppressed in K-G6pc-/--VD mice (Figure 4E).
As observed in Figure 2A,B, hepatic glycogen and G6P contents at 30 h of fasting were significantly higher in K-G6pc-/--Vh mice than in WT-Vh mice. In contrast, K-G6pc-/--VD mice exhibited no elevation in hepatic glycogen and G6P compared to WT-Vh (Figure 4F and G). Likewise, hepatic GK activity was increased in K-G6pc-/--Vh mice, but not in K-G6pc-/--VD (Figure 4H). Therefore, vitamin D injection was able to inverse the intestinal and hepatic metabolic changes promoted by kidney G6pc deficiency. Consistently, blood glucose level at 30 h of fasting in K-G6pc-/--VD mice was slightly higher than that in K-G6pc-/--Vh mice (K-G6pc-/--Vh: 79.2 ± 3.68 mg/dl, K-G6pc-/--VD: 90.6 ± 3.16 mg/dl, p = 0.047). These results suggest that, in K-G6pc−/− mice, vitamin D deficiency promotes the induction of intestinal gluconeogenesis, which results in the enhancement of GK activity and consequently in hepatic glycogen storage, contributing further to hypoglycemia during fasting.
Finally, it is noteworthy that intestinal AKT phosphorylation was significantly attenuated in K-G6pc-/--Vh mice compared to WT-Vh mice but was not changed in K-G6pc-/--VD mice compared to K-G6pc-/--Vh mice (Figure 4I). This suggests that vitamin D could improve intestinal insulin signaling, which is known to down-regulate the expression of intestinal gluconeogenesis genes [18]. Thus, the induction of intestinal gluconeogenesis consecutive to the vitamin D deficiency in K-G6pc-/- mice could be linked to a decrease in intestinal insulin signaling.
4. Discussion
The repartition of EGP is exquisitely regulated during starvation, where renal and intestinal gluconeogenesis mobilizing exergonic pathways are increased, while hepatic gluconeogenesis (endergonic) is decreased [12]. Fasting hypoglycemia is one of the most important complications in CKD since it is associated with a higher mortality [12]. Several lines of evidence indicate the possible involvement of various mechanisms in hypoglycemia associated with CKD, such as insufficient availability of hepatic gluconeogenesis substrates, impaired hepatic glycogenolysis, inadequate insulin degradation and also diminished renal gluconeogenesis [13], [14], [31]. We here tackle the pathophysiological complexity of fasting hypoglycemia from the viewpoint of the specific role of the kidney in the repartition of EGP.
Given that renal gluconeogenesis increasingly contributes to EGP during fasting, reducing renal G6Pase activity should exert a substantial influence on blood glucose level. On the contrary, blood glucose level in K-G6pc-/- mice was only slightly decreased during fasting. One of the reasons would be the insufficient reduction in renal G6Pase activity due to the only partial knockdown of G6pc in the kidney cortex and a compensatory mechanism of induction in the non-affected proximal tubular cells [16]. In addition, increased intestinal gluconeogenesis may also contribute to increased glucose production to avoid further decrease of blood glucose, as we previously showed in prolonged fasting [21]. What was especially intriguing is that hepatic glycogen is not exhausted in K-G6pc-/- mice, even after a long (30 h) fasting. In line with these data, nephrectomized rats reportedly showed higher hepatic glycogen contents [34], [35]. Since there was no difference in hepatic glycogen content at 6 h of fasting in WT and K-G6pc-/- mice, spared glycogen content at 30 h of fasting indicates impaired hepatic glycogenolysis and/or activated glycogen synthesis, thereby contributing to lower blood glucose level in K-G6pc-/- mice more than in WT mice. In line with this rationale, we found hepatic G6P level higher in K-G6pc-/- mice than in WT mice at 30 h of fasting. G6P is not only a precursor substrate of glycogenesis, it is also an allosteric activator of glycogen synthase and an inhibitor of glycogen phosphorylase in the liver [36]. When glycogenolysis becomes negligible in the late phase of fasting, the synthesis of hepatic G6P results from the fluxes of gluconeogenesis and of glucose phosphorylation via hepatic GK. Increased hepatic GK activity concomitant with unaltered G6Pase activity indicates that the flux from glucose to G6P should be augmented in K-G6pc-/- mice, in agreement with the interpretation that hepatic glucose output from glycogenolysis is consequently downregulated. Hepatic GK activity is regulated both by transcriptional and post-transcriptional mechanisms that are only partially understood. Insulin upregulates Gck mRNA expression and this effect is mediated by the phosphatidylinositol-3 kinase/Akt pathway [37]. We found increased hepatic Gck gene expression at 6 h of fasting and enhanced GK activity at 30 h of fasting in K-G6pc-/- mice, suggesting that increased GK expression in the early post-absorptive state promotes the enhanced GK activity as fasting goes on. Despite that the plasma insulin/glucagon ratio was not increased, AKT phosphorylation in the liver was enhanced in fasted K-G6pc-/- mice. Moreover, the plasma NEFA level, that is involved in hepatic insulin sensitivity, was not different in WT and K-G6pc-/- mice [38]. This suggests a potentiation in hepatic insulin signaling, which could be the consequence of increased intestinal gluconeogenesis [10], [39]. Indeed, we found decreased Gck mRNA expression and no difference in GK activity in KI-G6pc-/- mice compared to WT mice during fasting. We previously reported the decrease in hepatic insulin sensitivity of EGP in intestine-specific G6pc knockout mice [28]. These findings firmly indicated that upregulation of intestinal gluconeogenesis is required to enhance GK activity during fasting.
