ABSTRACT
Background
Multiple myeloma (MM) is a B-cell malignancy that is incurable for the majority of patients. New treatments are urgently needed. Recombinant immunotoxins (RITs) are chimeric proteins that are composed of the Fv or Fab portion of an antibody fused to a bacterial toxin. B-cell maturation antigen (BCMA) is a lineage-restricted differentiation protein and an ideal target for antibody-based treatments for MM.
Methods
RITs were produced by expressing plasmids encoding the components of the anti-BCMA RITs in Escherichia coli followed by inclusion body preparation, solubilization, renaturation, and purification by column chromatography. The cytotoxic activity of RITs was tested in vitro by WST-8 assays. We also measured their binding to human and mouse serum albumins and to BCMA and measured their serum half-life in mice.
Results
Using Fvs from different anti-BCMA antibodies, we produced RITs that specifically kill BCMA-expressing MM cells in vitro. To increase the serum half-life in vivo, we generated RITs that are fused with albumin-binding domains (ABDs). All RITs with ABDs have some decreased activity compared to the parent RIT, which is not due to decreased binding to BCMA.
Conclusions
Various new anti-BCMA immunotoxins were produced and evaluated. None of these were better than LMB-75 (anti-BCMA BM306-disulfide-stabilized Fv-LRggs) supporting the further preclinical development of LMB-75.
Keywords: mAb BM306, mAb C11D5.3, mAb J22.9, LMB-75, ABD fusion protein
Statement of Significance
We have developed new recombinant immunotoxins using the Fv sequences of different anti-BCMA antibodies and evaluated their cytotoxic activity. An albumin-binding domain protein was added to one of the RITs to increase the serum half-life of the immunotoxin for preclinical development.
INTRODUCTION
Multiple myeloma (MM) is a B-cell malignancy that originates in the bone marrow (BM). Major advances have been made in the treatment of MM in recent years with the introduction of protease inhibitors and immunomodulatory drugs [1]. While new treatment regimens have increased the length of time patients live after diagnosis, almost all patients eventually relapse [1]. MM remains a very resistant disease due to its high propensity for clonal heterogeneity and its complex interactions with the BM microenvironment [1]. Data from the American Cancer Society estimates there will be approximately 30 000 new cases of MM and 13 000 deaths in the United States in 2018 [2].
Several immunotherapy agents including antibody-based therapies and chimeric antigen receptor T-cell (CAR T-cell) therapies have shown impressive responses in some patients, suggesting that immunological approaches have great promise in the treatment of MM [1]. Recombinant immunotoxins (RITs) fall within this new approach. RITs are composed of an antibody variable fragment fused to a portion of the bacterial toxin Pseudomonas exotoxin A (PE) [3]. Several RITs are in preclinical development or in clinical trials [3]. The RIT Moxetumomab pasudotox, which targets CD22, recently completed a successful phase 3 clinical trial. It has a very high response rate in refractory hairy cell leukemia and produced complete responses in many patients [4]. An immunotoxin targeting mesothelin has also caused major tumor regressions in patients with chemotherapy-resistant malignant mesothelioma [5].
B-cell maturation antigen (BCMA), a member of the tumor necrosis receptor superfamily, is a lineage-restricted differentiation antigen present on normal and malignant plasma cells [6]. Given its high expression on malignant plasma cells and lack of expression on essential organs, BCMA is an attractive target for treatment of MM [7]. A recent publication from our lab shows that the BCMA-targeted RIT LMB-70, which has an anti-BCMA Fab of monoclonal antibody (mAb) BM306 fused to domain III of PE (Fig. 1A), has high cytotoxic activity in vitro against BCMA expressing cell lines and myeloma cells from patients [8]. In mice with myeloma cells implanted subcutaneously, LMB-70 caused shrinkage of subcutaneous tumors but did not produce complete responses as a single agent [8]. Here we describe the properties of several new RITs targeting BCMA that either contain other anti-BCMA Fvs or were engineered to contain albumin-binding domains (ABDs) to increase half-life.
Figure 1 .
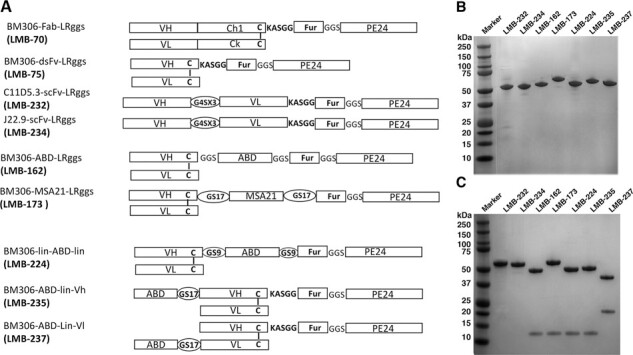
Immunotoxins targeting BCMA and albumin-binding fusion protein. (A) Schematics of various anti-BCMA immunotoxin constructs of Fab-RIT (LMB70), scFv RITs (LMB-232 and LMB-234), and dsFv RITs (LMB-75, LMB-162, LMB-173, LMB-224, LMB-235, and LMB-237). LMB-162 contains an ABD from Streptococcus, LMB-173 contains a single domain anti-albumin antibody from Llama, LMB-224 is similar to LMB-162 but has a 17 amino acids linker on either side of ABD, LMB-235 has the ABD attached to the amino-terminus of VH, and LMB-237 has the ABD attached to the amino terminous of VL. Fur: Furin cleavage site. (B) Non-reducing SDS-PAGE gel with various RIT proteins. (C) Reducing SDS-PAGE gel with various RIT proteins.
MATERIALS AND METHODS
Production of RITs
Cloning of RIT plasmids was done with a NEB Gibson Assembly reaction and NEB 5-α competent Escherichia coli. All disulfide-stabilized (ds)Fv RITs were expressed in E. coli by two separate expression vectors, one expressing VH-Toxin and the other one expressing VL. For single chain (sc)Fv RIT, a single expression vector encoding VH linked to VL by a 15 amino acid linker followed by the toxin domain was used to express the protein in E. coli. We used domain III of PE toxin (represented as PE24) as the toxin moiety because it is less immunogenic than PE38 and more active than the deimmunized version of domain III (also known as LO10). All proteins were made following the protocol described earlier from our laboratory [9]. Briefly, RITs were expressed as inclusion bodies in BL-21 competent E.coli. The inducible lac promoter was used to express the protein once an OD600 between two and three was reached. The cell pellets were lysed, and the inclusion bodies were washed with TES buffer (50 mM Tris-HCl, pH 8.0, 20 mM EDTA, 100 mM NaCl) containing 2.5% Triton X-100. Then 100 mg of protein was solubilized and denatured in GTE buffer (6 M guanidine HCl, 100 mM Tris-HCl, pH 8.0, 2 mM EDTA) with 100 mg of dithioerythritol. Next, the protein was refolded for 30–32 hours at 4°C (100 mM Tris-HCl, 1 mM EDTA, 0.5 M arginine, 0.9 mM oxidized glutathione, pH 9.5) and dialyzed for 16–20 hours at 4°C (20 mM Tris-HCl, pH 7.4, 100 mM urea). The dialysate was filter sterilized with a 0.45 μm Millipore filter and purified by anion exchange chromatography (Q Sepharose and Mono Q) followed by size exclusion chromatography (TSK).
Cell lines
H929 myeloma cells expressing American Type Culture Collection were obtained from ATCC and maintained following the guidelines provided by the supplier.
Cytotoxicity assays
WST-8 assays were used to assess the viability of myeloma cells treated with RITs [8,10]. Briefly, 2 × 104 H929 cells were plated in 96-well round bottom plates in Roswell Park Memorial Institute media containing 10% fetal bovine serum. Cells were treated with various concentrations of RIT for 72 hours. WST-8 was added to the plate and incubated for 3–4 hours before the plates were read on the spectrophotometer. Then the concentration of RIT causing a 50% inhibition of cell viability (IC50) was calculated.