Differential regulations between hepatic and intestinal G6Pase activity in K-G6pc-/- mice implies the occurrence of mechanisms that specifically enhance intestinal gluconeogenesis. Afferent renal nerve stimulation reportedly tended to increase noradrenaline activity in the muscle and intestine in rat, which could modulate intestinal G6Pase activity [40]. Moreover, total renal denervation performed in high-fat diet induced obese models, in which the sympathetic nervous activity is increased, showed a correction of hepatic gluconeogenesis [41], [42], [43]. This could also be associated with a change in intestinal G6Pase. However, renal sensitive denervation such as performed here had no effect on up-regulation of intestinal G6Pase activity observed in K-G6pc-/- mice. We previously reported that several humoral factors such as insulin and glucagon control intestinal gluconeogenesis [8], [44]. The absence of change in insulin and counter-regulatory hormones and the absence of effect of sensitive renal denervation on the induction of intestinal G6Pase activity prompted us to investigate whether a circulating kidney-derived factor might affect intestinal insulin sensitivity.
Vitamin D was reportedly decreased in various clinical kidney conditions, including CKD [17], [20], uninephrectomy [45] and glycogen storage disease type 1, the human deficiency in G6Pase [46]. K-G6pc-/- mice exhibited substantial decline in plasma 1,25 (OH)2 D3 during fasting. Since vitamin D administration to K-G6pc-/- mice increases intestinal AKT phosphorylation with decrease in intestinal G6Pase activity and gluconeogenic genes, the decreased level of vitamin D in K-G6pc-/- mice could result in blunting insulin signaling in the gut. The consequence would be the alleviation of the inhibitory tone on the expression of intestinal gluconeogenesis genes, leading to the up-regulation of intestinal gluconeogenesis and the hepatic modulations of glycogen metabolism deriving from the aforementioned gut-brain-liver neural communication. Thus, these data concur with the rationale that vitamin D could be involved in the changes in inter-organ repartition of EGP in response to deficient renal glucose production.
5. Conclusions
A diminution of renal gluconeogenesis promotes a novel repartition of EGP among glucose producing organs during fasting, featured by increased intestinal gluconeogenesis that leads to sparing glycogen stores in the liver. The decreased vitamin D circulating level accompanying renal gluconeogenesis deficiency could play a key role in this process, by alleviating the insulin inhibitory tone on intestinal gluconeogenesis, which activates a gut-brain-liver neural communication. These data shed a novel light on the inter-organ crosstalk among glucose-producing organs, which might take place in the situations of deficient renal glucose production, such as CKD.
Acknowledgement
We would like to thank the members of the Anipath Platform (Université Lyon 1 Laennec) for the histological experiments and of Animalerie Lyon Est Conventionnelle et SPF (Université Lyon 1, Laennec). Keizo Kaneko was supported by an EFSD/JDS Research Fellowship.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.06.010.
Contributor Information
Keizo Kaneko, Email: kayzo@oak.dti.ne.jp.