ELISAs
Enzyme-linked immunosorbent assays (ELISAs) were used to measure binding of RITs to mouse serum albumin (MSA), human serum albumin (HSA), and BCMA using methods previously described [11]. Briefly, 96-Well ELISA plates were coated with MSA, HSA, or BCMA-Fc overnight, and a casein blocking buffer was used. The RITs were added at increasing concentrations, and an antibody that binds to domain III of PE (IP12) followed by a goat anti-mouse IgG HRP was used to measure binding. Graphpad Prism was used to graph the results. The EC50 value, the concentration of a drug that gives half-maximal response, was calculated.
An ELISA was also used to measure RIT serum levels at varying time points. Mice were injected with 25 μg of LMB-162, and blood samples were taken at 5 minutes 1, 2, 4, 7, and 24 hours after injection. 96-Well ELISA plates were coated with BCMA-Fc overnight, and a blocking buffer containing bovine serum albumin was used. Serum was separated from the blood samples and added at increasing concentrations, and the IP12 antibody was used to determine how much RIT remained in the serum. Graphpad Prism was used to plot the results, and both one- and two-phase decay were utilized.
All animal experiments were performed in accordance with NIH guidelines and approved by the NCI Animal Care and Use Committee.
RESULTS
The anti-BCMA RIT LMB-70 (BM306-Fab-LRggs), previously described, contains the Fab portion of the BM306 mAb fused to domain III of PE (Fig. 1) (8). To assess other anti-BCMA RIT formats, we generated LMB-75 (BM306-dsFv-LRggs), which contains the dsFv of the BM306 mAb fused to domain III of PE (Fig. 1A). The dsFv format was used for RITs in the clinical trials previously described (4, 5). We chose to develop the dsFv format over the Fab for further preclinical development because the expression and refolding efficiency of the dsFv-RIT is much higher than the Fab-RIT with a final yield of 20 mg of pure monomeric protein starting from 100 mg of refolding material for this immunotoxin. We tested the in vitro efficacy of LMB-75 in a cytotoxicity assay with H929 myeloma cells, which express BCMA. Figure 2 shows a representative IC50 curve determined by WST-8 assay in H929 cells. LMB-75 was very active; the IC50 values range from 0.9 to 1.8 ng/ml, with an average of 1.3 ng/ml over eight assays (Table 1). Each assay was conducted in triplicate.
Figure 2 .
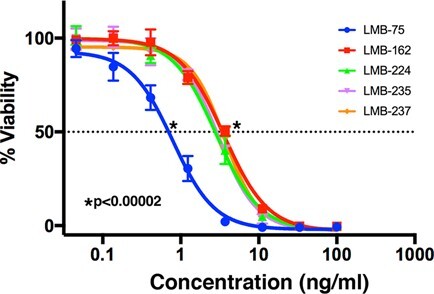
Representative cell killing curve of anti-BCMA immunotoxins. Cytotoxicity assays were performed on BCMA-positive H929 cells using WST-8 reagent after three days of incubation with LMB-75, LMB-162, LMB-224, LMB-235, and LMB-237.
Table 1.
Summary of IC50s in H929 cell line
| RIT | Description | IC 50 (ng/ml) |
|---|---|---|
| LMB-75 | BM306-dsFv-LRggs | 1.3 ± 0.3 |
| LMB-232 | C11D5.3-scFv-LRggs | 1.2 ± 0.4 |
| LMB-234 | J22.9-scFv-LRggs | 1.3 |
| LMB-162 | BM306-dsFv-ABD-LRggs | 5.4 ± 2.3 |
| LMB-173 | BM306-dsFv-MSA21-LRggs | 2.4 ± 0.6 |
| LMB-224 | BM306-dsFv-GS9-ABD-GS9-LRggs | 4.2 ± 2.0 |
| LMB-235 | BM306-ABD-VH-LRggs | 4.3 ± 1.8 |
| LMB-237 | BM306-ABD-VL-LRggs | 3.6 |
While LMB-75 is very active in vitro, we set out to explore if immunotoxins using Fvs from other anti-BCMA mAbs were more or less active. Ali et al. use the Fv from the C11D5.3 mAb that targets BCMA to make a CAR T-cell for the treatment of MM (clinical trial #NCT02215967) (12). Oden et al. describes the in vitro and in vivo antitumor activity of mAb J22.9, a high affinity antibody that targets BCMA (13). We used the Fvs from these mAbs to make two new RITs: LMB-232 (C11D5.3-scFv-LRggs) and LMB-234 (J22.9-scFv-LRggs), as shown in Figure 1A. After refolding 100 mg of each protein, a 4% and 6% yield of highly purified protein was obtained following anion exchange and size exclusion chromatography (profiles shown in Supplemental Figure S1) for LMB-232 and LMB-234, respectively. As demonstrated in a non-reducing and reducing SDS-PAGE gel in Figure 1B and C, we obtained highly purified LMB-232 and LMB-234 that migrated at the predicted molecular weights of 52.0 kDa and 51.8 kDa, respectively. We tested the in vitro cytotoxic activity of LMB-232 and LMB-234 against H929 cells in a WST-8 cytotoxicity assay. The IC50 values for LMB-232 range from 0.8 to 1.5 ng/ml over three assays, with an average IC50 of 1.2 ng/ml (Table 1). LMB-234 was assayed once, with an IC50 of 1.3 ng/ml (Table 1). All assays were conducted in triplicate. As shown in Table 1, the IC50 values for LMB-232 and LMB-234 do not differ from that of LMB-75, which has an average IC50 of 1.3 ng/ml. Thus, all three RITs are equally active.
LMB-75 has a molecular weight of 51 kDa. RITs of this size have a short half-life in mice, in the range of 12 minutes (11). To increase the serum half-life of LMB-75, we examined an ABD from Streptococcus and a single-domain antibody from Llama (11,14). As shown in Figure 1A, LMB-162 (BM306-dsFv-ABD-LRggs) contains an ABD from Streptococcus and LMB-173 (BM306-dsFv-MSA21-LRggs) contains a single-domain antibody from Llama that binds to serum albumin. LMB-162 was produced with a 17% yield, and LMB-173 was produced with a 5% yield after refolding of 100 mg of protein. A non-reducing SDS-PAGE gel in Figure 1B shows that we obtained highly purified LMB-162 and LMB-173 that migrated at the predicted molecular weights of 57 kDa and 64 kDa, respectively. The IC50 values for LMB-162 range from 2.2 to 8.3 ng/ml in H929 cells, with an average of 5.4 ng/ml over seven assays (Table 1). The IC50 values for LMB-173 range from 2.0 to 2.8 ng/ml over two assays, with an average of 2.4 ng/ml (Table 1). Both RITs were assayed in triplicate. While both LMB-162 and LMB-173 are active in vitro, they are less active than the parental RIT with no ABD, LMB-75, which has an average IC50 value of 1.3 ng/ml (Table 1).
To measure the affinity of LMB-162 and LMB-173 for HSA, an ELISA was performed. The EC50 value for LMB-162 with HSA is 4.2 ng/ml compared to 4.3 ng/ml for LMB-164, an RIT with the same format but containing the Fv of the SS1 antibody targeting mesothelin (Table 2). LMB-162 binds to HSA with a similar EC50 to that of the positive control, LMB-164 (Fig. 3A). LMB-75, which does not contain an ABD, was used as a negative control. Figure 3B shows that the affinity of LMB-162 for MSA is similar to its affinity for HSA; the EC50 value with MSA is 6.3 ng/ml for LMB-162 compared to 6.2 ng/ml for the positive control LMB-164.
Table 2 .