Gilles Mithieux, Email: gilles.mithieux@inserm.fr.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Soty M., Gautier-Stein A., Rajas F., Mithieux G. Gut-brain glucose signaling in energy homeostasis. Cell Metabolism. 2017;25(6):1231–1242. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Pillot B., Soty M., Gautier-Stein A., Zitoun C., Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology. 2009;150(2):616–624. doi: 10.1210/en.2008-0601. [DOI] [PubMed] [Google Scholar]
- 3.Owen O.E., Felig P., Morgan A.P., Wahren J., Cahill G.F., Jr. Liver and kidney metabolism during prolonged starvation. The Journal of Clinical Investigation. 1969;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekberg K., Landau B.R., Wajngot A., Chandramouli V., Efendic S., Brunengraber H. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes. 1999;48(2):292–298. doi: 10.2337/diabetes.48.2.292. [DOI] [PubMed] [Google Scholar]
- 5.Croset M., Rajas F., Zitoun C., Hurot J.M., Montano S., Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50(4):740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 6.Mithieux G., Gautier-Stein A., Rajas F., Zitoun C. Contribution of intestine and kidney to glucose fluxes in different nutritional states in rat. Comparative biochemistry and physiology Part B Biochemistry & Molecular biology. 2006;143(2):195–200. doi: 10.1016/j.cbpb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Mithieux G., Rajas F., Gautier-Stein A. A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. Journal of Biological Chemistry. 2004;279(43):44231–44234. doi: 10.1074/jbc.R400011200. [DOI] [PubMed] [Google Scholar]
- 8.Mutel E., Gautier-Stein A., Abdul-Wahed A., Amigo-Correig M., Zitoun C., Stefanutti A. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes. 2011;60(12):3121–3131. doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Backhed F., Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metabolism. 2016;24(1):151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 10.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Gerich J.E., Meyer C., Woerle H.J., Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24(2):382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 12.Moen M.F., Zhan M., Hsu V.D., Walker L.D., Einhorn L.M., Seliger S.L. Frequency of hypoglycemia and its significance in chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN. 2009;4(6):1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arem R. Hypoglycemia associated with renal failure. Endocrinology and Metabolism Clinics of North America. 1989;18(1):103–121. [PubMed] [Google Scholar]
- 14.Rubenfeld S., Garber A.J. Abnormal carbohydrate metabolism in chronic renal failure. The potential role of accelerated glucose production, increased gluconeogenesis, and impaired glucose disposal. The Journal of Clinical Investigation. 1978;62(1):20–28. doi: 10.1172/JCI109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kida K., Nakajo S., Kamiya F., Toyama Y., Nishio T., Nakagawa H. Renal net glucose release in vivo and its contribution to blood glucose in rats. The Journal of Clinical Investigation. 1978;62(4):721–726. doi: 10.1172/JCI109182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clar J., Gri B., Calderaro J., Birling M.C., Herault Y., Smit G.P. Targeted deletion of kidney glucose-6 phosphatase leads to nephropathy. Kidney International. 2014;86(4):747–756. doi: 10.1038/ki.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoccali C., Vanholder R., Massy Z.A., Ortiz A., Sarafidis P., Dekker F.W. The systemic nature of CKD. Nature Reviews Nephrology. 2017;13(6):344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 18.Veelken R., Schmieder R.E. Renal denervation–implications for chronic kidney disease. Nature Reviews Nephrology. 2014;10(6):305–313. doi: 10.1038/nrneph.2014.59. [DOI] [PubMed] [Google Scholar]
- 19.Mahfoud F., Schlaich M., Kindermann I., Ukena C., Cremers B., Brandt M.C. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123(18):1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima A., Yokoyama K., Yokoo T., Urashima M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World Journal of Diabetes. 2016;7(5):89–100. doi: 10.4239/wjd.v7.i5.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penhoat A., Fayard L., Stefanutti A., Mithieux G., Rajas F. Intestinal gluconeogenesis is crucial to maintain a physiological fasting glycemia in the absence of hepatic glucose production in mice. Metabolism Clinical and Experimental. 2014;63(1):104–111. doi: 10.1016/j.metabol.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Rajas F., Bruni N., Montano S., Zitoun C., Mithieux G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology. 1999;117(1):132–139. doi: 10.1016/s0016-5085(99)70559-7. [DOI] [PubMed] [Google Scholar]
- 23.Agius L., Peak M., Newgard C.B., Gomez-Foix A.M., Guinovart J.J. Evidence for a role of glucose-induced translocation of glucokinase in the control of hepatic glycogen synthesis. Journal of Biological Chemistry. 1996;271(48):30479–30486. doi: 10.1074/jbc.271.48.30479. [DOI] [PubMed] [Google Scholar]