Summary of albumin-binding properties and mouse serum half-life of ABD–immunotoxin fusion proteins
| LMB number | Descriptive name | MSA half-maximum binding (ng/ml) | HSA half-maximum binding (ng/ml) | Half-life in mouse serum (minutes) |
|---|---|---|---|---|
| LMB-162 | BM306-dsFv-ABD-LRggs | 6.3 | 4.2 | 148 |
| LMB-75 | BM306-dsFv-LRggs | None | None | 7.4 |
| LMB-164 | SS1-ds-Fv-ABD-LRggs | 6.2 | 4.3 | 194(11) |
Figure 3 .
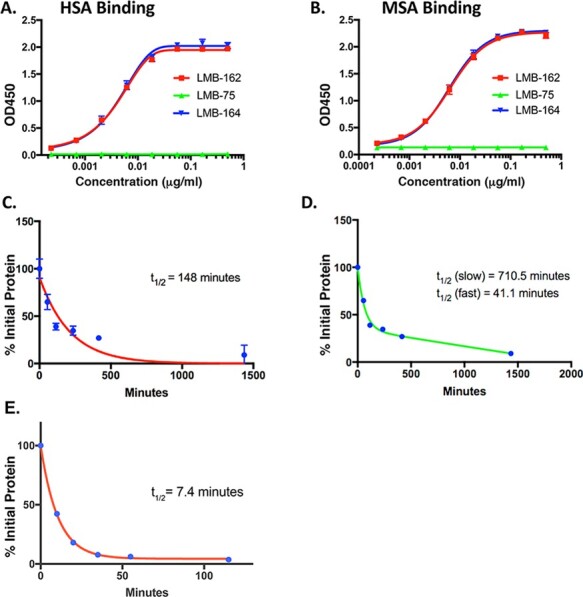
Characterization of anti-BCMA immunotoxin and ABD fusion proteins. Binding of LMB-162 with HSA (A) and MSA (B) by ELISA using LMB-164 as a positive control and LMB-75 as a negative control. (C) Half-life study with LMB-162 using a one-phase decay. (D) Half-life study with LMB-162 using a two-phase decay. (E) Half-life study of LMB-75 using a one-phase decay.
To determine the half-lives of the new RITs in mice, we carried out a pharmacokinetics study that shows that LMB-162 has a half-life of 148 minutes, according to a one-phase decay analysis (Fig. 3C). Using a two-phase decay model, LMB-162 has an α; of 41 minutes and a β; decay of 710 minutes (Fig. 3D). The half-life of LMB-162 is significantly longer than the parent immunotoxin LMB-75, which is about 8 minutes (Fig. 3E), or other similar dsFv RITs without ABDs, which have a half-life of 10 to 15 minutes (11).
While the ABD-fusion proteins greatly increase half-life, they have a slight decrease in cytotoxic activity compared to immunotoxins without ABDs. As shown in Table 1, the IC50 value of LMB-162 is 5.4 ng/ml compared to a 1.3 ng/ml IC50 for LMB-75, which does not have an ABD. We have not seen this decrease in activity with RITs targeting mesothelin (11). To try and overcome this loss in activity, we made proteins with different length linkers surrounding the ABD and placed the linker in different locations in the protein. LMB-224 (BM306-dsFv-GS9-ABD-GS9-LRggs) uses the same construct as LMB-162 but has a longer peptide linker on either side of the ABD (Fig. 1A). It was produced with a 20% yield from 100 mg of protein. A non-reducing SDS-PAGE gel in Figure 1B shows we obtained pure LMB-224, which migrated at the predicted molecular weight of 58 kDa. In cytotoxicity assays, IC50 values for LMB-224 range from 2.7 to 7.5 ng/ml, with an average of 4.2 ng/ml over five assays (Table 1). The assays were conducted in triplicate. With an average IC50 of 4.2 ng/ml, LMB-224 shows a marginal increase in activity compared to LMB-162, which has an average IC50 of 5.4 ng/ml (Table 1).
We engineered two other proteins, where the ABD was attached by a long 17 amino acid linker to the N-terminus of the Fv portion of the antibody. One of these RITs, LMB-235 (BM306-ABD-VH-LRggs), has the ABD attached to the N-terminus of the VH portion of the Fv of BM306, while the other, LMB-237 (BM306-ABD-VL-LRggs), has the ABD attached to the N-terminus of the VL portion of the Fv of BM306 (Fig. 1A). LMB-235 was produced with a 17% yield, and LMB-237 with a 14% yield. A non-reducing SDS-PAGE gel in Figure 1B shows we obtained pure LMB-235 and LMB-237 that migrated at the predicted molecular weight of 58 kDa. The IC50 values for LMB-235 range from 3 to 5.5 ng/ml over two assays, with an average IC50 of 4.3 ng/ml (Table 1). LMB-237 was assayed once, with an IC50 of 3.6 ng/ml (Table 1). All assays were conducted in triplicate. With average IC50 values of 4.3 ng/ml and 3.6 ng/ml, respectively, neither LMB-235 nor LMB-237 show significantly increased activity compared to LMB-162, which has an average IC50 value of 5.4 ng/ml (Table 1). Figure 2 shows an IC50 curve in H929 cells, which demonstrates similar cytotoxic activity for LMB-162, LMB-224, LMB-235, and LMB-237. While all ABD-containing BCMA-targeted RITs are active, they are significantly less active than LMB-75, which does not contain an ABD (Fig. 2).
To determine if the addition of the ABD interfered with binding of the RITs to BCMA and thereby decreased cytotoxic activity, we conducted a BCMA-binding ELISA. Figure 4 shows that LMB-75, LMB-162, LMB-224, LMB-235, and LMB-237 all bind to BCMA with similar affinities. Therefore, the decrease in cytotoxicity seen in BCMA-targeted RITs containing ABDs is not due to decreased binding to BCMA.
Figure 4 .
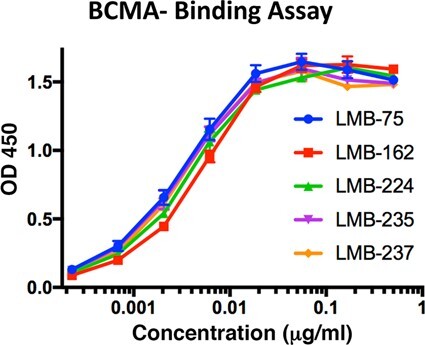
Effect of ABD domain on binding to BCMA antigen. Binding of anti-BCMA immunotoxin (LMB-75) and ABD fusion proteins (LMB-162, LMB-224, LMB-235, and LMB-237) to BCMA determined by ELISA assay.
DISCUSSION AND CONCLUSIONS
We have previously reported on the properties of an anti-BCMA immunotoxin made with an antibody isolated in our laboratory (8). Before undertaking clinical development of this immunotoxin, we wanted to be sure it was as active as immunotoxins containing other anti-BCMA antibodies in pre-clinical or clinical use. We report here that anti-BCMA immunotoxins made with three different antibodies have similar cytotoxic activities. We have extensively characterized the antibody used to make immunotoxin LMB-75 and have shown it does not react with other members of the TNF-receptor family and is suitable for clinical development (8). No such studies have been done with other reported anti-BCMA antibodies in the literature.