- 24.Foss J.D., Wainford R.D., Engeland W.C., Fink G.D., Osborn J.W. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. American Journal of Physiology Regulator Integrative and Comparative Physiology. 2015;308(2):R112–R122. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinko J.R., Land B.B., Solecki W.B., Wickham R.J., Tellez L.A., Maldonado-Aviles J. Vitamin D3: a role in dopamine circuit regulation, diet-induced obesity, and drug consumption. eNeuro. 2016;3(2) doi: 10.1523/ENEURO.0122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow E.C., Quach H.P., Vieth R., Pang K.S. Temporal changes in tissue 1alpha,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. American Journal of Physiology. Endocrinology and Metabolism. 2013;304(9):E977–E989. doi: 10.1152/ajpendo.00489.2012. [DOI] [PubMed] [Google Scholar]
- 27.Massa M.L., Gagliardino J.J., Francini F. Liver glucokinase: an overview on the regulatory mechanisms of its activity. IUBMB Life. 2011;63(1):1–6. doi: 10.1002/iub.411. [DOI] [PubMed] [Google Scholar]
- 28.Soty M., Penhoat A., Amigo-Correig M., Vinera J., Sardella A., Vullin-Bouilloux F. A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Molecular Metabolism. 2015;4(2):106–117. doi: 10.1016/j.molmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaere F., Duchampt A., Mounien L., Seyer P., Duraffourd C., Zitoun C. The role of sodium-coupled glucose co-transporter 3 in the satiety effect of portal glucose sensing. Molecular Metabolism. 2012;2(1):47–53. doi: 10.1016/j.molmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freisinger W., Schatz J., Ditting T., Lampert A., Heinlein S., Lale N. Sensory renal innervation: a kidney-specific firing activity due to a unique expression pattern of voltage-gated sodium channels? American Journal of Physiology Renal Physiology. 2013;304(5):F491–F497. doi: 10.1152/ajprenal.00011.2012. [DOI] [PubMed] [Google Scholar]
- 31.Kendrick J., Cheung A.K., Kaufman J.S., Greene T., Roberts W.L., Smits G. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2012;60(4):567–575. doi: 10.1053/j.ajkd.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S., Villalta S.A., Agrawal D.K. FOXO1 mediates vitamin D deficiency-induced insulin resistance in skeletal muscle. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research. 2016;31(3):585–595. doi: 10.1002/jbmr.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandgren M.E., Bronnegard M., DeLuca H.F. Tissue distribution of the 1,25-dihydroxyvitamin D3 receptor in the male rat. Biochemical and Biophysical Research Communications. 1991;181(2):611–616. doi: 10.1016/0006-291x(91)91234-4. [DOI] [PubMed] [Google Scholar]
- 34.Delmez J.A., Rutherford W.E., Klahr S., Blondin J. Studies on the role of the liver and splanchnic tissues in the production of carbohydrate intolerance in uremia. Metabolism Clinical and Experimental. 1981;30(7):658–665. doi: 10.1016/0026-0495(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 35.Mannan A., Noujaim A.A., Wiebe L.I., Secord D.C., Sanders E.J., Silverberg D.S. The influence of chronic uremia on hepatic glycogen in the rat. Clinical Biochemistry. 1975;8(1):44–51. doi: 10.1016/s0009-9120(75)90536-6. [DOI] [PubMed] [Google Scholar]
- 36.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochemical Journal. 2008;414(1):1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 37.Roth U., Curth K., Unterman T.G., Kietzmann T. The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. Journal of Biological Chemistry. 2004;279(4):2623–2631. doi: 10.1074/jbc.M308391200. [DOI] [PubMed] [Google Scholar]
- 38.Bergman R.N., Iyer M.S. Indirect regulation of endogenous glucose production by insulin: the single gateway hypothesis revisited. Diabetes. 2017;66(7):1742–1747. doi: 10.2337/db16-1320. [DOI] [PubMed] [Google Scholar]
- 39.Troy S., Soty M., Ribeiro L., Laval L., Migrenne S., Fioramonti X. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metabolism. 2008;8(3):201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Patel K.P., Knuepfer M.M. Effect of afferent renal nerve stimulation on blood pressure, heart rate and noradrenergic activity in conscious rats. Journal of the Autonomic Nervous System. 1986;17(2):121–130. doi: 10.1016/0165-1838(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 41.Iyer M.S., Bergman R.N., Korman J.E., Woolcott O.O., Kabir M., Victor R.G. Renal denervation reverses hepatic insulin resistance induced by high-fat diet. Diabetes. 2016;65(11):3453–3463. doi: 10.2337/db16-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W., Chang Y., He L., Jian X., Li L., Gao L. Effect of renal sympathetic denervation on hepatic glucose metabolism and blood pressure in a rat model of insulin resistance. Journal of Hypertension. 2016;34(12):2465–2474. doi: 10.1097/HJH.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 43.Young J.B., Landsberg L. Suppression of sympathetic nervous system during fasting. Obesity Research. 1997;5(6):646–649. doi: 10.1002/j.1550-8528.1997.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 44.Martine Croset F.R., Carine Zitoun, Jean-Marc Hurot, Sandrine Montano, Gilles Mithieux. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 45.Friedlander M.A., Segre G.V. Response to parathyroid hormone in normal human kidney donors before and after uninephrectomy. Journal of Clinical Endocrinology & Metabolism. 1988;66(5):896–902. doi: 10.1210/jcem-66-5-896. [DOI] [PubMed] [Google Scholar]
- 46.Banugaria S.G., Austin S.L., Boney A., Weber T.J., Kishnani P.S. Hypovitaminosis D in glycogen storage disease type I. Molecular Genetics and Metabolism. 2010;99(4):434–437. doi: 10.1016/j.ymgme.2009.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.