We recently reported that the addition of an ABD to an immunotoxin targeting mesothelin did not adversely affect cytotoxic activity in vitro and significantly enhanced anti-tumor activity in mice (11). When we added an ABD at the same location used to target mesothelin, the anti-BCMA immunotoxin has a two- to three-fold loss in activity. We attempted to solve this problem by creating longer linkers on either side of the ABD or attaching the ABD to either the N-terminus of the VH or the N-terminus of the VL. We usually attach the ABD to the C-terminus of the variable domain of the antibody and before the furin cleavage site (Fig. 1A). While these new constructs were active in vitro, they were not as active as an immunotoxin with no ABD. Binding studies showed that all the new immunotoxins with ABDs bound to BCMA with similar affinities, indicating the decreased activity is not due to decreased binding to BCMA. It is possible that the addition of the ABD affects other steps in immunotoxin action such as processing within the cell or transfer to the endoplasmic reticulum. It is also possible that the ABD fusion proteins may result in several rounds of FcRn-mediated endosomal recycling in non-target cells. That, in turn, may inactivate or release the toxin due to their exposer to the endosomal proteases.
The ABD-containing immunotoxins in our study have much longer half-life in mouse than the parent immunotoxins but have significantly shorter half-life compared to other published ABD-fusion proteins. Other studies using the same ABD domain from Streptococcus have shown the half-life of their fusion protein can be as long as 30 to 40 hours (15). We are now investigating the factors that influence this unexpected short half-life of our ABD–immunotoxin fusion proteins in mice.
Despite the small loss in activity of the immunotoxins with ABDs, they may be more active than LMB-75 in mice because of their increase in half-life. Further characterization of these RITs in mice is currently underway.
AUTHOR CONTRIBUTIONS
Z.S. and M.W. performed experiment, analyzed data, and wrote the manuscript, A.I. performed experiment, S.N. and T.I. provided reagents, and I.P. and T.K.B. designed experiment, analyzed data, and wrote the manuscript.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (BC008753). We thank the CCR Genomic Core Facility for DNA sequencing and Dr Mitchell Ho for technical advice.
REFERENCES
- 1. Varga, C, Laubach, JP, Anderson, KCet al. Investigational agents in immunotherapy: a new horizon for the treatment of multiple myeloma. Br J Haematol 2018; 181: 433–46. [DOI] [PubMed] [Google Scholar]
- 2. Siegel, RL, Miller, KD, Jemal, A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Pastan, I, Hassan, R, Fitzgerald, DJet al. Immunotoxin therapy of cancer. Nat Rev Cancer 2006; 6: 559–65. [DOI] [PubMed] [Google Scholar]
- 4. Kreitman, RJ, Tallman, MS, Robak, Tet al. Phase 1 trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012; 30: 1822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassan, R, Miller, AC, Sharon, Eet al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med 2013; 5: 208ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seckinger, A, Delgado, JA, Moser, Set al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 2017; 31: 396–410. [DOI] [PubMed] [Google Scholar]
- 7. Laabi, Y, Gras, MP, Carbonnel, Fet al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J 1992; 11: 3897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bera, TK, Abe, Y, Ise, Tet al. Recombinant immunotoxins targeting B-cell maturation antigen are cytotoxic to myeloma cell lines and myeloma cells from patients. Leukemia 2017; 32: 569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pastan, I, Beers, R, Bera, TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol 2004; 248: 503–18. [DOI] [PubMed] [Google Scholar]
- 10. Weldon, JE, Xiang, L, Zhang, Jet al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther 2013; 12: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei, J, Bera, TK, Liu, XFet al. Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc Natl Acad Sci USA 2018; 115: E3501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ali, SA, Shi, V, Maric, Iet al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016; 128: 1688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oden, F, Marino, SF, Brand, Jet al. Potent anti-tumor response by targeting B cell maturation antigen (BCMA) in a mouse model of multiple myeloma. Mol Oncol 2015; 9: 1348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nygren, PA, Eliasson, M, Abrahmsén, Let al. Analysis and use of the serum albumin binding domains of streptococcal protein G. J Mol Recognit 1988; 1: 69–74. [DOI] [PubMed] [Google Scholar]
- 15. Stork, R, Muller, D, Kontermann, ER. A novel tri-functional antibody fusion protein with improved pharmacokinetic properties generated by fusing a bispecific single-chain diabody with an albumin-binding domain from streptococcal protein G. Protein Eng Des Sel 2007; 20: 569–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


